Abstract
Hydatidosis is relatively uncommon entity and it rarely affects bone and joints. A rare case of primary hydatidosis (Echhinococcus granulosus infection) involving the distal femur and the knee joint in a 53 years old female is reported here. This presented as a pathological supracondylar fracture of femur. On establishment of a clinical diagnosis she was treated preoperatively with Albendazole 600 mg, daily for 3 cycles each of 21 days with a gap of 1 weeks between cycles. Two stage surgery was carried out, the first being a meticulous debridement and second a total knee replacement with cemented tumor mega-prosthesis. Postoperatively the wound healed completely without any evidence of infection and albendazole therapy was continued for three months following surgery. During the follow-up period of two and a half year, no recurrence of hydatidosis was noticed.
Keywords: Primary hydatidosis, Pathological fracture, Mega prosthesis
1. Introduction
Hydatid disease is a rare parasitic infestation and it rarely affects bones and joints in humans.
The primary infection is acquired by ingestion of echinococcus granulosus eggs excreted by infected carnivores, most common being domestic animals like dog and cats Infection may also be contracted by contact with definitive hosts, eggs contaminated soil or plants by direct hand to mouth transmission.
In an agriculture based country like India, female sex and farmers are more susceptible to acquire this infestation.1
Liver and Lungs are most common sites involved as they act as primary and secondary filter respectively for the organism in human body.2
Bone and joints are rarely involved (0.5–4%),3 the frequent sites being vertebrae, pelvic bones, upper end of long bones e.g. humerus, femur and tibia. The distal femur is a rare site to be involved.
