Abstract
Purpose
Variation exists in cooperative group recommendations for the dorsal border for the chest wall clinical target volume (CTV). We aimed to quantify the impact of this variation on doses to critical organs and examine patterns of chest wall recurrence relative to the pectoralis muscle.
Methods and Materials
We retrospectively assessed patterns of chest wall recurrence quantified to the recommended CTV borders for women treated between 2005 and 2017. We compared treatment plans for 5 women who were treated with left postmastectomy radiation therapy, with the chest wall contoured using varying dorsal borders for CTV: (1) Anterior pleural surface (Radiation Therapy Oncology Group), (2) anterior surface of pectoralis major (European Society for Radiotherapy and Oncology), and (3) anterior rib surface (institutional practice). Treatment plans were generated for 50 Gy in 25 fractions. Doses to organs-at-risk were compared using paired-sample t tests.
Results
Institutional patterns of chest wall recurrence were 64.7% skin and subcutaneous tissue, 23.5% both anterior to and between the pectoralis muscles, and 11.8% isolated to the tissue between the pectoralis major and minor. No chest wall recurrences were noted deep to pectoralis minor. When comparing the plans generated per the Radiation Therapy Oncology Group versus European Society for Radiotherapy and Oncology contouring guidelines, the mean lung V20Gy, heart mean dose, and left anterior descending artery mean dose were 33.5% versus 29.4% (P < .01), 5.2 Gy versus 3.2Gy (P = .02), and 27.3Gy versus 17.8Gy (P = .04), respectively.
Conclusions
The recommended variations in the dorsal chest wall CTV border have significant impact on doses to the heart and lungs. Although our study was limited by small numbers, our institutional patterns of recurrence would support a more anterior dorsal border for the chest wall CTV consistent with older literature.
Summary.
Significant variation exists in contouring guidelines for post-mastectomy radiotherapy. We examined institutional patterns of chest wall recurrence, showing recurrences are predominately in the skin and subcutaneous tissues. Inclusion of the ribs and intercostal muscles in the CTV as recommended by the RTOG guidelines significantly increases doses to the heart and lung as compared to the more anterior border of the pectoralis major recommended by the ESTRO guidelines which may miss recurrences within the pectoralis muscles.
Introduction
Radiation oncology has undergone a great renaissance transition from 2- to 3-dimensional planning to better conform dose to the target and spare organs at risk. However, the benefits of 3-dimensional planning hinge on the accurate identification of the clinical target volume (CTV). Cooperative groups have published contouring guidelines with recommendations for CTV design to better guide the application of postmastectomy radiation therapy.1, 2 These guidelines were predominately formulated based on expert opinion rather than clinical outcomes,3 and considerable variation exists in the recommended dorsal border of the chest wall CTV between the guidelines.
The Radiation Therapy Oncology Group (RTOG) recommends that the dorsal CTV border include the entire thickness of the chest wall to the pleural surface to target the skin, subcutaneous tissues, pectoralis major, pectoralis minor, ribs, and intercostal muscles.1 Conversely, the European Society for Radiotherapy and Oncology (ESTRO) recommends that the dorsal CTV border include the skin and subcutaneous tissue to the anterior surface of pectoralis major, and only recommends inclusion of the pectoralis muscles and ribs in the setting of documented invasion for T4a and T4c tumors.2 Similarly, the RADCOMP Consortium trial (NCT02603341) breast contouring guidelines recommend that the dorsal CTV border extends posteriorly to, but not including, the ribs.
