Abstract
The purpose of this manuscript is to provide a current concept review for the rehabilitative management of knee osteoarthritis (KOA) following regenerative medicine intervention. A proposed comprehensive regenerative rehabilitative program has been created, based on a literature review of the current best practices of rehabilitative methods and non-operative management in KOA patients with an emphasis on the goals of regenerative medicine: to optimize self-healing and functional tissue recovery. Regenerative medicine promotes regeneration and joint restoration by using blood-based procedures such as platelet rich plasma, stem cell and cell-based or tissue engineering. Regenerative medicine procedures are variable and lack of standardization in product preparation, administration, and different treatment protocols. The lack of standardization imposes challenges in regenerative rehabilitation. Over the last decade, there is growing evidence in regenerative medicine and its uses in non-operative management of various pathologies. Advances in regenerative medicine technologies brings radical innovations to establish new and effective rehabilitation protocols promoting restoration of function through tissue regeneration and repair optimizing the standard of care, specifically in rehabilitation when combined with regenerative protocols for patients with KOA is the most common degenerative disease in the knee and can affect any synovial joint in the body. It is a leading cause of disability affecting the quality of lives of millions of people world-wide. Conventional methods of mild to moderate KOA are focused on short-term symptomatic relief and do not promote joint homeostasis or regeneration of injured tissue. Regenerative medicine emphasizes a paradigm shift in patient-centered care promoting regeneration and joint restoration by using blood-based procedures such as platelet rich plasma, stem cell and cell-based or tissue engineering. The purpose of this current concept review is to outline a comprehensive post-regenerative rehabilitative program in the management of KOA based on the best available evidence. Our proposed regenerative rehabilitation program is intended to align the goals of regenerative medicine with the current, high-level evidence of non-operative management for KOA, to optimize self-healing and functional tissue recovery.
1. Introduction
Osteoarthritis (OA) is a common degenerative disorder affecting joint cartilage, underlying bone and is characterized by chronic structural and functional degeneration of synovial joint.1 Additionally, OA has a chronic cycle of aberrant attempts to repair joints involved leading to inflammation and tissue degradation.2 OA is the most common joint disorder in the United States. It accounts for 80% of the global burden of diseases3 and affects 10% of men and 16% of women aged 60 years and older.4 The knee joint is the most commonly affected joint and has increasing prevalence with age and weight.3,5 In recent decades, a rise in Body Mass Index (BMI) has become an epidemic in the United States and is a well-known risk factor for KOA.3 This is possibly due to the effects of joint overloading6 and adiposity-induced inflammation.7 More recently, OA has been defined as a multifactorial, complex disorder, which includes genetics, aging, obesity, biomechanics, joint laxity and malalignment.1,6 Studies show that excessive mechanical stress can alter the homeostatic balance by directly causing damage to the chondrocyte extracellular matrix, causing a shift in the balance between catabolic and anabolic activity, favoring catabolic activity.6 During the early stages of OA there is an initial increase in inflammation, which triggers the body's natural immune system to recognize the presence of damaged cells and irritants. This results in an influx of inflammatory mediators, matrix-degrading proteinases and stress-response factors in the cartilage.8 During the body's attempt to repair, it has been shown that phenotypic modulation of chondrocytes occurs with abnormal matrix molecules being produced and altered cell behavior.8 This aberrant attempt to repair the affect joint results in a homeostatic imbalance leading to further progression of the disease.2
Regenerative medicine, with the use of orthobiologics, is a rapidly growing field and one that is fairly new, specifically in rehabilitative settings. Orthobiologic intervention is a treatment option that is new to many rehabilitative clinicians and in order to achieve optimal outcomes the clinician must understand its involvement in the rehabilitation continuum.
The management of OA using various rehabilitative methods is fundamental for effective symptom relief, including pain management, improvement of functional limitations and quality of life. The future of regenerative rehabilitation offers unparalleled prospects in supporting the repair of degenerated, diseased, or damaged tissues. The ultimate goal of regenerative medicine is to effectively support the body's natural healing system. Regenerative rehabilitation interventions involve treating all structures that surround the affected joint to achieve pain relief and prevention from further deterioration. Research shows that non-pharmacological treatments have demonstrated only modest clinical benefits and minimal positive outcomes of early surgical intervention.2,9,10
This current concepts review will examine the field of regenerative medicine as it applies to patients with KOA and outline a comprehensive, post-regenerative, rehabilitative program based on the best available evidence.
The purpose of this manuscript is to provide a current concept review for the rehabilitative management of knee KOA following regenerative medicine intervention. A proposed comprehensive regenerative rehabilitative program has been created, based on a literature review of the current best practices of rehabilitative methods and non-operative management in KOA patients with an emphasis on the goals of regenerative medicine: to optimize self-healing and functional tissue recovery.
