Abstract
Background
Microscopic observation drug susceptibility (MODS) assay has been suggested as a low cost method for rapid, accurate detection of tuberculosis (TB) and multidrug resistant tuberculosis (MDR-TB).
Methods
A total of 2424 samples collected from 1063 eligible patients of suspected pulmonary or extrapulmonary TB were subjected to MODS assay. Performance of MODS was compared with culture and drug susceptibility testing (DST) by conventional solid Lowenstein–Jensen (LJ) media or liquid Mycobacteria Growth Indicator Tube (MGIT) culture.
Results
When compared to reference gold standard of positivity in either solid or liquid reference culture, the MODS assay had sensitivity, specificity, positive predictive value and negative predictive value of 91.3%, 98.2%, 96.0% and 95.9% respectively. MODS took a median time of 10.3 days to culture positivity as compared to 13.8 days using MGIT and 30.5 days using LJ culture. Culture and DST being concurrent in MODS, the median turnaround time for DST was the same as that for culture i.e. 10.3 days. The overall median turn around time for culture positivity and DST using manual MGIT and LJ medium was 23.6 days and 61.2 days respectively. The concordance between MODS culture and the reference susceptibility method was 97.7% for rifampicin, 95.6% for isoniazid, 98.5% for rifampicin and isoniazid. The cost of performing a single MODS assay was INR 200.
Conclusion
MODS is a rapid and sensitive, yet simple and inexpensive test that may be helpful to enhance diagnostic accuracy, and case detection of TB and MDR-TB in resource constrained settings.
Keywords: MODS assay, Tuberculosis, MDR tuberculosis
Introduction
Tuberculosis (TB) remains a major public health problem in developing countries where resources to diagnose the disease are scarce. India alone is estimated to have 2.2 million cases of the total global incidence of 9.6 million. The problem is further complicated by emergence and spread of the drug resistance. Globally 3.3% of new TB cases and 20% of previously treated cases are estimated to have multidrug resistant (MDR) TB-defined as resistant to both isoniazid (INH) and rifampicin.1 Rapid and accurate TB diagnosis and identification of drug resistance is required for timely initiation of correct treatment and control of the disease.
Smear microscopy for acid-fast bacilli (AFB) is the most commonly used diagnostic test for TB in developing countries. The test, although rapid, cheap and easy to perform, lacks sensitivity, cannot distinguish viable from nonviable bacteria and does not provide any information on drug resistance. Culture of Mycobacterium tuberculosis (MTB) remains the gold standard for both diagnosis and drug susceptibility testing (DST). Conventional culture on solid media like Lowenstein–Jensen (LJ) medium, while cheap and simple, have the major disadvantage of being very slow requiring 20–56 days for diagnosis and further 4–6 weeks for drug susceptibility testing.
Culture in liquid media (e.g. 7H9 Middlebrook media) is more sensitive and faster than conventional solid media. In 2007, the World Health Organization (WHO) endorsed use of liquid culture technology but due to high cost and complexity of commercial automated liquid culture systems its use is limited to few referral laboratories in developing countries. The microscopic-observation drug susceptibility (MODS) assay, a liquid culture based test, has been described as a rapid, sensitive and inexpensive test for detection of TB and MDR-TB directly from clinical specimen.2, 3, 4, 5, 6 Growth of MTB is detected by microscopic observation of characteristic spiral or comma shaped microcolonies of growing mycobacteria in liquid culture under an inverted microscope. Concurrent growth in drug-containing media indicates resistance to that drug. The assay was first described by the tuberculosis working group in Peru in the year 2000.7 The test was further refined by Moore et al.8 and a standard operating procedure (SOP) of the methodology is available at http://www.modsperu.org/. Introduction of such rapid and simple, yet inexpensive test in developing countries like India is timely for improving both patient management and infection control. The study was therefore undertaken to evaluate the performance of MODS assay in comparison to conventional methods.
Material and methods
This diagnostic study was conducted in a tertiary care respiratory centre of India. The clinical laboratory of the hospital is the referral laboratory for mycobacteriology. The proposal of this study was approved by Hospital Ethics Committee.