Recent pattern-of-care analyses have examined the impact of this variation of patterns of recurrence, and documented a significant number of recurrences that occurred outside of the smaller ESTRO, but within the larger RTOG volumes.4 However, despite modern planning techniques, the increase in the treatment volume from the RTOG CTV recommendations relative to traditional 2-dimensional postmastectomy radiation therapy comes at a cost of significant increases in the doses to the heart and lungs.5 Any benefits from postmastectomy radiation therapy in reducing locoregional recurrences that translate into improved overall survival have to be balanced against the potential risks of long-term sequelae, including cardiac-related mortality.6, 7 We have previously proposed a compromised dorsal border for the chest wall CTV of the anterior rib surface to include the skin, subcutaneous tissues, and pectoralis muscles based on a literature review of patterns of recurrence in older literature.3, 8
Contemporaneous to the improvements in radiation delivery, improvements in mammographic screening, systemic therapy, and image guided surgical techniques have reduced the risks of locoregional recurrence in breast cancer in the modern era. A reduction in the absolute risks of recurrence can also occur with a change in patterns of chest wall recurrence, which has strong implications for chest wall CTV design. Thus, we aimed to quantify the impact of the recommended variation of the dorsal chest wall CTV border on doses to critical organs and examined the patterns of chest wall recurrence relative to the pectoralis muscle. We hypothesized that the majority of recurrences in the modern era occur anterior to the rib surface, and a reduction in the dorsal chest wall CTV border might decrease normal tissue exposure to the heart and lungs.
Methods and Materials
After institutional review board approval, we used a prospectively maintained, institutional, breast cancer database and identified patients who were evaluated for surgical resection of chest wall recurrence between 2005 and 2017. We reviewed the location of the recurrence noted on the preoperative imaging or postoperative pathology results quantified in relation to the recommended dorsal CTV borders.
Patients with nodal recurrence in the absence of disease recurrence in the chest wall were excluded, but patients with chest wall recurrence in the setting of lymph node or distant metastases were included. If preoperative imaging or postoperative pathology results were not available to discern the location of chest wall recurrence relative to the recommended CTV borders, patients were also excluded. The baseline characteristics for patients with chest wall recurrences in the anterior skin and subcutaneous tissues were compared with those of patients with recurrences that involved the pectoralis muscles using an χ2 analysis with SPSS, version 24 (IBM Cooperation, Armonk, NY).
Subsequently, we retrospectively created treatment plans for 5 women treated with comprehensive postmastectomy radiation therapy to the left chest wall, undissected axilla, supraclavicular, and internal mammary lymph nodes. The chest wall was contoured using varying dorsal borders for CTV, as recommended in the literature: (1) Anterior pleural surface (per RTOG guidelines), (2) anterior surface of pectoralis major (per ESTRO guidelines), and (3) anterior rib surface to include both pectoralis major and minor (institutional practice). Treatment plans were generated for 50 Gy in 25 fractions with goals to meet the criteria for target volume coverage as recommended in the NSABP-B51 study. All plans were created using partially wide tangents with a single isocenter field-in-field technique. Priority was given to target coverage over critical organ dosimetry to allow for normalization of the plans across the varying CTV definitions. The doses to the organs at risk were compared between the plans using paired-sample t tests with SPSS, version 24 (IBM cooperation, Armonk, NY).
Results
From 2005 to 2017, we retrospectively reviewed 69 patients who were evaluated for surgical resection of chest wall recurrence, and identified 17 patients with chest wall recurrence that met our inclusion criteria. The baseline characteristics of the 17 patients identified with chest wall recurrences are detailed in Table 1. Most recurrences were invasive ductal carcinoma (71%), 82% were disease stage II or III, and the most common subtype was estrogen receptor + progesterone receptor + human epidermal growth factor receptor 2 negative (47%). The median age at the time of diagnosis was 57 years (interquartile range, 42-57 years). The median time to chest wall recurrence from the time of diagnosis was 2.25 years (interquartile range, 1.29-5.70 years).
Table 1.
