Abstract
Purpose
Volumetric modulated arc therapy (VMAT) has been shown by multiple planning studies to hold dosimetric advantages over intensity modulated radiation therapy (IMRT) in the management of brain tumors, including glioblastoma (GBM). Although promising, the clinical impact of these findings has not been fully elucidated.
Methods and Materials
We retrospectively reviewed consecutive patients with a pathologic-confirmed diagnosis of GBM who were treated between 2014 and 2015, a period that encompassed the transition from IMRT to VMAT at a single institution. After surgery, radiation with VMAT consisted of 2 to 3 coplanar arcs with or without an additional noncoplanar arc or IMRT with 5 to 6 gantry angles with concurrent and adjuvant temozolomide. Actuarial analyses were performed using the Kaplan Meier method.
Results
A total of 88 patients treated with IMRT (n = 45) and VMAT (n = 43) were identified. Patients were similar in terms of age, sex, performance status, extent of resection, and the high dose target volume. At a median follow-up time of 27 months (range, .7-32.3 months), the overall survival, freedom from progression, and freedom from new or worsening toxicity rates were not different between the 2 treatment groups (log-rank: P = .33; .87; and .23, respectively). There was no difference in incidences of alopecia, erythema, nausea, worsening or new onset fatigue, or headache during radiation, or temozolomide dose reduction for thrombocytopenia or neutropenia (all P > .05). Patterns of failure were different with more out of field failures in the IMRT group (P = .02). The mean time of treatment (TOT) was significantly reduced by 29% (P < .01) with VMAT (mean TOT: 10.3 minutes) compared with IMRT (mean TOT: 14.6 minutes).
Conclusions
For GBM, treatment with VMAT results in similar oncologic and toxicity outcomes compared with IMRT and may improve resource utilization by reducing TOT. VMAT should be considered a potential radiation modality for patients with GBM.
Summary.
Volumetric arc radiation therapy (VMAT) and intensity modulated therapy (IMRT) are highly conformal techniques used to treat glioblastoma; however, outcomes from these techniques have not been compared. In this retrospective review of 88 consecutive patients treated with either VMAT or IMRT, we observed no differences in overall survival, progression free survival, freedom from progression, or toxicity between the two techniques; however, there were differences in patterns of relapse, and treatment time was significantly shorter for VMAT. VMAT is a viable and more rapid treatment alternative to IMRT; however, differences in patterns of failure merit further investigation.
Introduction
Trimodality therapy that consists of maximal resection, concurrent chemoradiation, and adjuvant chemotherapy is the standard approach for the treatment of glioblastoma (GBM), which is the most common primary brain tumor in adults.1 Advances in patient immobilization, linear accelerator technologies, and the advent of magnetic resonance imaging (MRI) have led to improvements in radiation conformality with increased sparing of normal brain tissue. Volumetric modulated arc therapy (VMAT) is a highly conformal technique that has been shown to improve the sparing of normal tissue without compromising target coverage in comparison with intensity modulated radiation therapy (IMRT).2, 3
Despite improved dosimetry and an increased adoption of VMAT over IMRT, a comparison of clinical outcomes between IMRT and VMAT has not been reported. Our institution recently transitioned from IMRT to VMAT for the treatment of GBM, and we evaluated the outcomes of patients who were treated with these 2 techniques.
Methods and Materials
In this study, which was approved by an institutional review board, the medical records of 88 consecutive patients who were treated in 2014 and 2015 for pathologically confirmed World Health Organization grade IV GBM were reviewed. This period included the transition from IMRT to VMAT at our institution. All patients underwent upfront surgical resection followed by postoperative MRI of the brain with gadolinium contrast, which was used to assess the degree of resection and define treatment volumes. An experienced neuropathologist evaluated the surgical specimens.