2. Methodology
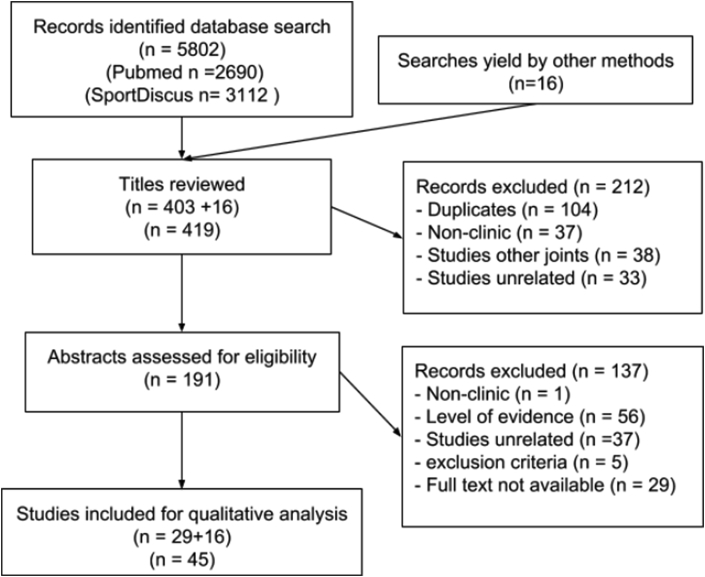
A literature search was performed in databases PubMed (January 1, 1970–June 30, 2018) and SPORTDiscus (through June 30, 2018) to identify the rehabilitation in KOA and their levels of evidence to ensure inclusion of pertinent data. Reviews and case studies were excluded, except for the purpose of background information on OA, regenerative rehabilitation and regenerative medicine (Fig. 1). Additionally non-English language articles and those not matching our inclusion criteria were excluded from the study (Table 1).
Fig. 1.
Systematic literature qualitative analysis.
Table 1.
Eligibility criteria for literature search analysis.
| Criteria | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Level of evidence | Level I-II studies assess for various rehabilitation interventions for KOA. | Level III-V evidence. |
| Subjects | Human subject aged ≥18 with primary or secondary confirmed KOA in one or both knees. | Pilot studies (n < 20 human participants), animal, cadaver studies. |
| Study design | Randomized controlled trials, systematic review of level-I randomized controlled trials, prospective cohort study poor-quality randomized controlled trials, systematic review such as level-II studies, nonhomogeneous and level-I studies. | Case-control, retrospective cohort studies, systematic reviews of Level-III studies, case series, ideas, opinions and expert opinions (level III-IV evidence). |
| Study outcome | Studies assess the management and treatment for KOA. | Non-clinical studies Studies on other joints (e.g. Hip) Studies unrelated to knees (injection-based therapies, pharmacology interventions) |
| Study language | Articles in English only. | Articles in languages other than English. |
Keywords used included knee osteoarthritis, non-operative, management, non-surgical, rehabilitation, blood flow restriction or therapy, strength, quadriceps, proprioception, sensorimotor, laser, shockwave, transcutaneous electric neuromuscular stimulation, regenerative medicine, regenerative rehabilitation, kinesio taping, acupuncture, flexibility, joint loading, soft-tissue therapy, brace, flexibility and weight loss. The articles were then reviewed for additional references and originality. Primarily, the methodology and results were extracted from each pertinent scientific article. Inclusion criteria are outlined (Table 1).
Literature search revealed 5802 studies involving rehabilitation of KOA (Fig. 1). These revealed studies were subjected to screening based on preferred reporting items for systematic review and meta-analysis.11
3. Regenerative rehabilitation for managing OA
3.1. Exercise, strengthening and weight management
Moderate physical exercise is associated with a decreased risk of severe KOA, suggesting that exercise has a protective effect against cartilage degradation.6 An exercise program that combines endurance work and strength in arthritic patients has been shown to increase functional capacity and reduce pain.9,12 Patients with KOA often present with quadriceps weakness, atrophy and strength training is associated with increasing cross-sectional area of muscle fibers and increased strength.9,13 Other benefits include improvement in bone mineral density, decreased risk of falling, increased walking speed, better balance, and increased stair-climbing ability.
Although physical exercise has been shown to be beneficial, increased mechanical loading may have adverse effects on the knee joint caused by wear and tear.14 Implementation of exercises should involve gradual loading, specificity and must be attainable.9 Longer training durations at increased loads have been shown to increase muscular strength overtime. Other benefits include improvement in bone mineral density, decreased risk of falling, increased walking speed, better balance, and increased stair-climbing ability. Excessive mechanical stress can directly damage the cartilage extracellular matrix and shift the balance in chondrocytes to favor catabolic activity.6 A change of −1% in body weight has been shown to have a significant association with slower loss of tibial cartilage volume and improvement in symptoms, suggesting a meaningful impact on cartilage health.15 The triad of education, exercise is the first-line treatment to reduce pain and improve function in individuals with KOA.6
Although the effectiveness of strength training in patients with KOA is widely recognized, the exact pathway by which muscle strengthening results in reduced pain and activity limitations, is still unclear.16, 17, 18, 19 Various mechanisms have been suggested, including neuromuscular control changes, peri-articular, intra-articular and psychosocial mechanisms or via general health. Exercise therapy typically focuses on training muscles intrinsic to the knee, but recent research has shown strengthening to hip and pelvis musculature can produce positive results in this population.12 As physiologic mechanical stimulation of the articular cartilage generates biochemical signals which increase the anabolic activity of chondrocytes.6 We can suggest that exercise therapy following interventional regenerative medicine is indicated based on the best available data in the management of KOA. Its inclusion in combination with a regenerative medicine/rehabilitation protocol is strongly recommended. This recommendation can be found in Table 1.
