Abstract
Purpose
Perineural invasion (PNI) is a histologic feature that is present in as many as 84% of patients with prostate cancer. The prognostic significance of PNI is controversial, with recent studies yielding contradictory results. This study aims to assess whether PNI, on the surgical pathology of patients with pT2N0M0 disease and with negative surgical margins, is an independent prognostic indicator of the risk of biochemical recurrence.
Methods and materials
We identified 1549 patients who received a diagnosis of margin-negative pT2N0M0 prostate cancer at 3 separate institutions between January 1, 2008 and December 31, 2014. We reviewed the electronic medical records of these patients and collected clinical and histologic data. A multivariable analysis was performed to assess the association between PNI and biochemical recurrence.
Results
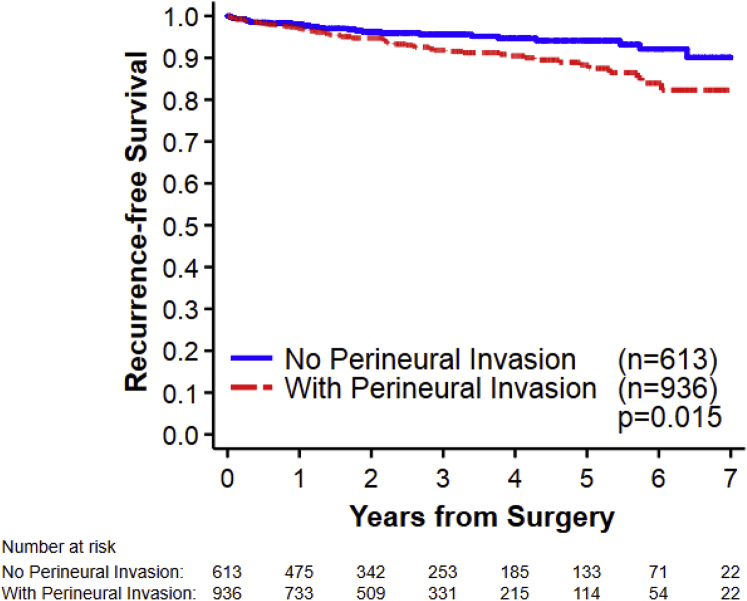
Of the 1549 patients identified, 936 (60.4%) had PNI and 96 (6.2%) had biochemical recurrence. The median time until recurrence was 16 months. The median follow-up in patients without recurrence was 26.5 months. PNI was associated with pT2c disease. The proportion of patients with pT2c was 89% in patients with PNI compared with 79% in patients without PNI (P < .001). PNI was also associated with a higher surgical Gleason score (of those with vs without PNI, 21% vs 50% had Gleason score 3 + 3; 62% vs 41% had a Gleason score 3 + 4, 12% vs 5% had a Gleason score 4 + 3; and 5% vs 3% had a Gleason score 8-10; P < .001). On univariate analysis, patients with PNI appeared to be more likely to have disease recurrence (hazard ratio: 1.7; 95% confidence interval, 1.1-2.6; P = .015). However, after adjusting for other variables, there was not a significant association between PNI and recurrence (hazard ratio: 1.1; 95% confidence interval, 0.70-1.8: P = .65).
Conclusions
We found that PNI was not an independent indicator of the risk of biochemical recurrence. Instead, PNI may be an indicator of unfavorable histology such as a high Gleason score or diffuse disease within the prostate in pT2N0 patients.
Introduction
Prostate cancer (PC) is the leading cancer diagnosis in men, with an estimated incidence of 164,690 in the United States in 2018.1 Of these patients, approximately 70.2% will have organ-confined (pT2) disease,2 of whom 4.6% to 13% will subsequently develop disease recurrence.3, 4, 5, 6, 7, 8 Improved identification of the subset of patients with organ-confined disease who are likely to experience disease recurrence is critical for providers to formulate more accurate and timely recommendations for adjuvant treatment.