Case selection
Consecutive patients with suspected pulmonary or extra-pulmonary tuberculosis were enrolled for the study. Study inclusion criteria were age ≥ 18 years and assessed by a chest physician who thought a diagnosis of tuberculosis was possible. Patients suspected to have relapse or treatment failure were also included. Both male and female patients from out patients department and inpatient wards of the hospital were enrolled. Specimens submitted for routine analysis in mycobacteriology laboratory were studied and were categorized as pulmonary or extrapulmonary based on site of involvement.
Clinical specimens
Patients with suspected pulmonary tuberculosis provided minimum of 2 sputum samples with at least one early morning specimen. All specimens were screened using Auramine O fluorescent stain followed by Ziehl Neelsen stain confirmation. Smear negative patients with high clinical suspicion of pulmonary tuberculosis were subjected to bronchoscopy and bronchioalveolar lavage (BAL) fluid/bronchoscopic biopsies were also submitted for mycobacterial evaluation. A minimum of 1 and a maximum of 3 samples for suspected extra pulmonary tuberculosis were included in the study.
Biosafety
Mycobacteriology procedures requiring biosafety, such as sample processing, smear preparation, inoculation of media, identification, inoculums preparation and drug susceptibility testing, were performed in two Class II Biosafety Cabinets dedicated for mycobacterial work. The laboratory staff was well trained in biosafety procedures and used protective clothing (gown, gloves, cap etc.) and respiratory protection (N 95 masks) at all times.
Sample processing
Samples were collected in a sterile 15 ml falcon tube with a screw cap. All sputum samples were assessed macroscopically and digested and decontaminated within 4 h of collection using NALC–NaOH–sodium citrate modified Petroff's method and pelleted by centrifugation at 3000 × g for 20 min. Samples other then sputum which were likely to be contaminated, such as BAL, urine and pus were also processed as sputum. Body fluids, such as pleural fluid, ascitic fluid, CSF etc. and lymphnode aspirates were collected aseptically and were inoculated directly without decontamination. Specimens larger than 10 ml in volume were concentrated by centrifugation at 3000 × g for 20 min and thick or mucoid specimens were liquefied by adding NALC powder. Pellet obtained after centrifugation was resuspended in 1 ml of phosphate buffer saline. Resuspended pellet was used for making smears and for inoculation of MODS media, LJ slopes and manual Mycobacterium growth indicator tubes (MGIT) (Becton Dickinson and Company, USA). Remaining pellets was stored at −20 °C as backup.
MODS assay
MODS assay protocol was standardized based on earlier publications and standard operating procedure (SOP) given in the MODS website http://www.modsperu.org/(MODS User guide v12.1 14082008). One modification to the protocol made was the addition of paranitrobenzoic acid (PNB) well for identification of MTB complex and non-tuberculous mycobacteria (NTM). 0.5 ml of resuspended sample pellet was added to MODS medium containing 4 ml of Middlebrook 7H9 broth base (Becton Dickinson and Company, USA) supplemented with 0.5 ml OADC (Oleic-Albumin-Dextrose-Catalase) (Becton Dickinson and Company, USA) and 0.1 ml of reconstituted PANTA (polymyxin B, amphotericin B, nalidixic acid, trimethoprim and azlocillin) (Becton Dickinson and Company, USA) antibiotic mixture.