3.2. Stability, proprioception and neuromuscular training
The majority (>60%) of patients with KOA report knee instability, which is associated with pain and activity limitations.16 The knee joint is stabilized by a combination of static and dynamic stabilizers.16 The deterioration of strength and proprioception seen in KOA patients may result in impaired balance and therefore neuromuscular training is needed.20 Although there is conclusive evidence that exercise improves physical function in subjects with KOA, the relationship between long-term exercise and stability in older adults remains inconclusive.20
Knoop et al. found that initial knee stabilization training may have added value over standard exercises (i.e. strength/functional training) in patients with strong muscles, but not in those with weak muscles.16 Patient with muscle weakness around the knee and instability, should focus on muscle strengthening, whereas in patients with knee instability and strong muscles, initial knee stabilization training can be beneficial.16 Maggo et al. demonstrated that the combination of lower extremity strengthening and proprioceptive training combined was superior when compared to strengthening alone in patients with KOA.21 As with strength training, the physiologic mechanical stimulation of the articular cartilage generates biochemical signals which increase the anabolic activity of chondrocytes.6
A combination of strength, stability, proprioception and neuromuscular training is recommended in post regenerative medicine/rehabilitation intervention due to its’ role in chondrocyte stimulation and role in improving function of patients with KOA. Refer to Table 2 for specific training protocols.
Table 2.
Rehabilitation protocols for KOA after regenerative medicine intervention(s).
| Immediately post intervention (day 0–3) | Day 3–14 post treatment | 2–4 weeks post treatment | 5–10 weeks post treatment: | |
|---|---|---|---|---|
| Goals |
|
|
|
|
| Precautions | No activity that causes pain | Weight bearing and exercise as tolerable | ||
| Suggested therapeutic exercise(s) or modality |
|
|
|
|
| Cardiovascular Exercises | None | Cycling, Swimming, walking- low impact Aerobic exercise. Can be accumulated in bouts of 10 min, aim for 150 min/week | ||
| Progression Criteria |
|
|
|
|
● If increased pain return to previous step until pain is controlled and full return to function required for that step.
● Abbreviations: knee osteoarthritis, KOA; range of motion, ROM; pro re nata, PRN (as circumstances require); low level laser therapy, LLLT; transcutaneous electric nerve stimulation, TENS; interferential current, IFC; whole body vibration, WBV; blood flow restriction therapy, BFRT.
3.3. Manual therapy
Manual therapy (MT) involves skilled, hands-on techniques that are diagnostic and interventional. Using MT, a clinician can treat soft tissue and joint dysfunction, increase ROM, modulate pain, decrease inflammation, alter muscle tone, and increase circulation that ultimately improves function and movement.22
Deyle et al. found that patients with KOA who were treated with MT and exercise experienced clinically and statistically significant, lasting improvements in self-perceptions of pain, stiffness, and functional ability when compared to a control group.22 According to Deyle et al., patients frequently reported 20%–40% relief of symptoms after only two to three clinical treatments of MT and exercise.22
MT in the treatment of KOA shows significant improvements with respects to pain, stiffness and functional ability.22 The mechanisms of MT are to improve tissue repair, stability and functions and its inclusion in a regenerative medicine/rehabilitation protocol for KOA is suggested. Refer to Table 2 for specific recommendations.
3.4. Blood flow restriction therapy
Blood Flow Restriction training (BFR) is a rapidly growing intervention for patients suffering from muscle atrophy, weakness, neuromuscular control deficits, pain, and traumatic injuries where sufficient loads required for muscle strengthening and hypertrophy cannot be tolerated.23 The clinician applies a tourniquet to the extremity of the patient and partially restricts blood flow as the patient undergoes mobilization, or exercise.23 To date this technique has been shown to be a safe and effective mechanism for patients, including those with the comorbidities associated with KOA.23
Ferraz et al. concluded that BFR and high interval-resistance training were similarly effective in increasing muscle strength, quadriceps muscle mass and functionality in KOA patients.24 Additionally, BFR was also able to improve pain while inducing less joint stress, emerging as a feasible and effective therapeutic adjunct in OA management.24
Research supports the use of BFR to increase muscle hypertrophy, increase strength, improve serum growth hormone, muscle endurance, increase peripheral stem cell count and increase protein synthesis with using less than recommended weight training intensities for those gains.23 For patients with KOA who concomitantly are receiving orthobiologic intervention this can be an essential rehab component to ensure optimal results and effectiveness of the orthobiologic are achieved. Refer to Table 2 for specific recommendations.
