Abstract
Purpose
Patients with oligometastatic colorectal cancer have demonstrated excellent clinical outcomes with surgical resection of hepatic and pulmonary metastases. Stereotactic ablative radiation therapy (SABR) has emerged as an alternative local therapy for nonsurgical candidates. Herein, we report the oncologic and patient-reported quality-of-life (PR-QoL) outcomes for a subset of patients with oligometastatic colorectal cancer who were treated in a prospective phase 2 multicenter clinical trial.
Methods and materials
Patients with a pathologically proven diagnosis of oligometastatic colorectal cancer were enrolled as part of a prospective study. SABR dose and fractionation schedules were dependent on the lesion location and size. Patient follow-up occurred 6 weeks after completion of SABR and at 3-month intervals for the following 3 years. Patients received the Functional Assessment of Cancer Therapy-General questionnaire at baseline and at each follow-up visit to assess PR-QoL. The total Functional Assessment of Cancer Therapy-General questionnaire scores were compared with those from baseline using the Wilcoxon signed rank test. Overall survival, local progression-free survival (PFS), and distant PFS were calculated using the Kaplan-Meier estimation to the date of the last follow-up visit/death or local/distant failure.
Results
A total of 31 patients with oligometastatic colorectal cancer with 1 (71.0%), 2 (16.1%), 3 (3.2%), 4 (3.2%), or 5 (6.5%) metastatic lesions were identified. After a median follow-up time of 50.1 months, the median OS from the time of completion of the SABR was 53.9 months (95% confidence interval, 23.2-84.6), and the 5-year OS, local PFS, and distant PFS were 45%, 83%, and 27%, respectively. Acute grade 2+ toxicity was 9.7% (pain, nausea, fatigue) and late grade 3+ toxicity (small bowel obstruction) was 3.2% with no significant change in PR-QoL in the year after SABR.
Conclusions
This subset analysis of a prospective phase 2 study demonstrates that SABR is a safe and effective treatment option for patients with unresectable oligometastic colorectal cancer. In addition, SABR of oligometastatic disease preserves PR-QoL.
Summary.
Here we report the initial outcomes of sub-set analysis of a multi-center phase II prospective trial assessing the role of stereotactic ablative radiotherapy (SABR) for oligometastatic colorectal cancer. Our results demonstrate SABR to be a safe and effective treatment modality demonstrating excellent overall survival and local control. Additionally, we report very low rates of acute and late grade 3 toxicity and show SABR to have no significant adverse effect on patient reported quality of life.
Introduction
Colorectal cancer (CRC) remains the third most common cancer in men and women, and the second most common cause of cancer-related mortality in the United States.1 Approximately 25% to 50% of patients with CRC will develop liver metastases within 3 years of the primary tumor resection.2 Historically, patients with metastases were considered incurable and not candidates for definitive local therapy. Recently, patients with limited volume metastatic disease (ie, oligometastatic disease) have demonstrated improved outcomes with aggressive local therapy.3 Surgical resection has since become a standard of care for oligometastatic hepatic or pulmonary lesions, and reports indicate excellent local control and overall survival (OS).4, 5 Surgical intervention is not feasible in 80% to 90% of patients, either owing to tumor location, medical comorbidities, or functional status.6, 7
Stereotactic ablative radiation therapy (SABR) provides a promising alternative treatment modality that is currently under investigation for the treatment of oligometastatic disease. SABR consists of high-dose, conformal radiation therapy delivered in 3 to 5 fractions.8 In select patients, SABR has demonstrated excellent local control, and limited radiation-related toxicity to surrounding normal tissue. Herein, we report on the patient outcomes, toxicity, and quality of life (QoL) of patients with oligometastatic CRC who were prospectively treated with SABR.
Methods and materials
Patient selection
Patients were recruited for a multicenter prospective phase 2 study to evaluate the safety and feasibility of SABR for patients with oligometastatic cancer of any histology. This report includes a subset analysis of patients with oligometastatic colorectal adenocarcinoma. Patients were included if they were age ≥18 years with a Zubrod performance status score of 0 to 1 and biopsy-proven colorectal adenocarcinoma. Oligometastatic disease was defined as ≤5 total sites of metastases in ≤3 organs on fludeoxyglucose-positron emission tomography/computed tomography (CT) scans within 8 weeks of enrollment.