In 1999, the College of American Pathologists published a consensus statement on prognostic factors for PC that could help better identify patients at risk of recurrence. In this statement, perineural invasion (PNI) was identified as a potential prognostic factor (category III) that needed additional study.9 PNI is defined as the infiltration of cancer cells into the perineural space where they track along or around a nerve10 and is found in 22.4% to 65.4% of PC specimens in patients with pT2 disease.2, 6, 11, 12, 13 The presence of PNI has been associated with inhibition of apoptosis of cancer cells, thereby allowing for increased proliferation of cancer cells.14
Since first being identified in 1999 as a potential prognostic indicator, the prognostic significance of PNI has remained controversial. Studies evaluating PNI on surgical pathology after prostatectomy have yielded varied results, with some studies suggesting PNI is an independent prognostic indicator for biochemical recurrence6, 12, 13, 15, 16, 17, 18, 19 and others indicating no prognostic utility for biochemical recurrence.8, 11, 20, 21, 22, 23 However, many of these reports included patients with both pT2 and pT3 disease, and the differing baseline recurrence risks of these groups may confound the results.2, 20, 21, 23
The primary goal of this retrospective study was to assess whether PNI on surgical pathology of patients with pT2N0M0 PC and with negative surgical margins is an independent prognostic indicator of the risk of biochemical recurrence.
Methods and materials
Patient selection
A retrospective institutional review board–approved chart review of the electronic medical record (EMR) was conducted at Los Angeles County hospital (LAC) (1), one of the largest safety-net hospitals in the country, USC Norris Comprehensive Cancer Center (Norris) (2), and the Hospital of the University of Pennsylvania (Penn) (3). USC Norris Comprehensive Cancer Center (Norris) and Hospital of the University of Pennsylvania (Penn) 2 and 3 are both university-based private cancer hospitals that are National Cancer Institute–designated comprehensive cancer centers. We obtained a list of patients who received diagnoses of PC between January 1, 2008 and December 31, 2014 who underwent prostatectomy and were found to have margin-negative pT2N0M0 disease.
Patient lists were obtained from multiple sources, including the Penn Medicine Data Analytics Center, Los Angeles County Cancer Surveillance Program, and the Los Angeles Registry (part of the National Cancer Institute's Surveillance, Epidemiology, and End Results cancer registry program). We reviewed the EMR and excluded patients based on (1) positive surgical margin status, (2) receipt of neoadjuvant radiation therapy or androgen deprivation therapy before surgery, (3) Gleason score <6, and (4) unknown PNI status or unknown Gleason score.
Data collection
The EMR of each patient was reviewed, and data were abstracted related to patient-level demographic data (age at diagnosis, race/ethnicity), tumor-specific data (clinical T stage, prostate-specific antigen [PSA] at diagnosis [defined as PSA 6 months before or 3 months after the date of diagnosis], surgical Gleason score, pathologic T stage, presence of PNI on surgical pathology, surgical margin status, and all postoperative PSA values), and development of biochemical recurrence. Biochemical recurrence was defined as a PSA >0.2 ng/mL after prostatectomy.
Patient data were abstracted through January 1, 2018 to determine (1) if the patient had biochemical recurrence or (2) the duration of biochemical progression-free survival (BPFS). Patients were considered as having a recurrence if they had a PSA >0.2 ng/mL after surgery. In our patient cohort, all patients had a PSA <0.2 ng/mL in the postoperative setting. The duration of BPFS was defined as the time between prostatectomy and the most recent postoperative PSA value in patients who did not meet the criteria for biochemical recurrence.
Data analysis
The primary endpoint for this study was BPFS. Patients who did not have a recurrence were censored at the date of the most recently reported PSA value. Patient characteristics at LAC, Norris, and Penn 1, 2, and 3 were summarized and compared using the Student 2-sample t test and Pearson's χ2 test.
Association between patient and disease characteristics and BPFS was assessed using univariate and multivariable Cox regression models. P values from the Cox analyses were based on likelihood ratio tests. Statistical analyses were performed using STATA software, version 11 (College Station, TX). All P-values were 2-sided, and P-values <.05 were considered statistically significant.