3.5. Vibration therapy
Whole body vibration (WBV) training involves placing a person on a vibrating platform.25,26 The amplitude is varied while the patient is either positioned orthostatically or performing dynamic movements.25 The vibratory stimulus strengthens the lower limbs and improves proprioception in elderly patients with KOA by inducing isometric, concentric, and eccentric contractions of the hip, knee extensor muscle groups and the plantar flexors thereby improving the control and execution of functional movements such as those required for static and dynamic balance and gait performance.25
Wang et al. concluded that WBV exercise in combination with quadriceps resistance exercise provided over a 24-week period improved symptoms, physical function, activities of daily living, and quality of life in patients with KOA to a great extent and was superior to quadriceps resistance exercise only in most outcomes.25 Simao et al. found that the addition of vibration training to squat exercise training improves static and dynamic balance and gait performance and that the association of the squat exercise with whole-body vibration was sufficient to induce significant alterations in the plasma concentrations of inflammatory markers, in elderly individuals with KOA, which could reflect a reduction in the inflammatory joint process of the knee.24
WBV in KOA patients may slow the progression of cartilage loss potentially due to modulation of skeletal tissue, increasing oscillation of chondrocytes, and augmenting thickness of the chondrocyte layer.26 Thus, WBV exercise is suggested in combination with a regenerative medicine and the regenerative rehabilitation program for patients with KOA. Refer to Table 2 for specific recommendations.
3.6. Electro modalities
Electro-modalities induce physiological actions in living tissues at the cellular level.27 These effects include cellular oxygenation, release of neurotransmitters associated with pain modulation, release of anti-inflammatory, endogenous mediators, augmentation of blood flow, increased capillary permeability, tissue metabolism and fibrous tissue extensibility, muscle relaxation, and elevation of pain threshold.27 Electro-modalities examined in this review included low level laser therapy (LLLT), therapeutic ultrasound (US), transcutaneous electrical nerve stimulation (TENS) and Interferential Current (IFC).
The study of Welch et al. compared therapeutic US to placebo and concluded that US therapy appears to have no benefit over placebo for patients with KOA.28 Atamaz et al. conducted a randomized controlled multicenter study comparing the effectiveness of TENS and IFC between groups in addition to exercise training and education in KOA.29 Their results showed that all assessment parameters significantly improved in all groups without a significant difference.29
Alfredo et al. found positive results with the application of a class 3b LLLT applied in a total dose of 27J per treatment after the completion of a 45-min exercise program, three times per week for 8 weeks.27 The combination of LLLT and exercises yielded pain reduction, improvement in activity and function compared to placebo group.27 Similar results were found by Stelian et al. who observed significant functional improvement and pain reduction in the laser group but not in placebo group in patients with OA.27,30 Hegedus et al. and Montes- Molina et al. carried out clinical trials according to the recommendations of the World Association of Laser Therapy, effective results were recorded in pain relief and improvements in microcirculation in the irradiated area in patients with KOA.31,32
With respects to regenerative medicine/rehabilitation protocols, there is insufficient evidence to suggest that the inclusion of US, TENS or IFC could be detrimental or beneficial in the treatment of KOA. However, the inclusion of LLLT in combination with a regenerative rehabilitation protocol could induce photochemical physiological actions at the cellular level to improve circulation and the renewing effects of regenerative medicine in relation to OA of the knee. Refer to Table 2 for specific recommendations.
3.7. Acupuncture and dry needling
Acupuncture and dry needling (DN) are treatment modalities that are often used in the treatment of painful skeletal muscle conditions that may produce sensitive, motor, or autonomic symptoms and signs.33 Clinical trials conducted by Scharf et al. and Chen et al. concluded that acupuncture as adjunct therapy to exercise in the treatment for KOA improved pain and function scores, however no significant difference was noted between puncturing (non-sham) needles and non-puncturing (sham) needles.34,35 Sanchez-Remero et al., concluded that DN intervention in conjunction with exercise was no more effective than the placebo intervention 3.
Although, there is a lack of evidence in the mechanisms and actions of acupuncture/DN in the treatment of KOA, evidence suggests that acupuncture/DN principles can positively alter sensitive, motor, and autonomic function in patients with KOA. In addition, evidence suggests that acupuncture/DN would be of benefit as an adjunct therapy to a regenerative medicine/rehabilitation protocol with respects to KOA. Refer to Table 2, for specific recommendations.
