Abstract
More than many other fields in medicine, cancer vaccine development has been plagued by a wide gap between the massive amounts of highly encouraging preclinical data on one hand, and the disappointing clinical results on the other. It is clear now that traditional approaches from the infectious diseases' vaccine field cannot be borrowed as such to treat cancer. This review highlights some of the strategies developed to improve vaccine formulations for oncology, including research into more powerful or “smarter” adjuvants to elicit anti-tumoral cellular immune responses. As an illustration of the difficulties in translating smart preclinical strategies into real benefit for the cancer patient, the difficult road of vaccine development in lung cancer is given as example. Finally, an outline is provided of the combinatorial strategies that leverage the increasing knowledge on tumor-associated immune suppressive networks. Indeed, combining with drugs that target the dominant immunosuppressive pathway in a given tumor promises to unlock the true power of cancer vaccines and potentially offer long-term protection from disease relapse.
Keywords: cancer vaccine, adjuvant, dendritic cell, TLR, STING, checkpoint
Introduction
The aim of a vaccine is to induce an in vivo adaptive immune response against a defined antigen or set of antigens. This implies leveraging specific functions of professional antigen-presenting cells in order to trigger T-helper cell responses to support production of antibody production and induce cytotoxic effector T-cells.
The remarkable clinical responses observed with immune checkpoint inhibitors and CAR-T cell therapy have put a definitive end to the discussion whether the human immune system, and T-cells in particular, is capable of controlling or even eradicating cancer. The problem is that vaccination approaches have largely been successful when it comes to inducing humoral immunity, while no major breakthrough has been reached in diseases where cellular responses are also required, such as tuberculosis, HIV, or cancer. For cancer, the bar is raised even higher as vaccines are primarily developed in a therapeutic setting, i.e., with the aim of controlling clinically evident or, at best, minimally residual disease.
The purpose of this review is not to give an exhaustive account of all attempts at cancer vaccination so far, but to provide the reader with the necessary concepts to understand where the field is going, specifically focusing on strategies to elicit clinically meaningful cellular immune responses. Finally, this review will give a perspective of potential combinatorial strategies that could unlock the unique power of vaccines in cancer.
In order for vaccination to deliver unequivocal clinical benefit for cancer patients, improvements must be achieved at two levels: (1) maximizing the induction of a T-cell response with optimal amplitude, specificity and effector profile, (2) ensuring that vaccine-induced T-cells can reach the tumor site and perform their function without any restraint.
The first level involves optimization of the choice of antigenic target(s), of adjuvant potency, and of delivery system. The main principles and some representative preclinical examples in this field will be highlighted in the following section, followed by clinical data (“reality check”) using lung cancer as an illustrative case. In a last section we will outline combinatorial strategies that could herald a revival of cancer vaccines. Molecular formulation of antigens and specific antigen delivery systems constitute a wide domain on their own and will not be handled in detail in this review.
Optimizing Antigenic Targets
The antigenic landscape in cancer is far more complex than that of viral or bacterial pathogens, where adaptive immunity to well-defined epitopes can drive long term disease protection. In cancer vaccines, it seems rational to target the broadest repertoire of antigens possible in order to avoid selection of escape variants. Approaches that can address this need are the use of autologous tumor lysates, whole tumor-derived mRNA, irradiated autologous tumor cells or allogeneic tumor cell lines (3, 4). All of these pose challenges in terms of logistics, standardization and compliance to regulatory demands including Good Manufacturing Practice (GMP) requirements. Many efforts have been devoted in developing vaccines targeting one or a restricted set of cancer antigens. These can be either differentiation antigens (e.g., MelanA, gp100, tyrosinase), cancer-testis antigens (e.g., MAGE/LAGE/XAGE family, NY-ESO1), or virus-derived antigens (e.g., HPV or EBV-derived proteins) (5). On one hand, this is motivated by practical considerations, including simplicity of vaccine manufacturing and monitoring of immune responses. On the other hand, it is anticipated that effective responses to one antigen, through tumor cell destruction, can lead to an immunogenic release of additional endogenous antigens and spark a broader immune response, a phenomenon known as “epitope spreading” (6).
Mutanome-derived epitopes are the most recent addition to defined tumor antigens for use in cancer vaccines. The idea originates from the observation that objective responses to immune checkpoint blockade are proportional to the mutational burden of a given tumor, a number which is the highest in carcinogen-induced cancers (7). This is why the top targets for immune checkpoint inhibition are melanoma, lung cancer and bladder cancer, along with tumors with DNA mismatch repair defects (8). It is now thought that among the total bulk of non-synonymous mutations, a subset that is clonally distributed within the tumor gives rise to mutation-containing peptides (neo-epitopes) that can be recognized by cytotoxic T-cells (9). In addition to single-nucleotide variants, indels have been shown to be strongly predictive of response to immune checkpoint inhibition as well (10). Complex bioinformatic pipelines have been developed to extract a list of candidate immunogenic neo-epitope for a given patient's cancer. This requires deep genomic sequencing of a tumor sample to list all single nucleotide variations (SNVs) and indels. In parallel, RNA sequencing on the same material allows to narrow down on the genomic aberrations that are effectively expressed. Next, in silico algorithms are called into action to predict which of the mutations will be presented to T-cells based on proteasome processing and binding affinity for human leucocyte antigen (HLA) molecules. The resulting coding sequences can be synthesized either as peptides as synthetic mRNA. This methodology has been validated in preclinical experiments, showing that vaccination with mutanome-derived neo-antigens can induce protective and therapeutic immune response to autologous tumors (11). Today, this ambitious approach, entirely patient-individualized has entered clinical development with recent phase 1 data demonstrating the feasibility, safety and immunogenicity of neo-antigen-targeted vaccine in metastatic melanoma (12). Notwithstanding the sophistication of this approach, two concerns can be brought forward: (1) several algorithms exist for the prediction of neo-epitopes, and the list of candidate antigens produced for a given tumor can be influenced by the bioinformatic pipeline used, (2) the whole process from next-generation sequencing until manufacturing and release of a GMP-compliant mutanome-derived mRNA vaccine currently takes around 100 days (12), implying that only patients with maximally debulked or relatively indolent tumors are optimally eligible.
The (Very Crowded) Road TOWARD Optimal Cancer Vaccine Adjuvants
The benefit of adjuvants are best described by the operational definition of Gaston Ramon, better known as the father of the diphtheria vaccine (13): “substances used in combination with a specific antigen that produce more immunity than the antigen alone.” Finding adjuvant formulations that can unlock clinically relevant immune responses against cancer antigens has remained a challenging task: for one, cancer antigens are often poorly immunogenic due to partial homology with self-antigens; on top of that, the optimal cancer vaccine adjuvant must succeed in driving a type 1-polarized, cell-mediated immunity rather than a type 2-polarized and/or humoral response.
