Abstract
Ultrasound (US) is an excellent imaging tool to evaluate most of the structures in the knee joint. US is useful in various applications of regenerative medicine, starting from the biomaterial harvesting stage of the procedures, it can thus/conveniently be used for the diagnosis and treatment of various forms of knee osteoarthritis (OA) where the interventions need to be carried out under US guidance. In this paper, we have reviewed US guided bioharvesting of venous blood, bone marrow and adipose tissue, the US evaluation of the knee joint and the relevant findings in knee OA along with US guided regenerative interventions for the knee joint.
1. Introduction
In recent decades, significant progress has taken place in the area of regenerative and cellular based medicine. Regenerative injection therapy (RIT) has been used for bone, cartilage, ligament and tendon conditions as the injectates can be useful in augmenting the natural healing process.1 US is a inexpensive, noninvasive, radiation-free imaging tool, with no known contraindications.
Ultrasound (US) imaging has many advantages such as being non-invasive, portable and radiation-free and allowing dynamic evaluation. Since US provides either real time or indirect guidance for interventional procedures - it has already gained a well-established role in the management of several musculoskeletal pathologies, and its use in various applications of regenerative medicine is a logical progression and advancement in personalized medicine.2, 3, 4, 5 This paper reviews the US evaluation of the knee joint with a focus towards relevant findings in knee OA. Biomaterial harvesting that maybe useful for US guided interventions for the knee have also been described.
2. Ultrasound use in biomaterial harvesting useful in knee osteoarthritis
The regenerative treatment modalities used in the treatment of knee OA include, but not limited to, platelet rich plasma (PRP), bone marrow aspiration concentrate (BMAC), adipose derived products, and mesenchymal stem cell (MSC) therapies. The peripheral blood, bone marrow and adipose tissue are the main sources of these products.
2.1. Peripheral venous access
Depending on the technique or the equipment used, peripheral blood with varying volume (10–60 ml) is needed to prepare PRP. In clinical routine, the venous puncture is mostly performed without a need for guidance (done by vessel visualisation and/or palpation). However, it can be challenging in some patients with deeper and thinner peripheral veins due to obesity, edema, chronic illness, etc. Furthermore, it can be sometimes be challenging for an inexperienced clinician in a private office setting. As, any damage to the vessel wall may lead early activation of the platelets as well. As such, US guidance might be preferred in patients with difficult venous access and previous failed attempts in order to avoid discomfort, anxiety or complications.6,7
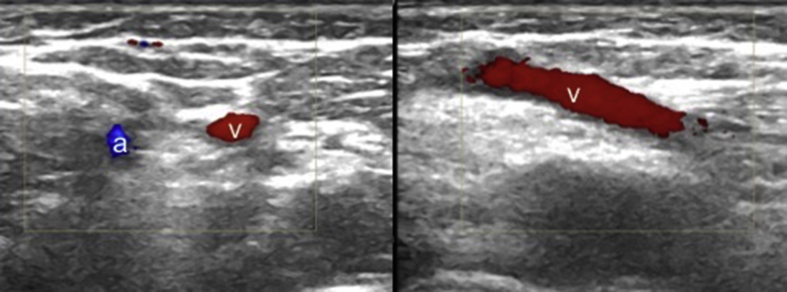
Experience in vascular imaging and proper technique for needle placement are required. Of note, even after a single training session, it has been reported that previously US-naïve medical students were able to use US to guide peripheral vascular access on real patients.8 The first step is identifying the arteries and veins; while the former are pulsatile and usually not compressible, the latter do not pulsate and become easily compressible. Doppler imaging can also be used either to recognize the vessels within the nearby soft tissues or to differentiate the arteries and veins with their flow patterns (Fig. 1).
Fig. 1.
Axial US image shows the brachial artery (a) and basilic vein (v) in the cubital fossa (left side). Longitudinal US image of the basilic vein (v)(right side).
The target vessel, the needle and - in some cases - the surrounding organs should be accurately depicted under real time imaging.9 Veins can be visualized either in the short or long axis views whereby the two techniques were found to be similar in terms of difficulty rated by the users, time to venipuncture and number of attempts.10 Likewise, the needle advancement can be performed using the in-plane or out-plane approaches. While the former technique refers to the identification of the needle throughout its long axis, the latter is only able to show a single hyperechoic dot which may always not precisely depict the needle tip though.
2.2. Bone marrow aspiration
The rationale of using US guidance in bone marrow aspiration (BMA) is to harvest high yield MSC containing bone marrow from the targeted location while avoiding peripheral structural damages, and providing a comfortable procedure for the clinicians and patients alike. BMA is otherwise/traditionally performed using palpation of bony landmarks.11
Muschler et al.12 reported that the first 2 ml of the BMA contains the highest concentration of nucleated cells, while the BMA will be diluted by peripheral blood as the volume of aspirate increases. Fennema et al.13 found that a second 10 ml aspiration from the same site decreases MSC yield. Furthermore, they reported that every aspiration site has its own volume depending on the shape of the trabecula and its connections with other trabeculae. Therefore, in order to obtain higher yield, the aspiration from one site should be limited. Aspiration from more sites increases the amount of subcortical MSCs and cortical and perivascular pericytes.14 Therefore, maintaining lower aspiration volumes from multiple sites may provide higher concentrations of stem cells. Alternative strategies would be advancing deeper and rotating the trocar in the marrow, manipulating the trocar using the same bone entry. However, there is no reliable data to support these techniques yet. Additionally, the volume of the syringe used for aspiration was reported to affect the BMA yields i.e. a 10 ml syringe compared with a 50 mL syringe provides a greater yield.15
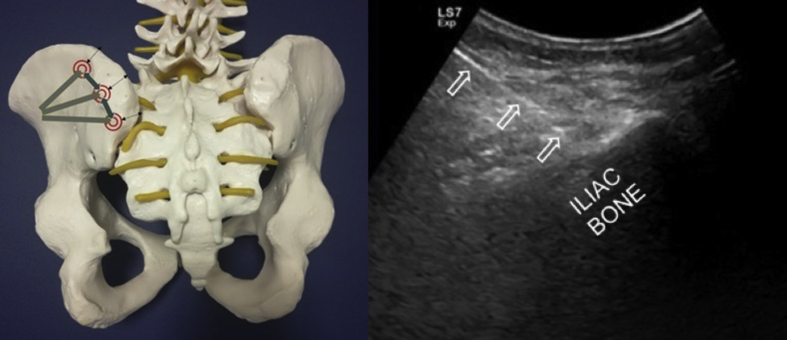
The most commonly used location for BMA is the posterior iliac crest (PIC) as it is found to be safe and having low rate of complications.16,17 In the study comparing the BMA yields obtained from the PIC, anterior iliac crest (AIC) and the tibial plateau; while multipotent/progenitor cell yields were found to be similar between the former two, less than the half was attained from the tibial plateau.18 Further, in the study comparing the concentration and yield of colony forming units of BMA obtained from AIC vs. PIC, a greater cellular yield were obtained from the latter.19 In general, if the patient is under spinal or general anesthesia and lying supine, AIC can be used for aspiration. In this section, we discuss how to perform US-guided BMA from the PIC instead.
