Abstract
Purpose
Stereotactic body radiation therapy (SBRT) is an effective treatment for oligometastatic or unresectable primary malignancies, although target proximity to organs at risk (OARs) within the ultracentral thorax (UCT) limits safe delivery of an ablative dose. Stereotactic magnetic resonance (MR)–guided online adaptive radiation therapy (SMART) may improve the therapeutic ratio using reoptimization to account for daily variation in target and OAR anatomy. This study assessed the feasibility of UCT SMART and characterized dosimetric and clinical outcomes in patients treated for UCT lesions on a prospective phase 1 trial.
Methods and Materials
Five patients with oligometastatic (n = 4) or unresectable primary (n = 1) UCT malignancies underwent SMART. Initial plans prescribed 50 Gy in 5 fractions with goal 95% planning target volume (PTV) coverage by 95% of prescription, subject to strict OAR constraints. Daily real-time online adaptive plans were created as needed to preserve hard OAR constraints, escalate PTV dose, or both, based on daily setup MR image set anatomy. Treatment times, patient outcomes, and dosimetric comparisons were prospectively recorded.
Results
All initial and daily adaptive plans met strict OAR constraints based on simulation and daily setup MR imaging anatomy, respectively. Four of the 5 patients received ≥1 adapted fraction. Ten of the 25 total delivered fractions were adapted. A total of 30% of plan adaptations were performed to improve PTV coverage; 70% were for reversal of ≥1 OAR violation. Local control by Response Evaluation Criteria in Solid Tumors was 100% at 3 and 6 months. No grade ≥3 acute (within 6 months of radiation completion) treatment-related toxicities were identified.
Conclusions
SMART may allow PTV coverage improvement and/or OAR sparing compared with nonadaptive SBRT and may widen the therapeutic index of UCT SBRT. In this small prospective cohort, we found that SMART was clinically deliverable to 100% of patients, although treatment delivery times surpassed our predefined, timing-based feasibility endpoint. This technique is well tolerated, offering excellent local control with no identified acute grade ≥3 toxicity.
Introduction
Stereotactic body radiation therapy (SBRT) is an established treatment modality for unresectable primary or metastatic thoracic malignancies. Increased radiation dose correlates with increased tumor control, and delivery of a biologically effective dose of 100 Gy or more has been found to improve survival outcomes in SBRT for early-stage lung cancers.1, 2, 3 However, within the central thorax, dose-escalated therapy has previously resulted in excess toxicity because of the proximity of critical organs at risk (OARs).4, 5 Inherent uncertainties in targeting precision and accuracy, attributable to both inter- and intrafraction OAR motion as well as setup and gating inaccuracies, may contribute to this risk.
Toxicity risks of SBRT may be highest in ultracentral thorax (UCT) malignancies. Here we define UCT tumors as those with gross tumor volumes (GTVs) directly abutting the mainstem bronchi, carina, or contents of the mediastinum, although some definitions also include tumors whose planning target volumes abut or overlap these structures.6, 7 Hypofractionated stereotactic radiation therapy is one reported approach to central and ultracentral lesions, predicated on the theory that a more fractionated course, using 6 to 15 fractions (rather than ≤5 as with SBRT), could enhance OAR protection.8, 9 However, in a reported series of hypofractionated stereotactic radiation therapy for ultracentral tumors, toxicity remained a concern and the radiobiologic evidence for improved OAR protection using this method is inconclusive.6
As an alternative method, SBRT approaches using improved image guidance and adjusting for changes in tumor and OAR anatomy through online adaptive radiation therapy could also improve OAR protection. Magnetic resonance (MR) image (MRI) guided radiation therapy (MR-IGRT) offers superior soft tissue visualization for sites such as the mediastinum and is increasingly practiced in a variety of disease sites.10, 11, 12 A recently published phase 1 trial of stereotactic MR-guided online adaptive radiation therapy (SMART) described application of this technique in the abdomen.13 A simulated study of SMART previously indicated potential dosimetric benefits in the UCT, including improvement of the therapeutic index through daily treatment plan reoptimization based on daily volumetric setup imaging and treatment monitoring with cine MR-guided gating during treatment delivery.14 Prospective clinical evaluation of this technique to improve the therapeutic index of SBRT for UCT disease has not previously been reported.
To prospectively assess the feasibility and safety of SMART, we a conducted a phase 1 clinical trial of this technique for oligometastatic and unresectable primary malignancies. The primary endpoint was feasibility, defined by delivery of adaptive treatment in <80 minutes of on-table time for >75% of cases. Here we report the results of this study in patients with UCT malignancies. We hypothesized that SMART would be feasible and deliver ablative radiation doses with acceptable rates of acute thoracic toxicity.
Methods and Materials
Patient eligibility
This protocol was approved by Washington University's institutional review board (NCT 02264886). Enrolled patients were considered clinical and technical SBRT candidates and had oligometastatic or unresectable primary UCT malignancies. We defined oligometastatic disease as ≤3 progressive disease sites. Eligible patients had ≥1 site amenable to UCT SBRT, were ≥18 years old, had a Karnofsky performance status score of ≥70, had the capacity to provide consent, and had disease of solid tumor (nonhematologic) classification, excluding small cell cancers; characteristics are provided in Table 1. Any preceding systemic therapy was held ≥1 week before planned start of SMART, with no plans to reinitiate systemic therapy for ≥1 week after SMART completion. Exclusion criteria comprised history of radiation within the projected treatment field, ongoing receipt of other investigational agents, uncontrolled intercurrent illness, pregnancy and/or breastfeeding, or any contraindication to MRI.