3.8. Taping and bracing
Kinesio Tape (KT) is used to increase regional circulation, increase afferent stimulation, reduce pain, decrease edema and assist in muscle inhibition when used correctly36,37 although its success as a treatment option for KOA remains inconclusive.36,38 Mutlu et al., demonstrated that KT resulted in short term benefits of taping on improving a walking task, decreasing pain, while increasing knee ROM compared to placebo taping in patients with KOA.38
Knee bracing has been advocated as an effective device for the management of patients with KOA,39 to achieve proprioceptive changes, muscle activation and pain reduction stemming from joint unloading.39 Ornetti et also showed that the bracing effectively reduced symptoms of medial compartment KOA in both short-term (6 weeks) and medium-terms (52 weeks).39
KT and bracing may be a beneficial adjunct to those who have undergone orthobiologic intervention especially in the acute phase/short term to decrease pain, improve ROM and function, and increase muscle activation. Refer to Table 2 for specific recommendations.
4. Comprehensive rehab program following regenerative medicine intervention
Phase 1 should begin immediately after procedure and can last up to a week depending on pain and inflammation (Table 2). In the acute phase it is wise to initiate loading as soon as pain permits, as early mobilization and tissue loading has shown to have a positive effect to promote collagen reorganization and tissue healing.40 Introductory loading should involve a return to full weight bearing, which can also be achieved through hydrotherapy or weight-assisted treadmills.40 Because of its pain inhibitory effects, isometric exercise makes another excellent option as the first line of tissue loading intervention.40 Despite their being only Level IV and Level V studies, low-intensity pulsed ultrasound and neuromuscular electrical stimulation are still used in the clinical setting in an attempt to manage inflammation and promote tissue healing.40
The rehabilitation program for an individual with KOA must pay special attention to the strength, stability, and inter-muscular balance of the muscles of the core, hip, knee and knee and ankle joints. Early implementation of kinetic chain exercises will prepare the body for further progression of more advanced joint loading exercises. Exercises should be designed to progress from single joint to multi-jointed kinetic chain exercises focusing on motions to address the core, hip, knee and ankle. Common potential deficits to examine include: (1) weakness of the quadriceps, hamstrings and hip muscles (2) reduced proprioception and joint kinesthesia. Phase one will focus on reducing pain and inflammation as well as early loading isometric exercises before attempting isotonic exercises. Kinetic chain exercises should include squats, lunges, single leg step downs, side planks, front planks, glute bridges. Exercise initiation and progressions will depend on individualized gains such as neuromuscular control, range of motion etc. Exercises positions will progress from supine or prone to half kneeling, quadruped and lastly standing. Phase 2 can begin once pain and inflammation diminish, and neuromuscular control and static stability reach adequate levels. Phase 3 can begin once the individual has achieved full ROM, at least 4/5 manual muscle test strength, and has minimal pain. Phase 4 is initiated once the individual attains non-painful ROM; satisfactory isokinetic test results is available (quadriceps and hamstring ratio); appropriate rehabilitation progress. The 2016 consensus statement on return to sport was used to inform the rehabilitation and return to sport protocols (Table 3).41 Return to participation, sport and performance will be a continuation of phase 1–4 to address any performance needs for athletic population (Table 3). The main treatment modalities included were active rehabilitation to promote tissue healing in conjunction with PRP or stem cell therapy. Early mobilizations and in acute phases loading as pain permits. A graded load progression and cardiovascular exercise are recommended.
Table 3.
Return to activity protocols for knee osteoarthritis after regenerative medicine intervention(s).
| Goals | Return to participation | Return to sport | Return to performance |
|---|---|---|---|
| Precautions | Gradual and progressive | Gradual and progressive | No limitations |
| Suggested therapeutic exercise(s) or modality |
|
|
|
| Cardiovascular Exercises | Light aerobic activity | Replicate sport or work specific energy demands | Perform at fitness level needed for sport |
| Progression Criteria | Modified or unrestricted | Individual has returned to his or her defined sport/activity, but is not performing at his/her desired performance level |
|
● If increased pain return to previous step until pain is controlled and full return to function required for that step.
● Abbreviations: knee osteoarthritis, KOA; range of motion, ROM; pro re nata, PRN (as circumstances require); low level laser therapy, LLLT; transcutaneous electric nerve stimulation, TENS; interferential current, IFC; whole body vibration, WBV; blood flow restriction therapy, BFRT.