The radiation simulation and treatment methods were previously described.2 Computed tomographic simulation with a customizable thermoplastic head mask was performed in the supine position. Images were coregistered with MRIs to facilitate normal tissue and target volume delineation. The gross tumor volume (GTV) consisted of the resection cavity and enhancing areas on the T1-weighted sequence. The clinical target volume (CTV) consisted of a 2-cm isometric expansion about the GTV that was subsequently modified to include any abnormalities on fluid-attenuated inversion recovery images, with respect for the normal anatomic barriers. The planning target volume (PTV) was prescribed a dose of 50 Gy in 30 daily fractions, and was generated by expanding the CTV by 3 to 5 mm. The boost PTV was prescribed a dose of 60 Gy in daily 30 fractions and was generated by expanding the GTV by 3 to 5 mm. Normal tissue avoidance structures included the optic chiasm, brain stem, eye, and cochlea. All treatment plans were reviewed for target coverage and dose volume constraints by radiation oncologists specializing in the treatment of central nervous system tumors. Treatment planning was performed in Pinnacle (Philips Health Care, Fitchburg, WI).
Nominal photon energy was 6 MV. IMRT consisted of 5 or 6 noncoplanar beams with 4 couch angles delivered using a step-and-shoot technique. VMAT consisted of 2 full or partial coplanar arcs with or without an additional noncoplanar arc. Radiation time of treatment (TOT) was defined as the start of imaging to the final beam off-time. During this 2-year period, optimization constraints and treatment planning algorithms did not change.
During the course of radiation, patients were assessed for toxicity on a weekly basis with a symptoms-focused history and physical examination. For toxicity reporting, only patients with new or worsening symptoms during radiation were recorded as having a toxicity event.
Systemic therapy was administered with oversight by neurooncologists. Temozolomide (TMZ) consisted of 75 mg/m2 given by mouth daily with doses adjusted for thrombocytopenia or neutropenia. A complete blood count was obtained weekly during radiation. Maintenance TMZ consisted of 150 mg/m2 for 5 consecutive days followed by 23 days without treatment for up to 6 cycles. TMZ was either dose adjusted or discontinued in the event of severe thrombocytopenia or neutropenia.
A brain MRI scan with contrast was obtained approximately 1 month after completion of radiation. Patients were monitored for recurrence with serial brain MRI scans with gadolinium contrast every 3 months for 3 years and then every 6 months thereafter. Progression within the 60-Gy isodose line was defined as local failure, progression outside the 60-Gy but within the 50-Gy isodose line as regional failure, and progression outside the 50-Gy isodose line as distant failure. Regional failures were confirmed by delineating progressive disease on T1-weighted sequences with contrast and fluid-attenuated inversion recovery MRI sequences, and subsequently those image sets were fused with the original treatment plans. Multifocal progression was defined as a combination of either local or regional failure and distant failure. In the event of recurrence or progression, salvage therapy was determined by the multidisciplinary consensus among the treating physicians.
Statistics
Differences between the 2 treatment groups were compared using the χ2 or Fisher’s exact test for categorical variables and the Student t test for continuous variables. The averages of the 5th, 15th, and 25th fraction treatment times for each patient were used to compare TOT between IMRT and VMAT with the t test for unequal variances. For overall survival (OS) and freedom from progression (FFP), t0 was defined as the last day of radiation. OS was defined as the length of time between t0 and death with living patients censored at the last follow-up. Follow-up was defined as the time between t0 and the last follow-up for patients alive at the time of the analysis. FFP was defined as the length of time between t0 and progression confirmed on MRI brain or biopsy. For FFP, patients without progression were censored on the last day of MRI imaging.
Progression free survival (PFS) was defined as the length of time between t0 and the earlier of either death or progression. Patients alive and without evidence of progression on MRI were censored at the time of the last follow up. For toxicity endpoints, t0 was defined as the first day of radiation. The Kaplan-Meier method was used for the actuarial analysis, and the log-rank test was used to test for differences between survival curves.
Results
Patients and treatment
A total of 88 patients were identified, with 45 patients treated with IMRT in 2014 and 43 patients treated with VMAT in 2015. Patient and treatment characteristics are summarized in Table 1. The patient characteristics were well balanced between the 2 treatment groups. Two of 6 patients treated with IMRT tested for O6-methylguanine DNA methyltransferase (MGMT) methylation status were found to be hypermethylated. Five of 10 VMAT patients had hypermethylated MGMT status. Alpha thalassemia/mental retardation syndrome X-linked mutation was identified in 1 patient treated with IMRT. The 1p19q status was tested in 2 patients treated with VMAT and was codeleted in 1 patient. There were no positively identified IDH1 mutations in the study population.
Table 1.