Baseline patient characteristics for patient with chest wall recurrence
| Baseline characteristics | N (%) |
|---|---|
| Laterality | |
| Left | 12 (71%) |
| Right | 5 (29%) |
| Histology | |
| Invasive ductal carcinoma | 12 (71%) |
| Invasive lobular carcinoma | 2 (12%) |
| Inflammatory | 3 (18%) |
| Age, years | |
| <57 | 10 (59%) |
| ≥58 | 7 (41%) |
| Initial disease stage | |
| I | 1 (6%) |
| II | 7 (41%) |
| III | 7 (41%) |
| Unrecorded | 2 (12%) |
| Subtype | |
| ER + PR + HER2- | 8 (47%) |
| ER + PR + HER2+ | 0 (0%) |
| ER-PR-HER2+ | 2 (12%) |
| ER-PR-HER2- | 5 (29%) |
| Unrecorded | 2 (12%) |
| Prior irradiation | |
| No | 8 (47%) |
| Yes | 4 (24%) |
| Unrecorded | 5 (29%) |
Abbreviations: ER = estrogen receptor; HER2 = human epidermal growth factor receptor 2; PR = progesterone receptor.
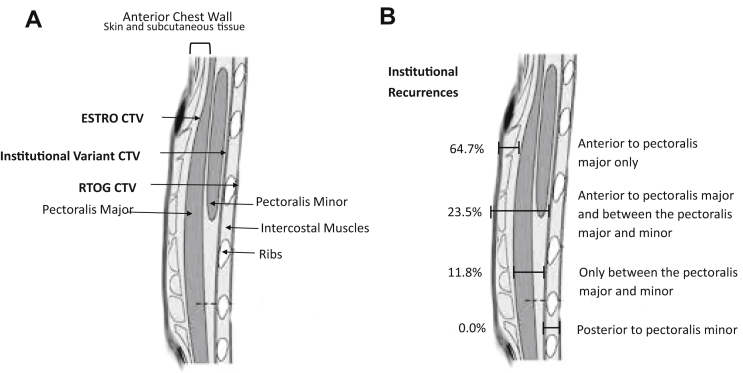
Recurrences were examined by comparing the anatomic borders used by the consensus guidelines: Anterior to the pectoralis major (ESTRO guidelines), below pectoralis major and minor but to the anterior rib surface (institutional variation), and anterior pleural surface (RTOG guidelines; Fig 1A). When examining the anatomic location of chest wall recurrences, 64.7% of recurrences were isolated to tissue anterior to the pectoralis muscle, 23.5% both anterior to and between the pectoralis muscles, and only 11.8% were isolated to the tissue between the pectoralis major and minor (Fig 1B). There were no chest wall recurrences noted deep to the pectoralis minor. There were no differences in patterns of recurrence by laterality, histology, age, stage, receptor subtype, or prior irradiation (Table 2).
Figure 1.
Chest wall anatomy relative to (A) contorting recommendations for clinical target volume design and (B) institutional patterns of chest wall recurrence postmastectomy. CTV, clinical target volume.
Table 2.
Comparative patterns of recurrence by baseline characteristics
| Anterior recurrence (n = 11) | Pectoralis recurrence (n = 6) | P-value | |
|---|---|---|---|
| Laterality | 0.39 | ||
| Left | 7 (64%) | 5 (83%) | |
| Right | 4 (36%) | 1 (17%) | |
| Histology | 0.36 | ||
| Invasive ductal carcinoma | 7 (64%) | 5 (83%) | |
| Invasive lobular carcinoma | 1 (9%) | 1 (17%) | |
| Inflammatory | 3 (27%) | 0 (0%) | |
| Age, years | 0.63 | ||
| <57 | 6 (55%) | 4 (67%) | |
| ≥58 | 5 (46%) | 2 (33%) | |
| Initial disease stage (n = 15) | 0.39 | ||
| I | 0 (0%) | 1 (17%) | |
| II | 4 (44%) | 3 (50%) | |
| III | 5 (56%) | 2 (33%) | |
| Subtype (n = 15) | 0.70 | ||
| ER + PR + HER2- | 5 (50%) | 3 (60%) | |
| ER + PR + HER2 + | 0 (0%) | 0 (0%) | |
| ER-PR-HER2 + | 1 (10%) | 1 (20%) | |
| ER-PR-HER2- | 4 (40%) | 1 (20%) | |
| Prior irradiation (n = 12) | 0.16 | ||
| No | 5 (56%) | 3 (100%) | |
| Yes | 4 (44%) | 0 (0%) |
Abbreviations: ER = estrogen receptor; HER2 = human epidermal growth factor receptor 2; PR = progesterone receptor.