Table 1.
Patient and disease characteristics
| Characteristic | Number or Median (range) |
|---|---|
| Median age (range), y | 64 (45-76) |
| Median tumor size (range), cm | 3.1 (1.1-5.6) |
| Median prior chemotherapy regimens (range) | 1 (0-9) |
| Median KPS (range) | 90 (70-90) |
| Disease histology | |
| Primary non-small cell lung cancer | 1 |
| Papillary thyroid carcinoma metastasis | 1 |
| Colorectal cancer metastasis | 1 |
| Spindle cell sarcoma metastasis | 1 |
| Renal cell carcinoma metastasis | 1 |
| Disease subsite | |
| Invading pericardium | 2 |
| Invading mainstem bronchus | 2 |
| Abutting esophagus | 1 |
| Acute (within 6 mo) grade 3+ toxicities | 0 |
| Late (>6 mo) treatment-related toxicities | 1 |
| Esophageal stricture 15 mo posttreatment | 1 |
Abbreviations: KPS = Karnofsky Performance Status.
Simulation and initial plan
The initial treatment planning process and details of treatment simulation, as well as the MR-IGRT treatment device, its built-in dedicated treatment planning system, and imaging characteristics, have been previously described.13, 15, 16, 17 Briefly, we used a commercially available MRIdian (ViewRay, Sunnyvale, CA) MR-IGRT unit, consisting of a low-field split-solenoid 0.35 Tesla MRI unit straddling a ringed gantry with 3 multileaf collimator–equipped 60Co heads spaced 120° apart. All patients underwent computed tomography (CT) and MRI simulation with custom immobilization, per standard clinical protocol. Exhale breath-hold simulation CT scans served as primary image sets for treatment planning density information, with registration of simulation MR image sets to aid physician delineation of target volumes and OARs.
Prescription dose for all plans was 50 Gy in 5 fractions, with goal 95% planning target volume (PTV) coverage by 95% of prescription dose (47.5 Gy). Prescription coverage was subject to hard OAR constraints, as delineated in Table 2, Table 3, using a strict isotoxicity approach. A 5-mm volumetric expansion on the GTV was used to generate the PTV. A strict isotoxicity approach was used such that if goal PTV coverage could not be achieved without OAR constraint violation, then coverage of the PTV was sacrificed to meet OAR constraints.
Table 2.
Organ-at-risk dosimetry
| Organ at risk | Hard constraint | No. of PI constraint violations | Mean ± SD | Median | Range |
|---|---|---|---|---|---|
| Uninvolved lung | 1500 cm3 <12.5 Gy | NA | NA | NA | NA |
| (lung GTV) | 1000 cm3 <13.5 Gy | NA | NA | NA | NA |
| Trachea max | V50 Gy <0.2 cm3 | 3 | 11.32 ± 1.83 cm3 | 10.97 cm3 | 9.68-13.30 cm3 |
| Bronchial tree max | V50 Gy <0.2 cm3 | NA | NA | NA | NA |
| Esophageal max | V32 Gy ≤0.5 cm3 | 3 | 3.24 ± 1.27 cm3 | 3.09 cm3 | 2.05-4.58 cm3 |
| Heart/pericardium | V32 Gy ≤15 cm3 | 3 | 23.38 ± 6.67 cm3 | 25.66 cm3 | 15.87-28.62 cm3 |
| Cord | V25 Gy <1.0 cm3 | 3 | 1.87 ± 0.09 cm3 | 1.84 cm3 | 1.72-1.89 cm3 |
| Stomach max | V33 ≤0.5 cm3 | 1 | 1.19 cm3 | 1.19 cm3 | NA |
Abbreviations: GTV = gross tumor volume; NA = not applicable; max = maximum; min = minimum; PI = initial nonadaptive plan; SD = standard deviation.
Table 3.
Target volume coverage
| Target volume | Goal coverage | Projected nonadaptive mean ± SD | Projected nonadaptive median (range) | Cumulative adaptive mean ± SD | Cumulative adaptive median (range) |
|---|---|---|---|---|---|
| PTV V50, % | NA | 54.9 ± 50.8 | 77.6 (0-98.9) | 73.1 ± 43.3 | 98.7 (0-100) |
| PTV V47.5, % | 95% | 57.4 ± 52.3 | 86.7 (0.1-100) | 75.6 ± 43.1 | 99.8 (0.5-100) |
| GTV V50, % | 100% | 59.3 ± 54.2 | 96.7 (0-100) | 76.2 ± 43.4 | 100 (0-100) |
| GTV V47.5, % | 100% | 60.0 ± 54.0 | 98.2 (0.5-100) | 78.8 ± 43.6 | 100 (1.1-100) |
| GTV V45, % | 100% | 80.7 ± 29.3 | 99.0 (33.0-100) | 86.3 ± 29.8 | 100 (33.0-100) |
| GTV max, Gy | NA | 58.5 ± 10.5 | 58.1 (47.8-72.3) | 62.5 ± 10.3 | 64.0 (49.1-76.5) |
| GTV min, Gy | NA | 48.5 ± 11.1 | 43.5 (37.9-61.0) | 53.0 ± 12.0 | 53.8 (40.3-70.1) |
Abbreviations: GTV = gross tumor volume; max = maximum; min = minimum; NA = not applicable; PI = initial nonadaptive plan; PTV = planning target volume; SD = standard deviation.