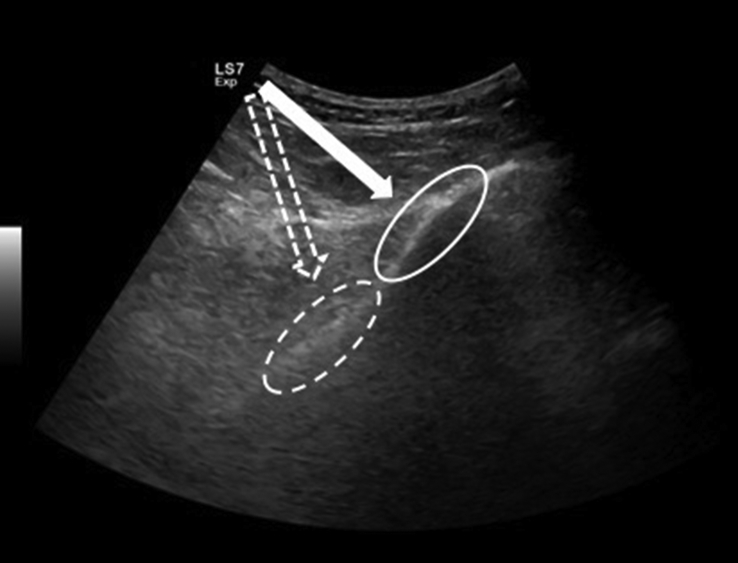
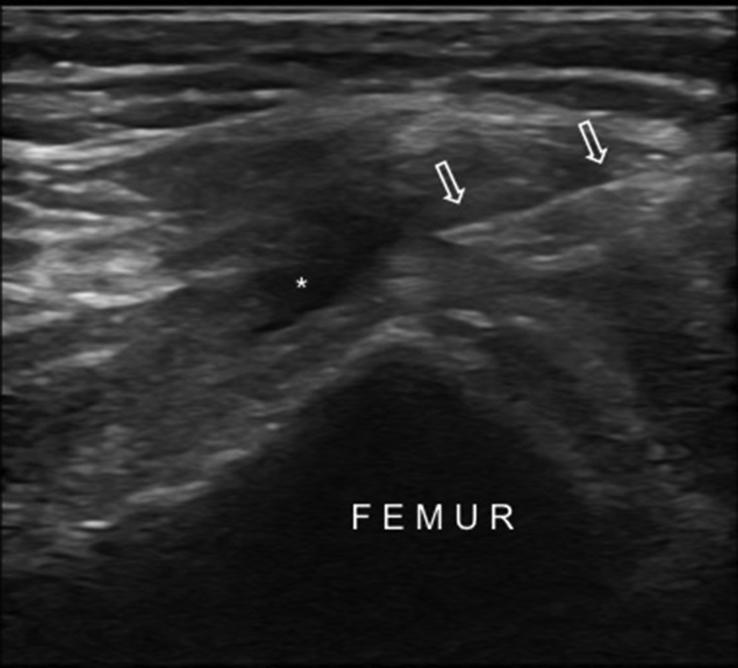
The iliac bone has thick and thin areas. The thick area is targeted because the risk of pelvic invasion with the trocar increases in thin areas (Fig. 2). The anatomical locations of the neural and vascular structures have been studied and the gluteal region is divided into six sections and three zones. ‘Zone 1’ was found to be the safest; as such vertical penetration to the thick part of ileum under US-guidance is recommended for BMA.13,20
Fig. 2.
US image shows the thick (white circle) and thin (dashed circle) areas on the iliac bone and the relevant needle trajectories (arrows).
During the procedure, a multifrequency linear probe is generally used; however a curvilinear probe can be preferred in obese patients. Patients lie in prone or lateral decubitus position. First, the skin and subcutaneous tissue is infiltrated with local anesthesic (LA) and a small incision (1–2 mm) is opened. The trajectory of the trocar and periosteum is infiltrated with appropriate amount of LA. US-guided BMA from the PIC can be performed using the direct or indirect technique.
2.2.1. Indirect guidance/Parallel method
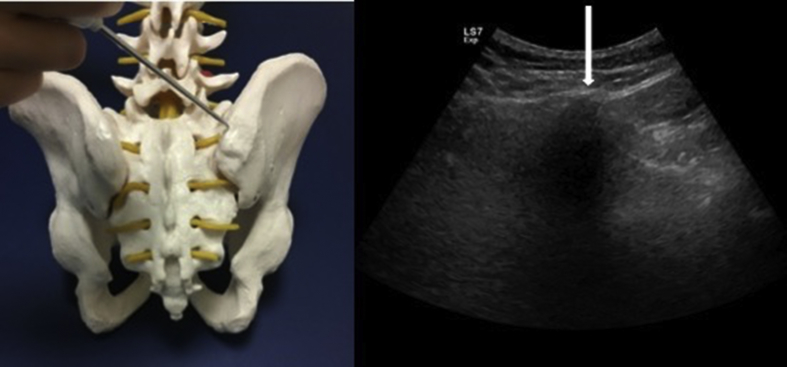
Indirect technique simply combines the conventially used manual method with the demonstration of bony landmarks with US. After the PIC is visualized, the depth of the aspiration site(s) and the routes for approach are determined. Then, in accordance with the imaging/measurements (without using real-time US imaging), the trocar is advanced until it penetrates the upper border of the iliac crest. After invasion into the iliac crest, it is further advanced paralel to the bone alignment (Fig. 3). If the trocar suddenly loses contact during aspiration, it should be kept in mind that penetration into the pelvic or gluteal regions may occur and that visceral injury might be unavoidable. Of note, the neighboring anatomical structures are superior gluteal, cluneal and sciatic nerves, and the external iliac artery.
Fig. 3.
The alignment of the needle and its entry site on the iliac bone on a model (left side). Axial US image shows the iliac crest and the white arrow indicates the needle targeting.