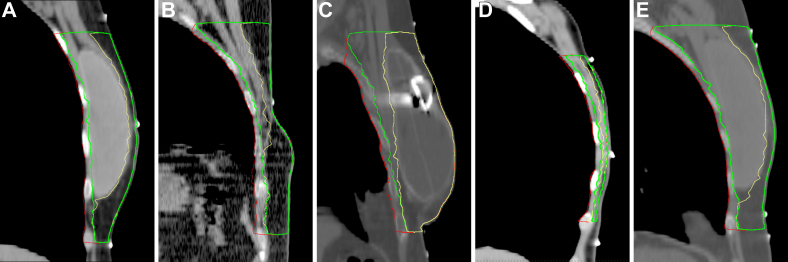
Next, we aimed to quantify the impact of the dorsal CTV border variation on doses to critical organs. We created treatment plans that covered the chest wall, undissected axilla, supraclavicular, and internal mammary lymph nodes for 5 women who underwent left-sided mastectomies. Table 3 summarizes the baseline characteristics for these patients. We varied the dorsal CTV border according to the RTOG, ESTRO, and institutional variation protocols (Figs 2A-E). Figure 3 summarizes the impact of the varying dorsal borders for critical organ dosimetry.
Table 3.
Baseline patient characteristics for chest wall planning patients
| Age | Pathology | TNM Stage | Clinical/pathologic Stage | ER | PR | HER2 | No. of positive LNs | Location of tumor | Size | Surgery | Reconstruction | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 40 | ILC | pT3 N1a M0 | pIIIa | + | + | - | 2/4 | Left | 7.0 cm | Left total mastectomy | Immediate implant-based |
| B | 59 | IDC | pT2 N0 Mx | pII | + | - | - | 0/15 | Left | 4.5 cm | Left modified radical mastectomy | None |
| C | 79 | mixed IDC/ILC | pT2 N2a M0 | pIIIa | + | + | - | 5/11 | Bilateral | 3.1 cm multifocal | Bilateral skin sparing mastectomy with left axillary dissection and right sentinel LN | Delayed immediate reconstruction with bilateral tissue expander placement |
| D | 41 | IDC | T1 N1 M0, ypT0N0M0 | cIIa | + | + | - | 0/16 | Left | no residual invasive carcinoma | Left modified radical mastectomy and right prophylactic mastectomy with sentinel lymph node | None |
| E | 59 | IDC | pT2N1a | pIIa | + | + | - | 1/1 | Left | 4.0 cm | Left simple mastectomy with sentinel LN | Immediate implant-based |
Abbreviations: ER = estrogen receptor; HER2 = human epidermal growth factor receptor 2; IDC = invasive ductal carcinoma; ILC = invasive lobular carcinoma; LN = lymph node; PR = progesterone receptor.
Figure 2.
Contouring examples for postmastectomy radiation therapy. Red: Radiation Therapy Oncology Group guidelines; yellow: European Society for Radiation therapy and Oncology guidelines; green: institutional variation.