Although compatible with long-term survival, the disease is not easy to eradicate and perhaps impossible to cure with drugs alone. A combination of high dose Albendazole therapy and surgery could be the most efficacious treatment. Intra-operative use of PMMA (Polymethyl methacrylate) bone cement as bone filler can serve the dual purpose of implant fixation as well as reduce the chance of recurrence owing to its local necrotizing effect on living cells.
This case report aims to describe management of primary hydatidosis of distal femur involving the knee joint using a combination of high dose albendazole chemotherapy and surgery that included radical excision, debridement of the bone and decompression of medullary cavity in first stage followed by reconstruction of the skeletal defect using cemented tumor mega prosthesis.
2. Case report
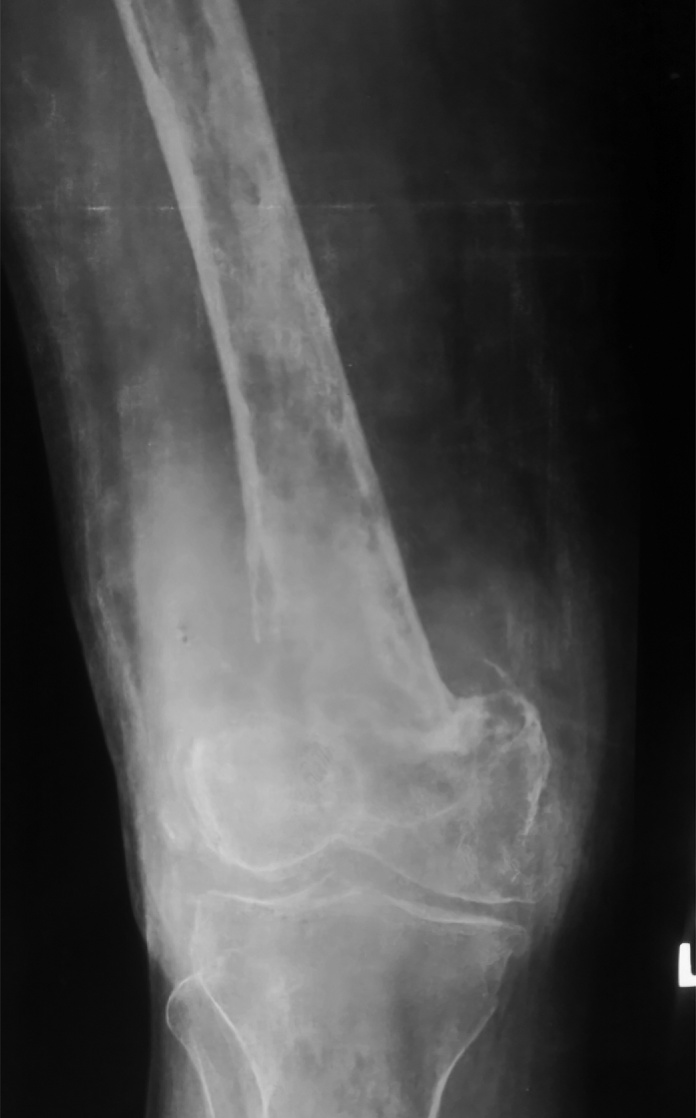
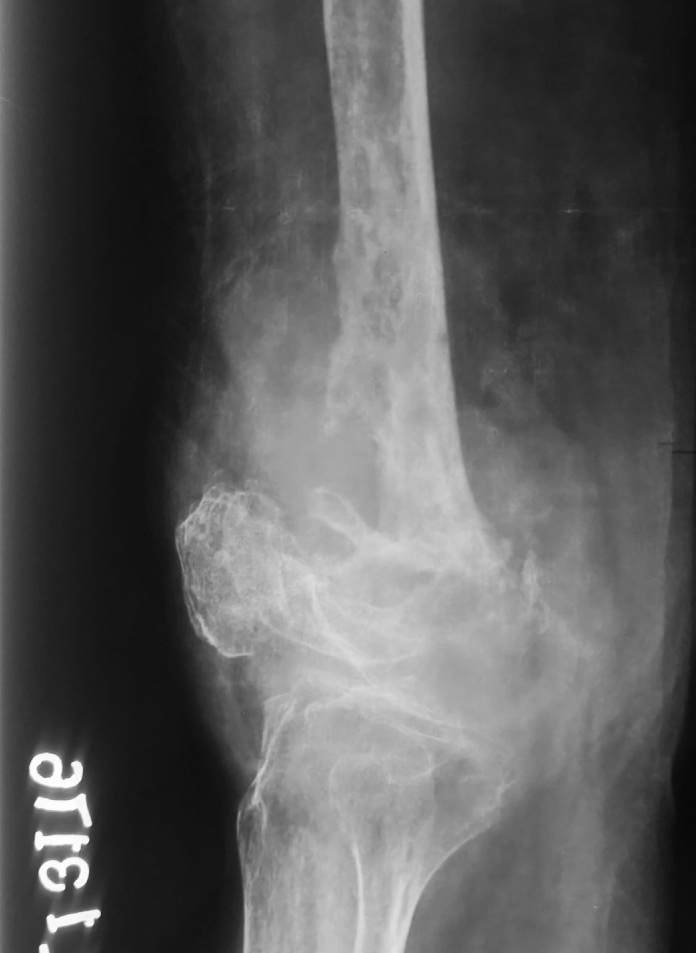
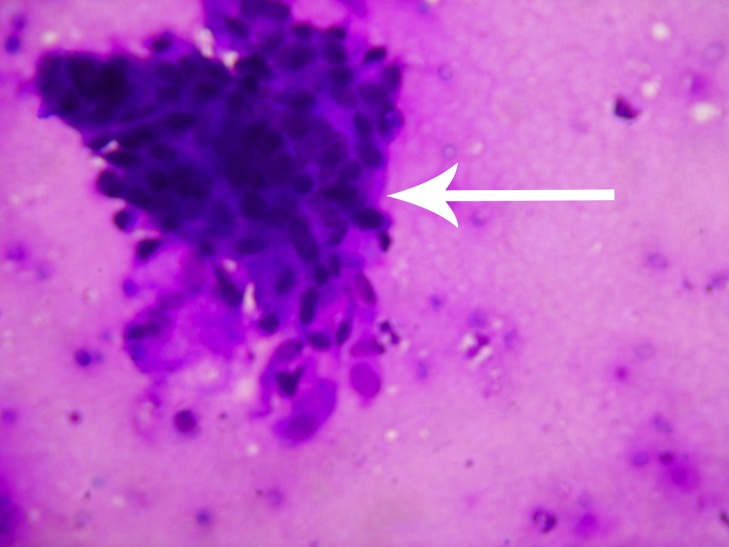
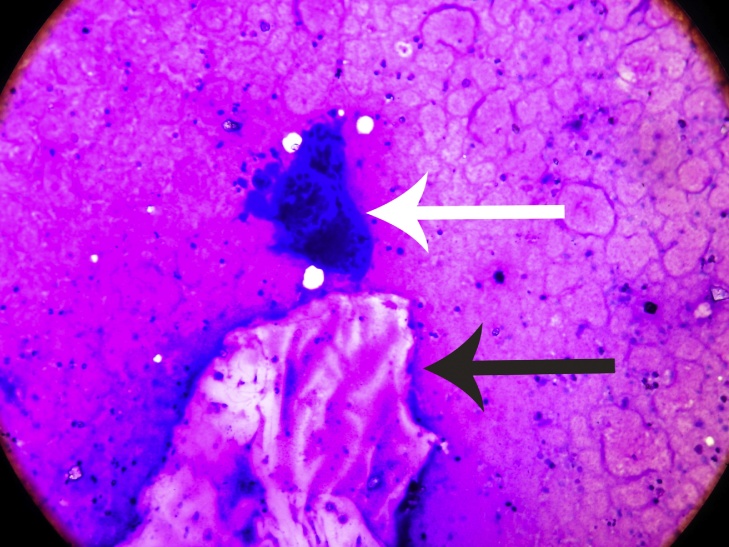
A 53 year old female presented with pain and swelling over her right knee region. A history of fall from bed following which she was unable to mobilize her right lower limb was given. Routine radiographs were ordered which exhibited multiple lytic lesions with thinned out cortices in lower half of femur and a fracture of the supracondylar region (33 C1 AO OTA classification) (Fig 1, Fig 2). All routine laboratory investigations were ordered which were reported as normal. Considering it to be a pathological fracture, a fine needle aspiration cytology (FNAC) was performed by the pathologist. It was later reported as a suspected malignancy most probably an osteosarcoma. A confirmatory open biopsy was