Any patient with another primary tumor diagnosed or treated within the last 3 years (other than cutaneous skin cancer), liver-only metastatic disease amenable to resection, diffuse metastatic spread confined to 1 organ (ie, leptomeningeal spread in the central nervous system or peritoneal carcinomatosis), metastatic disease sites not treatable via SABR, pregnancy, or severe active medical comorbidities were excluded. There were no exclusion criteria on the basis of treatment of the primary tumor. At the time of registration, demographic information, management of the primary tumor, and prior treatments for oligometastases were documented. The protocol was approved by the university institutional review board, and all study participants signed informed consent. This trial was prospectively registered with ClinicalTrials.gov (NCT01345552).
Treatment plan
All sites of disease were treated with SABR per the American College of Radiology and American Society for Therapeutic Radiology and Oncology guidelines.9 SABR was performed on either a CyberKnife Robotic Radiosurgery (Accuray Inc, Sunnyvale, CA) or linear, accelerator-based platforms (Trilogy, TrueBeam; Varian Medical Systems, Palo Alto, CA). All treatments were completed within 3 weeks of each other. The gross target volume was defined by CT scan, fludeoxyglucose-positron emission tomography scan, and clinical information. The planning treatment volume was defined as the gross target volume with a margin appropriate for location and surrounding normal tissue proximity. Dose and fractionation schedule for each metastatic site was based on location, size, and dose constraints of organs at risk according to the recommendations of national protocols (Table 1).10, 11 A minimum of 48 hours was required between SABR treatments for each treatment site.
Table 1.
Dose and fractionation regimens for metastatic sites based on size and location
| Location | Dose (Gy) | Fractions |
|---|---|---|
| Central nervous system | ||
| <2 cm | 24 | 1 |
| 2-3 cm | 18 | 1 |
| 3-4 cm | 15 | 1 |
| >4 cm | 30 | 3 |
| Lung | ||
| Central lesions | 48 | 4 |
| Noncentral lesions | 60 | 3 |
| Adrenal | 50 | 5 |
| Bone | 18-25 | 1 |
| Liver | 60 | 3-4∗ |
| Lymph nodes | ||
| 18-24 | 1 | |
| 60 | 3 | |
| 50 | 5 |
Preference was given to 60 Gy in 4 fractions.
Patient assessment and follow-up
Patients were seen in follow-up by study physicians 6 weeks after completion of SABR, and then at 3-month intervals for 3 years, and 6-month intervals thereafter. Toxicity was evaluated at each follow-up visit using the Common Terminology Criteria for Adverse Events, version 4.0. Follow-up imaging with CT was performed every 3 months for the first 2 years and then every 6 months until 5 years after completion of the therapy. The measurement of response was determined by the Response Evaluation Criteria in Solid Tumors as either complete response, partial response, stable disease, or progressive disease.
The primary endpoints included 2-year OS, local progression-free survival (LPFS), and distant progression-free survival (DPFS). OS was defined as the time from enrollment to death from any cause. LPFS was defined as the time from enrollment to the first documentation of local failure at the treated oligometastatic site. DPFS was defined as the time from enrollment to documentation of new distant metastases.
The secondary endpoints included toxicity and QoL analysis. QoL was assessed at baseline, completion of SABR, and at each follow-up using the 27-item Function Assessment of Cancer Therapy-General (FACT-G) questionnaire. The FACT-G questionnaire included the following 4 categories: physical, social/family, emotional, and functional well-being. The total FACT-G scores were calculated at each time point.
Statistical analysis
The median follow-up was calculated with the reverse Kaplan-Meier method; 3 survival endpoints were analyzed: OS, LPFS, and DPFS. The Kaplan-Meier survival curve was plotted for each survival endpoint. Predictive factors for OS, LPFS, and DPFS were determined with the use of a univariate log-rank test or Cox regression analysis. Variables deemed significant (P < .05) were incorporated into multivariable survival analyses using forward conditional selection in a Cox proportional hazards regression model.
Statistical significance was set with a 2-sided P-value of < .05. For the QoL analysis, the total FACT-G score was compared using a Wilcoxon signed rank test between baseline and each time point. The statistical analysis was performed using IBM SPSS Statistics, version 22.