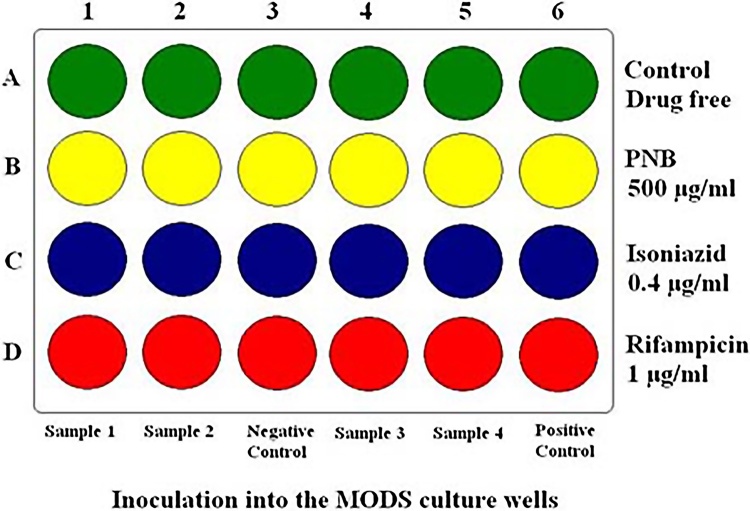
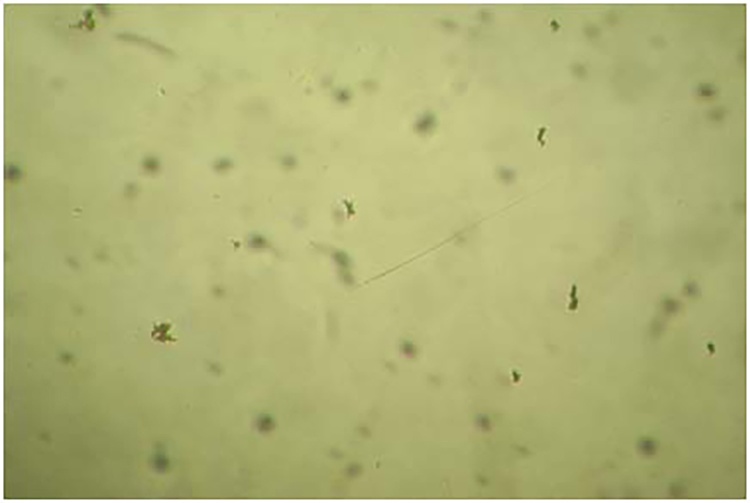
The MODS assay was performed using 24-well tissue culture plates. Each column of the plate was used to test one sample and four samples were run in a single plate. In each column 1 ml of drug-free broth was distributed in the first row. In the other three rows, 1 ml of broth with PNB at 500 μg/ml, INH at 0.4 μg/ml and rifampicin at 1 μg/ml, respectively, were distributed. Every plate included one column of four wells of negative control (no specimen) and four wells of positive control containing Mycobacterium tuberculosis H37Rv as a quality control for both culture and sensitivity testing (Fig. 1). The inoculated plate was labelled, sealed by scotch tape and placed in a transparent ziplock polyethylene bag. The plates were incubated at 37 °C and examined daily within the sealed bags from 4th to 21st day at 20× magnification under an inverted microscope. If no growth was observed by day 21, the final result was considered as negative. Positive cultures were identified by presence of microcolonies of mycobacteria in drug free control well in form of small curved commas or spiral which progress to form characteristic serpentine cord and later more irregular tangled growth (Fig. 2). Detection of two or more microcolonies (≥2 cfu) in drug free control well was considered as positive. When a positive result was observed, PNB, INH, and rifampicin containing wells were read on the same day. Absence of growth in PNB well combined with cord formation in drug free control well was considered MTB complex growth. Presence of growth in drug-containing wells indicated resistance to that drug. If bacterial or fungal contamination was detected, backup pellets were reprocessed and tested again.
Fig. 1.
Diagrammatic representation of the MODS procedure.
Fig. 2.
Positive MODS culture identified by mycobacterial growth in form of small curved commas or spiral (20×).
Solid and liquid culture, identification and DST
Solid culture was performed on LJ medium slants which were incubated at 37 °C and inspected weekly for 8 weeks or until growth of characteristic colonies was observed. Liquid culture was performed in 4 ml manual MGIT tubes as per manufacturer's instructions. Inoculated tubes were incubated at 37 °C. The smear positive specimen MGIT tubes were read daily using Micro MGIT fluorescence reader while smear negative specimen MGIT tubes were read weekly till they became positive or for a maximum of 6 weeks.
Cultures found AFB positive by microscopy were further identified as MTB complex by presumptive cord formation in liquid media and MPT 64 Antigen immunochromatography assay (SD Bioline TB Ag MPT64 rapid kit). For all cultures identified as MTB complex drug susceptibility testing was performed for INH and rifampicin on manual MGIT as per manufacturers guidelines. Resistant strains detected on manual MGIT liquid culture were confirmed by repeating DST on LJ medium by 1% proportion method.