planned and performed. The biopsy was reported as hydatid cyst of the bone. In view of contradictory findings of FNAC and biopsy, the case was discussed with pathologist. The FNAC slides were again examined and it was observed that the cytology slides contained cluster of atypical cells giving a giant cell appearance in a rich cellular background. On detailed histological and cytological analysis it was found to be indeed a foreign body giant cell reaction around the lamellated body part of Echinococcus granulosus. This was separated from the host bone by an acellular lamellated layer surrounding scolices (Fig 3, Fig 4, Fig 5).
Fig 1.
Preoperative X-Ray AP view of affected knee and femur showing pathological fracture and lytic lesions.
Fig 2.
Preoperative X-Ray Lateral view of affected knee and femur showing pathological fracture and lytic lesions.
Fig 3.
Histopathological slide showing a cluster of atypical cells seen (Giemsa Stain × 400).
Fig 4.
Histopathological slide showing foreign body giant cell reaction(white arrow) around the body part (black arrow) (Giemsa Stain × 200).
Fig 5.
Histopathological slide showing laminated body part (Giemsa Stain × 200).
The serology for hydatid disease (haemagglutinin test) was reported as positive with a value of 19.33 U/ml (Normal < 8.0 U/ml). The Casoni’s skin test was negative. CECT (Contrast enhanced Computed Tomography) of thorax,Ulrtasound abdomen and chest were eventually normal.
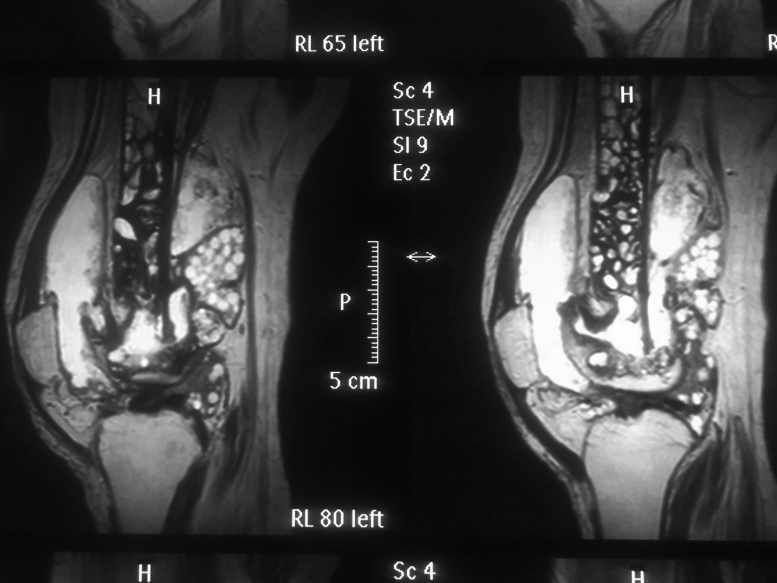
CT and MRI of affected region showed presence of multiple cysts in distal femur, knee joint with cortical breech in the posterior cortex of the distal femur and involvement of the soft tissues (Fig. 6). Intramedullary the cysts infiltrated till the mid shaft of the femur.
Fig 6.