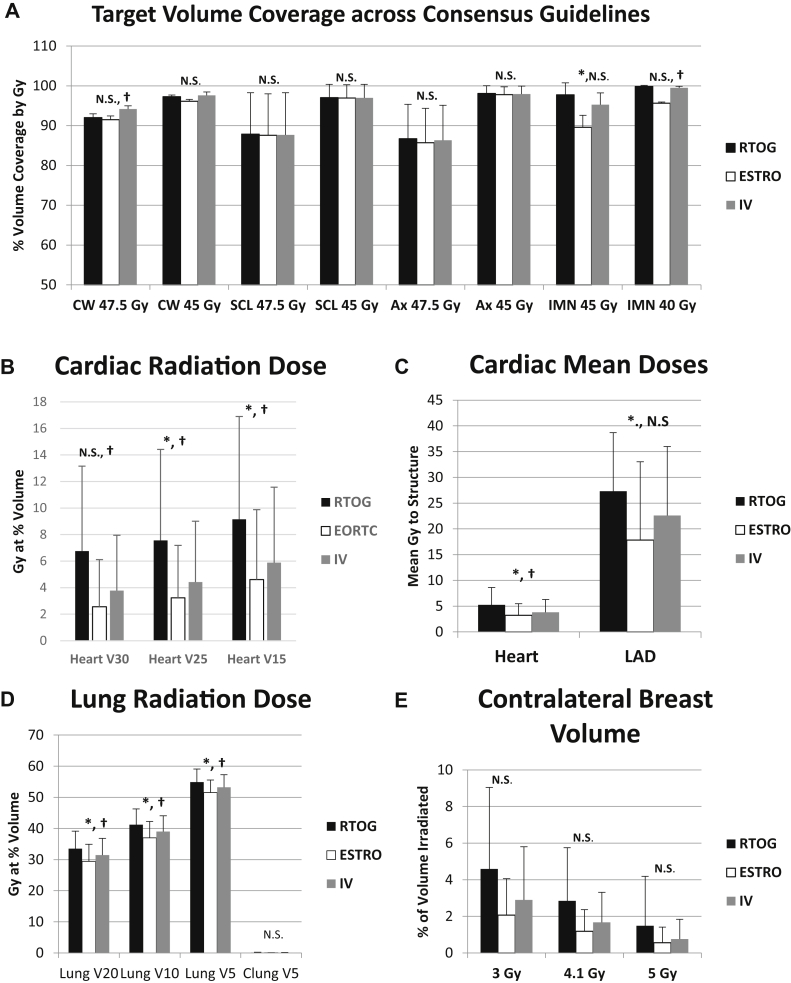
Figure 3.
Target volume coverage for treatment plans across consensus guidelines. NS, not statistically significant with P > .05. ∗Radiation Technology Oncology Group versus European Society for Radiation therapy and Oncology P < .05; †Radiation Technology Oncology Group versus institutional variation, P < .05. Ax, undissected axillary lymph nodes; Clung V5, contralateral lung volume receiving 5 Gy; CW, chest wall. IMN, internal mammary lymph nodes; LAD, left anterior descending artery; Lung V5, ipsilateral lung volume receiving 5Gy; Lung V10, ipsilateral lung volume receiving 10 Gy; lung V20, ipsilateral lung volume receiving 20 Gy; SCL, supraclavicular lymph nodes; V15, volume receiving 15 Gy; V25, volume receiving 25 Gy; V30, volume receiving 30 Gy.
When comparing the RTOG versus ESTRO-generated contouring guideline plans, we found that the mean lung V20Gy, heart mean dose, and left anterior descending artery mean doses were 33.5% versus 29.4% (P < .01), 5.2 Gy versus 3.2 Gy (P = .02), and 27.3 Gy versus 17.8 Gy (P = .04), respectively. When comparing the RTOG versus our institutional variation-generated contouring guideline plans, we found that the mean lung V20Gy, heart mean dose, and left anterior descending artery mean doses were 33.5% versus 31.4% (P = .01), 5.2 Gy versus 3.8 Gy (P = .03), and 27.3 Gy versus 22.6 Gy (P = .12), respectively.