Results
Of the 2243 patients initially identified with pT2N0M0 PC, 380 were excluded for positive margins, 309 were excluded for incomplete data, 3 were excluded for positive lymph nodes, and 2 were excluded for Gleason scores <6. Of the remaining 1549 patients, 936 (60.4%) had PNI and 96 (6.2%) had biochemical recurrence. BPFS by PNI status is presented in Figure 1. Of the 96 patients who had disease recurrence, 20 had detectable PSA after prostatectomy (median PSA in these 20 patients was 0.29 ng/mL). The median time until recurrence in the 96 patients was 16 months (range, 9 days to 92 months). The median follow-up in patients without recurrence was 26.5 months (range, 13 days to 102 months). The majority of patients in this study were non-Hispanic white (74.7%) and had cT1c disease (85.1%), a surgical Gleason score of 7 (80.5%), and pT2c disease (79.3%). Patient demographic and clinical information is presented in Table 1.
Figure 1.
Biochemical progression-free survival by perineural invasion.
Table 1.
Patient and disease characteristics
| Variables | Surgical perineural invasion |
P-value | |
|---|---|---|---|
| Yes (n = 936) | No (n = 613) | ||
| Institution | |||
| Norris | 128 (14%) | 59 (10%) | <.001∗ |
| LAC | 12 (1%) | 25 (4%) | |
| Penn | 796 (85%) | 529 (86%) | |
| Age at diagnosis (years) | |||
| Mean (range), y | 61 (41-85) | 59 (35-79) | <.001∗ |
| (35, 50) | 53 (6%) | 49 (8%) | |
| (50, 60) | 367 (39%) | 296 (48%) | |
| (60, 70) | 423 (45%) | 229 (37%) | |
| (70, 85) | 93 (10%) | 39 (6%) | |
| Race/ethnicity | |||
| Latino | 24 (3%) | 29 (5%) | .016† |
| Black | 148 (16%) | 93 (15%) | |
| Asian | 14 (2%) | 20 (3%) | |
| White | 715 (76%) | 442 (72%) | |
| Other/unknown | 35 (4%) | 29 (5%) | |
| Clinical T stage | |||
| T1c | 792 (85%) | 526 (86%) | .52† |
| T2 | 144 (15%) | 87 (14%) | |
| Prostate-specific antigen level at diagnosis | |||
| Mean (range) | 5.8 (0.2-63.4) | 5.3 (0.2-66.4) | .060† |
| (0.2, 5) | 447 (51%) | 307 (54%) | |
| (5, 10) | 364 (41%) | 224 (40%) | |
| (10, 20) | 60 (7%) | 30 (5%) | |
| (20, 66.4) | 10 (1%) | 4 (<1%) | |
| Unknown | 55 | 48 | |
| Pathologic tumor | |||
| p2a | 66 (7%) | 110 (18%) | <.001† |
| p2b | 35 (4%) | 15 (3%) | |
| p2c | 812 (89%) | 471 (79%) | |
| p2 (a, b, or c unknown) | 23 | 17 | |
| Gleason score per surgery pathology | |||
| 6 | 196 (21%) | 308 (50%) | <.001† |
| 7 (3 + 4) | 577 (62%) | 253 (41%) | |
| 7 (4 + 3) | 115 (12%) | 32 (5%) | |
| 8-10 | 48 (5%) | 20 (3%) | |
Student's 2-sample t test
Pearson's χ2d test
Patient demographics and associations with PNI
The mean age at diagnosis for patients with PNI was 61 years, compared with 59 years for patients without PNI (P < .001). Of the patients with PNI, 76% were non-Hispanic white, 16% were Black, 3% were Latino, 2% were Asian, and 4% were other/unknown compared with 77%, 15%, 5%, 3%, and 5%, respectively, for patients without PNI (P = .016).
Clinical information and associations with PNI
There was no statistically significant difference in clinical T staging between patients with or without PNI. Of the patients with PNI, 85% had cT1c disease and 15% had cT2 disease compared with 86% and 14%, respectively, for patients without PNI. There was a trend toward patients with PNI having a higher PSA value at diagnosis compared with patients without PNI (5.8 ng/mL vs 5.3 ng/mL; P = .06). PNI was found to be associated with pT2c disease, with 89% of patients with PNI having pT2c disease compared with 79% of patients without PNI having pT2c disease (P < .001). PNI was also associated with a higher surgical Gleason score (patients with vs without PNI: 21% vs 50% had Gleason score 3 + 3, 62% vs 41% had Gleason score 3 + 4, 12% vs 5% had Gleason score 4 + 3, and 5% vs 3% had Gleason score 8-10; P < .001).