MRI Pictures showing anterior and posterior cortical breach with massive soft tissue as well as proximal marrow infiltration by daughter cysts.
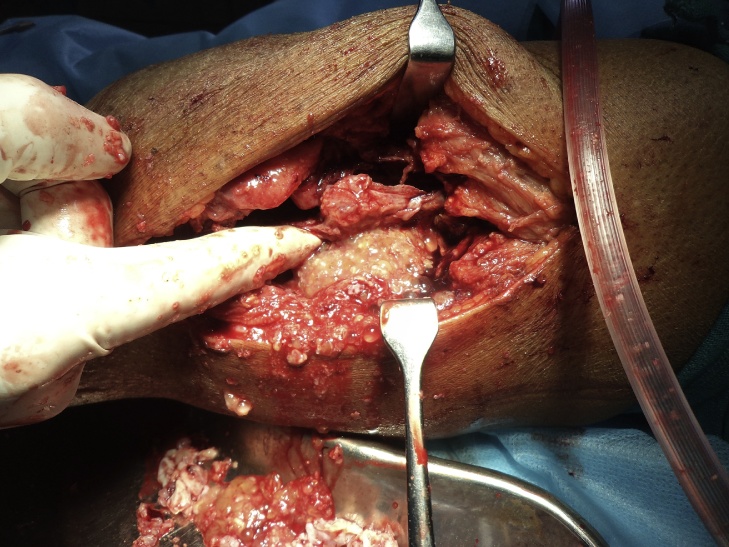
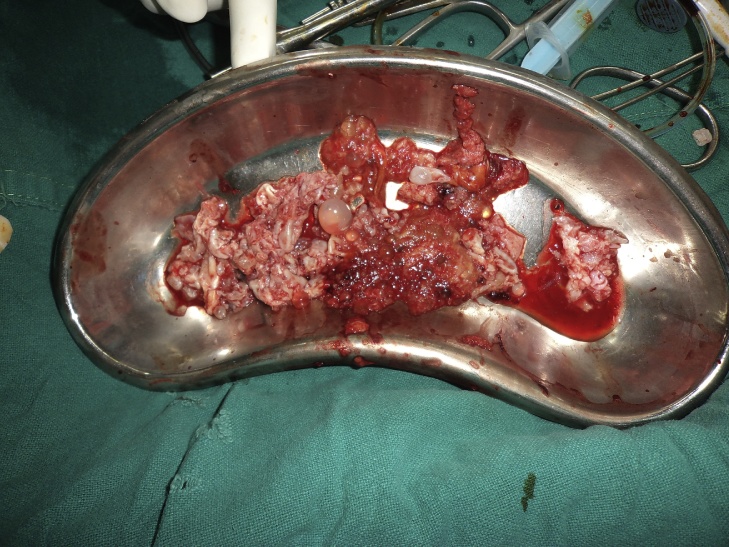
The patient was started on pharmacotherapy with high dose albendazole, 600 mg daily for 21 days. 3 such cycles were given with a gap of 1 week between them. For the preoperative evaluation the Knee Society score (modified by Insall 1993)4 was planned but it could not be evaluated due to an associated fracture. The patient was then posted for first stage of surgery which included a complete debridement of distal femur and knee region with a careful and gentle curettage of proximal extent of intramedullary involvement (Fig 7, Fig 8). This was done to avoid any bursting of the cyst and anaphylaxis. A thorough wound lavage was given with hypertonic saline which mechanically washed out the cysts. The wound was closed in layer over a suction drain. Postoperatively the Albendazole therapy was continued for a month.
Fig 7.
Intraoperative image of debridement showing multiple daughter cysts.
Fig 8.
Debrided soft tissue showing large daughter cyst.
After one month period the patient underwent a Total knee replacement using a cemented tumor mega prosthesis (Fig 9, Fig 10). During the procedure the tibial tuberosity suffered an avulsion for which an uneventful reattachment was done. The wound was closed in layers over a suction drain. Postoperative, Albendazole along with a third generation cephalosporin was given for 3 days, albendazole was then continued for a period for three months. Gentle passive ROM excersises were started after a period of 7 days considering the reattachment of the tibial tuberosity. Active ROM was started after 3 weeks postoperatively.