Results
Patient and treatment characteristics
From October 2011 through July 2017, 31 patients with colorectal adenocarcinoma were enrolled. The median age at the time of enrollment was 65.6 years (interquartile range [IQR], 55.9-75.1), and 54.8% of patients (n = 17) were male. The primary tumor was located either in the colon (64.5%) or rectum (35.5%) and treated with surgery (96.8%), chemotherapy (80.6%), or radiation (32.3%).
Before SABR, 67.7% of patients (n = 21) received treatment to separate metastatic sites. This included surgery (45.2%), chemotherapy (48.4%), and radiation therapy (6.5%) for disease recurrence/distant metastases at non-SABR treated sites. Patients had either 1 (71.0%), 2 (16.1%), 3 (3.2%), 4 (3.2%), or 5 (6.5%) metastatic sites treated with SABR. Of the total 49 metastases that were treated, the most common location was the lung (61.2%), followed by the liver (18.4%), lymph nodes (14.3%), bone (4.1%), and hilar mass (2.0%). SABR was delivered via either the Truebeam (49.0%) or Trilogy (20.4%) platform. The patient and treatment characteristics are summarized in Table 2.
Table 2.
Patient and treatment characteristics
| N = 31 Metastasis = 49 |
|
|---|---|
| Median age at time of diagnosis (IQR) | 59.7 (47.8-71.6) |
| Sex | |
| Male | 17 (54.8%) |
| Female | 14 (45.2%) |
| Location | |
| Colon | 20 (64.5%) |
| Rectum | 11 (35.5%) |
| Initial surgery | |
| Yes | 30 (96.8%) |
| No | 1 (3.2%) |
| Initial chemotherapy | |
| Yes | 25 (80.6%) |
| No | 6 (19.4%) |
| Initial radiation | |
| Yes | 10 (32.3%) |
| No | 21 (67.7%) |
| Median age at time of enrollment (IQR) | 65.6 (55.9-75.1) |
| Karnofsky performance status | |
| 100 | 17 (54.8%) |
| 90 | 5 (16.1%) |
| 80 | 4 (12.9%) |
| Unknown | 5 (16.1%) |
| Prior surgery for DM/recurrence | |
| Yes | 14 (45.2%) |
| No | 17 (54.8%) |
| Prior chemotherapy for DM/recurrence | |
| Yes | 15 (48.4%) |
| No | 16 (51.6%) |
| Prior radiation for DM/recurrence | |
| Yes | 2 (6.5%) |
| No | 29 (93.5%) |
| Number of lesions treated with SABR/SRS | |
| One | 22 (71.0%) |
| Two | 5 (16.1%) |
| Three | 1 (3.2%) |
| Four | 1 (3.2%) |
| Five | 2 (6.5%) |
| Lesion location | |
| Lung | 30 (61.2%) |
| Liver | 9 (18.4%) |
| Lymph node | 7 (14.3%) |
| Bone | 2 (4.1%) |
| Hilar mass | 1 (2.0%) |
| Median sum of lesions longest diameter cm (IQR) | 2.5 (1.5-4.9) |
| Treatment characteristics per lesion | |
| Median gross tumor volume cc (IQR) | 2.24 (0.98-5.85) |
| Median planning treatment volume cc (IQR) | 15.2 (8.0-24.9) |
| Median Isodose (IQR) | 89.5% (85%-91%) |
| Treatment platform | |
| Truebeam | 24 (49.0%) |
| Trilogy | 10 (20.4%) |
| Unknown | 15 (30.6%) |
Abbreviations: DM = distant metastases; IQR = interquartile range; SABR = stereotactic ablative body radiation therapy; SRS = stereotactic radiosurgery.