2.2.2. Direct guidance/Vertical method
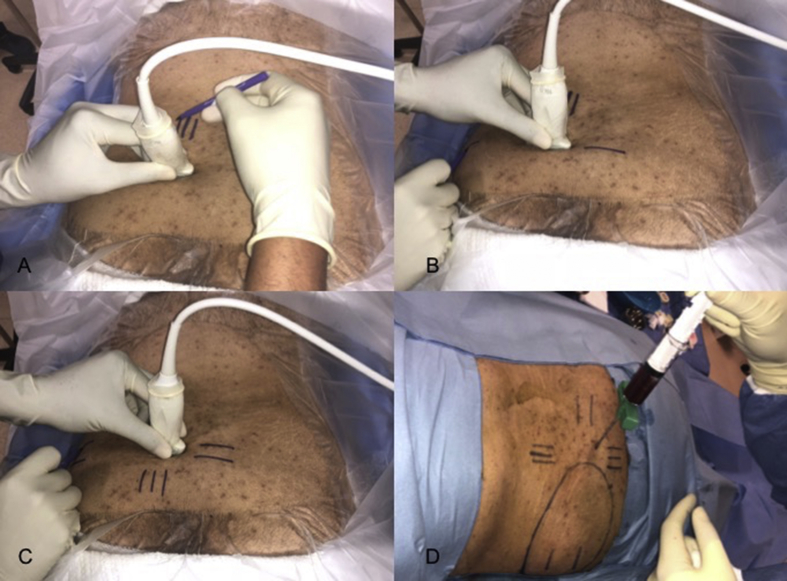
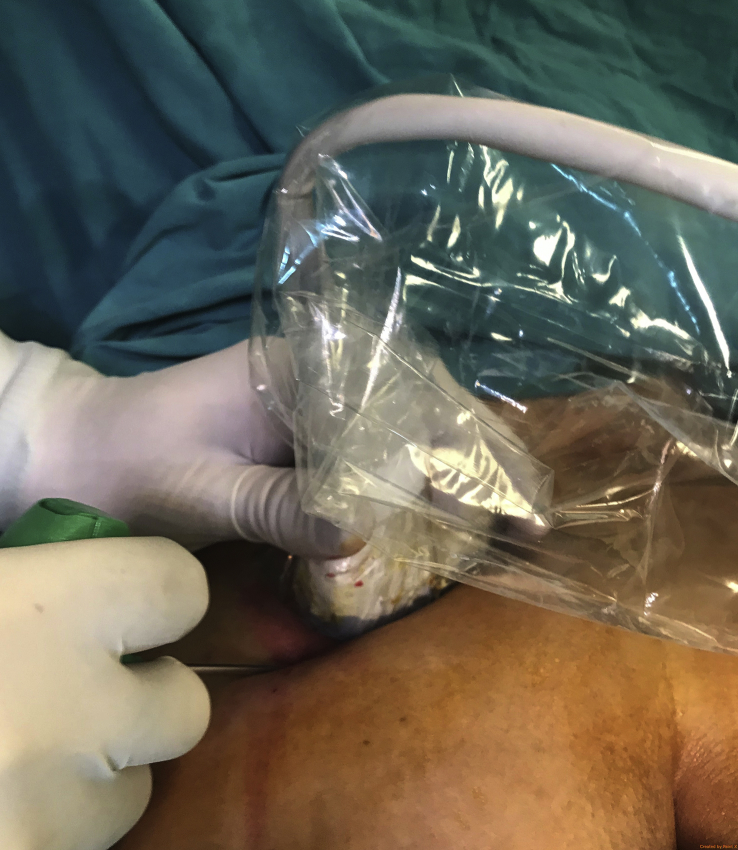
In order to minimize the risks during BMA with a trocar, direct guidance i.e. the advancement of the trocar under simultaneous US imaging is preferably applied. The US probe can be covered with a sterile glove or special sterile sheath (Fig. 4). The trocar is advanced from lateral to medial using the in-plane technique. The thick part of the iliac bone which appears like a mountain on the short-axis image is targeted (Fig. 2). The recommended target points on the iliac bone are located 1–2 cm inferior to the PIC, and 2 cm apart from each other14 (Fig. 5, Fig. 6). After aspiration from the first site, the trocar is pulled back (without getting out of the cutaneous entry site), and US probe and trocar are repositioned for the other aspiration site.
Fig. 4.
Determination of the penetration site under US guidance by marking the skin entry site while rotating the US probe 90° (A, B, C). The aspiration of the bone marrow (D).
Fig. 5.
Needle routes (grey lines) and target entry sites (circles) 2 cm apart from each other on the iliac crest border model (left side). Longitudinal US image shows the needle (arrows) while penetrating the thick part of the iliac bone (right side).
Fig. 6.
Placement of the US probe (covered with a sterile sheath) and advancement of the trocar using the in-plane technique.
2.3. Adipose tissue isolation
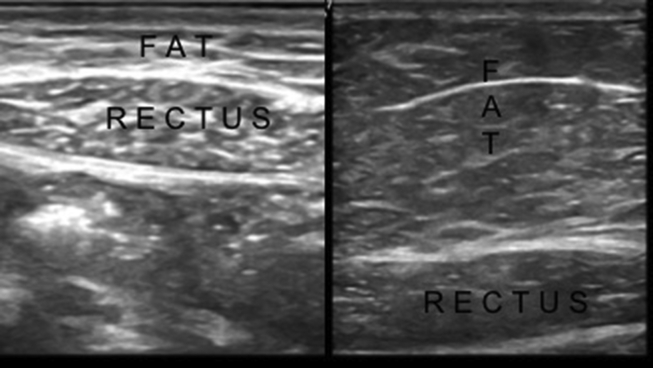
Adipose tissue is another source of MSCs. US guidance is particularly necessary in thin patients to determine the site of lipoaspiration and to measure the thickness of the fat tissue at the target location (Fig. 7). The most frequently used lipoaspiration sites are abdominal, gluteal and trochanteric regions. For special procedures, US imaging/guidance can help for lipoaspiration from tissues like suprapatellar, infrapatellar or other regions as well. For instance, some clinicians use BMAC and Adipose derived products together in the same procedure. In that case, the same entry site near the PIC can be used for harvesting both biomaterials.
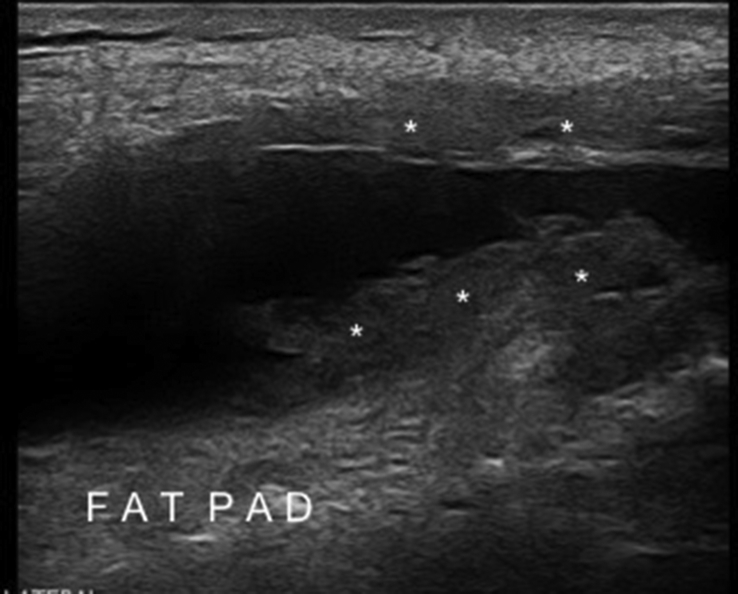
Fig. 7.