Patient, disease, and treatment characteristics
| All (n = 88) | IMRT (n = 45) | VMAT (n = 43) | P-value | |
|---|---|---|---|---|
| Mean age, years (range) | 55 (19-79) | 53 (21-79) | 56 (19-73) | .34 |
| Male sex | 63% | 60% | 65% | .62 |
| KPS score >80 | 86% | 84% | 88% | .20 |
| Seizures at presentation | 28% | 31% | 26% | .86 |
| Other neurologic symptoms at presentation | 93% | 98% | 88% | .83 |
| Gross total resection | 58% | 60% | 53% | .85 |
| Mean gross tumor volume (cm3) | 52.1 | 50.7 | 53.5 | .87 |
| Steroid medications during radiation | 50% | 49% | 51% | .83 |
| Steroid medication increased during radiation | 28% | 33% | 23% | .20 |
| Temozolomide dose adjusted | 12% | 14% | 10% | .16 |
| Adjuvant temozolomide | 93% | 88% | 97% | .72 |
Abbreviations: IMRT = intensity modulated radiation therapy; KPS = Karnofsky performance status; VMAT = volumetric modulated arc therapy.
Mean TOT was significantly different between patients treated with IMRT (mean: 14.3; range, 7.7-21.0 minutes) and VMAT (mean: 10.3; range, 6.3-15.3 minutes; t test for unequal variances: P < .001). Six VMAT patients were treated with an additional noncoplanar arc. Two patients were treated with tumor treating fields (TTF): one patient received TTF after completion of chemoradiation therapy with VMAT and expired after 23 months, and the other patient received TTF as salvage therapy (along with repeat radiation) after VMAT and expired after 19 months.
Survival
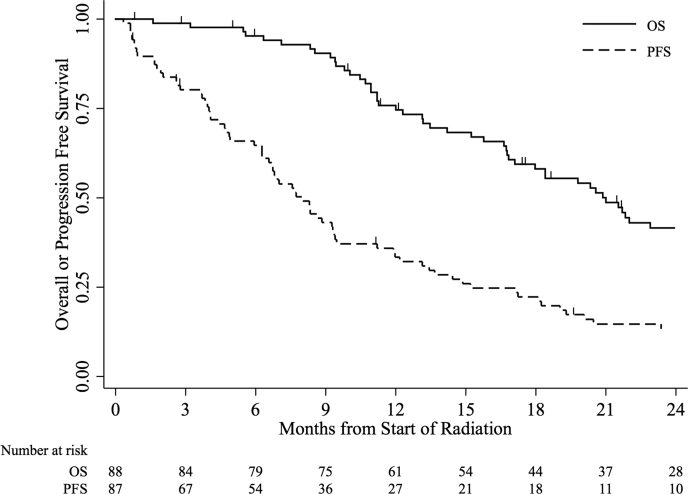
The median follow-up time was 27.4 months (range, 0.7-32.3 months). The median OS time was 21.0 months (95% confidence interval [CI], 16.8-26.4 months), the median PFS time was 8.0 months (95% CI, 6.3-9.4 months), and the median FFP time was 8.6 months (95% CI, 7.0-12.0 months). At the time of the analysis, 9 patients (10%) were alive and without evidence of progression, and 27 patients (31%) were alive with progressive disease. The OS and PFS curves for all patients are shown in Figure 1.
Figure 1.
Overall or progression-free survival for all patients.
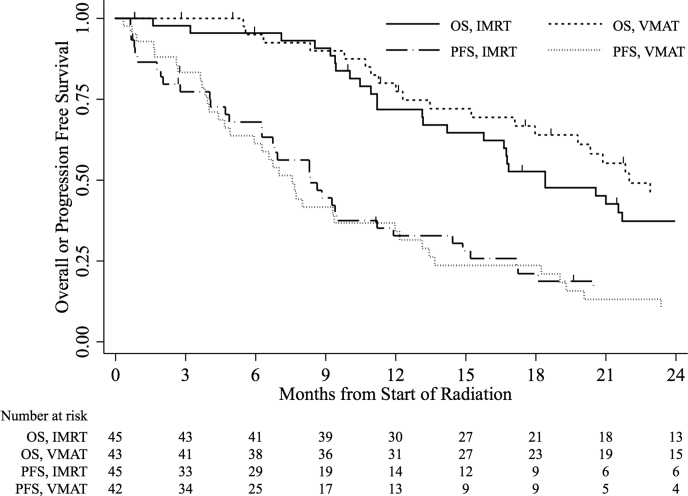
The median OS time was 18.4 months (95% CI, 14.2-27.1 months) and 22.0 months (95% CI, 17.1 months-not available) for patients treated with IMRT and VMAT, respectively. OS was not different between IMRT and VMAT (log-rank: P = .33). The median FFP time was 8.8 months (95% CI, 6.7-14.4 months) and 8.0 months (95% CI, 6.6-13.1 months) for IMRT and VMAT, respectively. Neither IMRT nor VMAT conferred a superior FFP (log-rank: P = .87). OS and PFS curves stratified by treatment are shown in Figure 2.