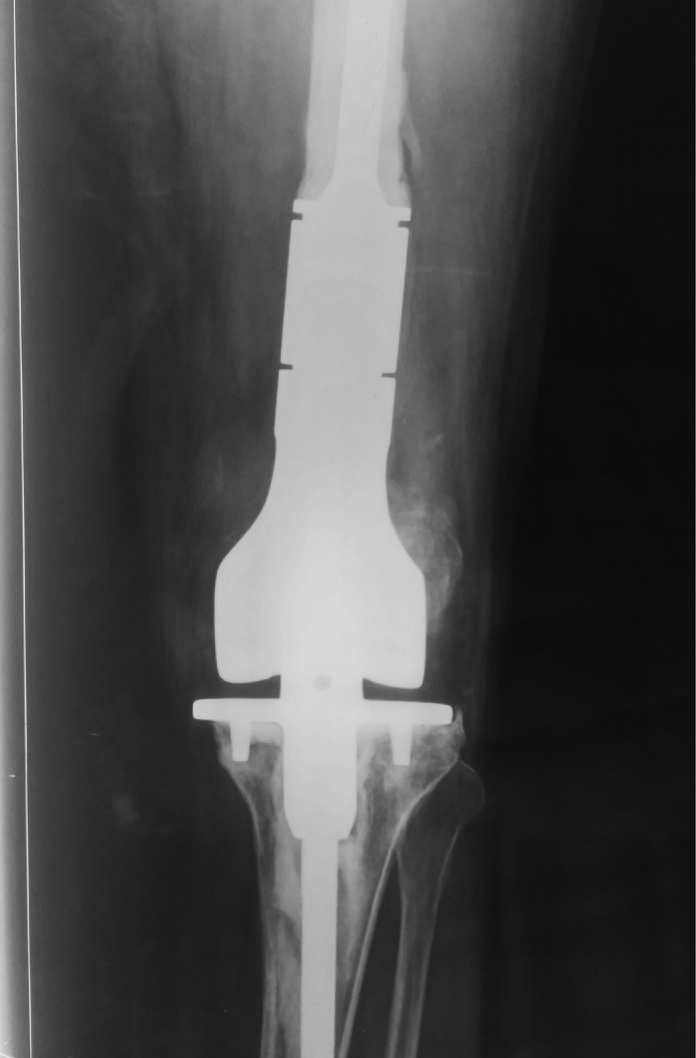
Fig 9.
Postoperative X Ray AP view of knee with mega prosthesis.
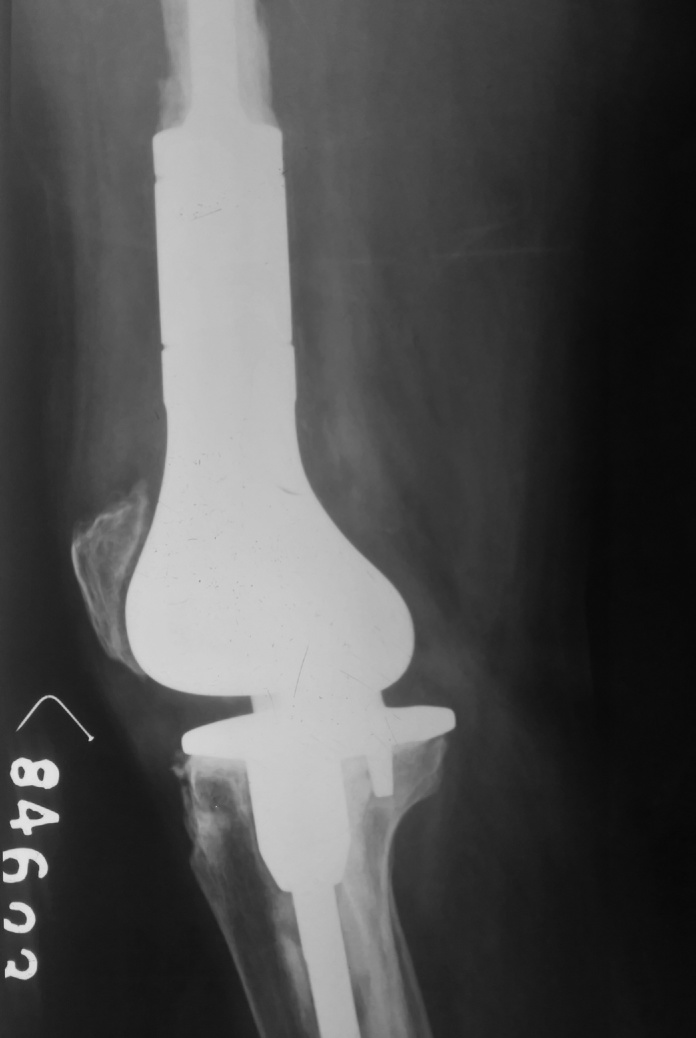
Fig 10.