Reference gold standard
Combined results of solid and liquid culture methods were taken as reference gold standard. A positive gold standard result was defined as a positive culture for MTB in at least one of the culture method. A negative gold standard result was defined as any sample in which both culture methods yielded negative results or in which one was negative and the other indeterminate owing to contamination. A patient was considered to have TB if at least 1 sample was positive by reference gold standard.
Statistical analysis
Performance of MODS was calculated as compared to a combination reference gold standard of positivity in either solid or liquid reference culture. Diagnostic parameters such as sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) were calculated using 2 × 2 contingency tables. For agreement between the drug susceptibility test results of MODS and MGIT Kappa (κ) statistic was applied. All costs were estimated based on purchase price of consumables for detection by the different culture techniques and did not include infrastructure, equipment, labour or overhead expenses.
Results
1283 suspected tuberculosis patients were enrolled found for the study. 220 of the OPD patients were excluded from the study as they failed to provide at least 2 sputum samples and were lost to follow up. A total of 1063 eligible patients were included in the study. Majority of them were male (84.1%). Median age was 34 years. 890 cases had suspected pulmonary tuberculosis (83.7%) while 173 (16.3%) had suspected extrapulmonary tuberculosis. A total of 2424 samples were collected from eligible patients of suspected pulmonary or extrapulmonary tuberculosis. 2144 (88.4%) samples were found to be smear negative and 280 (11.6%) samples were smear positive.
Of the 280 smear positive specimen 247 (88.2%) were culture positive by MGIT and/or LJ reference culture method while 32 (11.4%) were culture negative. Of 2144 smear negative patients 275 (12.8%) were culture positive and 1829 (85.3%) samples were culture negative by both the reference culture method. Culture positivity according to sample types and smear status is shown in Table 1.
Table 1.
Culture positivity according to smear status and sample types (n = 2424).
| Number | Culture positive |
|||
|---|---|---|---|---|
| Smear positive | Smear negative | Total (%) | ||
| Pulmonary | ||||
| Sputum | 1859 | 230 | 226 | 456 (24.5%) |
| BAL | 289 | 7 | 15 | 22 (7.6%) |
| Biopsies (TBLB, endobronchial) | 70 | 1 | 12 | 13 (18.6%) |
| Extra-pulmonary | ||||
| FNAC | 35 | 4 | 3 | 7 (20%) |
| Pus | 20 | 3 | 5 | 8 (40%) |
| Body fluid aspirates | 136 | 2 | 14 | 16 (11.8%) |
| Others (CSF, urine, bone marrow) | 15 | 0 | 0 | 0 (0%) |
Culture result of 1 smear positive sample and 40 smear negative samples were inconclusive as both reference cultures were contaminated and excluded from the analysis. Contamination rate for MGIT liquid culture and LJ solid culture was 8.4% (n = 204) and 5.2% (n = 126) respectively. The initial contamination rate of MODS was 7% (n = 171). The samples that were contaminated during first run of MODS were decontaminated and tested again from the remaining pellets that were stored at −20 °C as backup. Final contamination rate of MODS after repeat culture was 1.8% (n = 44). Of the 44 MODS culture which remained contaminated even after repeat inoculation 33 were also contaminated by both reference liquid and solid culture. Remaining 11 samples that were contaminated by MODS even after repeat culture were also excluded. A total of 2372 samples were thus included in the final analysis.
Comparison of culture results of reference liquid and solid culture vs MODS (n = 2372) is shown in Table 2. Culture positivity rate was significantly higher in MODS (91.5%) than the solid LJ culture (75.6%) but lesser than liquid culture in MGIT (93.9%) taking combined reference gold standard of positivity in either solid or liquid reference culture. Per subject performance of the MODS assay for detection of tuberculosis in comparison to reference gold standard is shown in Table 3. When compared to reference gold standard the MODS assay had sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of 91.3% (95% CI, 87.8–94.0%), 98.2% (95% CI, 96.9–99.0%), 96.0% (95% CI, 93.4–97.6%) and 95.9% (95% CI, 94.4–97.1%) respectively.
Table 2.