Discussion
Increasing the dorsal border for the chest wall CTV increases the doses to the heart and lungs as quantified herein, with an increase in the mean heart dose of 2 Gy and lung V20Gy of 4% for plans generated with the RTOG compared with the ESTRO-contouring guidelines. Although no recurrences deep to the pectoralis were identified, 35% of chest wall recurrences were noted in regions not routinely included in the ESTRO chest wall CTV, either between the pectoralis muscles or extending to both the pectoralis and subcutaneous tissues anterior to pectoralis major. However, 100% of recurrences would have been within a modified dorsal chest wall CTV border of the anterior rib surface, which reduced the mean heart dose by 1.4 Gy and mean lung V20Gy by 2% compared with the RTOG dorsal CTV border of the anterior pleural surface. Thus, with no recurrences deep to the pectoralis muscle, this dorsal CTV border might represent a potential compromise in maximizing the coverage of tissues at risk while minimizing exposure to normal organs.
We previously retrospectively analyzed patterns of recurrence based on a literature review of 278 cases identified from studies published between 1966 and 2003, and highlighted that 72% to 100% of chest wall recurrences occurred in the skin or subcutaneous tissues and no recurrences were identified deep to pectoralis.3 However, a number of questions remained in terms of how these patterns of recurrence held in the context of modern systemic therapy. For example, given that deep chest wall recurrences do occur, are these publications (predominantly surgical series) biased toward more superficial recurrences?9
More recently, a Korean multi-institutional analysis has shed further light on patterns of recurrence in the modern era.10 With 234 patients and 337 recurrences, the researchers identified 25 patients with 29 chest wall recurrences in patients undergoing mastectomy. The patterns of recurrence were identical to those previously identified in our literature review, with 76% in the skin or subcutaneous tissues, 24% within the pectoralis muscle, and 0% deep to the pectoralis in the ribs or intercostal muscles. Combined, these experiences are further validated by the patterns of chest wall recurrences noted herein (Fig 1B), and would potentially support the hypothesis that the epicenter for deep chest wall recurrences noted in series of full thickness chest wall resections is not in the ribs and intercostal spaces, but are a reflection of a direct invasion from recurrences starting in the skin, subcutaneous tissues, and pectoralis progressing in the setting of multiple prior recurrences, reirradiation, and large ulcerative lesions.11, 12, 13
Taken together, the prior literature review, multi-institutional Korean data, and patterns of recurrence presented herein are consistent in that patterns of recurrence do not support a need for the routine inclusion of the ribs and intercostal muscles in the chest wall CTV as recommended in the RTOG contouring guidelines for postmastectomy radiation therapy. However, 24% to 35% of recurrences that occur between the pectoralis major and minor might be potentially missed with the more anterior CTV border recommended by the ESTRO guidelines.3, 10
We recognized that increases in the depth of the CTV would have compromises in the doses to the heart and lungs (see Figure 3); therefore, we attempted to quantify the impact of these differences in CTV design on heart and lung exposure, and showed a significant increase in mean heart dose of 2 Gy and lung V20Gy of 4% when comparing the RTOG versus ESTRO-based plans. Thus, the question remains: Is this trade off in terms of increased risks of recurrence worth this difference in normal tissue exposure?
