Abstract
Purpose
We transitioned from a low-dose-rate (LDR) to a high-dose-rate (HDR) prostate brachytherapy program. The objective of this study was to describe our experience developing a prostate HDR program, compare the LDR and HDR dosimetry, and identify the impact of several targeted interventions in the HDR workflow to improve efficiency.
Methods and Materials
We performed a retrospective cohort study of patients treated with LDR or HDR prostate brachytherapy. We used iodine-125 seeds (145 Gy as monotherapy, and 110 Gy as a boost) and preoperative planning for LDR. For HDR, we used iridium-192 (13.5 Gy × 2 as monotherapy and 15 Gy × 1 as a boost) and computed tomography–based planning. Over the first 18 months, we implemented several targeted interventions into our HDR workflow to improve efficiency. To evaluate the progress of the HDR program, we used linear mixed-effects models to compare LDR and HDR dosimetry and identify changes in the implant procedure and treatment planning durations over time.
Results
The study cohort consisted of 122 patients (51 who received LDR and 71 HDR). The mean D90 was similar between patients who received LDR and HDR (P = .28). HDR mean V100 and V95 were higher (P < .0001), but mean V200 and V150 were lower (P < .0001). HDR rectum V100 and D1cc were lower (P < .0001). The HDR mean for the implant procedure duration was shorter (54 vs 60 minutes; P = .02). The HDR mean for the treatment planning duration dramatically improved with the implementation of targeted workflow interventions (3.7 hours for the first quartile to 2.0 hours for the final quartile; P < .0001).
Conclusions
We successfully developed a prostate HDR brachytherapy program at our institution with comparable dosimetry to our historic LDR patients. We identified several targeted interventions that improved the efficiency of treatment planning. Our experience and workflow interventions may help other institutions develop similar HDR programs.
Introduction
Brachytherapy is an important treatment modality for patients with prostate cancer. Multiple randomized trials have demonstrated improved biochemical control with combined external beam radiation therapy (EBRT) and brachytherapy boost compared with EBRT alone.1, 2, 3 Similarly, observational series suggest more durable biochemical control with brachytherapy monotherapy compared with dose-escalated EBRT.4, 5
Low-dose-rate (LDR) permanent interstitial implant is used by more brachytherapists and more frequently as the prostate brachytherapy modality for monotherapy and as a boost in the United States.6 There are multiple large series that define the long-term efficacy and safety of this approach.5, 7 However, increasing data support the use of high-dose-rate (HDR) prostate brachytherapy, and observational data suggest its potential dosimetric, radiobiologic, radiation safety, and toxicity profile advantages over LDR.8, 9, 10, 11, 12
Yet, aside from the debate should over the optimal type of brachytherapy and in juxtaposition with the growing evidence in support of brachytherapy-based treatment, the overall use of any type of brachytherapy in the treatment of prostate cancer in the United States is decreasing.13 Fewer centers and radiation oncologists have expertise in prostate brachytherapy. In addition, trainees in radiation oncology are gaining less prostate brachytherapy experience.14 These phenomena can make the development of brachytherapy programs challenging and daunting to practitioners, and raise concerns with regard to the future utilization of this important treatment modality in patients in the United States.
At our academic institution, we have had a history of an active LDR prostate brachytherapy program. In May 2015, we decided to develop an HDR prostate brachytherapy program to complement the LDR program. Through a collaboration with our multidisciplinary team, we treated our first patient in September 2015, and our program has grown and evolved since this time. This manuscript is meant to be a practical guide for centers that wish to develop an HDR prostate brachytherapy program. Specifically, our objectives were to describe our process to develop our program, assess HDR dosimetry in comparison with our prior LDR dosimetry, and describe how we improved our treatment planning efficiency through targeted workflow interventions.
Methods and Materials
Study design
We performed a retrospective cohort study of a prospectively maintained database of consecutive patients with localized prostate cancer who were treated with LDR or HDR brachytherapy by the same 2 prostate brachytherapy radiation oncologists. Institutional review board approval was obtained before conducting this study.