Modern rehabilitation methods are based on an active rehabilitation framework. It is important to keep the natural healing process in mind while constructing a rehabilitation program.40 Graded load progression plays a significant role in the rehabilitation model.40 Studies have shown that mechanical loading helps in initiating the anabolic cascade, helps in stem cell proliferation and osteogenic differentiation.42
Cyclical mechanical compression increases mineralization of scaffold seeded with osteogenic cell. These two studies document that external pressure stimulus might have an effect in guiding the fate of an stem cell.43 In tendon procedure using ECM (extracellular matrix) scaffold has been shown to help in scaffold remodeling, recruitment of circulating growth factors and progenitor cells.44
Cardiorespiratory training is recommended to maintain and improve aerobic capacity in conjunction with neuromuscular training to maintain overall muscle strength, flexibility, and proprioception.40 Sufficient loading will result in strength changes through neural adaptation and hypertrophy and may explain greater strength gains associated with high-intensity resistance programs.45 Low-intensity resistance programs may not elicit adequate muscle activity to promote neuromotor adaptation and hypertrophy. High-intensity resistance exercise showed low to moderate levels of quality of evidence for greater and more sustained benefits.45
5. Conclusions
Conventional methods of mild to moderate osteoarthritis of knee are focused on short term symptomatic relief and does not promote regeneration or restore the normal homeostasis of knee joints. Regenerative medicine is promoting a paradigm shift away from conventional methods towards promoting regeneration and joint restoration using blood based procedure like platelet rich plasma, stem cell and cell based or tissue engineering procedure. However, results of these regenerative procedure are variable due to lack of standardization in product preparation, administration, and different treatment protocols. This makes post regenerative rehabilitation more challenging and require well documented rehabilitation program to help in preparing the joints for regenerative procedures and supporting them until optimum tissue regeneration and joint restoration. The purpose of this current concept review was to outline a comprehensive post-regenerative rehabilitative program in osteoarthritis of knee joint based on the best available. Our proposed rehabilitation program is based on current evidence related to rehabilitation for individuals suffering from osteoarthritis. We evaluated various conventional methods that can promote healing to the knee joint. There are some interventions that appears to be promising to support the joint until regeneration or restoration. Further research and longer follow up studies are recommended to develop universally accepted post-regenerative rehabilitation protocols in osteoarthritis of knee joints.
Limitations of our study include a large portion of the studies reviewed were not done concurrently with orthobiologic intervention, there is minimal research in the field of rehabilitation of patients post orthobiologic intervention with KOA although there is significant literature in the use of regenerative medicine in patients with KOA.
Authors contributions
The authors’ responsibilities were as follows WDM concept of the paper, JM search design, JM, KF, NV, KH search and extraction, content writing and editing JM, KF, NV, AD, TO, Formatting and referencing KH, JM, KF and JM had primary responsibility for final draft and submission, all authors read and approved the final draft manuscript, WDM draft feedback.
Conflicts of interest
The authors have none to declare.
Funding statement
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Acknowledgements
Ashok Kumar for reviewing manuscript.
References
- 1.Yucesoy B., Charles L.E., Baker B., Burchfiel C.M. Occupational and genetic risk factors for osteoarthritis: a review. Work. 2015;50(2):261–273. doi: 10.3233/WOR-131739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts S., Genever P., McCaskie A., Bari C De. Prospects of stem cell therapy in osteoarthritis. Regen Med. 2011;6(3):351–366. doi: 10.2217/rme.11.21. [DOI] [PubMed] [Google Scholar]
- 3.Vos T., Flaxman A.D., Naghavi M. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y., Jordan J.M. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26(3):355–369. doi: 10.1016/j.cger.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Felson D.T. Osteoarthritis of the knee. N Engl J Med. 2006;354(23):2508–2509. [PubMed] [Google Scholar]
- 6.Sun H.B. Mechanical loading, cartilage degradation, and arthritis. Ann N Y Acad Sci. 2010;1211:37–50. doi: 10.1111/j.1749-6632.2010.05808.x. [DOI] [PubMed] [Google Scholar]
- 7.Wluka A.E., Lombard C.B., Cicuttini F.M. Tackling obesity in knee osteoarthritis. Nat Rev Rheumatol. 2013;9(4):225–235. doi: 10.1038/nrrheum.2012.224. [DOI] [PubMed] [Google Scholar]