Adjuvants can be subdivided in two major classes: (1) immunostimulatory molecules that trigger innate immune receptors, and (2) particulate adjuvants which mainly act either as antigen depots or as delivery systems. Immunostimulatory adjuvants mostly consist of molecules that mimic pathogen-associated molecular patterns and engage Toll-like receptors (TLRs) on antigen presenting cells (APCs) including B-cells, macrophages and most importantly dendritic cells (DCs). In the case of DCs this results in a complex and highly coordinated cellular response aimed at sparking adaptive immunity: (1) switch from antigen uptake mode to antigen processing and presentation, upregulation of a whole array of T-cell costimulatory molecules, upregulation of chemokine receptors mediating migration into T-cell areas of draining lymphoid tissues, and release of specific cytokines and chemokines to polarize the resulting T-cell response. Due to their immunostimulatory power and the capacity to prime naïve T-cells, properly activated DCs are also referred to as “nature's adjuvants.” The use of ex vivo-generated and antigen-loaded DCs as cellular vaccines will be reviewed in a different article of this Special Edition. The following paragraphs provide a non-exhaustive overview of some of the most notable acellular adjuvant systems optimized for use in cancer vaccines.
Immunostimulatory Adjuvants: TLR Ligands and Beyond
Among immunostimulatory adjuvants, TLR4 ligands constitute some of the most potent members in terms of APC activation. Lipopolysaccharide (LPS), the prototype TLR4-ligand, cannot be used as such in clinical formulations due to toxicity issues. MPL (3-O-desacyl-4′-monophosphoryl lipid A) is a chemically detoxified form of LPS derived from strain R595 of Salmonella minnesota, while still retaining immunostimulatory properties (14). It is the only defined TLR ligand approved as part of a vaccine in humans to this day and is a key ingredient of the AS04 adjuvant formulation used in the commercially available HPV and HBV vaccines. However, what makes MPL especially attractive with respect to anti-cancer vaccination is its capacity to induce robust Th1-polarized and cell-mediated immunity. MPL is also an ingredient of the DETOX adjuvant system, when combined with cell wall peptidoglycans from Mycobacteria (15). DETOX is the adjuvant used in the Melacine® vaccine formulation, which incorporates lysate from two allogeneic melanoma cell lines and has shown some modest clinical benefit in resected stage III melanoma patients (16). Likewise, CG-enriched oligodeoxynucleotides (CpG), by triggering the intracellular TLR9, have also been described as powerful inducers of Th1 and cytolytic T-cell responses. These properties have led the incorporation of MPL together with CpG as part of the proprietary adjuvant formula AS15 in the MAGE-A3-targeted cancer vaccine developed by GSK Biologicals (17). Because of biosynthetic variability in the structure of bacterial-derived LPS and downstream hydrolytic steps, MPL is a heterogenous mix of closely related structures (“congeners”). Hence, synthetic TLR4 agonists have been designed, i.e., aminoalkyl glucosaminide 4-phosphates (AGPs) such as glucopyranosyl lipid A and RC-529 (18). The latter has shown its capacity to induce Th1 responses equivalent to MPL, and still with much lesser in vivo toxicity than LPS (19). Several other extra- and intracellular TLR-ligands have been the subject of intensive research efforts [reviewed in (20)], and all have shown value to varying degrees in diverse preclinical tumor models. Although some molecules such as the TLR7/8 agonist imiquimod or the TLR2/4-stimulating preparation Bacille-Calmette-Guérin (BCG) are used routinely in the clinic as standalone therapies, no TLR agonist has so far successfully entered standard of care as an ingredient of a cancer vaccine.
It should be noted that triggering TLR signaling also activates homeostatic counterregulatory mechanisms. These include release of IL-10 by myeloid cells, induction of regulatory T-cells (Tr1), and upregulation of the T-cell checkpoint molecule programmed death ligand-1 (PD-L1) on APCs: all of which contribute to the further induction of T-regs and the dampening of anti-tumor cellular immune responses [reviewed in (21)]. The TLR ligands Pam2Cys (TLR2), LPS (TLR4), imiquimod (TLR7) and CpG (TLR9) all induce IL-10 production, and blockade of IL-10/IL10R axis in these settings augments immune responses (17, 18) Similarly, the TLR3-ligand poly I:C induces PD-L1 on DCs, while PD-L1 blockade boosts effector CD8+ T-cell expansion after a tumor vaccine involving poly I:C as adjuvant (22). Another counterregulatory mechanism after TLR stimulation is the upregulation of indoleamine 2,3-dioxigenase expression in DCs, a side-effect observed with CpG oligodeoxynucleotides (23). IDO is a well-described mediator of immunological tolerance: by depleting tryptophan and generating toxic catabolites, IDO enzymatic activity suppresses T-cell activation and promotes T-reg induction in the tumor micro-environment (discussed in more detail below).
A different class of immunostimulatory adjuvants does not belong to bacterial or viral pathogen-associated molecules but consists of extracts from plant origin. Saponins derived from the bark of the South American soapbark tree (Quillaja saponaria) contain a family of water-soluble, structurally diverse molecules with strongly pro-inflammatory properties. QS21 is one of the RP-HPLC fractions of Q. saponaria extracts that has been used the most in vaccine development (24). The triterpene aldehyde group is considered as the adjuvant active site, resulting in preclinical models in a strong mixed T-helper 1 (Th1), CD8 T-cell and humoral response. QS21 was shown to primarily activate the ASC/NALP3 inflammasome pathway, which converts pro-IL-1β and pro-IL-18 into their bioactive forms (25). This provides the rationale to combine with a TLR4 ligand in order to induce upstream expression of the pro-forms. Still, it appears that the magnitude and quality of the resulting immune response is not proportional to the degree of inflammasome activation, and high doses of QS21 can cause cell membrane lysis and apoptosis of APCs (25). QS21 has been tested extensively in therapeutic cancer vaccine formulations involving ganglioside antigens (GD2, GD3, or GM2) (24). Although robust and humoral responses were invariably observed, there was no convincing evidence of cell-mediated immunity in humans. QS21 is also combined with MPL as part of the AS01 and AS15 adjuvant formulation (GSK), as evaluated in the MAGE-A3 cancer vaccines (discussed below).