Low-dose-rate brachytherapy program
Our LDR workflow included a pre-implant volume study several weeks before the planned operative procedure, followed by target delineation and creation of a preoperative plan using stranded iodine-125 seeds to deliver a minimum peripheral dose of 145 Gy for monotherapy and 110 Gy for a boost with supplemental EBRT. On the day of the procedure, preloaded needles of stranded seeds were implanted jointly by the radiation oncologist and urologist using a transrectal, ultrasound-guided, perineal template approach and a stepper system. Patients were initiated on prophylactic tamsulosin, naproxen, and ciprofloxacin after the implant procedure, and typically received cefazolin in the operating room (OR) before the start of the procedure. Patients underwent Day 30 computed tomography (CT) simulation for postimplant dosimetry and implant quality assessment using the CT images for seed identification and target/normal tissue delineation. Our preplan and postimplant planning goals were based on American Brachytherapy Society guidelines.15
Preparation and planning logistics of the high-dose-rate program
We collaborated with our multidisciplinary genitourinary team to develop the practical aspects of the workflow. An important consideration for our team was to avoid hospitalization for the treatment delivery because this would limit the feasibility of HDR at our institution. Based on this, along with clinical data supporting the efficacy and safety, we selected 27 Gy in 2 fractions as monotherapy, delivered as a single fraction per implant performed 1 to 2 weeks apart and 15 Gy in 1 fraction/implant as a boost.16, 17 We believed that these dose/fractionations minimized the added operative procedures to the extent supported by the data available at the time. Although this dose/fractionation schedule avoided overnight hospitalizations, patients who received monotherapy had to undergo 2 operative procedures. Thus, our prostate caseload was limited by OR availability. Therefore, we worked with our ambulatory surgery center leadership to identify extra morning times to use the OR for our brachytherapy cases, which limited our schedule to 1 to 2 cases per day.
We adopted a free-hand, perineal, template-based approach for our ultrasound-guided catheter placement, similar to what was used at the institutions visited by the brachytherapy team. In addition, we used a CT-based postimplant treatment planning process. CT was readily available in our department and facilitated treatment planning in our usual brachytherapy workflow. The treatment planning system (Brachyvision, Varian Medical Systems, Palo Alto, CA) and the iridium-192 afterloader (GammaMed, Varian Medical Systems, Palo Alto, CA) were already commissioned and in use in our department.
The program was new; therefore, we also wanted to have robust quality management and peer review of cases. We previously reported on our department’s daily prospective contouring and planning rounds.18 We reviewed all HDR prostate brachytherapy cases at our daily contouring and planning round session before treatment delivery when possible. Cases were reviewed for appropriateness of treatment approach, implant quality, target delineation, and plan quality.
Patient selection
Patients with low-risk prostate cancer and those with intermediate-risk prostate cancer with multiparametric magnetic resonance imaging (MRI) evidence of no extracapsular extension (ECE) or seminal vesicle involvement (SVI) were typically treated with monotherapy. Patients with high-risk prostate cancer and those with intermediate-risk prostate cancer with ECE or SVI on multiparametric MRI underwent brachytherapy as a boost. Patients with intermediate-risk prostate cancer and ECE were treated with monotherapy if the ECE could be encompassed by the HDR implant; otherwise, these patients were candidates for HDR as a boost. Patients with a gland size ≥60 cc were assessed for anatomic suitability with a pre-implant volume study with transrectal ultrasound. Patients with International Prostate Symptom Score <20 were typically candidates, but exceptions to these criteria were made on a per-patient basis based on clinical discretion.
Workflow
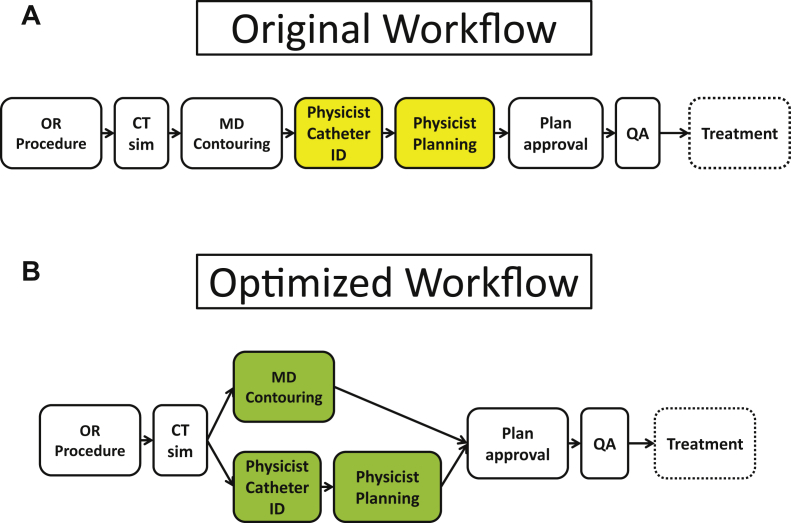
Figure 1A describes our original workflow. Briefly, patients presented to the ambulatory surgery center before the implant. Patients were instructed to take an enema the night before and the morning of the procedure as rectal preparation. In the OR, patients were administered general anesthesia, placed in the dorsal lithotomy position, and sterilely prepared and draped. A Foley catheter was placed with 7 cc of diluted contrast (1 cc iodinated contrast and 6 cc sterile water), and the scrotum was immobilized away from the perineum. A transrectal ultrasound was performed to ascertain the gland volume and help select the proper template size. Subsequently, catheters were placed through the perineum into the prostate with uniform distribution throughout the gland using the axial and sagittal planes on ultrasound.
Figure 1.
Institution's original (A) prostate high-dose-rate brachytherapy workflow and optimized workflow (B) after implementation of targeted workflow interventions from Table 1.