Figure 2.
Overall or progression free survival for all patients, stratified by treatment with intensity modulated radiation or volumetric modulated arc therapy.
Patterns of failure and salvage therapies
A total of 67 patients (77%) experienced progressive disease, and 58 of these (87%) were local failures at the site of the resection cavity. The patterns of failure differed significantly between patients treated with IMRT and VMAT (Fisher's exact: P = .017; Table 2). There were 29 local failures in each treatment group. Among patients treated with VMAT using only coplanar arcs, 3 regional failures were observed, 2 of which occurred in the plane orthogonal to the coplanar treatment arcs. One IMRT patient experienced regional progression.
Table 2.
Patterns of failure
| IMRT n (%) | VMAT n (%) | |
|---|---|---|
| Local failure | 29 (83) | 26 (81) |
| Regional failure | 1 (3) | 3 (9) |
| Distant failure | 5 (14) | 0 (0) |
| Multifocal (local and distant failure) | 0 (0) | 3 (9) |
Abbreviations: IMRT = intensity modulated radiation therapy; VMAT = volumetric modulated arc therapy.
Among patients treated with VMAT with an additional noncoplanar arc, 4 patients had local progression and 1 patient had multifocal progression with local failure. No patients treated with a noncoplanar arc were observed to have regional progression. Three patients in the VMAT group experienced distant progression, albeit as a component of multifocal progression, compared with 5 patients in the IMRT group.
In terms of salvage therapy, 36 patients in the IMRT arm and 30 patients in the VMAT arm underwent salvage therapy. The approaches to salvage therapy, which were based on a multidisciplinary consensus, were similar between the 2 groups. In the IMRT group, 23 patients received systemic therapy, 2 patients underwent salvage surgery, 1 patient received salvage radiation, and 10 patients received a combination of therapies. For patients treated with VMAT, 20 patients received salvage systemic therapy alone, 1 patient underwent salvage surgery, 1 patient received retreatment with radiation, and 8 patients received a combination of therapies. Systemic therapies varied and consisted of combinations of bevacizumab, lomustine, TMZ immunotherapy, or other investigational drugs.
Toxicity
The median time from the start of radiation to report of any new or worsening toxicity was 2 weeks for all patients, and the actuarial FFT did not differ between IMRT and VMAT patients (log-rank: P = .23). Skin toxicities were the most common between both groups; 81% of patients experienced alopecia and 58% erythema. New or worsening fatigue was reported by 57% of patients, and new or worsening headaches by 20% of patients. Three patients in each group were diagnosed with an extracranial venous thromboembolism during treatment. There were no differences in the incidence of toxicity between the 2 groups of patients as summarized in Table 3; however, more patients treated with IMRT required an increase in steroid dose for symptom management. In addition, patients treated with IMRT were slightly more likely to require a dose adjustment of TMZ because of thrombocytopenia; however, dose-adjusted TMZ did not appear to negatively affect OS or FFP (log-rank: P = .44 and P = .22, respectively).
Table 3.
Acute toxicities
| Toxicity (any grade) | IMRT n (%) | VMAT n (%) | X2P-value |
|---|---|---|---|
| Fatigue | 24 (53) | 26 (60) | .50 |
| Alopecia | 35 (78) | 36 (84) | .53 |
| Erythema | 25 (56) | 26 (58) | .55 |
| Headache | 8 (18) | 10 (23) | .52 |
| Nausea | 18 (40) | 13 (30) | .44 |
| Other neurologic symptoms | 8 (18) | 14 (33) | .11 |
Abbreviations: IMRT = intensity modulated radiation therapy; VMAT = volumetric modulated arc therapy.