- 8.Schroeppel J.P., Crist J.D., Anderson H.C., Wang J. Molecular regulation of articular chondrocyte function and its significance in osteoarthritis. Histol Histopathol. 2011;26(3):377–394. doi: 10.14670/HH-26.377. [DOI] [PubMed] [Google Scholar]
- 9.Hawker G.A., Mian S., Bednis K., Stanaitis I. Osteoarthritis year 2010 in review: non-pharmacologic therapy. Osteoarthritis Cartilage. 2011 doi: 10.1016/j.joca.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 10.Gay C., Chabaud A., Guilley E., Coudeyre E. Educating patients about the benefits of physical activity and exercise for their hip and knee osteoarthritis. Systematic literature review. Ann Phys Rehabil Med. 2016;59(3):174–183. doi: 10.1016/j.rehab.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 12.Lund H., Weile U., Christensen R. A randomized controlled trial of aquatic and land-based exercise in patients with knee osteoarthritis. J Rehabil Med. 2008;40(2):137–144. doi: 10.2340/16501977-0134. [DOI] [PubMed] [Google Scholar]
- 13.Mikesky A.E., Mazzuca S.A., Brandt K.D., Perkins S.M., Damush T., Lane K.A. Effects of strength training on the incidence and progression of knee osteoarthritis. Arthritis Care Res. 2006;55(5):690–699. doi: 10.1002/art.22245. [DOI] [PubMed] [Google Scholar]
- 14.Christensen R., Astrup A., Bliddal H. Weight loss: the treatment of choice for knee osteoarthritis? A randomized trial. Osteoarthritis Cartilage. 2005;13(1):20–27. doi: 10.1016/j.joca.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Waller B., Munukka M., Rantalainen T. Effects of high intensity resistance aquatic training on body composition and walking speed in women with mild knee osteoarthritis: a 4-month RCT with 12-month follow-up. Osteoarthritis Cartilage. 2017;25(8):1238–1246. doi: 10.1016/j.joca.2017.02.800. [DOI] [PubMed] [Google Scholar]
- 16.Knoop J., Steultjens M.P.M., Roorda L.D. Improvement in upper leg muscle strength underlies beneficial effects of exercise therapy in knee osteoarthritis: secondary analysis from a randomised controlled trial. Physiotherapy (United Kingdom) 2015;101(2):171–177. doi: 10.1016/j.physio.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Malas F.Ü., Özçakar L., Kaymak B. Effects of different strength training on muscle architecture: clinical and ultrasonographic evaluation in knee osteoarthritis. Pharm Manag PM R. 2013;5(8):655–662. doi: 10.1016/j.pmrj.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Anwer S., Alghadir A. Effect of isometric quadriceps exercise on muscle strength, pain, and function in patients with knee osteoarthritis: a randomized controlled study. J Phys Ther Sci. 2014;26(5):745–748. doi: 10.1589/jpts.26.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Topp R., Woolley S., Hornyak J., Khuder S., Kahaleh B. The effect of dynamic versus isometric resistance training on pain and functioning among adults with osteoarthritis of the knee. Arch Phys Med Rehabil. 2002;83(9):1187–1195. doi: 10.1053/apmr.2002.33988. [DOI] [PubMed] [Google Scholar]
- 20.Messier S.P., Royer T.D., Craven T.E., O'Toole M.L., Burns R., Ettinger W.H. Long-term exercise and its effect on balance in older, osteoarthritic adults: results from the fitness, arthritis, and seniors trial (FAST) J Am Geriatr Soc. 2000;48(2):131–138. doi: 10.1111/j.1532-5415.2000.tb03903.x. [DOI] [PubMed] [Google Scholar]
- 21.Maggo Aastha, Shobhit Saxena S.G. The effects of proprioceptive exercise and strengthening exercises in knee osteoarthritis. Indian J Physiother Occup Ther. 2010;4(3):144–148. [Google Scholar]
- 22.Deyle MPT G.D., Henderson N.E., Matekel R.L., Ryder M.G., Garber M.B., Allison S.C. Annals of internal medicine effectiveness of manual physical therapy and exercise in osteoarthritis of the knee a randomized. Controlled Trial. 2000;132(3) doi: 10.7326/0003-4819-132-3-200002010-00002. [DOI] [PubMed] [Google Scholar]
- 23.Loenneke J.P., Wilson J.M., Wilson G.J., Pujol T.J., Bemben M.G. Potential safety issues with blood flow restriction training. Scand J Med Sci Sports. 2011;21(4):510–518. doi: 10.1111/j.1600-0838.2010.01290.x. [DOI] [PubMed] [Google Scholar]
- 24.Ferraz R.B., Gualano B., Rodrigues R. Benefits of resistance training with blood flow restriction in knee osteoarthritis. Med Sci Sports Exerc. 2018;50(5):897–905. doi: 10.1249/MSS.0000000000001530. [DOI] [PubMed] [Google Scholar]
- 25.Simão A.P., Avelar N.C., Tossige-Gomes R. Functional performance and inflammatory cytokines after squat exercises and whole-body vibration in elderly individuals with knee osteoarthritis. Arch Phys Med Rehabil. 2012 doi: 10.1016/j.apmr.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 26.Wang P., Yang L., Liu C. Effects of Whole Body Vibration Exercise associated with Quadriceps Resistance Exercise on functioning and quality of life in patients with knee osteoarthritis: a randomized controlled trial. Clin Rehabil. 2016;30(11):1074–1087. doi: 10.1177/0269215515607970. [DOI] [PubMed] [Google Scholar]
- 27.Alfredo P.P., Bjordal J.M., Dreyer S.H. Efficacy of low level laser therapy associated with exercises in knee osteoarthritis: a randomized double-blind study. Clin Rehabil. 2012 doi: 10.1177/0269215511425962. [DOI] [PubMed] [Google Scholar]