The ultrasound was advanced and retracted to follow the catheters because they were placed to ensure that the urethra, rectum, and bladder were not perforated. Upon optimal placement of the catheters, the template was sutured to the perineum. When possible, upon completion of the catheter placement, a flexible cystoscopy with retroflexion to view the bladder neck was performed to ensure appropriate depth of the anterior catheters with advancement of needles to visualize bladder tenting. Subsequently, patients were transported to the postanesthesia care unit to recover and then transferred to the radiation oncology department in the same building. There, the bladder was filled with 30 cc diluted contrast (2 cc iodinated contrast, 28 cc normal saline), and patients underwent CT simulation including needle advancement if necessary (cystoscopy component of procedure was added after first 20 patients owing to more frequent need to adjust catheters at the time of CT simulation).
This was followed by treatment planning and subsequent treatment delivery. The implant was then removed, and patients were discharged home. Patients undergoing monotherapy presented for their second implant as planned 1 to 2 weeks later based on patient, physician, and OR availability. In terms of pharmacologic agents, cefazolin was typically used in the OR before the start of the operative procedure. Patients were also initiated on tamsulosin, naproxen, and ciprofloxacin after the first (and second) implants prophylactically (similar to LDR program). For pain management, the postanesthesia care unit staff delivered pain medications per their discretion until the patient presented to the radiation oncology department. In the radiation oncology department, the brachytherapy nurse checked vitals and assessed pain periodically throughout the treatment day. Hydromorphone and fentanyl were used for pain control at this time, and patients typically did not require narcotic pain medications at the time of discharge.
Treatment planning
In our initial HDR treatment-planning workflow, the physician first contoured the target and normal structures. The plan was then turned over to the physicist, who digitized the catheters and continued with plan optimization to meet the target coverage and dose constraints for critical structures. During the course of planning, a second physicist would double-check catheter positions and review the final dosimetry. In terms of planning goals, we initially used dose constraints based on those used by other centers of excellence.8
After the first several cases, our group determined that the treatment planning step was the most time-intensive step. Thus, we explored ways to improve our efficiency in this step over the course of several months. These targeted interventions are described in Table 1. As described in Figure 1B, our workflow was optimized to allow for simultaneous physician target delineation and physicist catheter identification in addition to the incorporation of targeted interventions using simplified dose constraints, adopting the built-in planning objective dashboard, and using the built-in planning objective dashboard in the treatment planning system.
Table 1.
Targeted interventions to improve treatment planning workflow
| Date | Intervention |
|---|---|
| January 2016 | Simplify planning goals to primarily those used in RTOG trials |
| February 2016 | Adopt use of built-in planning objective dashboard |
| November 2016 | Simultaneous physician contouring and physics catheter identification and planning using 2 imaging copies |
Abbreviation: RTOG = Radiation Therapy Oncology Group.
In our initial experience, we found that the automated catheter detection tool in our treatment planning software resulted in several errors and required extensive manual refinement, a process which took longer than complete manual detection. After the catheters were detected, a second physicist confirmed the catheter identification for correct spatial locations and catheter tip positions before further proceeding with planning. After the secondary check, the physicist manually loaded all the dwells with a set time and then used the dose shaper and point dose tools to refine the plan to meet the planning goals requested by the physician. With increased experience with the automatic applicator detection tool, we concluded that the inaccuracy of the algorithm was primarily due to the air gap between the patient and the template. We determined that by detecting the catheters on a shorter 3-dimensional CT scan that was generated in the treatment planning system and which excluded the slices that included air gap between the template and patient (so there was no discontinuity in the channel), the auto detection algorithm worked accurately with only moderate manual refinement needed.
Analysis
We performed analyses of our dosimetry and the procedural workflow to evaluate the progress of the new HDR program. To compare the quality of the dosimetry for our HDR patients, we compared the HDR dosimetry to that of previously treated LDR patients. We selected 51 consecutive LDR patients treated between 2012 and 2015 by the same two prostate brachytherapists AAS and MMH and compared the dosimetry between the LDR postimplant plans and HDR treatment plans. Dose-volume histogram data were compared for HDR (treatment plan) and LDR (postimplant plan) cases for structures used in planning for both approaches. The following target parameters were compared between HDR and LDR brachytherapy: target V200, V150, V100, V95, D90, rectum V100, rectum D2cc, and rectum D1cc. To account for within-observation correlation (eg, multiple implants/plans for HDR monotherapy patients), significance (P) was determined using multivariable, linear, mixed-effects models adjusted for patients’ T stage, prostate gland size, and treatment setting (ie, monotherapy or boost). In total, 120 observations were recorded for the 71 HDR brachytherapy patients.