Online plan adaptation
The online plan adaptation and plan quality assurance (QA) processes used in this study were summarized previously.13, 15 In short, patients underwent daily volumetric MRI for localization and setup. The initial plan, comprising either the simulation-based plan or the most recently used adapted plan, was loaded onto the image set of the day, and the treating physician edited contour volumes manually, as needed. The pre-existing plan was then assessed on the anatomy of the day. If OAR violation or an opportunity for PTV coverage improvement was identified, a new adaptive plan was generated while the patient remained on the table. The daily adaptive plan was then assessed and compared with the pre-existing, nonadaptive initial plan on the basis of dose to OARs and target volumes. The patient was then treated with the plan preferred by the treating physician. A fraction-by-fraction, strict isotoxicity approach was used to assess OAR sparing, without OAR point dose accumulation. Any delivered adaptive treatment plan became the default, nonadaptive plan for subsequent treatment days.
Treatment delivery and cine MR gating
Standard clinical practices for use of MR guidance and cine MR gating as performed in this study have been previously described.13, 18 Real-time MR guidance including planar sagittal cine MR gating on the GTV (based on the exhale phase during free breathing) was employed for all fractions. Targets for cine gating window construction and gating settings were chosen by the treating physician at the time of each treatment fraction.
Treatment time and dosimetry data collection
Feasibility, defined by delivery of adaptive treatment in <80 minutes of on-table time for >75% of cases, was the primary study endpoint. Study physicians subjectively designed this endpoint based on the clinical estimation that patients would not tolerate treatments lasting longer than 80 minutes. This inference was based on treating physician opinion of typical patient tolerance of daily immobilized treatment positioning within the bore of an MR-IGRT device; at the time of the study there was a paucity of preceding prospective radiation therapy data to better estimate patient timing tolerances for MR-IGRT. Timing data, including door-to-door patient treatment time and subcomponents such as imaging, recontouring, replanning, and plan QA times, were prospectively recorded by the treating radiation therapists. Dosimetry metrics, including fraction-by-fraction OAR dose, cumulative GTV/PTV dose, and projected dose that would have been delivered without plan adaptation, were also prospectively recorded. Current technology is insufficient to reproducibly identify point volumes of deformable OARs for dose accumulation. However, GTV/PTV dose accumulation was achieved by a previously described method, using rigid alignment of the daily dose distribution from each fraction to the first fraction, based on the centroid of the GTV volume, and addition of the dose from all fractions.13
Patient follow-up, outcomes, and statistical analysis
Patient demographic characteristics and baseline Response Evaluation Criteria in Solid Tumors (RECIST) target tumor measurements of the treated lesion were collected pretreatment and during scheduled study follow-up at 6 weeks, 3 months, and 6 months after treatment. Treatment response by RECIST was prospectively assessed by study physicians at 3 and 6 months after treatment. Acute thoracic toxicity, defined as toxicity within 6 months of radiation therapy completion, was assessed prospectively at 6 weeks, 3 months, and 6 months. Toxicity was graded according to the Common Terminology Criteria for Adverse Events Version 4.0 (CTCAE v4). Late toxicities were evaluated by treating physicians through routine clinical care and detailed retrospective chart review by study physicians. Disease-free, progression-free, and overall survival metrics were prospectively assessed at 3 and 6 months posttreatment. Longer-term outcomes were subsequently determined by study physicians through careful chart review and routine clinical appointments. The Kaplan-Meier method was used to estimate local progression-free survival. Statistical analyses were completed using SAS Version 9.4 (SAS Institute, Inc, Cary, NC).
Results
Patient and tumor characteristics
Five patients were enrolled and treated per protocol to UCT sites. Four of the 5 patients received treatment to oligometastatic lesions, and 1 had an unresectable primary non-small cell lung cancer. Median follow-up time from completion of therapy was 14 months (range, 8-31 months). A complete summary of patient demographics and disease characteristics is available in Table 1.
Treatment planning and delivery
All initial plans met hard OAR constraints (Table 2) based on CT/MRI simulation anatomy. Adaptive plans met identical hard constraints based on daily volumetric setup MRI anatomy. All 5 patients completed planned treatment using a 5-fraction course of SMART, for a total of 25 delivered fractions. Ten of 25 total treatment fractions were delivered using online adapted plans based on superior OAR sparing (7 of 10) or improved PTV coverage (3 of 10) compared with the initial, nonadaptive plan. Four out of 5 patients required adaptive planning for ≥1 fraction. One patient had a tumor invading and attached to the pericardium without other adjacent OARs; the fixed tumor–pericardium spatial relationship was not found to change, and adaptation was not deemed advantageous in that patient. Summarization of disease subsites treated and the clinical reasons for plan adaptation for each patient are available in Table 1, Table 4.
Table 4.