Risk of biochemical recurrence
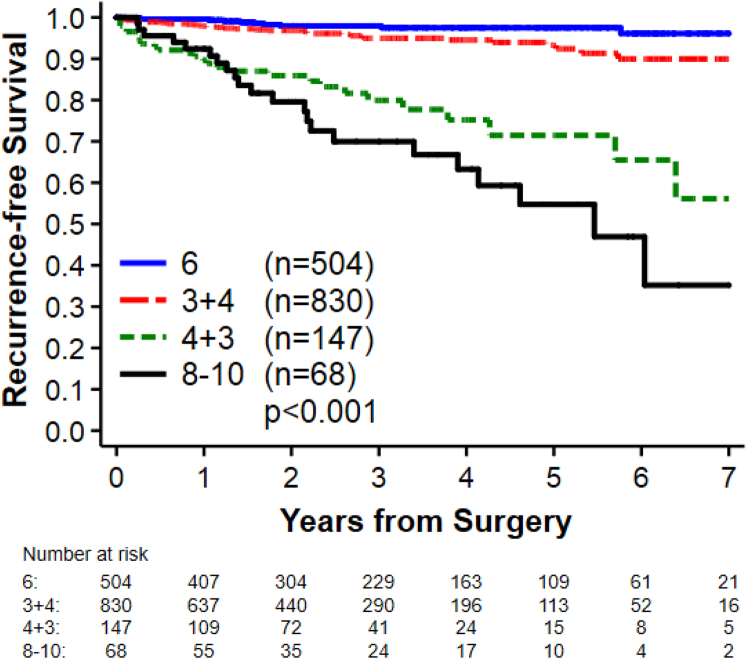
On univariate analysis, patients with PNI, a higher surgical Gleason score, higher PSA values at diagnosis, and age >70 years at diagnosis were more likely to have disease recurrence (Table 2). However, on multivariate analysis, after adjusting for pathologic tumor staging, Gleason score, and PSA at diagnosis, there was no longer a significant association between PNI and recurrence. Only an increasing Gleason score (Fig 2) and PSA >10 at diagnosis were found to be associated with an increased risk of biochemical recurrence (Table 2).
Table 2.
Association between disease characteristics and biochemical progression-free survival
| Variables | No. of patients (N = 1549) | No. recur (n = 96) | Univariate |
Multivariable∗ |
||
|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P-value | Hazard ratio (95% CI) | P-value | |||
| Surgical biopsy perineural invasion | ||||||
| No | 613 | 29 | 1.0 | .015 | 1.0 | .65 |
| Yes | 936 | 67 | 1.7 (1.1-2.6) | 1.1 (0.70-1.8) | ||
| Institution | ||||||
| Norris | 187 | 24 | 1.0 | <.001 | 1.0 | .069 |
| LAC | 37 | 3 | 0.58 (0.17-1.9) | 0.31 (0.09-1.1) | ||
| Penn | 1325 | 69 | 0.37 (0.23-0.60) | 0.63 (0.34-1.2) | ||
| Age at diagnosis (y) | ||||||
| 35-50 | 102 | 3 | 1.0 | .006† | 1.0 | .83 |
| 50-60 | 663 | 39 | 2.2 (0.67-7.0) | 1.5 (0.44-4.8) | ||
| 60-70 | 652 | 37 | 2.1 (0.65-6.9) | 0.91 (0.27-3.1) | ||
| 70-85 | 132 | 17 | 5.3 (1.6-18) | 1.5 (0.43-5.4) | ||
| Race/ethnicity | ||||||
| White | 1157 | 70 | 1.0 | .83 | Excluded | |
| Other | 392 | 26 | 1.1 (0.67-1.6) | |||
| Clinical t stage | ||||||
| T1c | 1318 | 81 | 1.0 | .83 | Excluded | |
| T2 | 231 | 15 | 1.1 (0.61-1.8) | |||
| Prostate-specific antigen level at diagnosis | ||||||
| 0.2-5 | 754 | 29 | 1.0 | <.001∗ | 1.0 | .002 |
| 5-10 | 588 | 36 | 1.9 (1.1-3.1) | 1.6 (0.95-2.6) | ||
| 10-66.4 | 104 | 16 | 5.3 (2.9-9.8) | 2.7 (1.4-5.2) | ||
| Missing‡ | 103 | 15 | ||||
| Pathologic tumor | ||||||
| p2a | 176 | 7 | 1.0 | .10† | 1.0 | .14 |
| p2b | 50 | 3 | 1.8 (0.45-7.0) | 0.54 (0.12-2.4) | ||
| p2c | 1283 | 82 | 1.9 (0.86-4.0) | 1.6 (0.70-3.5) | ||
| p2 ϯ | 40 | 4 | ||||
| Gleason score per surgery pathology | ||||||
| 6 | 504 | 11 | 1.0 | <.001 | 1.0 | <.001 |
| 7 (3 + 4) | 830 | 35 | 2.3 (1.2-4.5) | 2.1 (1.05-4.3) | ||
| 7 (4 + 3) | 147 | 28 | 11 (5.5-22) | 9.1 (4.3-19) | ||
| 8-10 | 68 | 22 | 17 (8.1-34) | 12 (5.6-27) | ||
Abbreviation: CI = confidence interval.