To analyze workflow efficiency, we recorded the times of the start and end of the OR case, CT simulation, and physician approval of the plan in the medical record. These times were used to assess the implant procedure duration (IPD; defined as the time from the start of the implant procedure until the end of the procedure) and treatment planning duration (TPD; defined as the time from CT simulation to physician approval of the plan). For both LDR and HDR, the IPD did not include radiation treatment planning by the physicist because this was done preoperatively for LDR patients and postoperatively for HDR patients. The IPD for HDR cases was compared with that of LDR cases, also using a linear mixed-effects model to allow for random intercepts for each patient to and assess differences in intraoperative time because available time in the OR was limited; thus, the length of cases had to be similar.
Within the HDR group, to assess for improvements in IPD and TPD efficiency, these variables were compared between chronological quartiles of cases to identify any changes over time. In each instance, univariable, linear, mixed-effects models with random intercepts for each patient were used to determine significance. All statistical analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC). An alpha error rate of P < .05 was considered statistically significant for all comparisons.
Results
Between September 21, 2015 and March 9, 2017, a total of 71 patients with prostate cancer were treated with HDR brachytherapy for a total of 120 implants. There were 50 monotherapy (70%) and 21 boost (30%) patients. One patient undergoing monotherapy received 13.5 Gy, but did not return for the second implant. We had an average of 3.3 patients per month for the first 6 months, 3.7 patients per month for the second 6 months, and 4.5 patients per month for the last 6 months of the cohort. Table 2 compares the unique clinical, demographic, and treatment characteristics of the HDR and LDR cohorts used for this analysis. Both groups had a similar age at the time of the consultation (P = .25), pretreatment gland size (P = .14), and T-stage (P = .15). HDR patients were more likely to have a higher pretreatment prostate-specific antigen level (P = .002) and Gleason score (P < .001) and to be intermediate or high risk (P < .001).
Table 2.
Clinical and demographic characteristics of low-dose-rate and high-dose-rate brachytherapy patients
| Patient characteristic | Low-dose-rate (n = 51) | High-dose-rate (n = 71) | P-value∗ |
|---|---|---|---|
| Mean age, years (SD) | 65.1 (6.4) | 66.4 (5.5) | .25† |
| Mean prostate gland size, mL (SD) | 34.2 (10.2) | 37.6 (14.5) | .14† |
| T stage | .15‡ | ||
| 1b | 1 (2.0) | 0 (0.0) | |
| 1c | 44 (86.3) | 54 (76.6) | |
| 2a | 6 (11.8) | 16 (23.0) | |
| 2c | 0 (0.0) | 1 (1.0) | |
| Gleason score | <.001 | ||
| 6 | 31 (60.8) | 34 (34.0) | |
| 7 | 20 (39.2) | 39 (55.0) | |
| 8-10 | 0 (0.0) | 8 (11.0) | |
| NCCN risk group | <.001 | ||
| Low risk | 25 (49.0) | 17 (24.0) | |
| Intermediate risk | 25 (49.0) | 41 (58.0) | |
| High risk | 1 (2.0) | 13 (18.0) | |
| Mean pre-treatment PSA (Median, IQR) | 5.80 (4.30-7.79) | 7.67 (5.59-11.00) | .002 |
| Setting | .02§ | ||
| Monotherapy | 46 (90%) | 50 (70%) | |
| Boost | 5 (10%) | 21 (30%) |
Abbreviations: IQR = interquartile range; NCCN = National Comprehensive Cancer Network; PSA = prostate-specific antigen; SD = standard deviation.
Wilcoxon rank sum test.
Two sample t test.
Fisher's exact test.
χ2 test.
Evaluating high-dose-rate dosimetry
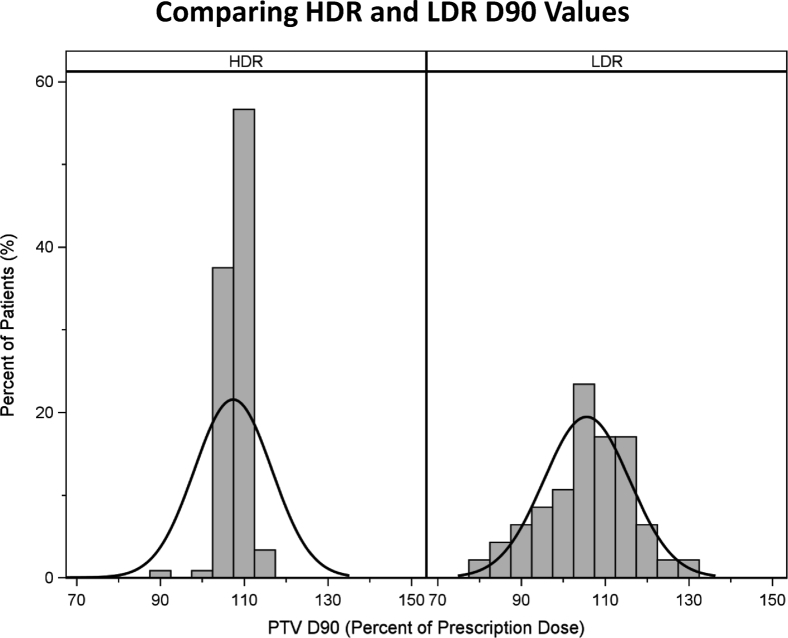
Table 3 compares the HDR and LDR dosimetry for patients treated with each modality adjusted for T stage, prostate gland size, and setting (ie, monotherapy vs boost). The mean D90 values were similar between LDR and HDR patients (P = .99), but there was less variability in the D90 values of HDR patients than those of LDR patients (Fig. 2). The mean V100 and V95 were higher in the HDR group (both P < .0001); the mean V200 and V150 were lower in the HDR group (both P < .0001). Rectum D2cc was similar (P = .34), but D1cc was lower in the HDR group (P < .0001), as was the V100 (0 for all HDR patients).