Treatment delivery parameters
| Characteristic | Number (%) or Median (range) |
|---|---|
| Total delivered fractions | 25 |
| Total adapted fractions (% of total) | 10 (40) |
| Adapted for ≥1 OAR violation, % | 7 (28) |
| Adapted only to increase PTV coverage, % | 3 (12) |
| Median on-table time (range), min | 69 (22-117) |
| Median imaging (volumetric and gating acquisitions) time (range), min | 2 (1-5) |
| Median recontour time (range), min | 8 (2-13) |
| Median replan time (range), min | 11 (8-20) |
| Median QA time (range), min | 4 (2-6) |
| Median beam-on time (range), min | 27 (13-52) |
Abbreviations: OAR = organ at risk; PTV = planning target volume; QA = quality assurance.
The primary feasibility endpoint of delivery of >75% of treatment fractions in ≤80 minutes was not met. Although median on-table treatment time was 69 minutes (range, 22-117 minutes), only 68% (17 of 25) of treatment fractions were delivered in ≤80 minutes. Instead, 76% (19 of 25) of fractions were delivered in ≤90 minutes. A detailed breakdown of treatment time components is provided in Table 4.
OAR constraints
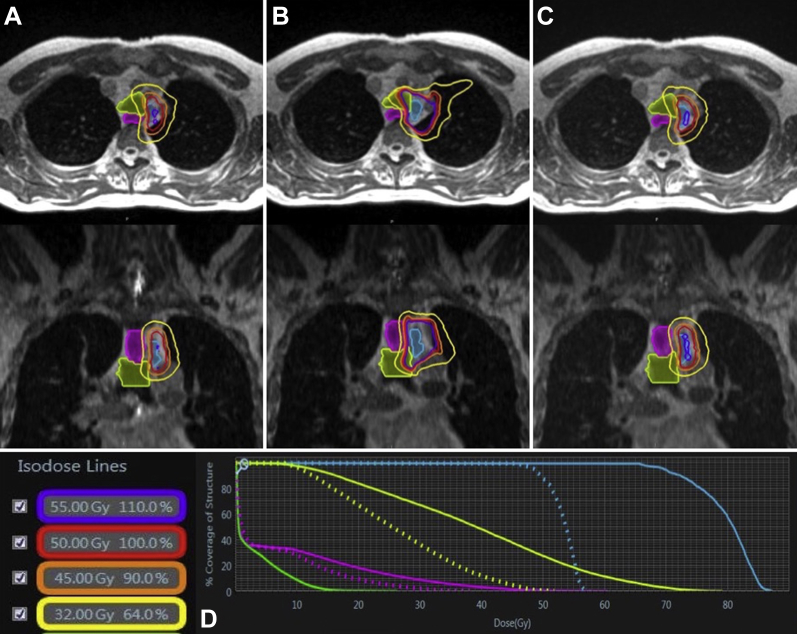
A total of 70% (7 of 10) of plan adaptations were due to need to reverse ≥1 hard OAR constraint violation that would have otherwise occurred had the initial, nonadaptive plan been delivered. An example case of plan adaptation to reverse OAR constraint violation that would have otherwise occurred is illustrated in Figure 1. OARs that would have received excess dose without adaptive planning included the esophagus (n = 3 averted violations), heart (n = 3), trachea (n = 3), stomach (n = 1), and spinal cord (n = 3). The magnitude by which constraints would have been exceeded was variable, as were the volumes of OARs that would have received excess dose; Figure 2 and Table 2 provide detailed summaries of OAR violations. With online adaptation, 100% of OAR violations that would have occurred were prevented.
Figure 1.
(A) A magnetic resonance (MR)–based, adaptive plan for fraction 1 (fx1) met all organ-at-risk (OAR) constraints based on daily setup anatomy from fx1. (B) Application of the fx1 plan to the fx2 MR image of a patient with an upper paratracheal metastasis (blue colorwash) abutting the esophagus (pink colorwash) resulted in violation of hard esophageal and trachea (green colorwash) constraints. (C) Daily adaptive planning for fx2 achieved OAR violation reversal while preserving target volume coverage, as determined by dose-volume histogram comparison (D). (A color version of this figure is available at https://dx.doi.org/10.1016/j.adro.2018.10.003.)
Figure 2.
Maximum scaled point doses projected to be delivered to constraint volumes of organs at risk (OARs) when initial nonadaptive plans were applied to daily anatomy. Blue circles indicate goal OAR constraints over 5 fractions. (A color version of this figure is available at https://dx.doi.org/10.1016/j.adro.2018.10.003.)