US images show the different tissue compartments in a thin (left side) and an obese (right side) subject.
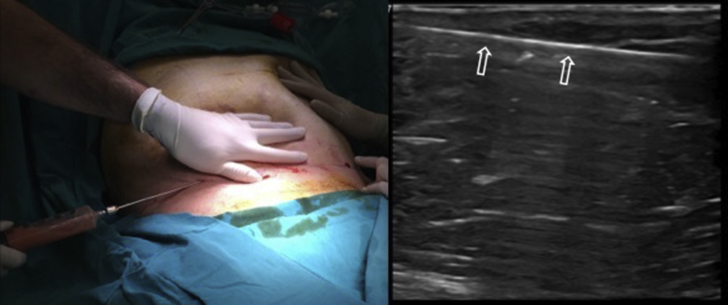
During the procedure, the lipoaspiration cannula should be held parallel to the skin in order to prevent neighboring/deep organ injuries (Fig. 8). If awake, the patient can feel pain when the cannula is too superficial (piercing the skin) or deep (penetrating the muscle). If the patient is under general anesthesia, US can help to determine the route(s) of the cannula (Fig. 8).
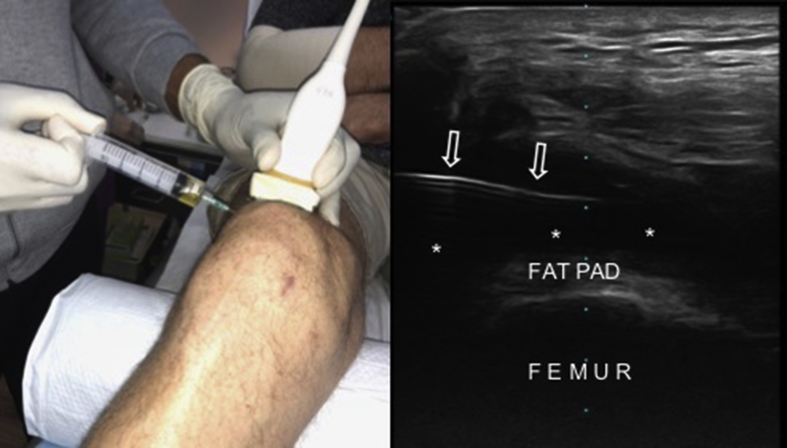
Fig. 8.
The lipoaspiration procedure under local anesthesia (left side) and the longitudinal US image (right side) of the lipo-aspiration cannula (open arrows).
3. Ultrasonographic evaluation of the knee joint
For soft tissue imaging, magnetic resonance imaging (MRI) is generally accepted to be a paramount standard.21 However, MRI is expensive, time consuming and not widely available for routine use in most countries. On the other hand, periarticular tendons, ligaments, bursae and the peripheral aspect of the bony structures and menisci can be evaluated by US.22,23
Knee joint imaging includes the assessment of anterior, medial, lateral and posterior aspects. The first three are performed while the patient either lies supine, the last one is performed while the patient lies prone with the knee extended on the examination bed. In order to better visualize particular structures or pathologies, mild flexion (to localize the joint fluid) or full flexion (femoral cartilage, anterior cruciate ligament) of the knee joint may be preferred joint positions.24
A multi-frequency linear probe is recommended. Starting with the anterior superior aspect of the knee, the probe is placed sagittally and moved mediolaterally over the suprapatellar recess. An axial view can also be obtained. During maximum knee flexion, the femoral trochlea and the overlying hyaline cartilage can be thoroughly assessed.24
In the infrapatellar zone, the probe is placed longitudinally on the midline for imaging inferior pole of the patella, patellar tendon and its insertion to the tibia. The medial and lateral aspects of the knee can be depicted with the probe placed longitudinally over the joint line femur and tibia on each side. While the medial meniscus, medial collateral ligament, pes anserine area can be scanned on the former; iliotibial band, lateral collateral ligament and the biceps tendon can be scanned on the latter.
On the posterior side, axial and longitudinal views can be used to image the semimembranosus and semitendinosus tendons, and the gastrocnemius semimembranosus bursa (as the origin of the Baker's cyst).