Discussion
Radiation is a cornerstone in the definitive management of GBM, and there has been continued interest in optimizing radiation treatment planning and delivery to reduce the radiation dose to the uninvolved brain. Many institutions have employed VMAT because of its decreased treatment delivery time as well as improved sparing of normal tissues compared with IMRT or 3-dimensional conformal radiation.2, 3, 4 Multiple planning studies have reported similar target coverage between IMRT and VMAT, and although anticipated to be similar, outcomes data comparing these 2 radiation techniques have not been published.
VMAT has many potential advantages over IMRT. Although it maintains its capability to achieve treatment planning goals for minimum target coverage (ie, PTV V95% ≥95%), VMAT has been shown to increase dose conformality and reduce dose to the brain stem, optic chiasm, hippocampi, or cochleae.2, 3, 4 As a result, VMAT is thought to be capable of providing comparative local control while reducing radiation toxicity. Although it is widely assumed that outcomes should be similar between patients treated with IMRT and VMAT, caution should be used when extrapolating dosimetric equivalence to clinical outcomes.
In an analogous study, Navarria et al. evaluated 341 patients (74% with GBM) who were treated with either VMAT or 3-dimensional conformal radiation therapy. There was no significant difference in PTV coverage between these 2 treatment techniques. However, at a median follow-up time of 1.3 years, VMAT was associated with a statistically significant PFS (15 months) and OS (19 months) benefit compared with 3-dimensional conformal radiation therapy (12 and 15 months, respectively). Patients treated with VMAT were also found to have a 10% absolute reduction in all recurrences.5
Although VMAT has been shown to be adept at meeting minimum target coverage goals, planning studies have also demonstrated the potential for lower mean and minimum doses to the PTV or the volume of dose receiving 95% of the prescribed dose, even among plans that are considered acceptable for target coverage.2, 6 Outcomes data are needed to establish the significance or perhaps nonsignificance of these differences. In our study, the incidence of and time to progression between IMRT and VMAT were nearly identical. However, the overall pattern of failure was different between the 2 groups. We observed more regional failures (defined as failures within the 50 Gy isodose volume but outside of the 60 Gy isodose volume) among patients treated with VMAT even though the CTV volume receiving at least 50 Gy was between 95.2% and 99.9%. As a whole, VMAT plans had a higher inhomogeneity index and significantly reduced the minimum, mean, maximum, and percentage of PTV receiving at least 50 Gy compared with IMRT (all P ≤ .05).2
Coplanar beam arrangements may have been a contributing factor as well because 2 of 3 cases of regional failure occurred within the narrowed penumbra orthogonal to the treatment arcs. Regardless of these differences, OS and PFS are similar between patients treated with IMRT and VMAT. Although not significant, the difference in the incidence of regional failure between the 2 techniques suggests that there may be potential room for improvement in VMAT treatment planning and delivery.
The dosimetry of VMAT may differ significantly from IMRT, and there was concern that our use of tighter volumetric expansions could lead to an increase in regional or marginal failures. These volume standards, which were maintained in our transition from IMRT to VMAT, are based on a pattern of failures analysis that demonstrated no local control benefit when including peritumoral edema residing >2 cm away from the regions of enhancement as well as a reduction in dose to the uninvolved part of the brain.7 Results from other series demonstrate a 72% to 93% recurrence rate within 2 cm of the primary tumor bed and support this approach.8, 9, 10, 11, 12
With respect to the relative increase in regional failures, treatment planning benchmarks, noncoplanar arcs, or modification to margins orthogonal to coplanar arcs may also merit further investigation, particularly when using smaller margin definitions than the Radiation Therapy Oncology Group.13 Ultimately, the necessary adjustments for treatment planning optimization may lead to longer treatment times and worse toxicity when using VMAT.
Although we would anticipate a decrease in certain side effects with increased sparing of brain stem, normal brain, and cochlea achieved with VMAT planning, there were no observed toxicity differences between the 2 groups. The most commonly reported toxicities were dermatitis followed by new or worsening fatigue. In a trial that compared adjuvant radiation with or without TMZ, Stupp et al. reported grade ≥2 fatigue as the most commonly observed adverse event present in up to 33% of patients.