Postoperative X Ray Lateral view of knee with mega prosthesis.
The patient was followed up for a period of two and a half year with 6 monthly follow up. At the final follow up of two and a half year the patient was asyptomatic with a Knee society Score of 78 (Fig. 11).
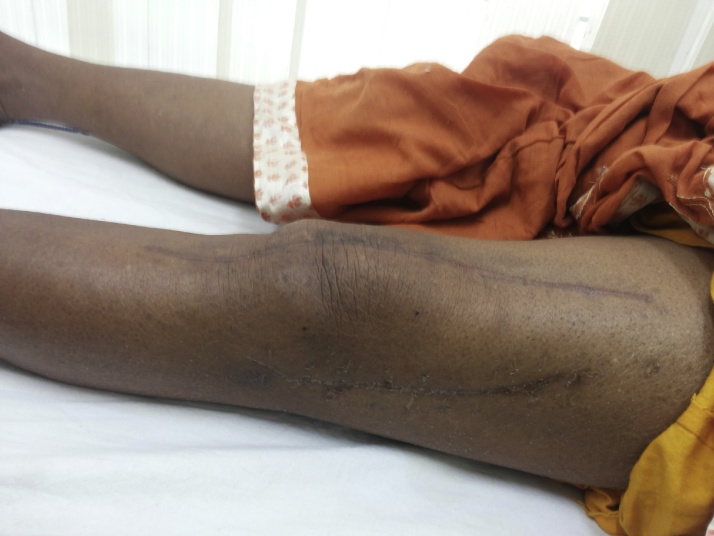
Fig 11.
Image showing healed surgical scar of both stage surgeries without any complications at two and a half year postoperative.
3. Results
The Patient was followed up for a period of two and a half year. At every follow-up she was evaluated using Knee Society Score(modified by Insall 1993)4. Serology was also done for hydatidosis on multiple occasions during the follow up. There were no signs of recurrence of echinococcal infection in the patient. Radiologically, there was no evidence of loosening. The knee was not painful and the range of motion was from 4° to 80°. At the final follow up the patient had a knee society score of 78.
4. Discussion
Hydatid disease is parasitic infestation caused by a tapeworm echinococcus. Although genus Echinococcus has different species including- E.vogeli, E.mulmultilocularis and E.granulosus. The latter is the most common causative species of hydatidosis in human.5
In recent times incidence of hydatid disease has decreased owing to education and control measures. It is still endemic in Iran, and sporadic cases are found in America, Europe, middle east and Asia.6 This condition may be included as a differential diagnosis of lytic lesions of the long bone diaphysis in endemic regions.
Primary Hydatidosis of bone is a very rare condition, and the incidence as compared to other organs range from 0.5 to 4% in literatures.3 About 60% cases of bone hydatidosis affects vertebrae and pelvis, 28% the long bones, and 8% the scapula and ribs.7 Distal femur involvement as seen in our case is very uncommon and is reported only in approximately 4 percent cases.
Primary hydatid disease of bone, due to Echinococcus granulosus, occurs when a blood-borne scolex settles in bone. In bone involvement, pericyst formation does not occur, thereby allowing aggressive proliferation in an irregular branching manner along the path of least resistance, especially the osseous canals. The parasite replaces the osseous tissue between trabeculae due to the slow growth of multiple vesicles. With time, the parasite reaches and destroys the cortex, with subsequent spread of the disease to surrounding tissues. Extraosseous cysts may calcify, whereas intraosseous disease rarely demonstrates calcification. Due to above mentioned characterstics of bone growth, the cysts are multiple and clustered rather than a single. Slow resorption of the trabeculae, without cortical expansion, occurs as a result of pressure. Usually the periosteum and articular cartilage are resistant to cyst extension when the cortex is breeched, then even expansion occurs in the surrounding soft tissues.8,9,10
In the long bones, primary cyst may either start in diaphysis or in the metaphysis with mutilocular cystic lesions leading to scalloping but with little sclerosis and periosteal reaction.