3.1. Ultrasonographic findings in knee osteoarthritis
These include bony changes, cartilage and soft tissue lesions. Femoral cartilage is an area of interest during US evaluation, as the cartilage lesions were found to be correlated with femoral-tibial joint osteophytes and arthroscopic findings.25, 26, 27, 28, 29, 30
3.1.1. Bony lesions
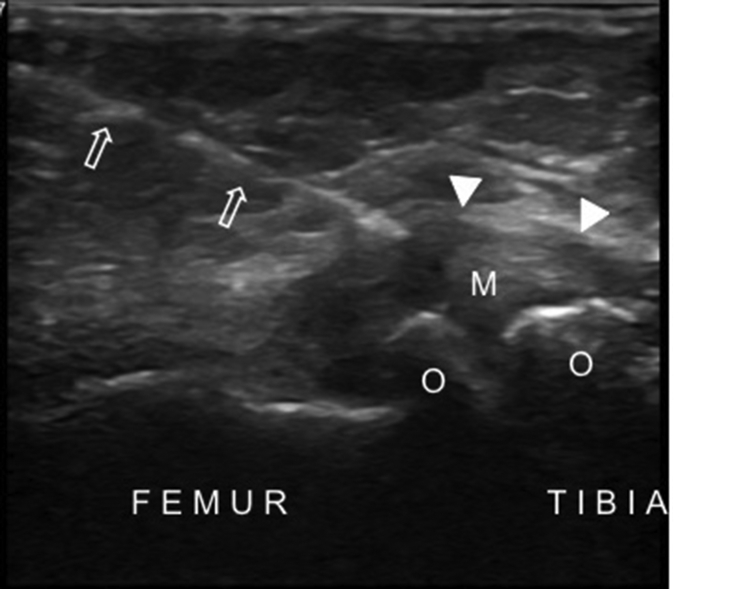
Osteophytes are the most prominent US findings in knee OA. These degenerative bony lesions can most frequently be seen at medial and lateral sides of the tibio-femoral joint (Fig. 9). US was found to be more sensitive in detecting osteophytes compared to plain radiographs at the medial compartment of the knee joint.25 A significant correlation was reported between osteophyte size and the articular cartilage degenerative changes at the medial compartment detected with arthroscopy.25
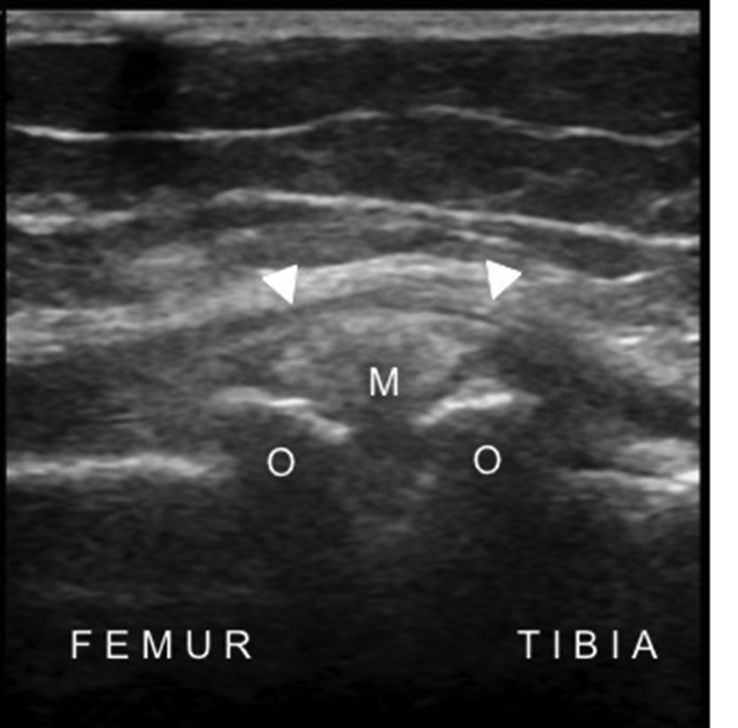
Fig. 9.
Longitudinal US image of the medial tibiofemoral joint shows the osteophytes (O) on each side, the protruded medial meniscus (M) and the medial collateral ligament (arrowheads) in a patient with knee osteoarthritis.
On the other hand, hidden osteophyte formation at intracondylar notch of femur which was found to be associated with an increased risk for incident radiographic OA, might not be detected by US. In such cases MRI should be the preferred imaging modality.31 The other locations of the bony lesions in degenerative osteoarthritic knee include cortical irregularity/enthesitis at the upper and lower poles of the patella, patellar tendon insertion to the tibial tuberosity, rarely insertion of the medial and lateral parapatellar ligaments to the patella, and at the femoral condylar cartilage surface.
3.1.2. Cartilage lesions
Femoral cartilage lesions found to be correlated with femorotibial joint osteophytes and arthroscopic findings while patellar cartilage and tibial cartilage are less studied25, 26, 27, 28, 29, 30 (Fig. 10). The semiquantitative grading system of the distal femoral cartilage using US has been demonstrated to correlate quite well with the histologic findings/grading.26 Herein, sonographic appearance of the cartilage is classified into 6 grades as regards clarity, echogenicity, sharpness of the edges, thickness of the femoral cartilage, cortical irregularities and osteophytes on the underlying bony surface.
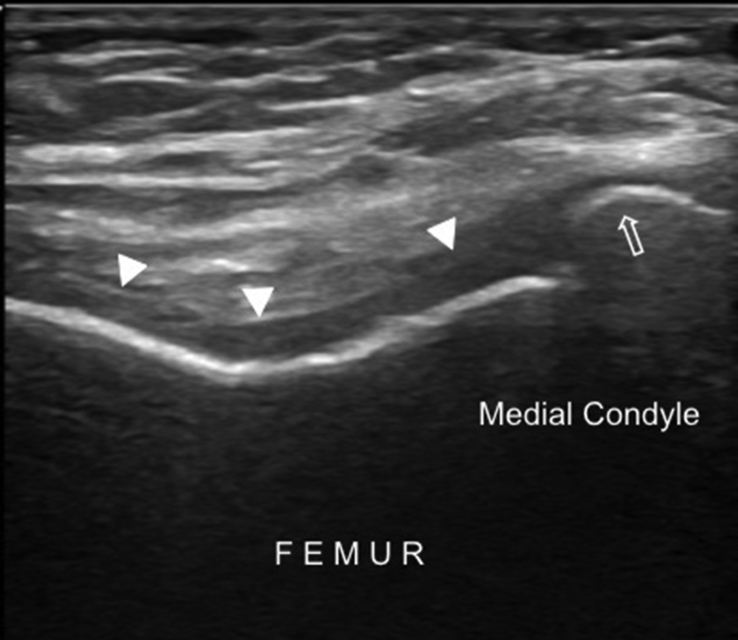
Fig. 10.
Transverse US imaging of the femoral condyles shows blurred margins and decreased clarity of the femoral cartilage (arrowheads), cortical irregularity and osteophyte on the bony surface (open arrow).
Saarakkala et al.28 evaluated the correlation between sonographic findings of femoral cartilage at medial/lateral condyles and the intercondylar notch, and arthroscopic Noyes’ grading for cartilage degeneration on 40 patients. They reported that positive US findings were strong indicators of arthroscopic degenerative cartilage findings, but that negative findings did not rule out degenerative changes. Further, significant associations between sonographic changes and knee pain severity assessed by VAS and pain upon motion assessed by WOMAC and Lequesne index questionnaire were reported in the study by Chen et al.30
Ultrasound (US) may provide useful information about meniscal tears, however it is not a reliable as a sole imaging modality for evaluating menisci. With a high frequency linear array US probe with good resolution, US was reported to have a sensitivity value as 85%, and specificity value as 86% for the evaluation of meniscal tears.32 Meniscal degeneration is observed as meniscal heterogeneity with possible extrusion and fragmentation, while meniscal tears will appear as hypoechoic line extending to the surface of the meniscus.
Ko et al.33 reported a mean of 4.8 to 1.7 mm medial meniscal subluxation (perpendicular distance between the outer edge of the meniscus and the line drawn as the extension of the medial border of the tibial plateau) in subjects with radiographic knee OA and 1.0 to 0.8 mm for knees without radiographic changes (Fig. 9). An advantage of US in meniscal extrusion measurement is the US evaluation can be carried out during weight bearing.
Timotijevic et al.34 reported that US was more sensitive and specific for chronic lateral meniscus injuries than for acute lateral meniscus injuries confirmed with magnetic resonance imaging and arthroscopy.