Variables with a P-value < .20 in univariate analyses were included in this multivariable model.
Test of trend.
Patients with missing/unknown values were included in the model as a missing category.
Figure 2.
Biochemical progression-free survival by surgical Gleason score.
Discussion
To our knowledge, this study is the largest to date analyzing the relationship between PNI on surgical pathology and BPFS in patients with pT2 disease. The primary goal of this retrospective study was to assess whether PNI on surgical pathology of patients with pT2N0M0 disease and negative surgical margins is an independent prognostic indicator of the risk of biochemical recurrence. We observed (1) a high incidence of PNI in margin-negative pT2N0M0 PC, (2) that patients with organ-confined disease and negative margins have a low rate of biochemical recurrence, and (3) that PNI is not an independent prognostic indicator of biochemical recurrence.
In this patient cohort, 60.4% of patients were found to have PNI after prostatectomy. This is consistent with other studies that have looked at patients with organ-confined disease and found rates of PNI on prostatectomy specimens to be between 22.4% and 65.4%.2, 6, 11, 12
The 6.2% recurrence rate in this cohort of patients was comparable with the 4.6% to 13% recurrence rate that has been reported in other studies of patients with organ-confined disease.3, 4, 5, 6, 7 Our recurrence rate is at the lower end of this range because the patients in this cohort had margin-negative disease. Nonetheless, the nonzero rate of biochemical recurrence in patients with apparent organ-confined disease status postprostatectomy illustrates the limitations of our current radiographic and pathologic staging methods and the need for more accurate personalized prognostic and predictive tools.
To our knowledge, 3 studies have found PNI on surgical pathology to be a prognostic indicator for biochemical recurrence in patients with pT2 disease. The first of these studies, performed by Endrizzi et al, looked at 131 patients with pT2 PC, of whom 48.1% had PNI and 13% had biochemical recurrence with a mean time until recurrence of 37 months.3 PNI was found to be more sensitive than a PSA value >10 ng/mL or Gleason score ≥7 in predicting biochemical recurrence. However, Endrizzi et al only looked at the sensitivity, specificity, and positive predictive value of PNI and did not perform a univariate or multivariate analysis on the association between PNI and biochemical recurrence.3
In comparison, studies performed by Jeon et al and Ozcan et al found PNI to be associated with biochemical recurrence on univariate and multivariate analysis; however, these 2 studies looked at a small cohort of patients.6, 13 Jeon et al performed a subgroup analysis of 145 patients in Korea with pT2 disease, of whom 24.8% had PNI on surgical pathology, and reported that PNI was associated with biochemical recurrence on both univariate and multivariate analysis.13 Ozcan et al also found PNI to be significantly associated with biochemical recurrence on multivariate analysis in their cohort of 178 patients with pT2N0 disease in Turkey, of whom 31.9% had PNI; the mean time until recurrence was 65 months.6
In contrast, 3 studies have shown that PNI on surgical pathology is not a prognostic indicator for biochemical recurrence in patients with pT2N0 PC. The largest of these studies was performed in Korea by Kang et al with 1481 patients, of whom 63% had PNI on surgical pathology, which was associated with biochemical recurrence on univariate but not multivariate analysis. Of note, PNI was found to be an independent prognostic indicator in the subgroup of patients with ≥pT3 disease.19
The most recent study to address the potential prognostic significance of PNI in pT2 PC patients was performed in Belgium by Aoun et al and comprised 910 patients, including 11.8% of patients with biochemical recurrence and 33.5% with PNI.5 Similar to our study, neither PNI nor pathologic stage was found to be associated with biochemical recurrence on univariate or multivariate analysis. Finally, Masieri et al looked at 239 patients with pT2 disease in Italy, of whom 65.7% had PNI on surgical pathology.4 The mean time until recurrence was 25 months. Masieri et al suggested a possible negative prognostic role of PNI but found no significant association with biochemical recurrence.