The lesion in bones may remain dormant for 10–20 years.7 The most common radiological finding in skeletal disease is a radiolucent expansile lesion with thinned out cortex. Clinically the disease presents with pain or pathological fracture following trivial trauma.11
Diagnosis is difficult due to rare nature of disease. Fine Needle Aspiration Cytology is also not always convincing. We realized this same in our case when fine neddle aspiration cytology gave us a presumptive diagnosis of malignancy due to abundance of giant cells on histo-pathological examination. In bones, microscopically the lamellated layers of echinococcus cysts are not well developed and so the appearance may be misleading and can be misdiagnosed as a metastatic growth along the canaliculi of bones. Histopathology could thus be misleading at many instances.12,13,14
Hydatidosis of bone should always be kept as a differential diagnosis of lytic lesions of bone like tuberculosis, chronic osteomyelitis, simple bone cyst, sub-acute arthritis, osteosarcoma, chondrosarcoma, giant cell tumour and myeloma.11,15
Plain radiograph, MRI, CT scan are commonly used investigations. MRI provides a good visualization of soft tissue involvement. It has been debated that spilling of cystic fluid may lead to anaphylactic reaction but FNAC has been used very safely in such cases.
The indirect haemagglutinition test is more reliable as compared to Casoni’s test or Weinberg’s complement fixation test for the diagnosis of the disease.
There has been many proponents like Mills16 who emphasized on total surgical removal of causative agents as the exclusive method when there is no effective treatment in absence of any efficient chemotherapy.
Mnaymneh and colleagues put forward idea of surgical removal of the involved bone with adequate margins of healthy bone and soft tissue as the only definitive treatment.17
Alldred and Nisbet proposed disarticulation in case the disease was in close proximity of a joint like hip or shoulder and also in case of a long bone if there is extensive involvement.18
Surgical excision and curettage alone can remove only macroscopic cysts, whereas most of the scolicidal agents as hypertonic saline and formalin cannot kill all microscopic cysts. Since there were no bone defect we chose to use a cemented megaprosthesis. Bone cement works by increasing the local temperature in the process of polymerization that causes necrosis of the daughter cysts. The monomers released by cement as well as the free radicals released during polymerization are toxic to the organism.
The effectiveness of chemotherapeutic treatment is related to the metabolism, absorption, and bioavailability of the drugs in the cyst and blood. In patients treated by benzimidazoles, it has been found that albendazole and mebendazole are not able to eliminate the disease completely. More recently Albendazole, owing to its higher blood plasma level (250 mg/l) than that of mebendazole (70–90 mg/l). The World Health Organization has regarded albendazole as drug of choice to treat hydatid disease.19
There has been a supporting literature evidence that states the role of antihelminthic drugs in hydatidosis using high dose mebendazole/albendazole. Though these drugs are not without adverse affects, but an extended course of high dose drugs should be given and the patient must be carefully monitored for any adverse affect.9,10,20
The reports on use of mega prosthesis with bone cement are rare, and hydatidosis of the bone can be contemplated as an outstretched use for mega prosthesis in addition to its use in limb salvage surgeries as in substantial trauma and tumor.21 In such a case where knee joint and approximately half of distal femur is involved reconstruction of the defect following wide resection using a cemented mega prosthesis can be best possible option to control the disease and salvage the limb with excellent function and without any complication as we did in our case.
The potential complications supposed to be associated with hydatid disease managed surgically are operative wound dehiscence, infection and recurrence but none of these complications occurred in our case.
5. Conclusion
We conclude that a two stage surgical procedure with careful debridement in first stage and reconstruction with cemented tumour megaprosthesis as a second stage procedure is worth considering in the management of primary hydatid disease of the bone. This would ensure good results and low incidence of recurrence of this disease.
Conflict of interest
None
Contributor Information
Sandeep Dathik, Email: 2951georgian@gmail.com.
Rajesh Kumar Chopra, Email: drrkchopra58@yahoo.com.
Jatin Talwar, Email: talwarjatin@gmail.com.
Mozammil Pheroz, Email: muzammilphrz@gmail.com.
Rajni Prasad, Email: drcrajni@rediffmail.com.