STING agonists are a recent addition to the arsenal of candidate vaccine adjuvants. STING (STimulator of INterferon Genes) is a transmembrane protein located in the endoplasmic reticulum that belongs to the family of nucleic acid sensors (26). STING activation triggers robust type 1 IFN responses in a TBK1-IRF3-dependent way as well as IKK/NFkB-dependent upregulation of inflammatory cytokines and chemokines. STING can be activated in two ways. The presence of cytosolic double-stranded DNA (e.g., originating from invading DNA viruses or self-DNA from stressed/damaged cells) is first detected by the cGAS molecule which generates cyclic 2′3′-GMP-AMP (cGAMP) from ATP and GTP. As a second messenger, 2′3′-cGAMP then goes on to bind and activate STING, triggering both IRF3- and NFkB-dependent immune/inflammatory gene expression. cGAS expression is by itself inducible by type I interferon, which provides a positive feedback mechanism when relevant ligands persist. Alternatively, STING can be directly triggered by bacterial cyclic dinucleotides such as c-di-GMP. In preclinical models, high doses of c-di-GMP injected intratumorally can directly induce caspase 3-dependent apoptosis of tumor cells and release of tumor-associated antigens, while lower exposure to c-di-GMP can lead to activation of DCs and promote CD8+ T-cell responses against those antigens (27). Other preclinical studies have demonstrated the value of STING agonists in the setting of therapeutic cancer vaccination (28). Caution must be paid however as among immune cells, STING expression is the highest in T lymphocytes. STING activation has been shown to lead to T-cell apoptosis, a phenomenon that appeared cell-specific as macrophages and DCs did not display such sensitivity (29). Hence, implementation of STING agonists in cancer vaccines should ideally be combined with adjuvant/antigen delivery systems that specifically target myeloid cells in vivo, as already reported (30). A potential bonus with this type of approach is that STING agonists can reprogram myeloid-derived suppressor cells toward a DC-like immune-stimulating phenotype expressing IL-12 and T-cell costimulatory molecules (27). Another difficulty in translating preclinical data to clinical development strategies is the fact that STING agonists can have differential binding properties in murine vs. human cells. The flavonoid compound DMXAA for instance can bind mouse STING and induced anti-tumor immunity, but fails to activate human STING (31). Still, based on its unique properties, the STING pathway has become a “hot” candidate in the pipeline of several biotech and larger pharmaceutical companies (IFM Therapeutics, Selvita, iTeos, MSD). To date few compounds have reached the stage of early clinical development: ADU-S100 (Novartis) and MK-1454 (MSD). Due to systemic toxicity, both require accessible lesions for intratumoral injection, and both are (quite rationally) combined with systemic administration of an immune checkpoint inhibitor (NCT03172936, NCT03010176).
Next to pathogen-derived molecules, specific host proteins have been shown to perform adjuvant-like functions as well. Immunostimulatory cytokines such as IL-2, IFN-γ, IL-12 and granulocyte-macrophage colony stimulating factor (GM-CSF) represent an obvious choice as an ingredient for a vaccine. By far the most used in clinical trials is GM-CSF. Based on preclinical studies, GM-CSF helps in the recruitment of dendritic cells to the vaccine injection site, promotes DC maturation and antigen-presentation, resulting in enhanced adaptive immune responses (32). GM-CSF is also the essential ingredient for the ex vivo generation of monocyte-derived DCs for vaccination purposes, as discussed elsewhere in this edition. GM-CSF has been incorporated in vaccine formulations either as a standalone adjuvant, or in the shape of allogeneic tumor cell lines engineered for stable expression of GM-CSF (GVAX®) (32). A concern still persists as to the optimal dosage of GM-CSF however, with preclinical studies indicating the potential of this cytokine to expand MDSCs, with paradoxical suppression of T-cell mediated anti-tumor responses in vivo as a consequence (33). This effect on MDSCs was also observed in clinical trials, where a low-dose GM-CSF added to a cancer vaccine caused a systemic expansion of an immunosuppressive CD14-positive HLA-DR-low/-negative myeloid cell subset. In an another controlled clinical trial, including GM-CSF as part of an incomplete Freund's adjuvant formula resulted in significantly lower T-cell responses to vaccine antigens compared to adjuvant without GM-CSF (34). Still, a surprisingly large number of trials using GM-CSF as an adjuvant component are active (listed in Supplementary Table); their results will need to be interpreted with caution.
A different class of endogenous proteins with immunogenic activity are heat-shock proteins (HSPs). HSPs are chaperones that are released from stressed or dying (cancer) cells, with the unique property of binding cell-derived peptides (35). These peptides can be delivered to DCs resulting in cross-presentation and induction of efficient CD8+ T-cell-mediated immunity (36). The transfer of peptides from HSPs to the APC's MHC class I molecules is not passive but requires uptake by the HSP receptor CD91 expressed by the APC and internal processing. The repertoire of peptides bound by the HSPs reflects the antigenic make-up of the cell of origin, a property which can be leveraged to induce a broad T-cell-mediated protective immunity. In addition, HSP carrier molecules by themselves act as innate immune stimuli, triggering essential events in APCs including release of TNF-α, IL-1β, IL-12, GM-CSF, inflammatory chemokines, and upregulation of costimulatory molecules (37). This effect could be due to binding of HSPs to TLR4, which reinforces the notion that HSPs constitute bona fide endogenous adjuvants. Immunization with tumor cell-derived HSPs such as HSP70 and GP96 has demonstrated impressive protective immunity in several preclinical studies [reviewed in (38)]. This has led to the clinical development of autologous HSP96-based vaccines formulation (e.g., vitespen / Oncophage®). Clinical trials have shown that this therapy is feasible and non-toxic, although clinical benefit was low except maybe in subset analyses including early-stage renal cell cancer (RCC) and a trend toward benefit in M1a/M1b melanoma patients (39, 40). With these results, vitespen failed to obtain approval from the European Medicines Agency (EMA). Also, one major limitation for further development of HSP-based vaccines is the manufacturing process itself which requires access to sufficient amounts of autologous tumor material. Still, a number of combination clinical trials implementing HSP-based vaccines are ongoing (Supplementary Table).
Particulate Matter Adjuvants
The most widely used particulate adjuvants historically have been aluminum salts, mostly in the shape of aluminum hydroxide (“alum”). Alum triggers innate immune responses in a TLR-independent way but rather stimulates the NALP3 inflammasome. Being very potent in inducing pure T-helper 2 (Th2) and antibody responses, alum salts are by themselves unfit for use in cancer vaccines. However, when associated with type-1 polarizing ingredients such as ISA 51 (Montanide, see below) and recombinant IL-12, alum was shown to enable a more sustained immune response to tumor-associated antigens probably due to a depot / slow release effect (41). Likewise, combining alum with MPL (GSK's AS04 adjuvant formula) enables a more sustained type-1 polarized cytokine response (42). Other particulate adjuvants have been tailored to better respond to the demands of a cancer vaccine (43). The oldest prototype, Freunds adjuvant, is a water-in-oil emulsion containing heat-killed Mycobacteria. Although being very immunogenic in preclinical models, it is much too toxic for human use. A less toxic formulation that incorporates squalene and oleate, Montanide ISA-51 (“Incomplete Freunds Adjuvant”) has been used in many therapeutic cancer vaccines. This includes a pivotal trial using the melanoma TAA gp100 as target, in which the clinical activity of ipilimumab alone or in combination with a vaccine vs. vaccine alone was assessed in metastatic melanoma patients (44). Despite induction of robust antibody and CTL responses and signals of clinical benefit in small patient cohorts, none of the Montanide-adjuvanted cancer vaccines has reached advanced clinical development in oncology so far. Adjuvants based on oil-in-water emulsions have been subsequently developed and show a superior safety profile, excellent depot properties, but produce strongly Th2-biased and humoral immune responses (15).