Target volume coverage
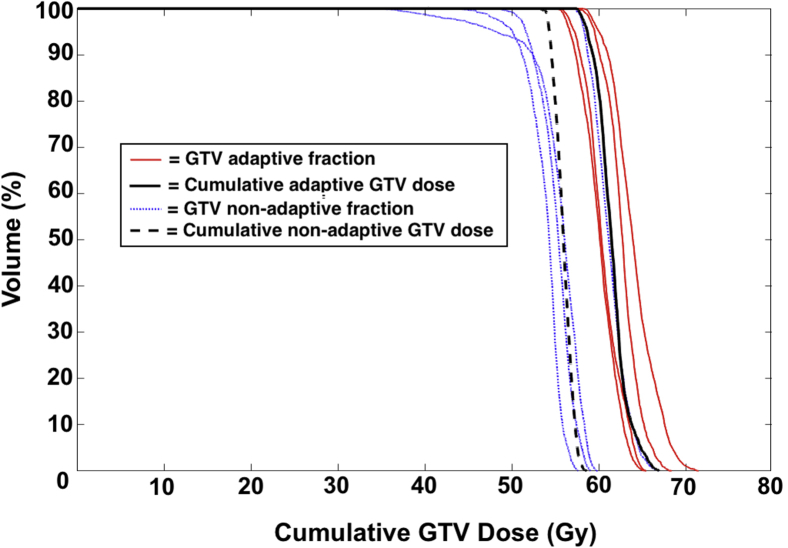
Online plan adaptation had variable effects on target coverage. Plan adaptation was performed in 3 of 10 adapted fractions for the primary indication of improved PTV coverage (Fig 1). By contrast, in most (5 of 7) fractions in which adaptation was indicated to reverse an OAR constraint violation, GTV and PTV coverage were reduced to protect OARs. An example of such reduction in target coverage to reverse multiple OAR constraints is demonstrated by the dose-volume histogram in Figure 1. In 2 fractions, OAR violations could be reversed concomitantly with improvements in PTV coverage. One patient treated for a spindle cell sarcoma metastasis invading the pericardium required dose reduction in the original CT simulation plan to 35 Gy to 95% of the PTV to meet the hard cardiac constraint. No change in tumor/OAR geometry was identified during daily MR-guided treatments, adaptation was not advantageous at any fraction, and the delivered dose remained 35 Gy to the PTV. Despite need to protect OARs, however, the mean and median cumulative GTV and PTV coverage were superior with SMART compared with the projected coverage that would have been delivered with nonadaptive SBRT. Improved cumulative coverage with SMART was found across all recorded metrics of GTV and PTV coverage (Table 3). In Figure 3, a dose-volume histogram demonstrates dose accumulation to the GTV for an example patient.
Figure 3.
Example dose-volume histogram (DVH) demonstrating the fraction-by-fraction delivered gross tumor volume (GTV) dose using online adaptation for a typical patient with an ultracentral right upper lobe lung metastasis. Proximity to the heart required plan adaptation for multiple fractions, with variable adaptive GTV dose escalation and de-escalation, but cumulative GTV dose remained at goal. Dose from adaptive fractions (red lines) and the projected dose from the original simulation plan that would have been delivered by nonadaptive fractions (blue dashed lines) are shown for comparison, as are the cumulative adaptive versus nonadaptive dose. The treatment fraction depicted here exemplifies the typical cumulative dose profile for all patients included in the study. (A color version of this figure is available at https://dx.doi.org/10.1016/j.adro.2018.10.003.)
Toxicity
Acute grade ≥3 CTCAE v4 treatment-related toxicity events, defined as occurring within 6 months of SMART completion, were not identified. With regard to late toxicity, 1 patient treated for an upper paratracheal spindle cell sarcoma nodal metastasis abutting the esophagus developed a CTCAE v4 grade 3 benign esophageal stricture 15 months after completion of radiation therapy, causing dysphagia and requiring elective endoscopic dilation. A second patient treated for a pericardial renal cell carcinoma metastasis (requiring a reduced dose of 35 Gy as per earlier) had regional progression 8 months after treatment, including pericardial studding and a metastasis in the left ventricular outflow tract, and developed a symptomatic pericardial effusion requiring pericardial window placement with subsequent heart failure. This grade 4 toxicity 8 months after radiation therapy completion could not be ruled out as possibly related to radiation treatment but was deemed likely secondary to disease progression.
Tumor control and survival
Local control of the treated lesion, defined as stable disease, partial response, or complete response by RECIST criteria, was 100% at 3 and 6 months. Local progression–free survival was 100% at 3 and 6 months and 80% at 12 months by Kaplan-Meier estimate. One locoregional failure outside the high-dose region was identified at 8-month follow-up. Overall survival was 100% at 6 months and 60% at 12 months. At last follow-up, 2 patients had no evidence of disease (19-month and 12-month follow-up), including the patient treated for a primary non-small cell lung cancer (adenocarcinoma). One patient had locoregional progression resulting in death from disease (9-month follow-up), and 2 patients had both regional and distant metastatic progression resulting in cancer-related death (14- and 16-month follow-up).
Discussion
In this prospective trial, we evaluated the feasibility of delivering SMART to UCT tumors. Although our subjective, clinician-determined timing endpoint was not met, we found that SMART was clinically deliverable and treatment was tolerated by 100% of patients. SMART offered dosimetric gains, including improved OAR sparing and greater target coverage. Acute grade 3 or higher toxicities within 6 months of radiation therapy were not identified, and control of the treated lesion was 100% at 3 and 6 months. Our findings suggest that SMART may offer a safe and novel approach to deliver ablative doses of radiation therapy to lesions within the UCT.