Our study underlines the conclusions of these last 3 studies, demonstrating that PNI on surgical pathology is not an independent prognostic indicator of biochemical recurrence. The patient cohort in our study is the largest study of patients with pT2 disease and represents a multi-institutional cohort. Although we found PNI on surgical pathology to be associated with biochemical recurrence on univariate analysis, this association was lost on multivariate analysis, as seen in the study by Kang et al.19 Masieri et al suggested that the lack of prognostic significance of PNI on multivariate analysis was due to the close correlation between tumor volume and PNI. This association between PNI and tumor volume has been demonstrated in multiple studies and increases the risk of a patient having positive margins.2, 24
PNI on surgical pathology has also been shown to be associated with a number of markers for more aggressive disease, including Gleason score, pathologic T staging, lymph node metastasis, lymphovascular invasion preoperative PSA, extracapsular extension, and seminal vesicle invasion.2, 6, 9, 11, 12, 13, 18, 19, 22, 23 Our study confirms the finding that PNI on surgical pathology is associated with elevated preoperative PSA levels, higher pathologic tumor staging, and a higher Gleason score on surgical pathology. The association may be between PNI and these aggressive disease markers rather than PNI itself, which may explain the association of PNI with biochemical recurrence on univariate but not multivariate analysis. Of note, PNI on biopsy specimens has also been found to be associated with clinical stage, pretreatment PSA level, and biopsy Gleason score.25 Unlike PNI on surgical pathology, a recent study at John Hopkins showed that PNI on biopsy specimens can serve as an independent prognostic indicator for adverse survival outcomes in patients who received definitive radiation therapy.26
When comparing the studies that found PNI on surgical pathology to be an independent prognostic indicator of biochemical recurrence with those that did not, a few trends emerge. The studies indicating that PNI is not associated with biochemical recurrence are more recent, with larger patient cohorts, longer follow-up, and generally higher rates of PNI. The differences in rates of PNI on surgical pathology in these studies may be due to institutional variation in patient populations and/or histologic criteria. In theory, institutions that have a higher threshold for determining the presence of PNI on surgical pathology may find that PNI has prognostic value.
Rather than looking at PNI as a binary variable, some studies have attempted to quantify various histologic parameters of PNI, including focality and diameter of PNI, to determine when PNI is clinically significant.12, 17, 24 Maru et al looked at patients with pT2 disease and found that those patients with a PNI diameter >0.25 mm had a significantly worse prognosis compared with patients with a PNI diameter <0.25 mm and that PNI >0.25 mm was an independent prognostic indicator for biochemical recurrence on multivariate analysis.12 Additionally, a study by Sun et al found that multifocal PNI, rather than unifocal PNI, is an independent prognostic indicator for biochemical recurrence in patients with high-risk PC. Sun et al recommended that patients with multifocal PNI on surgical pathology may benefit from initiating androgen deprivation therapy immediately after surgery.17 However, there is still controversy regarding the use of PNI diameter, density, and distance from the excisional margin because another study by Merrilees et al found no association between these variables and biochemical recurrence.20
This study is retrospective and thus has a number of limitations. Prostatectomy specimens did not undergo a centralized review by 1 pathologist, so there may be variability in the reporting of PNI among the 3 facilities. PNI was not always reported on pathology reports, and we excluded patients with unknown PNI status to minimize the potential for misclassification bias. Additionally, we were only able to report PNI as a binary variable because quantitative measures of PNI were not consistently included in pathology reports. Thus, we are unable to comment on how various histologic parameters of PNI on surgical pathology may affect prognostic outcomes.