It has been observed by many research groups that a key to induce cellular immunity is the capacity to exploit the cross-presentation capacity of dendritic cells. An efficient way to achieve this goal is by packaging antigens in non-soluble particles, such as virosomes, liposomes, ISCOMs, and microspheres (45). Virosomes and virus-like particles (VLP) are 20–100 nm size and consist of the membrane envelop of a virus (including embedded proteins) but devoid of a replication-competent genome. Nevertheless, VLPs can efficiently fuse with the membrane of the target cell (ideally an APC), simultaneously delivering an antigenic cargo and any PAMP that can be incorporated in the design. A successful VLP-based vaccine is Gardasil®, which contains capsid proteins of HPV serotypes 6, 11, 16, and 18. The vaccine uses aluminum hydroxide phosphate sulfate as adjuvant and is hence a potent inducer of long-lasting and very protective humoral immune responses.
Considerable experience has also been gathered with ISCOMs, which are 40 nm micellar structures in which a saponin adjuvant (QS21) and protein antigen is incorporated. ISCOMATRIX consists of just the micellar components and adjuvant, with the flexibility of adding an antigen of choice. ISCOMs differ from liposomes as the latter contain an internal aqueous space confined by a lipid bilayer. As a consequence of the built-in saponin, ISCOMs exert their adjuvant activity by activating the NALP3 inflammasome, while delivering antigenic cargo to dendritic cells to cross-prime CD8+ T-cells (46). In vivo, tumor antigen-specific cellular and humoral immune responses were observed after vaccination with NY-ESO1-containing ISCOMs (47). Further intensive research efforts are being devoted to engineer novel synthetic particles with the aims of maximizing vaccine potency while specifically targeting cross-presenting APCs. The wide spectrum of physico-chemical parameters that can be varied in the manufacturing such next-generation nanoparticles offers great flexibility in terms of targeting and immunostimulatory properties (see (48) for a comprehensive overview).
Optimizing Cancer Vaccine Formulations: a Reality Check
The solid preclinical rationale upon which several types of vaccine designs are based stands in sharp contrast to the sobering clinical results observed. Here, we summarize vaccine development in non-small cell lung cancer (NSCLC) as a good example of the limited clinical benefit of cancer vaccines as monotherapy. Many of the strategies described in the previous section have been tested clinically in lung cancer, be it protein-, liposome-, VLP-based or genetically engineered whole cell vaccine platforms.
One of the largest clinical trials ever undertaken in NSCLC was a randomized, double-blind, placebo-controlled phase 3 study using GSK Biological's recombinant MAGE-A3 vaccine (49). The formulation contains full-length recombinant MAGE-A3 protein, a cancer-testis antigen expressed in about 40% of NSCLC patients, combined with the AS15 adjuvant system described earlier. Despite the cancer-specificity of MAGE-A3, notwithstanding the strong type-1 polarizing activity of the AS15 adjuvant formulation and promising phase 2 trial data, the phase 3 trial showed no benefit at all in terms of overall and disease-free survival in early-stage NSCLC patients vaccinated after surgical resection (49). Moreover, an “immune-activated” predictive gene expression signature identified in the melanoma MAGE-A3 vaccine trials failed to identify a MAGE-A3+ NSCLC patient subset who might benefit from vaccination. The vaccine produced strong and long-lasting antibody responses, in line with early clinical data (50), but no convincing evidence for the induction of cytotoxic T-cell responses was provided in this trial. In part due to these results, development of a similar vaccine targeting the cancer-testis antigen PRAME in NSCLC was stopped prematurely (51).
L-BLP25 (Stimuvax®) is a liposomal formulation incorporating as antigen a synthetic lipopeptide coding for 25 amino acids of the Muc-1 protein (tecemotide), and MPL as adjuvant. Muc-1 is a glycoprotein that is overexpressed and typically aberrantly glycosylated in a several adenocarcinomas, among which a large subset of NSCLC. L-BLP25 failed to demonstrate a benefit in overall survival in the intention to treat population in a phase III trial involving locoregionally advanced NSCLC patients after chemo-radiotherapy (START trial, NCT00409188) (52). However, a major increase in median OS was observed in the subgroup of patients who received concurrent rather than sequential chemoradiotherapy. These results were meant to be verified in a follow-up phase 3 trial (START2, NCT02049151), however based on negative results of a trial in Asian NSCLC patients (INSPIRE, NCT01015443) (53) the sponsor decided to stop development of L-BLP25 (“Stimuvax”) in all indications.
TG4010 is another Muc-1-targeting vaccine evaluated in NSCLC. It consists of a replication-deficient viral vector, modified vaccinia Ankara (MVA), expressing both Muc-1 as well as IL-2 to support T-cell proliferation. In preclinical models, MVA induces expression of the incorporated antigen sequence in target tissues at equivalent levels compared to replication-competent virus, albeit with a faster kinetic (54). MVA can trigger type-1 IFN production in a TLR-independent fashion. This, combined with the induction of not only humoral but also of type-1-polarized cellular immune response makes MVA theoretically an attractive tool for cancer vaccination purposes. A first trial in advanced NSCLC gave indication of benefit when combined with 1st line chemotherapy, vs. chemotherapy + placebo (55). This prompted a confirmatory phase 2b/3 trial that included a candidate predictive biomarker (the percentage of activated NK-cells in peripheral blood). Results of the phase 2b part showed a significant increase in progression-free survival (PFS; primary endpoint) that was most pronounced in non-squamous NSCLC (where Muc-1 expression is expected to be the highest) and with biomarker value in the lower 3 quartiles (56). Results of the phase 3 part are still pending.
As a final example, in an attempt to target a broad repertoire of antigens, a vaccine was designed containing four irradiated NSCLC allogeneic cell lines (belagenpumatucel-L, Lucanix®). In addition, the cell lines where genetically engineered to express an antisense gene vector that inhibits TGF-β2 expression. TGF-β2, along with IL-10, is a prototypical mediator of tumor-induced immune suppression and T-reg induction, and introduction of TGF-β2 antisense plasmid was shown to increase vaccine immunogenicity in preclinical studies (57). It must be stressed though that while the production of TGF-β2 by the vaccine cells themselves is suppressed, this does not affect the levels of this suppressive cytokine emanating from the tumor microenvironment. Belagenpumatucel-L has been evaluated as consolidation therapy in locally advanced and metastatic NSCLC patients that had not progressed on their last line of chemotherapy. Data from a phase 2 trial appeared promising with a clear dose-dependent increase in overall survival (58). However, in a follow-up phase 3 study, no benefit in OS was observed except in a subgroup of patients that had received radiation and chemotherapy < 6 months prior to randomization (59). Patient numbers in this subgroup were very small though and to this day it remains unsure whether this analysis will prompt a confirmatory phase 3 study focusing on this subpopulation.