Table 3.
Comparison of dosimetric goals for high-dose-rate and low-dose-rate brachytherapy patients
| Structure (SE) | Low-dose-rate (%) | High-dose-rate (%) | P-value∗ |
|---|---|---|---|
| Target V200 (SE) | 13.08 (1.81) | 7.20 (1.67) | <.0001 |
| Target V150 (SE) | 40.15 (3.74) | 27.45 (3.45) | <.0001 |
| Target V100 (SE) | 81.73 (5.11) | 95.87 (4.75) | <.0001 |
| Target V95 (SE) | 93.57 (0.98) | 98.39 (0.89) | <.0001 |
| Target D90 (SE) | 105.93 (3.60) | 107.78 (3.32) | .99 |
| Rectum D2 cc (SE) | 68.92 (3.33) | 67.18 (3.03) | .34 |
| Rectum D1 cc (SE) | 82.82 (3.72) | 72.75 (3.38) | <.0001 |
| Rectum V100 (SE) | 0.45 (0.11) | - |
Abbreviation: SE = standard error.
Linear mixed-effects model adjusted for T stage, prostate gland size, and setting (monotherapy vs boost).
Figure 2.
D90 values for low-dose-rate (n = 51) and high-dose-rate (n = 71) prostate brachytherapy patients.
Gains in high-dose-rate efficiency
The mean IPD was statistically significantly shorter for the HDR group compared with LDR patients. The mean implant procedure duration was 60 minutes (standard error: 2.0 minutes) for LDR patients and 54 minutes (standard error: 1.6 minutes) for HDR patients (P = .02). For HDR patients, the implant procedure duration did not change over time (P = .14) despite the incorporation of cystoscopy after 20 cases.
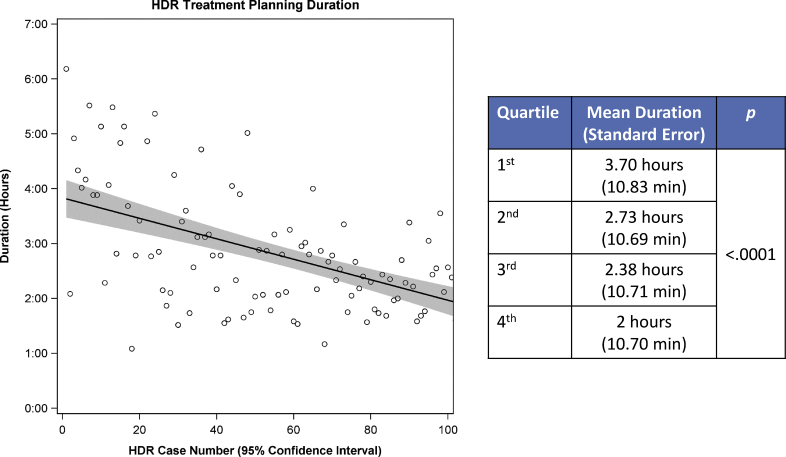
Figure 3 depicts the changes in time over TPD for the HDR group. Over the course of the study period, the treatment planning duration dramatically improved with the implementation of targeted interventions. The mean treatment planning duration was 3.7 hours for the first quartile, and 2.0 hours for the final quartile (P < .0001).
Figure 3.
Changes in treatment planning duration over time and chronological quartiles in high-dose-rate brachytherapy group.
Discussion
Data increasingly suggest that the unique characteristics of prostate brachytherapy allow for the maximization of the therapeutic ratio to deliver ablative radiation therapy for prostate cancer. HDR brachytherapy has multiple theoretical advantages to LDR brachytherapy but is less commonly used by brachytherapists compared to LDR brachytherapy in the United States.6 We successfully developed a prostate HDR brachytherapy program to complement the LDR program at our academic institution. We found comparable dosimetry for LDR patients, with some dosimetric advantages to HDR brachytherapy, including improved target coverage, more consistent D90 values, and a lower rectal dose. In addition, despite being the most time-consuming step of the process, we improved the CT-based program’s treatment planning duration through various targeted workflow interventions. Continuous efforts to improve efficiency and quality caused the program to rapidly grow over the first 1.5 years and become a major referral center for HDR in our region.