The primary endpoint of this study was feasibility of SMART. Before study initiation, the criteria for feasibility were subjectively designed by study physicians based on the perception that patients would not tolerate >80-minute treatment sessions. Given that this was the first prospective clinical trial of MR-IGRT and of SMART, there were no available preceding data regarding patient tolerance of remaining immobilized within an MRI bore in the treatment position, and thus this endpoint was chosen. Our endpoint of delivery of >75% of fractions in ≤80 minutes was unmet, although we did achieve delivery of >75% of fractions in ≤90 minutes, with a median treatment time of 69 minutes. Nevertheless, although the subjective feasibility criteria set for this study were unmet, we still conclude that SMART is clinically deliverable to the UCT. This is based on the observation that although treatment required more time than patients were anticipated to tolerate, treatment was successfully delivered for all patients, and all patients tolerated all treatment fractions, despite the length of time for delivery. We note that first implementations of multiple therapy modalities (that have since become standard of care in radiation oncology), including intensity modulated radiation therapy and nonadaptive robotic SBRT, initially required much longer delivery times.19, 20 Since the time of the present study, multiple improvements to the online adaptive process have been implemented at our institution, resulting in reduced treatment times. These include hiring of advanced radiation therapists to assist with recontouring, a more focused resegmentation window, improved instructions for covering physicians, and streamlining of QA procedures.21, 22 In addition, we recently installed a commercially available MR-guided linear accelerator, which may offer reduced beam-on times compared with the tri-cobalt source used in this study. Although treatment times were somewhat longer than anticipated in this early application of SMART, it is reasonable to anticipate that organized efforts toward process and technology improvements will improve future time demands of MR-IGRT.23
We also found that SMART offered dosimetric gains, both for OAR sparing and tumor coverage, compared with nonadaptive SBRT, with no observed grade 3+ acute toxicity. Online adaptive planning uncovered multiple unintended OAR constraint violations that would have otherwise occurred with nonadaptive SBRT. Although some constraints were minimally exceeded (Fig 2) and may not have resulted in toxicity, other violations were severe and may represent the historic portion of patients experiencing high-grade acute toxicities in SBRT to the central thorax.4 SMART reversed 100% of these constraint violations, including multiple point dose violations to the heart and proximal airways. Only 1 patient did not gain dosimetric advantage for OAR protection through online adaptive planning; in this patient, the tumor was fixed to the adjacent critical OAR (the heart). This particular case may highlight a key concept of online adaptive planning: Therapeutic gains through adaptation are likely to be most pronounced in clinical scenarios in which the spatial relationship between tumors and OARs is variable, whether by tumor response during therapy or mobility of the OAR relative to the tumor.
Although the size of our cohort (5 patients) is limited, zero grade 3+ acute toxicities and only 1 radiation-related reversible late grade 3 toxicity were identified, despite the high-risk disease site treated. Although the cohort in this pilot study is small, a prior study evaluating similarly ablative dose schedules with SBRT near the central bronchi reported acute grade 3 or higher toxicity rates of 33% for central thorax patients.24 Similarly, in the classic report by Timmerman et al,4 46% of patients experienced severe toxicity within 2 years of treatment. Although these experiences of substantial toxicity have discouraged use of highly ablative regimens in the UCT, it is possible that use of SMART may mitigate some toxicity risks.
Importantly, improvements in OAR sparing across the cohort did not compromise cumulative target coverage. Both cumulative GTV and PTV coverage were improved, on average, with SMART compared with projected nonadaptive plans. In several fractions, favorable daily anatomy was observed such that online adaptive planning could be performed specifically to increase target coverage. Local control of the treated lesion was excellent and compares favorably with other reports of SBRT to the central and peripheral thorax, with 100% of patients having control by RECIST criteria at 3 and 6 months posttreatment.25 The combined dosimetric and clinical findings of treatment safety and tumor control with improved target coverage suggest that SMART may offer a widened therapeutic index for ablative radiation therapy treatment in the UCT.
We acknowledge several limitations to this study. First, although fraction-by-fraction OAR dose was reduced using SMART in this small initial cohort, point dose accumulation to OARs was not performed. Dose deformation and accumulation of dose to OARs is the subject of ongoing research at our institution. However, reproducible methods to identify point volumes of deformable OARs to cumulatively track dose are not offered by current technology. Our fraction-by-fraction isotoxicity approach is therefore conservative, although it does beneficially ensure that no point volume of OAR can receive excess dose. Additionally, the on-board imaging component of the MR-IGRT device used in this study allowed for cine MR monitoring and gating in a single 2-dimensional plane, and it is possible that unobserved tumor/OAR motion in other planes during treatment delivery could diminish the therapeutic gains achieved through daily online adaptive planning. Although 3-dimensional volumetric real-time MR guidance and techniques to manage and adjust for intrafraction motion are of interest and may represent future standards of care, they are not clinically available at this time.26, 27 The imaging component of the device used in this study is also not of diagnostic quality, with magnet strength of 0.35 T. However, this low-field MRI component has been found to be sufficient and effective for clinical use in several clinics, including for SBRT and online adaptive applications.12, 21, 28 Finally, although new prospective evidence is emerging to support a survival benefit of ablative treatment for oligometastatic disease (as performed for the majority of patients in this study), use of oligoablation remains debatable in many tumor histologic types and is the subject of ongoing study.29, 30, 31 However, this clinical question is beyond the scope of our study, which primarily sought to determine feasibility of SMART as a novel radiotherapeutic approach to the UCT.
Conclusions
We found that SMART is a clinically deliverable, dosimetrically advantageous, and well-tolerated technique for ablation of UCT malignancies. SMART results in increased OAR sparing and simultaneous improvements in target coverage compared with traditional SBRT. Our future interests include further validation of SMART to the UCT in an expanded patient cohort as well as process improvements to streamline the clinical integration of online adaptive techniques for widespread clinical use.