The impossibility or at best difficulty to demonstrate unequivocal clinical benefit in these vaccination trials raises many questions. When it comes to cancer immunotherapy, the avalanche of robust and positive data coming from the immune checkpoint inhibitor field represents today's benchmark. Patient outcomes after vaccination highlight the difficulty of inducing productive cytolytic responses against cancer in humans. It is clear that a careful choice of antigenic target, adjuvant formula and delivery platform are not sufficient to elicit therapeutic or protective immunity against cancer. This warrants more attention to the tumor-associated tolerogenic or immunosuppressed climate that reigns in the cancer patient.
Unleashing Immune Effector Mechanisms Downstream of Vaccine Action
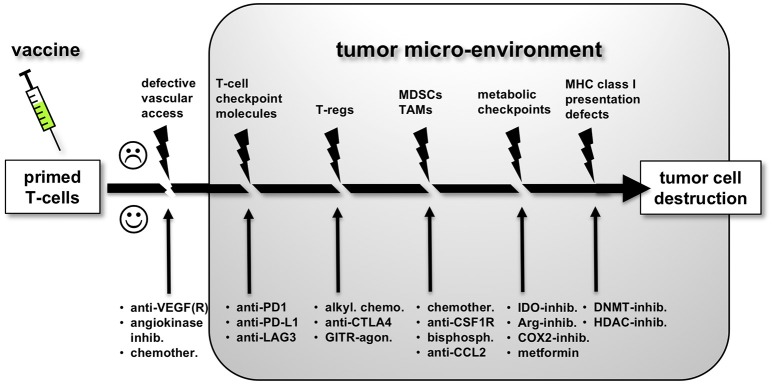
The immune response against cancer cells is a series of critical steps, also described as the “cancer immunity cycle” (60). As a consequence, the strength of the response at the end of this chain of events will be determined by its weakest link (see Figure 1). Each of the obstacles to successful antitumor immune responses have been studied in detail and offers opportunity for therapeutic modulation. Clinical trials exploring combinatorial strategies are summarized in Table 1. The underlying principles will be discussed below.
Figure 1.
The multiple obstacles to effective anti-tumor immune responses following successful priming of tumor antigen-specific T-cells by a vaccine. Each obstacle offers opportunities for therapeutic intervention in order to increase vaccine efficacy, as discussed in more detail in the main text.
Table 1.
Current clinical trial landscape exploring combinatorial approaches to improve therapeutic cancer vaccine efficacy.
| Clinical trial I.D. | Study title | Interventions | Phase |
|---|---|---|---|
| (A) Cancer Vaccine + Angiogenesis-Targeting | |||
| NCT03050814 | Standard of Care Alone or in Combination With Ad-CEA Vaccine and Avelumab in People With Previously Untreated Metastatic Colorectal Cancer QUILT-2.004 | Drug: Avelumab|Biological: Ad-CEA vaccine|Drug: Bevacizumab|Drug: 5-FU|Drug: Leucovorin|Drug: Oxaliplatin|Drug: Capecitabine | Phase 2 |
| NCT02754362 | A Toll-like Receptor Agonist as an Adjuvant to Tumor Associated Antigens (TAA) Mixed With Montanide ISA-51 VG With Bevacizumab for Patients With Recurrent Glioblastoma | Drug: Bevacizumab|Biological: Peptide Vaccine|Drug: Poly-ICLC as immune adjuvant|Drug: Keyhole limpet hemocyanin (KLH) | Phase 2 |
| NCT02432846 | Intratumoral Vaccination With Intuvax Pre-nephrectomy Followed by Sunitinib Post-nephrectomy vs. Sunitinib Post-nephrectomy in Newly Diagnosed Metastatic Renal Cell Carcinoma (mRCC) | Biological: Intuvax (ilixadencel)|Drug: Sunitinib | Phase 2 |
| NCT02010606 | Phase I Study of a Dendritic Cell Vaccine for Patients With Either Newly Diagnosed or Recurrent Glioblastoma | Biological: Dendritic cell vaccination, in addition to standard temozolomide chemotherapy and involved field radiation therapy|Biological: Dendritic cell vaccination, with optional bevacizumab treatment for patients previously treated with bevacizumab | Phase 1 |
| NCT01814813 | Vaccine Therapy With Bevacizumab vs. Bevacizumab Alone in Treating Patients With Recurrent Glioblastoma Multiforme That Can Be Removed by Surgery | Biological: HSPPC-96|Drug: bevacizumab | Phase 2 |
| NCT01551745 | Salvage Ovarian FANG Vaccine + Bevacizumab | Biological: Vigil,Ñc Vaccine|Drug: Bevacizumab | Phase 2 |
| NCT01312376 | Autologous T-Cells Combined With Autologous OC-DC Vaccine in Ovarian Cancer | Biological: OC-DC vaccine|Drug: Bevacizumab|Drug: cyclophosphamide 300 mg/m2/d for 3 days|Drug: fludarabine 30 mg/m2/d for 3 days|Drug: ex vivo CD3/CD28-costimulated vaccine-primed peripheral blood autologous T cells | Phase 1 |
| NCT01223235 | Polyvalent Vaccine-KLH Conjugate + Opt-821 Given in Combination With Bevacizumab | Biological: bevacizumab and the polyvalent vaccine-KLH conjugate + OPT-821 | N/A |
| NCT00913913 | Bevacizumab, Autologous Tumor/DC Vaccine, IL-2 and IFNα-2b in Metastatic Renal Cell Carcinoma (RCC) Patients | Biological: DC vaccine|Drug: Bevacizumab|Biological: IL-2|Biological: IFN | Phase 2 |
| NCT00874588 | Peptide Vaccine Targeting to Cancer Specific Antigen Combined With Anti-angiogenic Peptide Antigen in Treating Patients With Non-small Cell Lung Cancer | Biological: HLA-A*2402restricted URLC10, CDCA1, VEGFR1, and VEGFR2 | Phase 1 |
| NCT00828009 | BLP25 Liposome Vaccine and Bevacizumab After Chemotherapy and Radiation Therapy in Treating Patients With Newly Diagnosed Stage IIIA or Stage IIIB Non-Small Cell Lung Cancer That Cannot Be Removed by Surgery | Biological: bevacizumab|Biological: emepepimut-S|Drug: carboplatin|Drug: cyclophosphamide|Drug: paclitaxel|Radiation: radiation therapy | Phase 2 |
| (B) Cancer Vaccine + TAM/MDSC-Targeting | |||
| NCT02544880 | PDE5 Inhibition Via Tadalafil to Enhance Anti-Tumor Mucin 1 (MUC1) Vaccine Efficacy in Patients With HNSCC | Drug: Tadalafil|Biological: Anti-MUC1 Vaccine|Biological: Anti-Influenza Vaccine|Other: Tadalafil Placebo|Other: Vaccine Placebo|Procedure: Peripheral Blood Collection|Procedure: DTH Skin Test|Procedure: Tumor specimen collection | Phase 1/2 |