Few studies have compared the implant quality and dosimetry of LDR versus HDR brachytherapy in prostate cancer. White et al. performed a retrospective cohort study to evaluate the dosimetric properties of patients treated with HDR at the California Endocurietherapy Cancer Center.19 Similar to our study, the researchers found consistent D90 values with little variability. White et al. then compared the mean D90 from multiple HDR and LDR series and found similar results to those observed in our study: significant variability in mean LDR D90 values not seen with HDR patients.19
One potential rationale for this finding is that the inherent ability to adjust dwell times with HDR planning, rather than fixed seed strength in LDR, may lead to substantial improvements in dosimetry. Another possibility is that there are multiple points during the workflow to ensure measures for optimal dosimetry: at the time of transrectal ultrasound-guided catheter implantation, at the time of CT simulation when assessing catheter depth and distribution, and during treatment planning when adjusting the dwell times and dwell positions. Multiple randomized trials are currently accruing internationally to compare LDR and HDR monotherapy (NCT02692105, NCT02960087, and NCT02258087), and these studies will hopefully shed light on the dosimetric, and most importantly, clinical differences between the modalities.
The differences in patient experience are important to consider when transitioning from an LDR to an HDR prostate brachytherapy program. The overall time investment that is required for treatment from a patient is increased in the HDR workflow. In contrast with the volume study and operative procedure only with LDR (after which therapy is complete), HDR days are longer because they require operative procedures and then postimplant TPD and treatment, which takes several hours. In addition, for monotherapy, HDR consists of 2 implants over several weeks as opposed to 1 with LDR.
Ultimately, our practice is to objectively and comprehensively discuss the logistic, radiation-related, and clinical differences of each of these approaches with patients to help them make an informed decision regarding the optimal treatment approach. In our experience, most patients elect HDR over LDR for the potential benefits described, despite 2 implant procedures and longer treatment days, The optimal modality for HDR treatment planning is also controversial. We chose CT-based planning, but other centers have used ultrasound-based planning. There are several advantages to ultrasound-based planning, including avoiding catheter displacement and having to plan and deliver treatment after the patient wakes from anesthesia. In addition, prostate delineation can sometimes be easier on ultrasound images. Dosimetric comparisons suggest a relatively similar ability to meet planning goals between both planning modalities.20
We elected a CT-based approach for several reasons. Initially, part of the rationale was that this approach was used at the centers where we trained in HDR. Therefore, we could have a framework as we began our program. In addition, the workflow was similar to our gynecologic HDR patient workflow, which facilitated staff training and shared programmatic considerations. Over time, we also found that there was some degree of variability in the image quality of ultrasound imaging between patients and sometimes between implants, particularly after catheter insertion, that made a clear delineation of the prostate more difficult. Thus, using the information from the anatomic relationship of the catheters to the bladder neck and prostate could be combined with the information on CT scans to allow for a more precise target delineation.
To the best of our knowledge, there are a limited number of studies that describe interventions to the CT-based HDR planning workflow to improve efficiency. We found subjective and objective improvements in our treatment planning duration, which is the longest step in our workflow, through the adoption of these interventions. This improved the experience for both the patient and the care team. Other institutions who are attempting to develop an HDR prostate brachytherapy program may be able to use these interventions and our experience in developing our program to help navigate hurdles they may encounter.
Our program continues to evolve. In the last 6 months, we have been using knowledge-based planning for HDR patients and previously reported on our preliminary experiences.21 In addition, we have incorporated MRI obtained after CT simulation and before treatment planning to help refine our delineation of the prostate and normal structures for treatment planning. As part of the development of the MRI-guided planning, we have moved toward a prospective peer review of treatment targets and normal tissue organs-at-risk whenever possible. To prospectively evaluate disease control, toxicity, and impact of the HDR program on patient quality, all patients are enrolled in a prospective database and evaluated for posttreatment prostate-specific antigen levels, Common Terminology Criteria for Adverse Events version 5.0, and Expanded Prostate Cancer Index Composite-26 scores at all follow-up visits. We have conduced multiple retrospective analyses of dosimetric and clinical characteristics to predict for acute toxicity,22, 23, 24, 25 and once sufficient follow-up data are obtained, we will analyze predictors of late toxicity and disease control outcomes.
Our study must be interpreted in the context of several important limitations. The retrospective nature of the study design makes these findings hypothesis-generating. Also, we did not place a Foley catheter at the time of the postimplant CT scans for LDR patients, which made a comparison of urethral doses for LDR and HDR patients in this series not possible. Importantly, our LDR program used preoperative volume study and planning. Thus, the findings of the comparison between HDR and LDR IPD is likely not applicable to centers that perform intraoperative LDR planning. However, because many centers currently use a preoperative planning approach to LDR, and our key emphasis in this study was the development of the HDR program and the optimization of this workflow, we feel that the findings of our study are valuable to centers that use both types of LDR planning.