3.1.3. Soft tissue changes
Synovitis and effusion are two common sonographic findings detected in patients with knee OA (Fig. 11). Synovitis and/or effusion was detected between 47% and 100% of patients with symptomatic knee OA.35,36 The suprapatellar US scanning at 30° flexed knee was found to be the most sensitive position to detect effusion.37 An association was found between synovitis and the future development of medial cartilage loss, suggesting that ultrasonographic depiction of synovitis may predict structural progression of knee OA.38 The fluid distribution may vary in the suprapatellar bursa, with the greatest fluid mostly observed in the lateral recess. US can provide valuable information in MCL injuries with a reported accuracy of 94%.39 The US findings include ligamentous thickenning, heterogenous echogencity or loss of contiunity. Dynamic imaging of the medial joint space during valgus stress might also be valuable.39
Fig. 11.
Longitudinal US imaging of the suprapatellar bursa shows anechoic effusion and synovial thickenings/hypertrophy (asterisks) in a patient with knee osteoarthritis.
In a recent study, Riecke et al.40 developed a scoring system for detecting knee OA which included five domains: morphological changes including osteophyte and meniscus extrusion, inflammatory markers such as synovial hypertrophy and Doppler activity at the medial/lateral compartments and effusion. In the study by Ahmad et al.41 US showed improved synovial hypertrophy and vascularity scores, and less effusion after PRP injection.
4. Ultrasound guided interventions in knee osteoarthritis
Anatomical landmark (or palpation) guided knee injections can be challenging in obese patients, complex postoperative or posttraumatic anatomy, and in cases with previous failure of landmark guidance.42 For sure, accurate injections to certain articular (e.g. anterior cruciate ligament) or extraarticular structures (e.g. collateral ligaments) may require further guidance. The accuracy of the injection procedure is particularly important for regenerative treatments for better clinical outcome. Sibbit et al.43 compared US— and landmark palpation-guided steroid injections with regards to their cost-effectiveness in 94 knees without effusion. US guidance resulted with 48% reduction in procedural pain, 42% reduction in pain scores at outcome, 36% increase in therapeutic duration and 58% reduction in cost per-responder per-year.44
A multifrequency linear probe and standart 18–25 G needles are sufficient for US-guided interventions. For all the interventions; it is suggested that an initial diagnostic scanning is performed. This would definitely help decide whether or not to intervene, how exactly to target and what exactly to inject.45 The neighboring vulnerable structures (e.g. articular cartilage, nerves and vessels) must be avoided. In this sense, the direct in-plane or long-axis view is usually the recommended technique. The brightness of the needle can be optimized by maintaining the probe paralel to the needle.5
4.1. Intraarticular injections
The suprapatellar approach could be recommended for knee injections as it provides easy access to the joint.44,46 The injection can be performed from lateral to medial or medial to lateral using the in-plane approach with the ultrasound probe placed axially on the suprapatellar recess (Fig. 12, Fig. 13) The knee is in extended or mildly flexed position. An effusion can help to better delineate the pouch; however synovial space dilation with saline can also help to visualize the suprapatellar joint recess in patients without effusion. After the correct placement of needle, the delivery of the injectate and synovial space dilation can be observed. Patellofemoral approach can be performed alternatively if suprapatellar recess is difficult to visualize.46 The US probe is placed axially between the medial/lateral side of the patella and femur while the knee is in extended position. Using in-plane approach, the needle is advanced deep to the patellofemoral retinaculum into the patellofemoral recess or directly into the patellofemoral joint. Walk-down technique can be used during out-plane technique. The intraarticular flow should be confirmed by visualizing injectate flow into the suprapatellar recess.42
Fig. 12.
US-guided suprapatellar bursa injection from the lateral side using the in-plane approach (left side). The corresponding US image shows effusion (asterisks) and the needle (arrows).
Fig. 13.
US image shows the in-plane approach for the suprapatellar bursa injection from the medial side in a patient with knee osteoarthritis. Asterisk shows the mild effusion and arrows indicate the needle.
4.2. Extraarticular injections
4.2.1. Medial collateral ligament injection
Traumatic medial collateral ligament edema was reported to serve as a marker for medial knee OA or degenerative menisceal tear.47 Since chronic injuries can be treated with PRP, it can be reasonable to perform MCL injections in knee OA.48
Patient lies supine with externally rotated hip and extended or mildly flexed knee. In-plane technique is recommended. After the probe is placed longitudinally on the medial femorotibial joint, the needle is advanced from superior to inferior or inferior to superior to the target portion(s) of the ligament (Fig. 14).
Fig. 14.
US image shows the medial collateral ligament injection in a patient with knee osteoarthritis. The needle (arrows) is advanced into the medial collateral ligament (arrowheads). Also note the narrowed tibiofemoral joint space, protruded medial meniscus (M) and osteophytes (O).
4.2.2. Pes anserine tendon and bursa
The accuracy rate of US-guided pes anserine bursa injections was found as 92% in a cadaveric study.49 The probe is placed on the inferior anteromedial site of the knee in an oblique coronal position, while the hip is externally rotated and the knee is mildly flexed. The injection can be performed using long axis, in-plane technique.
4.2.3. Popliteus tendon sheath
Smith et al.50 investigated the popliteus tendon sheath injections using longitudinal and transverse approaches. They concluded that the longitudinal approach is potentially more accurate; however that both approaches may result in injectate overflow into the knee joint. Patient lies in lateral recumbent position over the contralateral leg, with the symptomatic knee slightly flexed and the leg internally rotated. The probe is kept obliquely while its proximal end is placed on the lateral femoral epicondyle anterosuperiorly. The lateral collateral ligament overlying on the popliteus tendon and the sulcus can be visualized. The in-plane technique is recommended whereby damage to the neighboring structures (e.g. peroneal nerve) must be avoided.
5. Conclusion
Ultrasound (US) is an excellent imaging tool to evaluate most of the structures in the knee joint. Additionally, starting from the biomaterial harvesting stage of the procedures, it can thus/conveniently be used for the diagnosis and treatment of various forms of knee OA where the interventions need to be carried out under US guidance.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
The authors have no conflict of interest.