Comparison of culture results of reference liquid and solid culture vs MODS assay (n = 2372).
| MGIT |
LJ |
MGIT/LJ |
Total | ||||
|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Positive | Negative | ||
| MODS positive | 409 | 23 | 326 | 106 | 419 | 13 | 432 |
| MODS negative | 34 | 1906 | 31 | 1909 | 53 | 1887 | 1940 |
| Total | 443 | 1929 | 357 | 2015 | 472 | 1900 | 2372 |
Table 3.
Per subject performance of the MODS assay for detection of tuberculosis in comparison to reference gold standard (n = 1063).
| Reference gold standard (LJ/MGIT) |
Total | ||
|---|---|---|---|
| Positive | Negative | ||
| MODS positive | 314 | 13 | 327 |
| MODS negative | 30 | 706 | 736 |
| Total | 344 | 719 | 1063 |
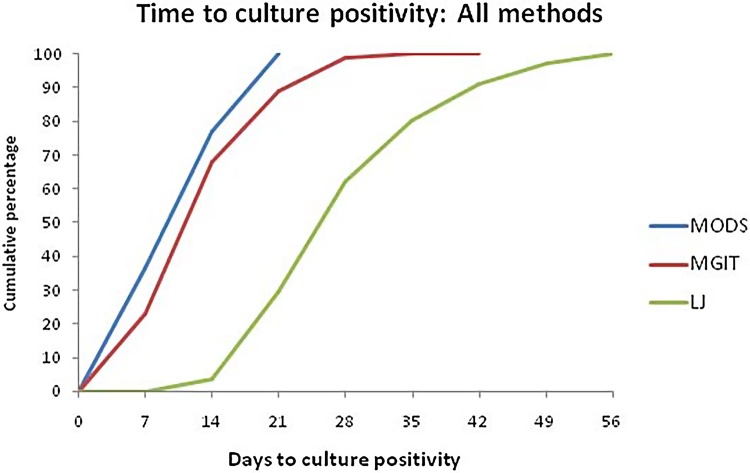
MODS took a median time of 10.3 days to culture positivity as compared to 13.8 days using MGIT and 30.5 days using LJ culture. The percentages of cultures that were positive at days 7, 14, and 21 were 36.4%, 77.2%, and 100%, respectively in MODS culture; 22.78%, 68.03%, and 88.92% in MGIT liquid culture; and 0%, 3.48%, and 29.43% in LJ solid culture (Fig. 3). The cumulative percentages of smear positive culture positive cases at days 7, 14, and 21 were 53.6%, 89.4%, and 100%, respectively, in MODS culture; 32.8%, 85%, and 96.6% in MGIT liquid culture; and 0%, 5.3%, and 40.6% in LJ solid culture while that of smear negative culture positive cases at days 7, 14, and 21 were 3.7%, 54.1%, and 100%, respectively, in MODS culture; 3.7%, 35.8%, and 74.3% in MGIT liquid culture; and 0%, 0%, and 8.3% in LJ solid culture.
Fig. 3.
Cumulative percentages of the time to culture positivity for 316 culture positive samples according to culture methods.
A total of 75 MTB isolates from 33 patients were found to be MDR strains using MGIT liquid culture. MODS correctly detected 71 of these strains confirming diagnosis of MDR-TB in 32 patients. False negative results occurred in 3 specimens from new cases and 1 specimen from relapse cases. The concordance between MODS culture and the reference susceptibility method was 97.7% for rifampicin, 95.6% for isoniazid, 98.5% for rifampicin and isoniazid. Overall MODS assay failed to detect only one new MDR case but misclassified 3 isolates as MDR that MGIT classified as susceptible (Table 4).
Table 4.