In addition, there are likely institution-specific environmental and personnel issues that may not make these results reproducible at other institutions, and other clinicians who attempt to develop an HDR program may have different challenges and opportunities at their own institutions. However, the challenges we dealt with are relatively broad and likely apply to other institutions.
Finally, we have not identified a satisfactory statistical method to assess how experiential learning and targeted workflow interventions contributed, individually or concurrently, to the decreases in the treatment planning times observed at our institution.
Conclusions
We successfully transitioned from an LDR to an HDR prostate brachytherapy program at our institution. Our HDR patients had dosimetry that was comparable with our LDR patients, with a few parameters that favored HDR. Through targeted interventions in the workflow, we improved our HDR planning efficiency. Our experience in the development of an HDR program and workflow refinements can help other institutions to develop and optimize prostate HDR brachytherapy programs.
Acknowledgments
The authors appreciate the guidance and assistance of Dr D. Jeffrey Demanes and the team at the California Endocurietherapy Center of the University of California, Los Angeles; Dr Mitchell Kamrava, now at Cedars-Sinai Hospital; Drs John Hayes and Thomas Skidmore and the Gamma West Cancer Services team; and Drs James and Mackenzie McGee and the OSF St. Francis team. All were instrumental in the successful development of our high-dose-rate prostate brachytherapy program. The authors also appreciate the statistical support from Dr Cara Joyce in the revisions of this manuscript.
Footnotes
Sources of support: This work had no specific funding.
Conflicts of interest: Dr Solanki has received travel expense payments and honoraria, and is on the advisory board for Blue Earth Diagnostics. Dr Harkenrider is on the advisory board for Varian Medical Systems Brachytherapy. Dr Roeske has accepted grants and personal fees from Varian Medical Systems. Dr Surucu has accepted research grants from Varian Medical Systems. Dr Small is on the advisory board for Varian Medical Systems, and has received honoraria and travel expense payments from Zeiss. None of these disclosures have any actual conflicts of interest for the material presented in this manuscript.
References
- 1.Dayes I.S., Parpia S., Gilbert J., Julian J.A., Davis I.R., Levine M.N. Long-term results of a randomized trial comparing iridium implant plus external beam radiation therapy with external beam radiation therapy alone in node-negative locally advanced cancer of the prostate. Radiat Oncol Biol. 2017;99:90–93. doi: 10.1016/j.ijrobp.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Hoskin P.J., Rojas A.M., Bownes P.J., Lowe G.J., Ostler P.J., Bryant L. Randomized trial of external beam radiation therapy alone or combined with high-dose-rate brachytherapy boost for localized prostate cancer. Radiother Oncol. 2012;103:217–222. doi: 10.1016/j.radonc.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Morris W.J., Tyldesley S., Rodda S. Androgen suppression combined with elective nodal and dose escalated radiation therapy (the ASCENDE-RT Trial ): An analysis of survival endpoints for a randomized trial comparing a low-dose-rate brachytherapy boost to a dose-escalated external beam boost. Radiat Oncol Biol. 2017;98:275–285. doi: 10.1016/j.ijrobp.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 4.Zelefsky M.J., Yamada Y., Pei X. Comparison of tumor control and toxicity outcomes of high-dose intensity modulated radiation therapy and brachytherapy for patients with favorable risk prostate cancer. Urology. 2011;77:986–993. doi: 10.1016/j.urology.2010.07.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grimm P., Billiet I., Bostwick D. Comparative analysis of prostate-specific antigen free survival outcomes for patients with low, intermediate and high-risk prostate cancer treatment by radical therapy. Results from the Prostate Cancer Results Study Group. BJU Int. 2012;109:22–29. doi: 10.1111/j.1464-410X.2011.10827.x. [DOI] [PubMed] [Google Scholar]
- 6.Buyyounouski M.K., Davis B.J., Prestidge B.R. A survey of current clinical practice in permanent and temporary prostate brachytherapy: 2010 update. Brachytherapy. 2012;11:299–305. doi: 10.1016/j.brachy.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Taira A.V., Merrick G.S., Butler W.M. Long-term outcome for clinically localized prostate cancer treated with permanent interstitial brachytherapy. Int J Radiat Oncol Biol Phys. 2011;79:1336–1342. doi: 10.1016/j.ijrobp.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Hauswald H., Kamrava M., Fallon J.M. High-dose-rate (HDR) monotherapy for localized prostate cancer: 10 year results. Int J Radiat Oncol Biol Phys. 2016;94:667–674. doi: 10.1016/j.ijrobp.2015.07.2290. [DOI] [PubMed] [Google Scholar]