Furthermore, a major limitation of this study was the relatively short median follow-up in patients without recurrence (26.5 months). More recurrences would likely be detected during a longer follow-up period. Of the studies that reported longer median lengths of follow-up, 2 studies noted a mean time until recurrence of 25 months4 and 37 months.3 That said, our observed recurrence rate falls within the range in these prior reports.
Conclusions
This study is the largest multi-institutional study published to date that specifically analyzed patients with pT2 PC. We found that PNI on surgical pathology was not an independent indicator of the risk of biochemical recurrence in patients with pT2 disease. PNI may instead be an indicator of aggressive disease because of its association with a high Gleason score or more widespread disease. We recommend that PNI continue to be reported in prostatectomy specimen reports because longer follow-up is needed to more accurately confirm whether PNI is an indicator of biochemical recurrence in other pathologic T stages. Additionally, it may be beneficial to determine whether PNI on surgical pathology is associated more with locoregional versus distant recurrence because this may partially explain why the literature has documented conflicting results on the clinical significance of PNI.
Footnotes
Sources of support: This work had no specific funding.
Conflicts of interest: The authors have no conflicts of interest to disclose.
References
- 1.Siegel R., Miller K., Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Lee J.T., Lee S., Yun C.J. Prediction of perineural invasion and its prognostic value in patients with prostate cancer. Korean J Urol. 2010;51:745–751. doi: 10.4111/kju.2010.51.11.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Endrizzi J., Seay T. The relationship between early biochemical failure and perineural invasion in pathologic T2 prostate cancer. BJU Int. 2000;85:696–698. doi: 10.1046/j.1464-410x.2000.00518.x. [DOI] [PubMed] [Google Scholar]
- 4.Masieri L., Lanciotti M., Nesi G. Prognostic role of perineural invasion in 239 consecutive patients with pathologically organ-confined prostate cancer. Urol Int. 2010;85:396–400. doi: 10.1159/000315491. [DOI] [PubMed] [Google Scholar]
- 5.Aoun F., Albisinni S., Henriet B., Tombal B., Van Velthoven R., Roumeguère T. Predictive factors associated with biochemical recurrence following radical prostatectomy for pathologic T2 prostate cancer with negative surgical margins. Scand J Urol. 2017;51:20–26. doi: 10.1080/21681805.2016.1263237. [DOI] [PubMed] [Google Scholar]
- 6.Ozcan F. Correlation of perineural invasion on radical prostatectomy specimens with other pathologic prognostic factors and PSA failure. Eur Urol. 2001;40:308–312. doi: 10.1159/000049791. [DOI] [PubMed] [Google Scholar]
- 7.Budäus L., Isbarn H., Eichelberg C. Biochemical recurrence after radical prostatectomy: multiplicative interaction between surgical margin status and pathologic stage. J Urol. 2010;184:1341–1346. doi: 10.1016/j.juro.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 8.Kinčius M., Matjošaitis A.J., Trumbeckas D., Mickevičius R., Milonas D., Jievaltas M. Independent predictors of biochemical recurrence after radical prostatectomy: A single center experience. Cent European J Urol. 2011;64:21–25. doi: 10.5173/ceju.2011.01.art4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bostwick D.G., Grignon D.J., Hammond M.E. Prognostic factors in prostate cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Laboratory Med. 2000;124:995–1000. doi: 10.5858/2000-124-0995-PFIPC. [DOI] [PubMed] [Google Scholar]
- 10.Batsakis J.G. Nerves and neurotropic carcinomas. Ann Otol Rhinol Laryngol. 1985;94:426–427. [PubMed] [Google Scholar]
- 11.Dere Y., Altinboga A.A., Bal K., Calli A., Ermete M., Sari A.A. The Histopathologic parameters affecting biochemical recurrence in radical prostatectomies. J Coll Physicians Surg Pak. 2017;27:213–217. [PubMed] [Google Scholar]