Survival
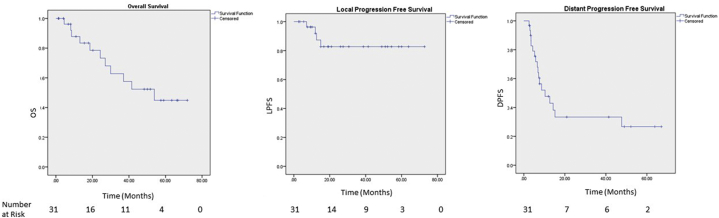
With a median follow-up time of 50.1 months (95% confidence interval [CI], 13.0-87.1 months), the median OS time was 53.9 months (95% CI, 23.2-84.6 months) with 1- and 5-year OS of 88% and 45%, respectively (Fig 1). The univariate analysis identified pre-SABR radiation for distant metastasis (hazard ratio [HR]: 11.29; 95% CI, 1.02-124.71; P = .05), 2 versus 1 metastasis (HR: 6.23; 95% CI, 1.42-27.23; P = .02), and 4 versus 1 metastasis (HR: 39.12; 95% CI, 2.22-688.25; P = .01) associated with worse OS. Superior OS was associated with pre-SABR surgery for distant metastases (HR: 0.17; 95% CI, 0.03-0.84; P = .03). On multivariate analysis, only 2 versus 1 (HR: 6.23; 95% CI, 1.42-27.23; P = .02) and 4 versus 1 (HR: 39.12; 95% CI, 2.22-688.25; P = .01) were significantly associated with worse OS (Table 3).
Figure 1.
Kaplan-Meier curves for overall survival and local and distant progression-free survival after stereotactic ablative radiation therapy for oligometastatic colorectal cancer.
Table 3.
Multivariate analysis of overall survival, LPFS, and DPFS
| Factor | Hazard ratio (95% confidence interval) | P-value |
|---|---|---|
| Overall survival | ||
| 2 vs 1 metastases | 6.23 (1.42-27.23) | .02 |
| 4 vs 1 metastases | 39.12 (2.22-688.25) | .01 |
| LPFS | ||
| No significant factors | ||
| DPFS | ||
| 4 vs 1 metastases | 16.04 (1.34-192.58) | .03 |
Abbreviations: DFPS = distant progression-free survival; LPFS = local progression-free survival.
Local and distant progression-free survival
The 1- and 5- year LPFS were 92% and 83%, respectively (median LPFS was not reached; Fig 1). The response to treatment per the Response Evaluation Criteria in Solid Tumors included complete response (29.0%), partial response (22.6%), stable disease (22.6%), and progressive disease (16.1%; Table 4). The univariate analysis identified 5 metastatic lesions (HR: 11.95; 95% CI, 1.66-86.35; P = .01) as associated with inferior LPFS. No multivariate model could be generated (Table 3).
Table 4.
Post-SABR treatment and RECIST response of treated metastases
| Treatments | N = 148 |
|---|---|
| Post-SABR surgery | |
| Yes | 6 (19.4%) |
| No | 25 (80.6%) |
| Post-SABR chemotherapy | |
| Yes | 13 (41.9%) |
| No | 18 (58.1%) |
| Post-SABR radiation | |
| Yes | 22 (71.0%) |
| No | 9 (29.0%) |
| Post-SABR immunotherapy | |
| Yes | 2 (6.5%) |
| No | 29 (93.5%) |
| RECIST response | |
| Complete response | 9 (29.0%) |
| Partial response | 7 (22.6%) |
| Stable disease | 7 (22.6%) |
| Progressive disease | 5 (16.1%) |
| Indeterminate | 3 (9.7%) |
Abbreviations: RECIST = Response Evaluation Criteria in Solid Tumors; SABR = stereotactic ablative body radiation therapy.
The median DPFS was 10.4 months (95% CI, 3.2-17.6) with 48% and 27% DPFS at 1- and 5-years, respectively. The univariate analysis demonstrated a trend toward significance with pre-SABR chemotherapy for distant metastases (HR: 0.39; 95% CI, 0.15-1.04; P = .06) and 4 metastases versus 1 metastasis (HR: 8.91; 95% CI, 0.90-87.90; P = .06). The multivariate analysis identified 4 metastases (HR: 16.04; 95% CI, 1.34-192.58; P = .029) as associated with inferior DPFS (Table 3).
Quality of life and toxicity
For the entire cohort, acute grade 2 toxicity was 9.7% (n = 3) with no grade 3+ toxicity observed. Three patients experienced acute grade 2 toxicity, which included pain, nausea, and fatigue. The median FACT-G score was 72 (IQR, 35-129) at baseline and 84.0 (IQR, 71.5-98.5) at the time of completion of the treatment. The Wilcoxon signed rank test showed that there was no difference in FACT-G total score at the time of completion, 6 weeks, 3 months, 6 months, 9 months, or 12 months after treatment compared with the baseline score (Table E5).