- 28.Kapci Yildiz S., Ünlü Özkan F., Aktaş İ., Şilte A.D., Yilmaz Kaysin M., Bilgin Badur N. The effectiveness of ultrasound treatment for the management of kneeosteoarthritis: a randomized, placebo-controlled, double-blind study. Turk J Med Sci. 2015;45:1187–1191. doi: 10.3906/sag-1408-81. [DOI] [PubMed] [Google Scholar]
- 29.Atamaz F.C., Durmaz B., Baydar M. Comparison of the efficacy of transcutaneous electrical nerve stimulation, interferential currents, and shortwave diathermy in knee osteoarthritis: a double-blind, randomized, controlled, multicenter study. Arch Phys Med Rehabil. 2012;93(5):748–756. doi: 10.1016/j.apmr.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 30.Stelian J., Gil I., Habot B. Improvement of pain and disability in elderly patients with degenerative osteoarthritis of the knee treated with narrow-band light therapy. J Am Geriatr Soc. 1992;40(1):23–26. doi: 10.1111/j.1532-5415.1992.tb01824.x. http://www.ncbi.nlm.nih.gov/pubmed/1727843 [DOI] [PubMed] [Google Scholar]
- 31.Montes-Molina R., Madronero-Agreda M.A., Romojaro-Rodriguez A.B. Efficacy of interferential low-level laser therapy using two independent sources in the treatment of knee pain. Photomed Laser Surg. 2009 doi: 10.1089/pho.2008.2315. [DOI] [PubMed] [Google Scholar]
- 32.Hegedus B., Viharos L., Gervain M., Gálfi M. The effect of low-level laser in knee osteoarthritis: a double-blind, randomized, placebo-controlled trial. Photomed Laser Surg. 2009 doi: 10.1089/pho.2008.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sánchez-Romero E.A., Pecos-Martín D., Calvo-Lobo C., Ochoa-Sáez V., Burgos-Caballero V., Fernández-Carnero J. Effects of dry needling in an exercise program for older adults with knee osteoarthritis. Medicine (Baltim) 2018;97(26) doi: 10.1097/MD.0000000000011255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scharf HP, Mansmann U, Streitberger K, et al. Acupuncture and knee osteoarthritis: a three-armed randomized trial. Ann Intern Med. 10.7326/0003-4819-145-1-200607040-00005 [DOI] [PubMed]
- 35.Chen D., Shen J., Zhao W. Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Res. 2017 doi: 10.1038/boneres.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anandkumar S., Sudarshan S., Nagpal P. Efficacy of kinesio taping on isokinetic quadriceps torque in knee osteoarthritis: a double blinded randomized controlled study. Physiother Theory Pract. 2014;30(6):375–383. doi: 10.3109/09593985.2014.896963. [DOI] [PubMed] [Google Scholar]
- 37.Aydoğdu O., Sari Z., Yurdalan S.U., Polat M.G. Clinical outcomes of kinesio taping applied in patients with knee osteoarthritis: a randomized controlled trial. J Back Musculoskelet Rehabil. 2017;30(5):1045–1051. doi: 10.3233/BMR-169622. [DOI] [PubMed] [Google Scholar]
- 38.Mutlu E.K., Mustafaoglu R., Birinci T., Ozdincler A.R. Does kinesio taping of the knee improve pain and functionality in patients with knee osteoarthritis?: a randomized controlled clinical trial. Am J Phys Med Rehabil. 2017;96(1):25–33. doi: 10.1097/PHM.0000000000000520. [DOI] [PubMed] [Google Scholar]
- 39.Ornetti P., Fortunet C., Morisset C. Clinical effectiveness and safety of a distraction-rotation knee brace for medial knee osteoarthritis. Ann Phys Rehabil Med. 2014;58(3):126–131. doi: 10.1016/j.rehab.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Dhillon H., Dhilllon S., Dhillon M. Current concepts in sports injury rehabilitation. Indian J Orthop. 2017;51(5):529. doi: 10.4103/ortho.IJOrtho_226_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ardern C.L., Glasgow P., Schneiders A. Consensus statement on return to sport from the first World congress in sports physical therapy, bern. Br J Sports Med. 2016;50(14):853–864. doi: 10.1136/bjsports-2016-096278. 2016. [DOI] [PubMed] [Google Scholar]
- 42.Capilla E., Rosen C., Gilsanz V., Pessin J., Judex S., Rubin C. Mechanical stimulation of mesenchymal stem cell proliferation and differentiation promotes osteogenesis while preventing dietary-induced obesity. Fac Res. January 2009;1(24) doi: 10.1359/JBMR.080817. https://mouseion.jax.org/stfb2000_2009/1935 2000 - 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duty A.O., Oest M.E., Guldberg R.E. Cyclic mechanical compression increases mineralization of cell-seeded polymer scaffolds in vivo. J Biomech Eng. 2007;129(4):531. doi: 10.1115/1.2746375. [DOI] [PubMed] [Google Scholar]
- 44.Hodde J.P., Badylak S.F., Shelbourne K.D. The effect of range of motion on remodeling of small intestinal submucosa (SIS) when used as an achilles tendon repair material in the rabbit. Tissue Eng. 1997;3(1):27–37. [Google Scholar]
- 45.Zacharias A., Green R.A., Semciw A.I., Kingsley M.I.C., Pizzari T. Efficacy of rehabilitation programs for improving muscle strength in people with hip or knee osteoarthritis: a systematic review with meta-analysis. Osteoarthritis Cartilage. 2014;22(11):1752–1773. doi: 10.1016/j.joca.2014.07.005. [DOI] [PubMed] [Google Scholar]