- 12.Maru N., Ohori M., Kattan M.W., Scardino P.T., Wheeler T.M. Prognostic significance of the diameter of perineural invasion in radical prostatectomy specimens. Hum Pathol. 2001;32:828–833. doi: 10.1053/hupa.2001.26456. [DOI] [PubMed] [Google Scholar]
- 13.Jeon H.G., Bae J., Yi J.S., Hwang I.S., Lee S.E., Lee E. Perineural invasion is a prognostic factor for biochemical failure after radical prostatectomy. Int J Urol. 2009;16:682–686. doi: 10.1111/j.1442-2042.2009.02331.x. [DOI] [PubMed] [Google Scholar]
- 14.Ayala G.E., Dai H., Ittmann M. Growth and survival mechanisms associated with perineural invasion in prostate cancer. Cancer Res. 2004;64:6082–6090. doi: 10.1158/0008-5472.CAN-04-0838. [DOI] [PubMed] [Google Scholar]
- 15.Meng Y., Liao Y.B., Xu P., Wei W.R., Wang J. Perineural invasion is an independent predictor of biochemical recurrence of prostate cancer after local treatment: A meta-analysis. Int J Clin Exp Med. 2015;8:13267–13274. [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L.J., Wu B., Zha Z.L. Perineural invasion as an independent predictor of biochemical recurrence in prostate cancer following radical prostatectomy or radiation therapy: A systematic review and meta-analysis. BMC Urol. 2018;18:5. doi: 10.1186/s12894-018-0319-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun G., Huang R., Zhang X. The impact of multifocal perineural invasion on biochemical recurrence and timing of adjuvant androgen-deprivation therapy in high-risk prostate cancer following radical prostatectomy. Prostate. 2017;77:1279–1287. doi: 10.1002/pros.23388. [DOI] [PubMed] [Google Scholar]
- 18.Reeves F., Hovens C.M., Harewood L. Does perineural invasion in a radical prostatectomy specimen predict biochemical recurrence in men with prostate cancer? Can Urol Assoc J. 2015;9:E252–E255. doi: 10.5489/cuaj.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang M., Oh J.J., Lee S., Hong S.K., Lee S.E., Byun S.S. Perineural invasion and lymphovascular invasion are associated with increased risk of biochemical recurrence in patients undergoing radical prostatectomy. Ann Surg Oncol. 2016;23:2699–2706. doi: 10.1245/s10434-016-5153-z. [DOI] [PubMed] [Google Scholar]
- 20.Merrilees A.D., Bethwaite P.B., Russell G.L., Robinson R.G., Delahunt B. Parameters of perineural invasion in radical prostatectomy specimens lack prognostic significance. Mod Pathol. 2008;21:1095–1100. doi: 10.1038/modpathol.2008.81. [DOI] [PubMed] [Google Scholar]
- 21.Freedland S.J., Csathy G.S., Dorey F., Aronson W.J. Percent prostate needle biopsy tissue with cancer is more predictive of biochemical failure or adverse pathology after radical prostatectomy than prostate specific antigen or Gleason score. J Urol. 2002;167:516–520. doi: 10.1016/S0022-5347(01)69076-1. [DOI] [PubMed] [Google Scholar]
- 22.Miyake H., Sakai I., Harada K., Eto H., Hara I. Limited value of perineural invasion in radical prostatectomy specimens as a predictor of biochemical recurrence in Japanese men with clinically localized prostate cancer. Hinyokika Kiyo. 2005;51:241–246. [PubMed] [Google Scholar]
- 23.Ng J.C., Koch M.O., Daggy J.K., Cheng L. Perineural invasion in radical prostatectomy specimens: lack of prognostic significance. J Urol. 2004;172:2249–2251. doi: 10.1097/01.ju.0000143973.22897.f8. [DOI] [PubMed] [Google Scholar]
- 24.Olar A., He D., Florentin D., Ding Y., Wheeler T., Ayala G. Biological correlates of prostate cancer perineural invasion diameter. Hum Pathol. 2014;45:1365–1369. doi: 10.1016/j.humpath.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeLancey J.O., Wood D.P., He C. Evidence of perineural invasion on prostate biopsy specimen and survival after radical prostatectomy. Urology. 2013;81:354–357. doi: 10.1016/j.urology.2012.09.034. [DOI] [PubMed] [Google Scholar]
- 26.Peng L.C., Narang A.K., Gergis C. Effects of perineural invasion on biochemical recurrence and prostate cancer-specific survival in patients treated with definitive external beam radiation therapy. Urol Oncol. 2018;36:309.e307–309.e314. doi: 10.1016/j.urolonc.2018.02.008. [DOI] [PubMed] [Google Scholar]