References
- 1.Murrell W.D., Anz A.W., Badsha H., Bennett W.F., Boykin R.E., Caplan A.I. Regenerative treatments to enhance orthopedic surgical outcome. PM and R. 2015;7(4 Suppl):S41–S52. doi: 10.1016/j.pmrj.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 2.Ulasli A.M., Kara M., Özçakar L. Publications of physical and rehabilitation medicine physicians concerning musculoskeletal ultrasonography: an overview. J Rehabil Med. 2011;43:681–683. doi: 10.2340/16501977-0834. [DOI] [PubMed] [Google Scholar]
- 3.Özçakar L., Tok F., Murat Ulasli A., Kara M., Bayram Çarlı A., Akarsu S. What actually changed after the use of musculoskeletal ultrasound? An international survey study in PRM. Eur J Phys Rehabil Med. 2014;50:469–471. [PubMed] [Google Scholar]
- 4.Özçakar L., Kara M., Chang K.V. Nineteen reasons why physiatrists should do musculoskeletal ultrasound: EURO-MUSCULUS/USPRM recommendations. Am J Phys Med Rehabil. 2015;94:e45–e49. doi: 10.1097/PHM.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 5.De Muynck M., Parlevliet T., De Cock K., Vanden Bossche L., Vanderstraeten G., Özçakar L. Musculoskeletal ultrasound for interventional physiatry. Eur J Phys Rehabil Med. 2012;48:675–687. [PubMed] [Google Scholar]
- 6.Pitts S.R., Niska R.W., Xu J., Burt C.W. National hospital ambulatory medical care survey. Natl Health Stat Report. 2006;7:1–38. [PubMed] [Google Scholar]
- 7.Liu Y.T., Alsaawi A., Bjornsson H.M. Ultrasound-guided peripheral venous access: a systematic review of randomized-controlled trials. Eur J Emerg Med. 2014;21:18–23. doi: 10.1097/MEJ.0b013e328363bebc. [DOI] [PubMed] [Google Scholar]
- 8.Osborn S.R., Borhart J., Antonis M.S. Medical students benefit from the use of ultrasound when learning peripheral IV techniques. Crit Ultrasound J. 2012;4:2. doi: 10.1186/2036-7902-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reusz G., Csomas A. The role of ultrasound guidance for vascular Access. Curr Opin Anaesthesiol. 2015;28:710–716. doi: 10.1097/ACO.0000000000000245. [DOI] [PubMed] [Google Scholar]
- 10.Griffits J., Carnegie A., Kendall R., Madan R. A randomised crossover study to compare the crosssectional and longitudinal approaches to ultrasoundguided peripheral venepuncture in a model. Crit Ultrasound J. 2017;9:9. doi: 10.1186/s13089-017-0064-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewandowski K., Kowalik M.M., Pawlaczyk R., Rogowski J., Hellmann A. Microscopic examination of bone marrow aspirate in healthy adults— comparison of two techniques of slide preparation. Int J Lab Hematol. 2012;34:254–261. doi: 10.1111/j.1751-553X.2011.01387.x. [DOI] [PubMed] [Google Scholar]
- 12.Muschler G.F., Boehm C., Easley K. Aspiration to obtain osteoblast progenitor cells from human bone marrow: the influence of aspiration volume. J Bone Joint Surg Am. 1997;79:1699–1709. doi: 10.2106/00004623-199711000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Fennema E.M., Renard A.J.S., Leusink A., van Blitterswijk C.A., de Boer J. The effect of bone marrow aspiration strategy on the yield and quality of human mesenchymal stem cells. Acta Orthop. 2009;80:618–621. doi: 10.3109/17453670903278241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedlis M.F., Centeno C.J. Performing a better bone marrow aspiration. Phys Med Rehabil Clin N Am. 2016;27:919–939. doi: 10.1016/j.pmr.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Hernigou P., Homma Y., Flouzat Lachaniette C.H. Benefits of small volume and small syringe for bone marrow aspirations of mesenchymal stem cells. Int Orthop. 2013;37:2279–2287. doi: 10.1007/s00264-013-2017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernigou P., Desroches A., Queinnec S. Morbidity of graft harvesting versus bone marrow aspiration in cell regenerative therapy. Int Orthop. 2014;38:1855–1860. doi: 10.1007/s00264-014-2318-x. [DOI] [PubMed] [Google Scholar]
- 17.Centeno C.J., Al-Sayegh H., Freeman M.D., Smith J., Murrell W.D., Bubnov R. Correction to: a multi-center analysis of adverse events among two thousand, three hundred and seventy two adult patients undergoing adult autologous stem cell therapy for orthopaedic conditions. Int Orthop. 2016;40:1755–1765. doi: 10.1007/s00264-016-3162-y. [DOI] [PubMed] [Google Scholar]
- 18.Marx R.E., Tursun R. A qualitative and quantitative analysis of autologous human multipotent adult stem cells derived from three anatomic areas by marrow aspiration:tibi a, anterior ilium, and posterior ilium. Int J Oral Maxillofac Implants. 2013;28:e290–e294. doi: 10.11607/jomi.te10. [DOI] [PubMed] [Google Scholar]
- 19.Pierini M., Di Bella C., Dozza B. The posterior iliac crest outperforms the anterior iliac crest when obtaining mesenchymal stem cells from bone marrow. J Bone Joint Surg Am. 2013;95:1101–1107. doi: 10.2106/JBJS.L.00429. [DOI] [PubMed] [Google Scholar]
- 20.Hernigou J., Picard L., Alves A., Silvera J., Homma Y., Hernigou P. Understanding bone safety zones during bone marrow aspiration from the iliac crest: the sector rule. Int Orthop. 2014;38:2377–2384. doi: 10.1007/s00264-014-2343-9. [DOI] [PubMed] [Google Scholar]
- 21.Ghozlan R., Vacher H. Where is imaging going in rheumatology? Baillieres Best Pract Res Clin Rheumatol. 2000;14:617–633. doi: 10.1053/berh.2000.0103. [DOI] [PubMed] [Google Scholar]
- 22.Van Holsbeeck M., Introcaso J.H. Musculoskeletal ultra- sonography. Radiol Clin North Am. 1992;30:907–925. [PubMed] [Google Scholar]
- 23.Friedman L., Finlay K., Jurriaans E. Ultrasound of the knee. Skeletal Radiol. 2001;30:361–377. doi: 10.1007/s002560100380. [DOI] [PubMed] [Google Scholar]
- 24.Özcakar L., Kara M., Chang K.V. Euro-musculus/USPRM Basic scanning protocols for knee. Eur J Phys Rehabil Med. 2015;51:641–646. [PubMed] [Google Scholar]
- 25.Koski J.M., Kamel A., Waris P. Atlas-based knee osteophyte assessment with ultrasonography and radiography: relationship to arthroscopic degeneration of articular cartilage. Scand J Rheumatol. 2016;45:158–164. doi: 10.3109/03009742.2015.1055797. [DOI] [PubMed] [Google Scholar]