- 9.Krauss D.J., Ye H., Martinez A.A. Favorable preliminary outcomes for men with low- and intermediate-risk prostate cancer treated with 19-Gy single-fraction high-dose-rate brachytherapy. Int J Radiat Oncol Biol. 2017;97:98–106. doi: 10.1016/j.ijrobp.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Grills I.S., Martinez A.A., Hollander M. High dose rate brachytherapy as prostate cancer monotherapy reduces toxicity compared with low dose rate palladium seeds. 2004;171:1098–1104. doi: 10.1097/01.ju.0000113299.34404.22. [DOI] [PubMed] [Google Scholar]
- 11.Hoskin P., Rojas A., Ostler P., Hughes R., Alonzi R., Lowe G. Single-dose high-dose-rate brachytherapy compared with 2 and 3 fractions for locally advanced prostate cancer. Radiother Oncol. 2017;124:56–60. doi: 10.1016/j.radonc.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 12.Jawad M.S., Dilworth J.T., Gustafson G.S. Outcomes associated with three treatment schedules of high-dose-rate brachytherapy monotherapy for favorable-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2016;94:657–666. doi: 10.1016/j.ijrobp.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Mahmood U., Pugh T., Frank S. Declining use of brachytherapy for the treatment of prostate cancer. Brachytherapy. 2014;13:157–162. doi: 10.1016/j.brachy.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Orio P.F., III, Nguyen P.L., Buzurovic I., Cail D.W., Chen Y.W. Prostate brachytherapy case volumes by academic and nonacademic practices: Implications for future residency training. Int J Radiat Oncol Biol Phys. 2017;96:624–628. doi: 10.1016/j.ijrobp.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Davis B.J., Horwitz E.M., Lee W.R. American Brachytherapy Society consensus guidelines for transrectal ultrasound-guided permanent prostate brachytherapy. Brachytherapy. 2012;11:6–19. doi: 10.1016/j.brachy.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Morton G., Loblaw A., Cheung P. Is single fraction 15 Gy the preferred high dose-rate brachytherapy boost dose for prostate cancer? Radiother Oncol. 2011;100:463–467. doi: 10.1016/j.radonc.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 17.Ghilezan M., Martinez A., Gustason G. High-dose-rate brachytherapy as monotherapy delivered in 2 fractions within 1 day for favorable/intermediate-risk prostate cancer: Preliminary toxicity data. Int J Radiat Oncol Biol Phys. 2012;83:927–932. doi: 10.1016/j.ijrobp.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Surucu M., Small W., Roeske J.C. Implementation of a Prospective Contouring and Planning Rounds Using the Workflow Tools Available in Our Record and Verify System. Int J Radiat Oncol. 2016;96:E546–E547. [Google Scholar]
- 19.White E.C., Kamrava M.R., Demarco J. High-dose-rate prostate brachytherapy consistently results in high quality dosimetry. Radiat Oncol Biol. 2013;85:543–548. doi: 10.1016/j.ijrobp.2012.03.035. [DOI] [PubMed] [Google Scholar]
- 20.Batchelar D.L., Chung H.T., Loblaw A., Law N., Cisecki T., Morton G.C. Intraoperative ultrasound-based planning can effectively replace postoperative CT-based planning for high-dose-rate brachytherapy for prostate cancer. Brachytherapy. 2016;15:399–405. doi: 10.1016/j.brachy.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Plypoo A., Roeske J., Surucu M., Solanki A., Harkenrider M., Kang H. 2017. Development of a Clinical Knowledge-Based Model for High Dose Rate (HDR) Prostate Brachytherapy Planning. In: Proceedings of the 59th Annual Meeting of the American Association of Physicists in Medicine (AAPM) SU-H4-GePD-T-1. [Google Scholar]
- 22.Korpics M., Hentz C., Martin B. Comparing Acute Toxicity Profiles for High Dose Rate Prostate Brachytherapy Patients Receiving Two Implants Separated by a One-Week Versus Two-Week Interval. Int J Radiat Oncol Biol Phys. 2017;99:E249. [Google Scholar]
- 23.Hentz C., Mark K., Martin B. HDR Prostate Brachytherapy is Associated With Lower Urinary Toxicity and More Rapid Resolution Over the First Year Compared to LDR Brachytherapy. Int J Radiat Oncol Biol Phys. 2017;99:E238. [Google Scholar]
- 24.Korpics M., Bajaj A., Mysz M. Comparing Low Dose Rate and High Dose Rate Prostate Brachytherapy Implant Dosimetry. Brachytherapy. 2017;16:S113–S114. [Google Scholar]
- 25.Jordan G.A., Stang K., Harris A. MRI-Based Treatment Planning for Prostate High Dose Rate Brachytherapy Leads to Decreased Target Size and Rectal Dose. Brachytherapy. 2018;17:S83. [Google Scholar]