Discussion
Surgical series have demonstrated durable local control and improved OS after resection of liver and pulmonary oligometastases. Despite excellent clinical outcomes, 80% to 90% of hepatic and pulmonary metastases are unresectable.7 Our findings suggest that SABR may be a safe and effective noninvasive alternative treatment for patients.
Surgical resection of hepatic metastases from CRC have yielded an actual 10-year cure rate in 17% of patients.5 In addition, numerous reports have demonstrated that hepatic resection is associated with 5-year OS ranging from 27% to 60%.12, 13, 14, 15, 16 After this success, pulmonary metastectomy gained momentum as Suzuki et al. reported a 5-year OS of 45.5% in 94 patients who underwent complete resection of pulmonary metastases from CRC.17 Assessing the role of pulmonary metastatectomy in conjunction with hepatic metastectomy, Matsui et al. reported on 186 patients who underwent hepatic or pulmonary resections for colorectal metastases.4 Within this cohort, 25 patients received both hepatic and pulmonary resection and demonstrated a 5-year OS of 38%. Patients who received pulmonary resection only (n = 61) demonstrated a 5-year OS of 63%.4 Finally, Sourrouille et al. reviewed 69 patients who were treated with either pulmonary metastectomy only (n = 38) or pulmonary and hepatic metastatectomy (n = 31), and reported a 5-year OS of 36%.18
Multiple reports have evaluated whether aggressive local therapy via SABR could yield parallel findings in nonsurgical oligometastatic candidates. Comito et al. prospectively enrolled 83 patients with 1 to 3 metastases from CRC confined to 1 organ (liver or lung). Patients received SABR in 60 Gy/3 fractions or 48 Gy/4 fractions to lung metastases, or 75 Gy/3 fractions to liver metastases. With a median follow-up time of 24 months, the median OS was 32 months and 3-year OS of 43%. The 3-year local control was 75% with no differences observed between lung and liver metastases.19
Agolli et al. retrospectively reviewed 44 patients with 1 to 4 pulmonary metastases from CRC treated with SABR. Patients received either 30 Gy/1 fraction, 23 Gy/1 fraction, or 45 Gy/3 fractions. With a median follow-up time of 36 months, the median OS was 38 months, and 3-year OS was 50.8%. The 3-year LPFS was 54.2%.20 Our study compares favorably with the 5-year OS and LPFS of 45% and 83%, respectively.
Within the earlier reports, no patients experienced grade 3+ toxicity; however, 70%19 and 11.4%20 developed acute grade 2 toxicity, and 13.6%20 developed late grade 2 toxicity. Our results demonstrated comparable acute toxicity with 9.7% and 0% of patients who developed grade 2 and grade 3+, respectively. These data indicate that SABR is very well-tolerated with minimal acute or late morbidity.
The present study demonstrates that SABR is a safe and effective modality to deliver aggressive local control to oligometastatic CRC. Furthermore, we demonstrated comparable OS and local control in patients who undergo surgical resection. This study was limited by a small sample size and nonrandomized nature. In addition, patients were enrolled either at the time of presentation of the oligometastatic disease or development of oligometastatic disease after numerous prior therapies. Because of these shortcomings, future multi-institution randomized phase 3 trials should be developed to further evaluate these results.
Conclusions
This subset analysis of a multicenter prospective phase 2 study demonstrates the feasibility and efficacy of SABR for oligometastatic CRC. This treatment regimen was well tolerated with limited grade 3+ acute and late toxicity, and no significant detriment on patient-reported QoL. Our results demonstrate comparable long-term survival and local control to surgical series. Future randomized controlled trials are needed to clarify the role of SABR for oligometastatic CRC.
Footnotes
Sources of support: None.
Conflicts of interest: None.
Supplementary material for this article can be found at https://doi.org/10.1016/j.adro.2018.09.001.