DST performance of the MODS assay in reference to DST on liquid MGIT culture for detection of MDR cases (n = 472).
| MGIT |
Total | |||
|---|---|---|---|---|
| Non MDR | MDR | |||
| MODS | Non MDR | 394 | 4 | 398 |
| MDR | 3 | 71 | 74 | |
| Total | 397 | 75 | 472 | |
Culture and DST being concurrent in MODS, the median turnaround time for DST was the same as that for culture i.e. 10.3 days. In comparison median turn around time for DST for smear positive and smear negative cases using manual MGIT was 20.2 and 30.1 days respectively. The overall median turn around time for culture positivity and DST using manual MGIT and LJ medium was 23.6 days and 61.2 days respectively. DST results for 89.4% of smear positive specimens and 54.1% of smear negative specimens by MODS assay were available within 14 days. MODS assay correctly identified 14 of 15 MDR cases amongst new cases and all 18 MDR cases amongst patients of TB relapse or treatment failure. The cost of performing a single MODS assay was INR 200, compared to INR 800 for LJ and INR 1750 for smear positive cases and INR 2050 for smear negative cases for culture and DST by manual MGIT.
Discussion
The MODS assay has been described as a rapid, inexpensive, and more sensitive test than other conventional solid or liquid culture-based tests.7, 8 We have tested the performance of MODS assay in comparison to combined reference gold standard of positivity in either solid LJ media or liquid MGIT reference culture. The study was conducted in clinical setting on patients suspected to have pulmonary or extra-pulmonary tuberculosis in a high TB-burden country with limited resources. In our study, culture positivity rate was significantly higher in MODS (91.5%) than the conventional solid LJ culture (75.6%) taking combined reference gold standard of positivity in either solid or liquid reference culture. Reagents used in MODS assay are same as that used in commercial MGIT system, hence it had comparable culture positivity rate (91.5%) as manual MGIT culture (93.9%). Overall MODS assay had sensitivity, specificity, PPV and NPV of 91.3%, 98.2%, 96.0% and 95.9% respectively. Similar results were also described in independent studies undertaken worldwide comparing MODS assay with conventional solid culture and/or automated mycobacterial liquid culture systems.2, 3, 7, 9, 10, 11, 12
In our study there were 13 false positive cases by MODS assay which were negative by both reference culture methods. Among six reference culture negative MODS-positives cases, one case was smear-positive with radiological features suggestive of tuberculosis, and five others were smear negative suspected cases of tuberculosis who responded to empirical ATT, suggesting a false-negative reference culture in these six cases. Moore et al. have also reported better sensitivity of MODS then either LJ or automated mycobacterial liquid culture system.2 Balance seven false positive results likely represent cross-contamination from control strain or another positive specimen at the time of inoculation. This may have important implications on patient management and underscores the importance of adequate training of laboratory personnel before implementing MODS assay in laboratories.
In the current study 11.4% of smear positive samples were culture negative by both the reference methods. Theoretically, the percentage of smear positive culture negative specimens should be less than 1%.13 However, our centre being a referral centre for Tuberculosis patients receiving cases from all over India, patients are often transferred from peripheral hospitals after initiating Anti tuberculosis treatment (ATT) based on clinical or radiological features. Large percentage of smear-positive culture-negative specimens seen in our study is likely due to patients being already on ATT.
In the present study, the time to culture positivity was significantly shorter by MODS (10.3 days) when compared to reference manual MGIT liquid culture (13.8 days) and LJ solid culture (30.5 days). Moore et al. have also demonstrated faster growth rate of MODS than that of the automated MGIT liquid culture and LJ solid culture.2 In a study from Ethiopia the turn-around time of smear positive sputum samples by MODS culture was 9 days.4 In other Indian studies, the time to positivity by MODS has ranged from nine to ten days.9, 10, 11, 12 Our finding about time to culture positivity in reference MGIT liquid culture is rather different from those previous findings in smear negative cases because as per our study protocol smear negative MGIT tubes were being read only weekly.