- 26.Lee C.L., Huang M.H., Chai C.Y., Chen C.H., Su J.Y., Tien Y.C. The validity of in vivo ultrasonographic grading of osteoarthritic femoral condylar cartilage: a comparison with in vitro ultrasonographic and histologic gradings. Osteoarthritis Cartilage. 2008;16:352–358. doi: 10.1016/j.joca.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 27.Pritzker K.P., Gay S., Jimenez S.A. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14:13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 28.Saarakkala S., Waris P., Waris V. Diagnostic performance of knee ultrasonography for detecting degenerative changes of articular cartilage. Osteoarthritis Cartilage. 2012;20:376–381. doi: 10.1016/j.joca.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Grassi W., Lamanna G., Farina A., Cervini C. Sonographic imaging of normal and osteoarthritic cartilage. Semin Arthritis Rheum. 1999;28:398–403. doi: 10.1016/s0049-0172(99)80005-5. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y.J., Chen C.H., Wang C.L., Huang M.H., Chen T.W., Lee C.L. Association between the severity of femoral condylar cartilage erosion related to knee osteoarthritis by ultrasonographic evaluation and the clinical symptoms and functions. Arch Phys Med Rehabil. 2015;96:837–844. doi: 10.1016/j.apmr.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Katsuragi J., Sasho T., Yamaguchi S. Hidden osteophyte formation on plain X-ray is the predictive factor for development of knee osteoarthritis after 48 months – data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2015;23:383–390. doi: 10.1016/j.joca.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 32.Wareluk P., Szopinski K.T. Value of modern sonography in the assessment of meniscal lesions. Eur J Radiol. 2012;81:2366–2369. doi: 10.1016/j.ejrad.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 33.Ko C.H., Chan K.K., Peng H.L. Sonographic imaging of meniscal subluxation in patients with radiographic knee osteoarthritis. J Formos Med Assoc. 2007;106:700–707. doi: 10.1016/S0929-6646(08)60031-5. [DOI] [PubMed] [Google Scholar]
- 34.Timotijevic S., Vukasinovic Z., Bascarevic Z. Correlation of clinical examination, ultrasound sonography, and magnetic resonance imaging findings with arthroscopic findings in relation to acute and chronic lateral meniscus injuries. J Orthop Sci. 2014;19:71–76. doi: 10.1007/s00776-013-0480-4. [DOI] [PubMed] [Google Scholar]
- 35.Kristoffersen H., Torp-Pedersen S., Terslev L. Indications of inflammation visualized by ultrasound in osteoarthritis of the knee. Acta Radiol. 2006;47:281–286. doi: 10.1080/02841850600551508. [DOI] [PubMed] [Google Scholar]
- 36.D'Agostino M.A., Conaghan P., Le Bars M. EULAR report on the use of ultrasonography in painful knee osteoarthritis. Part 1: prevalence of inflammation in osteoarthritis. Ann Rheum Dis. 2005;64:1703–1709. doi: 10.1136/ard.2005.037994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mandl P., Brosard M., Aegerter P. Ultrasound evaluation of fluid in knee recesses at varying degrees of flexion. Arthritis Care Res (Hoboken) 2012;64:773–779. doi: 10.1002/acr.21598. [DOI] [PubMed] [Google Scholar]
- 38.Ayral X., Pickering E.H., Woodworth T.G., Mackillop N., Dougados M. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis—results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis Cartilage. 2005;13:361–367. doi: 10.1016/j.joca.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 39.Dai H., Huang Z.G., Chen Z.J., Liu J.X. Diagnostic accuracy of ultrasonography in assessing meniscal injury: meta-anal- ysis of prospective studies. J Orthop Sci. 2015;20:675–681. doi: 10.1007/s00776-015-0728-2. [DOI] [PubMed] [Google Scholar]
- 40.Riecke B.F., Christensen R., Torp-Pedersen S., Boesen M., Gudbergsen H., Bliddal H. An ultrasound score for knee osteoarthritis: a cross-sectional validation study. Osteoarthritis Cartilage. 2014;22:1675–1691. doi: 10.1016/j.joca.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 41.Ahmad H.S., Farrag S.E., Okasha A.E. Clinical outcomes are associated with changes in ultrasonographic structural appearance after platelet-rich plasma treatment for knee osteoarthritis. Int J Rheum Dis. 2018;21:960–966. doi: 10.1111/1756-185X.13315. [DOI] [PubMed] [Google Scholar]
- 42.Lueders D.R., Smith J., Sellon J.L. Ultrasound-guided knee procedures. Phys Med Rehabil Clin N Am. 2016;27:631–648. doi: 10.1016/j.pmr.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 43.Sibbitt W.L., Band P.A., Kettwich L.G., Chavez-Chiang N.R., DeLea S.L., Bankhurst A.D. A randomized controlled trial evaluating the cost-effectiveness of sonographic guidance for intra-articular injection of the osteoarthritic knee. J Clin Rheumatol. 2011;17:409–415. doi: 10.1097/RHU.0b013e31823a49a4. [DOI] [PubMed] [Google Scholar]
- 44.Bum Park Y., Ah Choi W., Kim Y.K., Chul Lee S., Hae Lee J. Accuracy of blind versus ultrasound- guided suprapatellar bursal injection. J Clin Ultrasound. 2012;40:20–25. doi: 10.1002/jcu.20890. [DOI] [PubMed] [Google Scholar]
- 45.Özçakar L., Onat Ş.Ş., Gürçay E., Kara M. Are blind injections ethical or historical? Think twice with ultrasound. Am J Phys Med Rehabil. 2016;95:158–160. doi: 10.1097/PHM.0000000000000377. [DOI] [PubMed] [Google Scholar]
- 46.Park Y., Lee S.C., Nam H.S., Lee J., Nam S.H. Comparison of sonographically guided intraarticular injections at 3 different sites of the knee. J Ultrasound Med. 2011;30:1669–1676. doi: 10.7863/jum.2011.30.12.1669. [DOI] [PubMed] [Google Scholar]
- 47.Wen D.Y., Propeck T., Kane S.M., Godbee M.T., Rall K.L. MRI description of knee medial collateral ligament abnormalities in the absence of trauma: edema related to osteoarthritis and medial meniscal tears. Magn Reson Imaging. 2007;25:209–214. doi: 10.1016/j.mri.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 48.Yoshida M., Marumo K. An Autologous leukocyte-reduced platelet-rich plasma therapy for chronic injury of the medial collateral ligament in the knee: a report of 3 successful cases. Clin J sports Med. 2017 Nov 29 doi: 10.1097/JSM.0000000000000515. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 49.Finnoff J.T., Nutz D.J., Henning P.T., Hollman J.H., Smith J. Accuracy of ultrasound-guided versus unguided pes anserinus bursa injections. PM R. 2010;2:732–739. doi: 10.1016/j.pmrj.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 50.Smith J., Finnoff J.T., Santaella-Sante B., Henning T., Levy B.A., Lai J.K. Sonographically guided popliteus tendon sheath injection: techniques and accuracy. J Ultrasound Med. 2010;29:775–782. doi: 10.7863/jum.2010.29.5.775. [DOI] [PubMed] [Google Scholar]