Our toxicity rate was significantly higher, although we included any new or worsening toxicity (including grade 1 toxicity) as an adverse event. Thrombocytopenia was also a common event and occurred in 14% of patients, which resulted in an adjustment to the TMZ dose. This finding is also consistent with the 12% grade 3 to 4 thrombocytopenia reported by Stupp et al.1 Efforts to continue reducing radiation-related toxicity are merited because a significant proportion of patients in our study are still living at the time of publication, and for this study cohort, the median OS times for both patients treated with IMRT and VMAT exceed those reported in the literature.14
Others have reported the need for additional treatment planning time for VMAT over IMRT.13 At our institution, the amount of active planning for either treatment technique was similar. Daily TOT with VMAT is shorter than with IMRT, which improves patient comfort and, in certain clinical settings, machine throughput. VMAT may also be less costly to the health care system. Based on the 2017 Medicare fee schedule for our geographic region, the allowable cost for a 2-arc VMAT treatment delivery over 30 fractions is approximately $9100 compared with approximately $27,200 for an IMRT treatment delivery using 6 noncoplanar gantry angles. Collectively, these differences are operationally and financially significant, which has resulted in a greater interest in exploring VMAT as a potential treatment option for tumors in the brain.15, 16, 17, 18
Our study is retrospective in nature, and draws upon a data set of patients treated at a single, tertiary cancer hospital. We do believe that patient selection between the 2 treatment techniques is minimized because patients were treated sequentially, and there was a set date when the transition between the 2 techniques occurred.
Our use of distinct volume definitions and dose-fractionation and the delayed integration of TTF into our practice (because patients were all treated before the publication of the phase 3 study) suggests a benefit of adjuvant TTF therapy.14 Together, these factors preclude broadly generalizing the results of this study to patients treated in other settings; although the issue of treatment volume definitions in relatively untested treatment techniques is an important consideration for any practice considering the adoption of VMAT in the management of GBM.
Our study cohort was selected from patients who were treated over a 2-year period during the transition from IMRT to VMAT, and VMAT patients who were treated later could have potentially benefited from advances in salvage therapies. Nevertheless, the general salvage approaches were similar between the 2 groups. Finally, biomarker status was only available for a limited number of patients in each cohort, which limited a meaningful interpretation of impact on outcomes between the groups.
Conclusions
VMAT holds many potential logistical advantages that can improve patient comfort while reducing costs and resource utilization. With many reports of an identical or even superior dosimetric profile with VMAT along with shorter treatment times, the practical transition to VMAT-based treatment delivery should only be done after careful consideration of the potential consequences of using a new technology in the context of preexisting standards. Volume definitions, dose distributions with different beam arrangements, and treatment planning goals should be considered with care.
Footnotes
Meeting information: Portions of this manuscript were presented at the 100th Annual Meeting of the American Radium Society in Orlando, Florida on May 5, 2018.
Sources of Support: This work had no specific funding.
Conflicts of interest: The authors have no conflicts of interest to disclose.