Footnotes
Sources of support: This study was funded by an industry research grant from ViewRay, Inc. The funding source had no role or involvement in study design, data collection, data analysis, interpretation of results, the writing of the manuscript, or the choice to submit the manuscript for publication. The content is solely the responsibility of the authors.
This publication was additionally supported by the Washington University Institute of Clinical and Translational Sciences grant UL1T2000448 from the National Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of interest: The authors listed here report the following financial relationships (authors not listed reported no relevant financial relationships). Dr Henke reports grants from ViewRay, Inc, during the conduct of the study; and grants and other from ViewRay, Inc, outside the submitted work. Dr Green reports personal fees and other from ViewRay, Inc, outside the submitted work. Dr Mutic reports grants and other from ViewRay, Inc; grants and other from Varian Medical Systems; other from Philips Healthcare; other from Siemens; other from TreatSafely,LLC; and other from Radialogica,LLC, outside the submitted work. Dr Roach reports grants and personal fees from Varian, outside the submitted work. Dr Bradley reports grants and other from ViewRay, Inc; grants from Mevion Medical Systems, Inc; and other from Varian, outside the submitted work. Dr Parikh reports grants from Philips Healthcare; grants and other from Varian Medical Systems; other from Holaira,Inc; other from Medtronic/Covidien; and grants from ViewRay, Inc, outside the submitted work. Dr Kashani reports grants and personal fees from ViewRay, Inc; and grants and personal fees from Varian Medical Systems, outside the submitted work. Dr Robinson reports grants and other from Varian Medical Systems; and other from Radiologica LLC, outside the submitted work.
References
- 1.Salama J.K., Hasselle M.D., Chmura S.J. Stereotactic body radiotherapy for multisite extracranial oligometastases: Final report of a dose escalation trial in patients with 1 to 5 sites of metastatic disease. Cancer. 2012;118:2962–2970. doi: 10.1002/cncr.26611. [DOI] [PubMed] [Google Scholar]
- 2.Onishi H., Araki T., Shirato H. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: Clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer. 2004;101:1623–1631. doi: 10.1002/cncr.20539. [DOI] [PubMed] [Google Scholar]
- 3.Grills I.S., Hope A.J., Guckenberger M. A collaborative analysis of stereotactic lung radiotherapy outcomes for early-stage non-small-cell lung cancer using daily online cone beam computed tomography image guided radiotherapy. J Thorac Oncol. 2012;7:1382–1393. doi: 10.1097/JTO.0b013e318260e00d. [DOI] [PubMed] [Google Scholar]
- 4.Timmerman R., McGarry R., Yiannoutsos C. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833–4839. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- 5.Senthi S., Haasbeek C.J.A., Slotman Ben J. Outcomes of stereotactic ablative radiotherapy for central lung tumors: A systematic review. Radiother Oncol. 2013;106:276–282. doi: 10.1016/j.radonc.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Tekatli H., Haasbeek N., Dahele M. Outcomes of hypofractionated high-dose radiotherapy in poor-risk patients with “ultracentral” non-small cell lung cancer. J Thorac Oncol. 2016;11:1081–1089. doi: 10.1016/j.jtho.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhuri A.A., Tang C., Binkley M.S. Stereotactic ablative radiotherapy (SABR) for treatment of central and ultracentral lung tumors. Lung Cancer. 2015;89:50–56. doi: 10.1016/j.lungcan.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 8.Li Q., Swanick C.W., Allen P.K. Stereotactic ablative radiotherapy (SABR) using 70 Gy in 10 fractions for non-small cell lung cancer: exploration of clinical indications. Radiother Oncol. 2014;112:256–261. doi: 10.1016/j.radonc.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 9.Lagerwaard F.J., Haasbeek C.J.A., Smit E.F. Outcomes of risk-adapted fractionated stereotactic radiotherapy for stage I non-small-cell lung cancer. Radiat Oncol Biol. 2008;70:685–692. doi: 10.1016/j.ijrobp.2007.10.053. [DOI] [PubMed] [Google Scholar]
- 10.Noel C.E., Parikh P.J., Spencer C.R. Comparison of onboard low-field magnetic resonance imaging versus onboard computed tomography for anatomy visualization in radiotherapy. Acta Oncol. 2015;54:1474–1482. doi: 10.3109/0284186X.2015.1062541. [DOI] [PubMed] [Google Scholar]
- 11.Mutic S., Dempsey J.F. The ViewRay system: Magnetic resonance-guided and controlled radiotherapy. Semin Radiat Oncol. 2014;24:196–199. doi: 10.1016/j.semradonc.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Fischer-Valuck B.W., Henke L.E., Green O. Two-and-a-half-year clinical experience with the world's first magnetic resonance image guided radiation therapy system. Adv Radiat Oncol. 2017;2:485–493. doi: 10.1016/j.adro.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henke L., Kashani R., Robinson C.G. Phase I trial of stereotactic MR-guided online adaptive radiation therapy (SMART) for the treatment of oligometastatic or unresectable primary malignancies of the abdomen. Radiother Oncol. 2018;126:519–526. doi: 10.1016/j.radonc.2017.11.032. [DOI] [PubMed] [Google Scholar]