Our institutional practice of a compromised dorsal border of the anterior rib surface to include both pectoralis major and minor would have included 100% of the recurrences in our prior literature review, the Korean multi-institutional data, and the patterns of recurrence presented herein. We believe that increasing the mean heart dose by only 0.6 Gy and lung V20Gy by 2% relative to the ESTRO-based plans represents a reasonable clinical compromise (Figs 1 and 3). Others have suggested that the application of a deeper dorsal border be individualized based on patients’ perceived risks of heart and lung toxicity relative to comorbidities such as smoking and other preexisting cardiopulmonary risk factors.14, 15
This may also represent a reasonable clinical application of this data. More work is needed to better elucidate which subgroups might be at the highest risk of deep chest wall recurrence, such as inflammatory breast cancer or triple negative subtypes, because no differences were noted in patterns of recurrence by baseline characteristics.16, 17 Furthermore, this study cannot speak to the implications for use of skin bolus in the postmastectomy setting, which varies at our institution, but is commonly applied, usually in an every-other-day fashion or daily until a Grade 2 reaction develops.18
This study is limited by inherent biases in retrospective design and small patient numbers, which limit the power of the study to detect small differences between groups. The patients included in our patterns-of-recurrence analysis were identified from a surgical database, and this might have biased the inclusion toward smaller chest wall recurrences. However, patients with large recurrences, even in the setting of metastatic disease who were evaluated for palliative resection, were included and identified in this database, and the patterns of recurrence were consistent with those of other larger series, which suggests that the impact of this bias is small, if any.3, 10
This analysis specifically does not take into account other modern radiation therapy techniques, such as deep-inspiration breath hold (routinely used at our institution in postmastectomy radiation therapy to achieve a mean heart dose <2 Gy but not used in the presented study) or proton beam radiation therapy, which might be able to further reduce the doses to the heart and lungs and thereby widen the therapeutic ratio toward the CTV coverage of deeper chest wall targets.19, 20
Ongoing trials, such as the RADCOMP Consortium trial (NCT02603341), that compare proton versus photon radiation therapy in breast cancer will help better elucidate if such techniques might indeed translate into clinically meaningful reductions in doses to the heart and lungs. In the interim, given the salient differences that exist in international consensus contouring definitions and the implications of these variations on target coverage relative to patterns of chest wall recurrence and normal tissue exposure, more studies are clearly needed to help better reach an international consensus for CTV design. Until such a consensus is reached, the current variation in CTV will challenge clinical practice and the interpretation of prospective clinical studies across groups.
Conclusions
Patterns of chest wall recurrence in the modern era are predominately in the skin and subcutaneous tissues, followed by recurrences within the pectoralis muscle. Isolated recurrences deep to the pectoralis are uncommon, and routine inclusion of the ribs and intercostal muscles in the chest wall CTV might not be necessary for routine applications of postmastectomy radiation therapy. Inclusion of the ribs and intercostal muscles in the chest wall CTV as recommended by the RTOG contouring guidelines significantly increase the doses to the heart and lungs compared with the more anterior border of the pectoralis major as recommended by the ESTRO contouring guidelines, which may miss chest wall recurrences within the pectoralis muscles.
Thus, we recommend a compromised dorsal border of the anterior rib surface, which will significantly reduce doses to the heart and lungs relative to the RTOG definition and be more consistent with patterns of chest wall recurrence than those per the ESTRO definition. Continued study and an international consensus are needed to help better guide clinical practice and trial design.
Footnotes
Conflicts of interest: Dr. John A. Vargo receives speaking honoraria from BrainLAB.
References
- 1.White J., Tai A., Arthur S. Breast cancer atlas for radiation therapy planning: Consensus definitions. www.rtog.org Available at: Accessed February 7, 2018.