Delay in diagnosis of MDR-TB leads to poor clinical outcome, increased mortality and continued transmission of drug resistant strains.14 Rapid detection of MDR-TB was possible by MODS assay with the median turnaround time of 10.3 days only. For the 472 culture positive specimen, drug susceptibility agreement between MODS and MGIT reference susceptibility method was excellent. For testing of INH and Rifampicin resistance, breakpoint concentrations 0.4 μg/ml and 0.1 μg/ml respectively were used. The concordance between MODS culture and the reference susceptibility method was 97.7% for rifampicin, 95.6% for INH and 98.5% for rifampicin and INH both. Minion et al. analyzed 9 studies published on MODS for the detection of drug resistance in a systemic review and reported similar pooled estimates for detection of INH and rifampicin resistance.15 Bwanga et al., in their meta-analysis, also reported good performance of MODS despite variable pooled sensitivity and specificity in detection of INH resistance.16
In our study MODS assay correctly identified 14 of 15 MDR cases amongst new cases and all 18 MDR cases amongst patients of TB relapse or treatment failure. Early detection of these cases is important as these patients would probably require a change in regimen. Three isolates misclassified as MDR by direct DST-MODS could be due to high bacterial load present in the processed sample as all had initial smear grading of 4+.
In the present study the estimated cost of consumables per sample for MODS assay was INR 200. This was substantially cheaper than conventional culture and drug susceptibility methods. Estimated working cost of the MODS in other studies has been reported to be approximately 2 USD per sample.2, 7, 17 Lazarus et al., in the year 2012, reported running cost of MODS to be INR 250 per sample.10
In this study, MODS assay detected MTB in both pulmonary and extrapulmonary specimens with good sensitivity and better speed and correctly identified MDR strains in less time than did solid LJ or liquid MGIT cultures. Negative MODS culture plates are required to be incubated for only 21 days rather than 42 days as in liquid MGIT or 8–12 weeks as in solid LJ.
The liquid media are more prone to contamination. The present study showed a contamination rate of 7% with MODS assay, 8.4% for MGIT and 5.2% with LJ medium. The contamination rate in our study is similar to that reported in literature.18, 19 Biosafety level II facilities combined with stringent individual protection is required for MODS assay. The risk of aerosol generation in MODS assay is mainly during sample processing and plate inoculation. Thereafter edges of plate are sealed by scotch tape, placed in transparent ziplock bags and never opened.18 Any spillage due to mishandling can only lead to spoiled culture. On completion of 21 days sealed plates are discarded by autoclaving at 121 °C for 60 min.
Once standardized, it is feasible to perform MODS assay in resource constrained settings. All consumables and reagents are easily available and non proprietary. Apart from an inverted light microscope the other equipment required for MODS assay would usually be available in an existing TB culture laboratory.
Based on the results of the present study and available literature, it can be stated that MODS assay can be used for early and accurate detection of MTB and MDRTB. Given its simplicity, low cost and reduced turnaround time, it would be an excellent method for routine tuberculosis testing in developing countries.
Conflicts of interest
The authors have none to declare.
Acknowledgements
This paper is based on Armed Forces Medical Research Committee Project No 4077/2010 granted and funded by the office of the Directorate General Armed Forces Medical Services and Defence Research Development Organization, Government of India.
References
- 1.WHO. Global Tuberculosis Report 2015. 20th ed. WHO/HTM/TB/2015.22. Geneva: World Health Organization. http://www.who.int/tb/publications/global_report/en/ Accessed 26.12.15.