References
- 1.Stupp R., Mason W.P., van den Bent M.J. Radiation therapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Briere T.M., McAleer M.F., Levy L.B. Sparing of normal tissues with volumetric arc radiation therapy for glioblastoma: a single institutional experience. Radiat Oncol. 2017;12:79. doi: 10.1186/s13014-017-0810-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaffer R., Nichol A.M., Vollans E. A comparison of volumetric modulated arc therapy and conventional intensity modulated radiation therapy for frontal and temporal high-grade gliomas. Int J Radiat Oncol Biol Phys. 2010;76:1177–1184. doi: 10.1016/j.ijrobp.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 4.Adeberg S., Harrabi S.B., Bougatf N. Intensity modulated proton therapy, volumetric-modulated arc therapy, and 3D conformal radiation therapy in anaplastic astrocytoma and glioblastoma: A dosimetric comparison. Strahlenther Onkol. 2016;192:770–779. doi: 10.1007/s00066-016-1007-7. [DOI] [PubMed] [Google Scholar]
- 5.Navarria P., Pessina F., Cozzi L. Can advanced new radiation therapy technologies improve outcome of high-grade glioma (HGG) patients? Analysis of 3D-conformal radiation therapy (3DCRT) versus volumetric-modulated arc therapy (VMAT) in patients treated with surgery, concomitant and adjuvant chemo-radiation therapy. BMC Cancer. 2016;16:362. doi: 10.1186/s12885-016-2399-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davidson M.T., Masucci G.L., Follwell M. Single arc volumetric modulated arc therapy for complex brain gliomas: Is there an advantage compared with intensity modulated radiation therapy or by adding a partial arc? Technol Cancer Res Treat. 2012;11:211–220. doi: 10.7785/tcrt.2012.500289. [DOI] [PubMed] [Google Scholar]
- 7.Chang E.L., Akyurek S., Avalos T. Evaluation of peritumoral edema in the delineation of radiation therapy clinical target volumes for glioblastoma. Int J Radiat Oncol Biol Phys. 2007;68:144–150. doi: 10.1016/j.ijrobp.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Wallner K.E., Galicich J.H., Krol G., Arbit E., Malkin M.G. Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int J Radiat Oncol Biol Phys. 1989;16:1405–1409. doi: 10.1016/0360-3016(89)90941-3. [DOI] [PubMed] [Google Scholar]
- 9.Brandes A.A., Tosoni A., Franceschi E. Recurrence pattern after temozolomide concomitant with and adjuvant to radiation therapy in newly diagnosed patients with glioblastoma: correlation With MGMT promoter methylation status. J Clin Oncol. 2009;27:1275–1279. doi: 10.1200/JCO.2008.19.4969. [DOI] [PubMed] [Google Scholar]
- 10.Buglione M., Pedretti S., Poliani P.L. Pattern of relapse of glioblastoma multiforme treated with radical radio-chemotherapy: Could a margin reduction be proposed? J Neurooncol. 2016;128:303–312. doi: 10.1007/s11060-016-2112-2. [DOI] [PubMed] [Google Scholar]
- 11.McDonald M.W., Shu H.K., Curran W.J., Jr., Crocker I.R. Pattern of failure after limited margin radiation therapy and temozolomide for glioblastoma. Int J Radiat Oncol Biol Phys. 2011;79:130–136. doi: 10.1016/j.ijrobp.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 12.Paulsson A.K., McMullen K.P., Peiffer A.M. Limited margins using modern radiation therapy techniques does not increase marginal failure rate of glioblastoma. Am J Clin Oncol. 2014;37:177–181. doi: 10.1097/COC.0b013e318271ae03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panet-Raymond V., Ansbacher W., Zavgorodni S. Coplanar versus noncoplanar intensity modulated radiation therapy (IMRT) and volumetric-modulated arc therapy (VMAT) treatment planning for fronto-temporal high-grade glioma. J Appl Clin Med Phys. 2012;13:3826. doi: 10.1120/jacmp.v13i4.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stupp R., Taillibert S., Kanner A. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: A randomized clinical trial. JAMA. 2017;318:2306–2316. doi: 10.1001/jama.2017.18718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sood S., Pokhrel D., McClinton C. Volumetric-modulated arc therapy (VMAT) for whole brain radiation therapy: not only for hippocampal sparing, but also for reduction of dose to organs at risk. Med Dosim. 2017;42:375–383. doi: 10.1016/j.meddos.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Chatterjee A., Serban M., Abdulkarim B. Performance of knowledge-based radiation therapy planning for the glioblastoma disease site. Int J Radiat Oncol Biol Phys. 2017;99:1021–1028. doi: 10.1016/j.ijrobp.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Smyth G., Evans P.M., Bamber J.C. Non-coplanar trajectories to improve organ at risk sparing in volumetric modulated arc therapy for primary brain tumors. Radiother Oncol. 2016;121:124–131. doi: 10.1016/j.radonc.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 18.Uto M., Mizowaki T., Ogura K., Hiraoka M. Noncoplanar volumetric-modulated arc therapy (VMAT) for craniopharyngiomas reduces radiation doses to the bilateral hippocampus: a planning study comparing dynamic conformal arc therapy, coplanar VMAT, and noncoplanar VMAT. Radiat Oncol. 2016;11:86. doi: 10.1186/s13014-016-0659-x. [DOI] [PMC free article] [PubMed] [Google Scholar]