- 14.Henke L., Kashani R., Yang D. Simulated online adaptive magnetic resonance-guided stereotactic body radiation therapy for the treatment of oligometastatic disease of the abdomen and central thorax: Characterization of potential advantages. Int J Radiat Oncol Biol Phys. 2016;96:1078–1086. doi: 10.1016/j.ijrobp.2016.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Acharya S., Fischer-Valuck B.W., Kashani R. Online magnetic resonance image guided adaptive radiation therapy: First clinical applications. Int J Radiat Oncol Biol Phys. 2016;94:394–403. doi: 10.1016/j.ijrobp.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 16.Wooten H.O., Green O., Yang M. Quality of intensity modulated radiation therapy treatment plans using a 60Co magnetic resonance image guidance radiation therapy system. Int J Radiat Oncol Biol Phys. 2015;92:771–778. doi: 10.1016/j.ijrobp.2015.02.057. [DOI] [PubMed] [Google Scholar]
- 17.Hu Y., Rankine L., Green O.L. Characterization of the onboard imaging unit for the first clinical magnetic resonance image guided radiation therapy system. Med Phys. 2015;42:5828–5837. doi: 10.1118/1.4930249. [DOI] [PubMed] [Google Scholar]
- 18.Green O.L., Rankine L.J., Cai B. First clinical implementation of real-time, real anatomy tracking and radiation beam control. Med Phys. 2018 doi: 10.1002/mp.13002. [Epub ahead of print]. Accessed August 1, 2018. [DOI] [PubMed] [Google Scholar]
- 19.Chao K.S., Majhail N., Huang C.J. Intensity modulated radiation therapy reduces late salivary toxicity without compromising tumor control in patients with oropharyngeal carcinoma: A comparison with conventional techniques. Radiother Oncol. 2001;61:275–280. doi: 10.1016/s0167-8140(01)00449-2. [DOI] [PubMed] [Google Scholar]
- 20.Whyte R.I., Crownover R., Murphy M.J. Stereotactic radiosurgery for lung tumors: Preliminary report of a phase I trial. Ann Thorac Surg. 2003;75:1097–1101. doi: 10.1016/s0003-4975(02)04681-7. [DOI] [PubMed] [Google Scholar]
- 21.Bohoudi O., Bruynzeel A.M.E., Senan S. Fast and robust online adaptive planning in stereotactic MR-guided adaptive radiation therapy (SMART) for pancreatic cancer. Radiother Oncol. 2017;125:439–444. doi: 10.1016/j.radonc.2017.07.028. [DOI] [PubMed] [Google Scholar]
- 22.Altman M.B., Kavanaugh J.A., Wooten H.O. A framework for automated contour quality assurance in radiation therapy including adaptive techniques. Phys Med Biol. 2015;60:5199–5209. doi: 10.1088/0031-9155/60/13/5199. [DOI] [PubMed] [Google Scholar]
- 23.Kerkmeijer L.G.W., Fuller C.D., Verkooijen H.M. The MRI-Linear Accelerator Consortium: Evidence-based clinical introduction of an innovation in radiation oncology connecting researchers, methodology, data collection, quality assurance, and technical development. Front Oncol. 2016;6:215. doi: 10.3389/fonc.2016.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song S.Y., Choi W., Shin S.S. Fractionated stereotactic body radiation therapy for medically inoperable stage I lung cancer adjacent to central large bronchus. Lung Cancer. 2009;66:89–93. doi: 10.1016/j.lungcan.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 25.Stephans K.L., Woody N.M., Reddy C.A. Tumor control and toxicity for common stereotactic body radiation therapy dose-fractionation regimens in stage I non-small cell Lung cancer. Int J Radiat Oncol Biol Phys. 2018;100:462–469. doi: 10.1016/j.ijrobp.2017.10.037. [DOI] [PubMed] [Google Scholar]
- 26.Kontaxis C., Bol G.H., Lagendijk J.J.W. A new methodology for inter- and intrafraction plan adaptation for the MR-linac. Phys Med Biol. 2015;60:7485–7497. doi: 10.1088/0031-9155/60/19/7485. [DOI] [PubMed] [Google Scholar]
- 27.Raaymakers B.W., de Boer J.C., Knox C. Integrated megavoltage portal imaging with a 1.5 T MRI linac. Phys Med Biol. 2011;56:N207–N214. doi: 10.1088/0031-9155/56/19/N01. [DOI] [PubMed] [Google Scholar]
- 28.Wojcieszynski A.P., Rosenberg S.A., Brower J.V. Gadoxetate for direct tumor therapy and tracking with real-time MRI-guided stereotactic body radiation therapy of the liver. Radiother Oncol. 2016;118:416–418. doi: 10.1016/j.radonc.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 29.Iyengar P., Wardak Z., Gerber D.E. Consolidative radiotherapy for limited metastatic non–small-cell lung cancer. JAMA Oncol. 2018;4:e173501–e173508. doi: 10.1001/jamaoncol.2017.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chmura S.J., Winter K.A., Salama J.K. NRG BR002: A phase IIR/III trial of standard of care therapy with or without stereotactic body radiotherapy (SBRT) and/or surgical ablation for newly oligometastatic breast cancer. J Clin Oncol. 2016 34:15_suppl, TPS1098-TPS1098. [Google Scholar]
- 31.Herfarth K.K., Debus J., Lohr F. Stereotactic single-dose radiation therapy of liver tumors: results of a phase I/II trial. J Clin Oncol. 2001;19:164–170. doi: 10.1200/JCO.2001.19.1.164. [DOI] [PubMed] [Google Scholar]