| NCT02479230 | Type I-Polarized Autologous Dendritic Cell Vaccine With Tumor Blood Vessel Antigen-Derived Peptides in Metastatic Breast Cancer Patients | Biological: tumor blood vessel antigen peptide-pulsed alpha-type-1 polarized dendritic cell vaccine|Drug: gemcitabine hydrochloride | Phase 1 |
| NCT02432378 | Intensive Locoregional Chemoimmunotherapy for Recurrent Ovarian Cancer Plus Intranodal DC Vaccines | Biological: Cisplatin + celecoxib + DC vaccine|Biological: Cisplatin + CKM + Celecoxib + DC Vaccine | Phase 1/2 |
| NCT02275039 | p53MVA Vaccine and Gemcitabine Hydrochloride in Treating Patients With Recurrent Ovarian Epithelial Cancer | Biological: modified vaccinia virus ankara vaccine expressing p53|Drug: gemcitabine hydrochloride|Other: laboratory biomarker analysis | Phase 1 |
| NCT01876212 | Dendritic Cell Vaccines + Dasatinib for Metastatic Melanoma | Biological: DC vaccine|Drug: Dasatinib | Phase 2 |
| NCT01803152 | Dendritic Cell Vaccine With or Without Gemcitabine Pre-Treatment for Adults and Children With Sarcoma | Biological: Dendritic Cells Vaccine|Biological: Lysate of Tumor|Drug: Gemcitabine|Drug: Imiquimod|Procedure: Leukapheresis | Phase 1 |
| NCT01697800 | A Phase II Trial of Tadalafil in Patients With Squamous Cell Carcinoma of the Upper Aero Digestive Tract | Drug: Tadalafil|Drug: Placebo | Phase 2 |
| NCT02616185 | A Phase 1 Study To Evaluate Escalating Doses Of A Vaccine-Based Immunotherapy Regimen For Prostate Cancer (PrCa VBIR) | Biological: PF-06755992|Biological: PF-06755990|Device: TDS-IM Electroporation Device|Biological: Tremelimumab|Drug: Sunitinib|Biological: PF-06801591 | Phase 1 |
| NCT02432846 | Intratumoral Vaccination With Intuvax Pre-nephrectomy Followed by Sunitinib Post-nephrectomy vs. Sunitinib Post-nephrectomy in Newly Diagnosed Metastatic Renal Cell Carcinoma (mRCC) | Biological: Intuvax (ilixadencel)|Drug: Sunitinib | Phase 2 |
| NCT03153410 | Pilot Study With CY, Pembrolizumab, GVAX, and IMC-CS4 (LY3022855) in Patients With Borderline Resectable Adenocarcinoma of the Pancreas | Drug: Cyclophosphamide|Drug: GVAX|Drug: Pembrolizumab|Drug: IMC-CS4 | Early Phase 1 |
| NCT02432378 | Intensive Locoregional Chemoimmunotherapy for Recurrent Ovarian Cancer Plus Intranodal DC Vaccines | Biological: Cisplatin + celecoxib + DC vaccine|Biological: Cisplatin + CKM + Celecoxib + DC Vaccine | Phase 1/2 |
| (C) Cancer Vaccine + T-Reg-Targeting | |||
| NCT03203005 | IMA970A Plus CV8102 in Very Early, Early and Intermediate Stage Hepatocellular Carcinoma Patients | Drug: IMA970A plus CV8102 and Cyclophosphamide | Phase 1/2 |
| NCT03066947 | SV-BR-1-GM in Metastatic or Locally Recurrent Breast Cancer | Biological: SV-BR-1-GM|Drug: Cyclophosphamide|Biological: Interferon-alpha-2b | Phase 1/2 |
| NCT02709993 | Consolidation Therapy in Patients With Hematologic Malignancies | Biological: TAPA-pulsed DC vaccine | Phase 1/2 |
| NCT02705703 | Consolidation Therapy in Patients With Metastatic Solid Malignancies | Biological: TAPA-pulsed DC vaccine | Phase 1/2 |
| NCT02390063 | Vaccination in Prostate Cancer (VANCE) | Biological: ChAdOx1.5T4|Biological: MVA.5T4|Drug: Cyclophosphamide | Phase 1 |
| NCT02224599 | Treatment of Patients With Progressive and/or Refractory Solid Malignancies | Biological: TAPA-pulsed DC vaccine | Phase 1/2 |
| NCT02223312 | Therapy for Progressive and/or Refractory Hematologic Malignancies | Biological: TAPA-pulsed DC vaccine | Phase 1/2 |
| NCT01696877 | A Neoadjuvant Study of Androgen Ablation Combined With Cyclophosphamide and GVAX Vaccine for Localized Prostate Cancer | Drug: degarelix acetate|Drug: Cyclophosphamide|Drug: GVAX | Phase 1/2 |
| NCT01192555 | Allogeneic Tumor Cell Vaccination With Oral Metronomic Cytoxan in Patients With High-Risk Neuroblastoma | Biological: Neuroblastoma Vaccine (unmodified SKNLP, with gene-modified SJNB-JF-IL2 and SJNB-JF-LTN neuroblastoma cells)|Drug: Cytoxan | Phase 1/2 |
| NCT00703105 | Ovarian Dendritic Cell Vaccine Trial | Biological: Ontak DC|Biological: DC vaccination|Drug: Ontak | Phase 2 |
| NCT00626483 | Basiliximab in Treating Patients With Newly Diagnosed Glioblastoma Multiforme Undergoing Targeted Immunotherapy and Temozolomide-Caused Lymphopenia | Biological: RNA-loaded dendritic cell vaccine|Drug: basiliximab | Phase 1 |
| NCT00515528 | Vaccination Plus Ontak in Patients With Metastatic Melanoma | Drug: 4-peptide melanoma vaccine|Drug: 4-peptide melanoma vaccine plus Ontak|Drug: ontak | Phase 2 |
| (D) Cancer Vaccine + Checkpoint inhibition | |||
| NCT03548467 | A Study to Evaluate Safety, Feasibility, Efficacy of Multiple Dosing With VB10.NEO Immunotherapy in Patients With Locally Advanced or Metastatic Cancer | Drug: VB10.NEO | Phase 1/2 |
| NCT03532217 | Neoantigen DNA Vaccine in Combination With Nivolumab/Ipilimumab and PROSTVAC in Metastatic Hormone-Sensitive Prostate Cancer | Biological: PROSTVAC-V|Biological: PROSTVAC-F|Drug: Nivolumab|Drug: Ipilimumab|Biological: Neoantigen DNA vaccine|Device: TriGrid Delivery System|Procedure: Tumor biopsy|Procedure: Peripheral blood|Procedure: Fecal samples | Phase 1 |