- 2.Moore D.A., Evans C.A., Gilman R.H. Microscopic-observation drug-susceptibility assay for the diagnosis of TB. N Engl J Med. 2006;355:1539–1550. doi: 10.1056/NEJMoa055524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore D.A., Mendoza D., Gilman R.H. Microscopic observation drug susceptibility assay, a rapid, reliable diagnostic test for multidrug-resistant tuberculosis suitable for use in resource-poor settings. J Clin Microbiol. 2004;42:4432–4437. doi: 10.1128/JCM.42.10.4432-4437.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ejigu G.S., Woldeamanuel Y., Shah N.S., Gebeyehu M., Selassie A., Lemma E. Microscopic observation drug susceptibility assay provides rapid and reliable identification of MDR-TB. Int J Tuberc Lung Dis. 2008;12:332–337. [PubMed] [Google Scholar]
- 5.Shiferaw G., Woldeamanuel Y., Gebeyehu M., Girmachew F., Demessie D., Lemma E. Evaluation of microscopic observation drug susceptibility assay for detection of multidrug resistant Mycobacterium tuberculosis. J Clin Microbiol. 2007;45:1093–1097. doi: 10.1128/JCM.01949-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arias M., Mello F.C., Pavon A. Clinical evaluation of the microscopic observation drug susceptibility assay for detection of tuberculosis. Clin Infect Dis. 2007;44:674–680. doi: 10.1086/511639. [DOI] [PubMed] [Google Scholar]
- 7.Caviedes L., Lee T.S., Gilman R.H. Rapid, efficient detection and drug susceptibility testing of Mycobacterium tuberculosis in sputum by microscopic observation of broth cultures. The Tuberculosis Working Group in Peru. J Clin Microbiol. 2000;38:1203–1208. doi: 10.1128/jcm.38.3.1203-1208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caviedes L., Moore D.A. Introducing mods: a low-cost, low-tech tool for high-performance detection of tuberculosis and multidrug resistant tuberculosis. Indian J Med Microbiol. 2007;25:87–88. doi: 10.4103/0255-0857.32711. [DOI] [PubMed] [Google Scholar]
- 9.Limaye K., Kanade S., Nataraj G., Mehta P. Utility of microscopic observation of drug susceptibility (MODS) assay for Mycobacterium tuberculosis in resource constrained settings. Indian J Tuberc. 2010;57:207–212. [PubMed] [Google Scholar]
- 10.Lazarus R.P., Kalaiselvan S., John K.R., Michael J.S. Evaluation of the microscopic observational drug susceptibility assay for rapid and efficient diagnosis of multi-drug resistant tuberculosis. Indian J Med Microbiol. 2012;30:64–68. doi: 10.4103/0255-0857.93039. [DOI] [PubMed] [Google Scholar]
- 11.Michael J.S., Daley P., Kalaiselvan S. Diagnostic accuracy of the microscopic observation drug susceptibility assay: a pilot study from India. Int J Tuberc Lung Dis. 2010;14:482–488. [PMC free article] [PubMed] [Google Scholar]
- 12.Solomon S., Balakrishnan P., Vignesh R. A rapid and low-cost microscopic observation drug susceptibility assay for detecting TB and MDR-TB among individuals infected by HIV in South India. Indian J Med Microbiol. 2013;31:130–137. doi: 10.4103/0255-0857.115225. [DOI] [PubMed] [Google Scholar]
- 13.Lipsky B.A., Gates J., Tenover F.C., Plorde J.J. Factors affecting the clinical value for acid-fast bacilli. Rev Infect Dis. 1984;6:214–222. doi: 10.1093/clinids/6.2.214. [DOI] [PubMed] [Google Scholar]
- 14.Dowdy D.W., Chaisson R.E., Moulton L.H., Dorman S.E. The potential impact of enhanced diagnostic techniques for tuberculosis driven by HIV: a mathematical model. AIDS. 2006;20:751–762. doi: 10.1097/01.aids.0000216376.07185.cc. [DOI] [PubMed] [Google Scholar]
- 15.Minion J., Leung E., Menzies D., Pai M. Microscopic-observation drug susceptibility and thin layer agar assays for the detection of drug resistant tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:688–698. doi: 10.1016/S1473-3099(10)70165-1. [DOI] [PubMed] [Google Scholar]
- 16.Bwanga F., Hoffner S., Haile M., Joloba M. Direct susceptibility testing for multi drug resistant tuberculosis: a meta-analysis. BMC Infect Dis. 2009;9:67. doi: 10.1186/1471-2334-9-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mengatto L., Chiani Y., Imaz M.S. Evaluation of rapid alternative methods for drug susceptibility testing in clinical isolates of Mycobacterium tuberculosis. Mem Inst Oswaldo Cruz. 2006;101:535–542. doi: 10.1590/s0074-02762006000500009. [DOI] [PubMed] [Google Scholar]
- 18.Moore D.A. Future prospects for the MODS assay in multidrug-resistant tuberculosis diagnosis. Future Microbiol. 2007;2:97–101. doi: 10.2217/17460913.2.2.97. [DOI] [PubMed] [Google Scholar]
- 19.Oberhelman R.A., Soto-Castellares G., Caviedes L. Improved recovery of Mycobacterium tuberculosis from children using the microscopic observation drug susceptibility method. Pediartrics. 2006;118:100–106. doi: 10.1542/peds.2005-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]