- 2.Offersen B.V., Boersma L.J., Kirkove C. ESTRO consensus guidelines on target volume delineation for elective radiation therapy of early stage breast cancer. Radiother Oncol. 2015;114:3–10. doi: 10.1016/j.radonc.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 3.Vargo J.A., Beriwal S. RTOG chest wall contouring guidelines for post-mastectomy radiation therapy: is it evidence based? Int J Radiat Oncol Biol Phys. 2015;93:266–267. doi: 10.1016/j.ijrobp.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Chang J.S., Byun H.K., Kim J.W. Three-dimensional analysis of patterns of locoregional recurrence after treatment in breast cancer patients: Validation of the ESTRO consensus guidelines on target volume. Radiother Oncol. 2017;122:22–29. doi: 10.1016/j.radonc.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 5.Fontanilla H.P., Woodward W.A., Lindberg M.E. Current clinical coverage of Radiation Therapy Oncology Group defined target volumes for postmastectomy radiation therapy. Prac Radiat Oncol. 2012;2:201–209. doi: 10.1016/j.prro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Early Breast Cancer Trialists’ Collaborative Group. McGale P., Taylor C. Effects of radiation therapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta-analysis of individual patient data for 8135 women in 22 randomized trials. Lancet. 2014;383:2127–2135. doi: 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darby S.C., Ewertz M., McGale P. Risk of ischemic heart disease in women after radiation therapy for breast cancer. N Engl J Med. 2015;373:307–316. [Google Scholar]
- 8.Vargo J.A., Beriwal S. In reply to Chang et al: Contouring guidelines for post-mastectomy radiotherapy a cry for international consensus. Radiother Oncol. 2017;123:483–484. doi: 10.1016/j.radonc.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 9.White J. Defining target volumes in breast cancer radiation therapy for the future: Back to basics. Int J Radiat Oncol Biol Phys. 2015;93:277–280. doi: 10.1016/j.ijrobp.2015.06.027. [DOI] [PubMed] [Google Scholar]
- 10.Chang J.S., Lee J., Chun M. Mapping patterns of locoregional recurrence following contemporary treatment with radiation therapy for breast cancer: A multi-institutional validation study of the ESTRO consensus guideline on clinical target volume. Radiother Oncol. 2018;126:139–147. doi: 10.1016/j.radonc.2017.09.031. [DOI] [PubMed] [Google Scholar]
- 11.Friedel G., Kuupers T., Engel C. Full thickness chest wall resection for locally recurrent breast cancer. Thorac Surg Sci. 2005;2:Doc01. [PMC free article] [PubMed] [Google Scholar]
- 12.Van der Pol C.C., Van Geel A.N., Menke-Pluymers M.B. Prognostic factors in 77 curative chest wall resection for isolated breast cancer recurrences. Ann Surg Oncol. 2009;16:3414–3421. doi: 10.1245/s10434-009-0662-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faber D., Fadel E., Kolb F. Outcomes of full thickness chest wall resection for isolated breast cancer recurrences. Eur J Cardiothorac Surg. 2013;44:637–642. doi: 10.1093/ejcts/ezt105. [DOI] [PubMed] [Google Scholar]
- 14.Chang J.S., Byun H.K., Kim Y.B. Reply to the letter to the editor by Vargo et al. Radiother Oncol. 2017;123:485. doi: 10.1016/j.radonc.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Taylor C., Correa C., Duane F.K. Estimating the risks of breast cancer radiation therapy: Evidence from modern radiation doses to the lungs and heart and from previous randomized trials. J Clin Oncol. 2017;35:1641–1649. doi: 10.1200/JCO.2016.72.0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kyndi M., Sorensen F.B., Knudsen H. Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiation therapy in high-risk breast cancer: The Danish Breast Cancer Cooperative Group. J Clin Oncol. 2008;26:1419–1426. doi: 10.1200/JCO.2007.14.5565. [DOI] [PubMed] [Google Scholar]
- 17.Cristofanilli M., Valero V., Buzdar A.U. Inflammatory breast cancer (IBC) and patterns of recurrence: Understanding the biology of a unique disease. Cancer. 2007;110:1436–1444. doi: 10.1002/cncr.22927. [DOI] [PubMed] [Google Scholar]
- 18.Turner J.Y., Zeniou A., Williams A., Jyothirmayi R. Technique and outcome of postmastectomy adjuvant chest wall radiation therapy–the role of tissue equivalent bolus in reducing risk of local recurrence. Br J Radiol. 2016;89:20160060. doi: 10.1259/bjr.20160060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel S.A., Lu H.M., Nyamwanda J.A. Postmastectomy radiation therapy technique and cardiopulmonary sparing: A dosimetric comparative analysis between photons and protons with free breathing versus deep inspiration breath hold. Pract Radiat Oncol. 2017;7:e377–e384. doi: 10.1016/j.prro.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Nissen H.D., Appelt A.L. Improved heart, lung, and target dose with deep inspiration breath hold in a large clinical series of breast cancer patients. Radiother Oncol. 2013;106:28–32. doi: 10.1016/j.radonc.2012.10.016. [DOI] [PubMed] [Google Scholar]