| NCT03422094 | Neoantigen-based Personalized Vaccine Combined With Immune Checkpoint Blockade Therapy in Patients With Newly Diagnosed, Unmethylated Glioblastoma | Biological: NeoVax|Biological: Nivolumab|Biological: Ipilimumab|Procedure: Research blood draw|Procedure: Leukapheresis for research | Phase 1 |
| NCT03362060 | PVX-410 Vaccine Plus Pembrolizumab in HLA-A2+ Metastatic Triple Negative Breast Cancer | Drug: Pembrolizumab|Biological: PVX-410 | Phase 1 |
| NCT03311334 | A Study of DSP-7888 Dosing Emulsion in Combination With Immune Checkpoint Inhibitors in Adult Subjects With Advanced Solid Tumors | Drug: DSP-7888 Dosing Emulsion|Drug: Nivolumab|Drug: Atezolizumab | Phase 1 |
| NCT02654587 | Study of OSE2101 vs. Standard Treatment as 2nd or 3rd Line in HLA-A2 Positive Patients With Advanced NSCLC After Failure of Immune Checkpoint Inhibitor | Drug: OSE2101|Drug: Docetaxel|Drug: Pemetrexed | Phase 3 |
| NCT03113487 | P53MVA and Pembrolizumab in Treating Patients With Recurrent Ovarian, Primary Peritoneal, or Fallopian Tube Cancer | Other: Laboratory Biomarker Analysis|Biological: Modified Vaccinia Virus Ankara Vaccine Expressing p53|Biological: Pembrolizumab | Phase 2 |
| NCT02977156 | Immunization Strategy With Intra-tumoral Injections of Pexa-Vec With Ipilimumab in Metastatic / Advanced Solid Tumors. | Biological: Pexa-Vec|Drug: Ipilimumab | Phase 1 |
| NCT02506114 | Neoadjuvant PROSTVAC-VF With or Without Ipilimumab for Prostate Cancer | Biological: PROSTVAC V/F|Drug: Ipilimumab | Phase 2 |
| NCT02432963 | Vaccine Therapy and Pembrolizumab in Treating Patients With Solid Tumors That Have Failed Prior Therapy | Other: Laboratory Biomarker Analysis|Biological: Modified Vaccinia Virus Ankara Vaccine Expressing p53|Biological: Pembrolizumab | Phase 1 |
| (E) Cancer Vaccine + Costimulation Agonists | |||
| NCT03258008 | Utomilumab and ISA101b Vaccination in Patients With HPV-16-Positive Incurable Oropharyngeal Cancer | Drug: Utomilumab|Biological: ISA101b | Phase 2 |
| NCT01898039 | Modified Melanoma Vaccine for High Risk or Low Residual Disease Patients | Biological: A2/4-1BBL melanoma vaccine|Procedure: DNP sensititzation|Drug: Cyclophosphamide | Phase 1/2 |
| NCT01861938 | Modified Melanoma Vaccine for High Risk or Low Residual Disease Patients | Biological: Melanoma vaccine modified to express HLA A2/4-1BB ligand | Phase 2/3 |
| NCT01644968 | Phase 1 Study of Anti-OX40 in Patients With Advanced Cancer | Drug: Cohort 1 anti-OX40|Drug: Cohort 2 anti-OX40|Drug: Cohort 3 anti-OX40|Biological: Tetanus Day 29|Biological: Tetanus Day 1|Biological: KLH Day 1|Biological: KLH Day 29 | Phase 1 |
| NCT00534209 | Vaccine Therapy in Patients With Stages IIIB/IV Non-Small Cell Lung Cancer Who Have Finished First-Line Chemotherapy | Biological: Allogeneic B7.1/HLA-A1|Other: Placebo | Phase 1/2 |
| NCT00031564 | Phase II Study of a B7-1 Gene-Modified Autologous Tumor Cell Vaccine and Systemic IL-2 | Biological: Interleukin-2|Biological: B7-1 | Phase 2 |
| (F) Cancer Vaccine + IDO-Inhibition | |||
| NCT02166905 | DEC-205/NY-ESO-1 Fusion Protein CDX-1401, Poly ICLC, and IDO1 Inhibitor INCB024360 in Treating Patients With Ovarian, Fallopian Tube, or Primary Peritoneal Cancer in Remission | Biological: DEC-205/NY-ESO-1 Fusion Protein CDX-1401|Drug: Epacadostat|Other: Laboratory Biomarker Analysis|Other: Pharmacological Study|Drug: Poly ICLC | Phase 1/2 |
| NCT03047928 | Combination Therapy With Nivolumab and PD-L1/IDO Peptide Vaccine to Patients With Metastatic Melanoma | Drug: Nivolumab|Biological: PD-L1/IDO peptide vaccine | Phase 1/2 |
| (G) Cancer Vaccine + Epigenetic Modulation | |||
| NCT02166905 | DEC-205/NY-ESO-1 Fusion Protein CDX-1401, Poly ICLC, and IDO1 Inhibitor INCB024360 in Treating Patients With Ovarian, Fallopian Tube, or Primary Peritoneal Cancer in Remission | Biological: DEC-205/NY-ESO-1 Fusion Protein CDX-1401|Drug: Epacadostat|Other: Laboratory Biomarker Analysis|Other: Pharmacological Study|Drug: Poly ICLC | Phase 1/2 |
| NCT02886065 | A Study of PVX-410, a Cancer Vaccine, and Citarinostat +/- Lenalidomide for Smoldering MM | Drug: Hiltonol|Drug: Citarinostat|Drug: Lenalidomide|Biological: PVX-410 | Phase 1 |
Combinations were structured in line with discussion in the text. Database searches were focused on combinations with agents that target (A) angiogenesis, (B) MDSCs/TAMs, (C) T-regs, (D) immune checkpoint molecules, (E) costimulatory molecules, (F) IDO, and (G) epigenetic modifications. No trials were found combining vaccines with interventions targeting immunosuppressive cytokines (IL-10, TGF-β, IL-6), arginase activity, hypoxic metabolism or adenosine signaling. Notes: Database search restricted to clinical trials that are active or will be activated in the near future. Only antigen-specific vaccination protocols were retained (e.g., the use of radiotherapy or intratumoral injections of checkpoint inhibitors was excluded). Combinations with anti-CTLA4 were listed onder “Vaccine + checkpoint inhibition” even though CTLA-4 blockers such as ipilimumab may also directly deplete T-regs. Interventions targeted at hematological malignancies were omitted.