References
- 1.Rao S.S., Mehra B., Narang R. The spectrum of hydatid disease in rural central india: an 11 yr experience. Annals Trop Med Public Health. 2012;5(May (3)):225. [Google Scholar]
- 2.Khandakar H.H., Islam S., Begum B.A., Khatun M., Alam M.A. Hydatid disease in femur: a case report. Dinajpur Med Col J. 2012;5:56–57. [Google Scholar]
- 3.Yildiz Y., Bayrakci K., Altay M., Saglik Y. The use of polymethylmethacrylate in the management of hydatid disease of bone. Bone Joint J. 2001;83(September (7)):1005–1008. doi: 10.1302/0301-620x.83b7.12105. [DOI] [PubMed] [Google Scholar]
- 4.Insall J.N., Dorr L.D., Scott R.D., Scott W.N. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;248(November (248)):13–14. [PubMed] [Google Scholar]
- 5.Babitha F., Priya P.V., Poothiode U. Hydatid cyst of bone. Ind J Med Microbiol. 2015;33:442–444. doi: 10.4103/0255-0857.158587. [DOI] [PubMed] [Google Scholar]
- 6.Zlitni M., Ezzaouia K., Lebib H., Karray M., Kooli M., Mestiri M. Hydatid cyst of bone: diagnosis and treatment. World J Surg. 2001;25(January (1)):75–82. doi: 10.1007/s002680020010. [DOI] [PubMed] [Google Scholar]
- 7.Hooper J.O., McLean I. Hydatid disease of the femur: report of a case. JBJS. 1977;59(October (7)):974–976. [PubMed] [Google Scholar]
- 8.Ferrandez H.D., Gomez-Castresana F., Lopez-Duran L., Mata P., Brandau D., Sanchez-Barba A. Osseous hydatidosis. JBJS. 1978;60(July (5)):685–690. [PubMed] [Google Scholar]
- 9.Booz M.K. The management of hydatid disease of bone and joint. Bone Joint J. 1972;54(November (4)):698–709. [PubMed] [Google Scholar]
- 10.Senyuz O.F., Yesildag E., Celayir S. Albendazole therapy in the treatment of hydatid liver disease. Surg Today. 2001;31(May (6)):487–491. doi: 10.1007/s005950170106. [DOI] [PubMed] [Google Scholar]
- 11.Tüzün M., Hekimolu B. Various Locations of cystic and alveolar hydatid disease: CT appearances. J Comput Assist Tomography. 2001;25(January (1)):81–87. doi: 10.1097/00004728-200101000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Sapkas G.S., Stathakopoulos D.P., Babis G.C., Tsarouchas J.K. Hydatid disease of bones and joints: 8 cases followed for 4–16 years. Acta Orthop Scand. 1998;69(January (1)):89–94. doi: 10.3109/17453679809002364. [DOI] [PubMed] [Google Scholar]
- 13.Markakis P., Markakis S., Prevedorou D., Bouropoulou V. Echinococcosis of bone: clinico laboratory findings and differential diagnostic problems. Archives D'Anatomie Et De Cytologie Pathologiques. 1990;38(3):92–94. [PubMed] [Google Scholar]
- 14.Kalinova K., Proichev V., Stefanova P., Tokmakova K., Poriazova E. Hydatid bone disease: a case report and review of literature. J Orthopaed Surg. 2005;13(December (3)):323–325. doi: 10.1177/230949900501300321. [DOI] [PubMed] [Google Scholar]
- 15.Merkle E., Schulte M., Vogel J. Musculoskeletal involvement in cystic echinococcosis. Report of eight cases and review of the literature. AJR. Am J Roentgenol. 1997;168(June (6)):1531–1534. doi: 10.2214/ajr.168.6.9168719. [DOI] [PubMed] [Google Scholar]
- 16.Mills T.J. Paraplegia due to hydatid disease. Bone Joint J. 1956;38(November (4)):884–891. doi: 10.1302/0301-620X.38B4.884. [DOI] [PubMed] [Google Scholar]
- 17.Mnaymneh W.A., Yacoubian V.A., Bikhazi K.A. Hydatidosis of the pelvic girdle—treatment by partial pelvectomy. A case report. JBJS. 1977;59(June (4)):538–540. [PubMed] [Google Scholar]
- 18.Alldred A.J., Nisbet N.W. Hydatid disease of bone in Australasia. Bone Joint J. 1964;46(May (2)):260–267. [PubMed] [Google Scholar]
- 19.Song X.H., Ding L.W., Wen H. Bone hydatid disease. Postgrad Med J. 2007;83(August (982)):536–542. doi: 10.1136/pgmj.2007.057166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yildiz K., Gurbuz Y., Buluc L., Cirpici Y. Diagnostic clues in fine needle aspiration biopsy of hydatid disease of bone. ActaCytologica. 2005;50(December (3)):353. doi: 10.1159/000325969. [DOI] [PubMed] [Google Scholar]
- 21.Natarajan M.V., Kumar A.K., Sivaseelam A., Iyakutty P., Raja M., Rajagopal T.S. Using a custom mega prosthesis to treat hydatidosis of bone: a report of 3 cases. J Orthopaed Surg. 2002;10(December (2)):203–205. doi: 10.1177/230949900201000216. [DOI] [PubMed] [Google Scholar]