Supplementary data
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Khatri V.P., Petrelli N.J., Belghiti J. Extending the frontiers of surgical therapy for hepatic colorectal metastases: Is there a limit? J Clin Oncol. 2005;23:8490–8499. doi: 10.1200/JCO.2004.00.6155. [DOI] [PubMed] [Google Scholar]
- 3.Hellman S., Weichselbaum R.R. Oligometastases. J Clin Oncol. 1995;13:8–10. doi: 10.1200/JCO.1995.13.1.8. [DOI] [PubMed] [Google Scholar]
- 4.Matsui T., Kitamura T., Ozawa H., Matsuguma H., Kotake K. Analysis of treatment that includes both hepatic and pulmonary resections for colorectal metastases. Surg Today. 2014;44:702–711. doi: 10.1007/s00595-013-0769-0. [DOI] [PubMed] [Google Scholar]
- 5.Tomlinson J.S., Jarnagin W.R., DeMatteo R.P. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575–4580. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 6.Misiakos E.P., Karidis N.P., Kouraklis G. Current treatment for colorectal liver metastases. World J Gastroenterol. 2011;17:4067–4075. doi: 10.3748/wjg.v17.i36.4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim M., Son S.H., Won Y.K., Kay C.S. Stereotactic ablative radiotherapy for oligometastatic disease in liver. BioMed Res Intl. 2014;2014:340478. doi: 10.1155/2014/340478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dilling T.J., Hoffe S.E. Stereotactic body radiation therapy: Transcending the conventional to improve outcomes. Cancer Control. 2008;15:104–111. doi: 10.1177/107327480801500202. [DOI] [PubMed] [Google Scholar]
- 9.Potters L., Steinberg M., Rose C. American Society for Therapeutic Radiology and Oncology and American College of Radiology practice guideline for the performance of stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2004;60:1026–1032. doi: 10.1016/j.ijrobp.2004.07.701. [DOI] [PubMed] [Google Scholar]
- 10.Shaw E., Scott C., Souhami L. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: Final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47:291–298. doi: 10.1016/s0360-3016(99)00507-6. [DOI] [PubMed] [Google Scholar]
- 11.Timmerman R., Paulus R., Galvin J. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith J.J., D'Angelica M.I. Surgical management of hepatic metastases of colorectal cancer. Hematol Oncol Clin North Am. 2015;29:61–84. doi: 10.1016/j.hoc.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Arru M., Aldrighetti L., Castoldi R. Analysis of prognostic factors influencing long-term survival after hepatic resection for metastatic colorectal cancer. World J Surg. 2008;32:93–103. doi: 10.1007/s00268-007-9285-y. [DOI] [PubMed] [Google Scholar]
- 14.Andres A., Majno P.E., Morel P. Improved long-term outcome of surgery for advanced colorectal liver metastases: Reasons and implications for management on the basis of a severity score. Ann Surg Oncol. 2008;15:134–143. doi: 10.1245/s10434-007-9607-1. [DOI] [PubMed] [Google Scholar]
- 15.House M.G., Ito H., Gonen M. Survival after hepatic resection for metastatic colorectal cancer: Trends in outcomes for 1,600 patients during two decades at a single institution. J Am Coll Surg. 2010;210 doi: 10.1016/j.jamcollsurg.2009.12.040. 744-752, 752-755. [DOI] [PubMed] [Google Scholar]
- 16.Rees M., Tekkis P.P., Welsh F.K., O'Rourke T., John T.G. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: A multifactorial model of 929 patients. Ann Surg. 2008;247:125–135. doi: 10.1097/SLA.0b013e31815aa2c2. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki H., Yoshino I. Approach for oligometastasis in non-small cell lung cancer. Gen Thorac Cardiovasc Surg. 2016;64:192–196. doi: 10.1007/s11748-016-0630-7. [DOI] [PubMed] [Google Scholar]
- 18.Sourrouille I., Mordant P., Maggiori L. Long-term survival after hepatic and pulmonary resection of colorectal cancer metastases. J Surg Oncol. 2013;108:220–224. doi: 10.1002/jso.23385. [DOI] [PubMed] [Google Scholar]
- 19.Comito T., Cozzi L., Clerici E. Stereotactic ablative radiotherapy (SABR) in inoperable oligometastatic disease from colorectal cancer: A safe and effective approach. BMC Cancer. 2014;14:619. doi: 10.1186/1471-2407-14-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agolli L., Valeriani M., Nicosia L. Stereotactic ablative body radiotherapy (SABR) in pulmonary oligometastatic/oligorecurrent non-small cell lung cancer patients: A new therapeutic approach. Anticancer Res. 2015;35:6239–6245. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.