Improving Effector T-Cell Access Into the Tumor
Following successful expansion and adequate polarization of tumor-antigen specific T-cells, the latter acquire the capacity of exiting the lymph node and recirculate through the bloodstream to scan for antigens in peripheral tissues. Unfortunately, penetration of effector lymphocytes into tumoral beds is hampered in many ways. Tumor-induced angiogenesis results in a network of aberrant blood vessels in which proper adhesion and extravasation of cytolytic T-cells is impaired. The endothelium of tumoral vasculature is known to be poor in leukocyte adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1). Overactivity of the endothelin-endothelin receptor axis on tumoral endothelia further limits T-cell extravasation by decreasing ICAM-1 expression while further boosting the production of angiogenetic factors such as vascular-endothelial growth factor (VEGF) (61). Similar to physiological immune-privileged organs, the endothelium of tumoral vessels also overexpresses T-cell checkpoint ligands including PD-L1, death receptors such as FasL and TRAIL, and IDO. All of these factors do not seem to hamper the recruitment of T-regs, and together contribute in shielding tumor cells from immune attack. Hence, the clinical benefit obtained with commonly used anti-angiogenic compounds such as the VEGF blocker bevacizumab potentially relies on boosting immune infiltration into tumors (62). Also, inhibition of endothelin receptor signaling has been shown to restore endothelial ICAM-1 expression, increase T-cell infiltration and importantly, act synergistically together with a cancer vaccine (63). Regardless of its prototypical role in angiogenesis, VEGF is also known as a cytokine that suppresses T-cell function and DC activation. Hence VEGF-targeted anti-angiogenic therapy can also exert positive immunomodulatory effects in a cancer immunotherapy setting (64–66).
Fighting Suppressive Immune Cells in the Tumor Microenvironment
A next obstacle for vaccine elicited T-cells is the influence of several immune suppressive leukocytes that populate the tumor micro-environment, foremost regulatory T-cells (T-regs) and myeloid-derived suppressor cells (MDSCs). T-regs are known to be preferentially recruited into tumors and inhibit the functions of antitumoral T-cells by producing immunosuppressive mediators such as interleukin-10 (IL-10), transforming growth factor-beta (TGF-β) and adenosine or by consuming interleukin-2 (IL-2) which is critical for cytolytic T-lymphocyte (CTL) proliferation. In a clinical trial involving a NY-ESO1-ISCOMATRIX vaccine in melanoma, absence of clinical efficacy and cellular immune responses was correlated to increased T-reg activity in metastatic compared to early stage patients (67). Preclinical exploration of this phenomenon in a mouse model of pancreatic cancer showed that impaired responses to ISCOM vaccine can be restored by anti-CD25 mAb-mediated depletion of T-regs, or interestingly by adding low-dose CpG-ODN to the ISCOM formulation (68). Numerous other preclinical studies have shown that therapeutic vaccine efficacy can be boosted by depleting T-regs in vivo (69). However, selectively eliminating T-regs in a clinical setting is not a straightforward task. As an example the alkylating agent cyclophosphamide can decrease the number of T-regs in cancer patients (70), however this effect is not easily reproducible and is only achieved within a narrow dose range (“metronomic scheduling”). The development of new clinical-grade compounds that can specifically interfere with the suppressive function of T-regs enables interesting combinatorial approaches with vaccines. T-regs typically express high levels of CTLA-4, and the anti-CTLA4 checkpoint inhibitor ipilimumab, being an IgG1-class antibody, can mediate Fc-dependent depletion of these cells in the tumor micro-environment (TME) (71). Glucocorticoid-induced tumor necrosis factor (TNF) receptor related gene (GITR) is another receptor that is highly expressed on T-regs. Engaging GITR with an agonist has the capacity to shut down the immunosuppressive functions of T-regs, while also stimulating CD8+ T-cell function (72). GITR agonists are currently in clinical development as an add-on to anti-PD-1 checkpoint blockade. Preclinical experiments also indicate a clear synergism between GITR agonists and therapeutic cancer vaccines (73, 74), yet to date no clinical trials are investigating this avenue in cancer patients.
MDSCs constitute another potential obstacle to vaccine success. This heterogenous population of immature monocytic and granulocytic leukocytes are released from the bone marrow in advanced cancer patients and can severely disrupt CD8+ T-cell function through several mechanisms. For instance, MDSCs produce high levels of nitrogen monoxyde (NO) and reactive oxygen species (ROS), combining to form nitrosamines that impair TCR function (75). MDSCs also typically overexpress arginase 1 which depletes arginine in the TME, thereby depriving effector T-cells with an essential “fuel” for proliferation (76). Tumor-associated macrophages are myeloid cells which share several T-cell suppressive properties with MDSCs. Tumor-associated macrophages (TAMs) release TGF-β, IL-10, pro-fibrogenic, and pro-angiogenetic factors (77).
Several classes of compounds can be “repurposed” to achieve a reduction of MDSCs both systemically and intratumorally, and/or interfere with these cell's suppressive capacity (78). In many cases this results in enhancement of T-cell responses in a therapeutic cancer vaccine setting. This is true for myeloablative chemotherapeutics such as platinum salts, taxanes, and anti-metabolites (gemcitabine, 5-FU) (79–81), which are known to decrease systemic MDSC numbers in metastatic cancer patients. In preclinical vaccination models, this has been shown to translate into a boosted in T-cell response to vaccination (82, 83). Alternative strategies to target suppressive myeloid cells include administration of all-trans retinoic acids, triterpenoids, phosphodiesterase inhibitors (e.g., sildenafil), tyrosine kinase inhibitors (e.g., sunitinib), amino-bisphosphonates, recombinant IL-12 and anti-IL-6R monoclonal antibodies (84–89). Anti-CSF-1R and anti-CCL2 can both reduce the recruitment of MDSCs and monocyte-derived TAMs into the tumor bed and also contribute to revert the immunosuppressive climate within tumors (90, 91).
Finally, as noted earlier, next to their adjuvant property in itself, STING agonists have the interesting property of being able to reprogram MDSCs from a T-cell suppressive into a type-1 immune polarizing leukocyte (27).
Freeing T-Cells From Negative Checkpoint Signals
On a molecular level, tumor beds also maintain a climate of tolerance and immune suppression through the abundant expression of T-cell checkpoint ligands and a relative lack of costimulatory molecules. Fortunately, the field of immuno-oncology is currently driven forward by the development of several compounds that can disrupt this inhibitory climate: in a first wave of clinical trials, immune checkpoint inhibitors (ICIs) such as CTLA-4, PD-1 and PD-L1 blocking antibodies have demonstrated unequivocal clinical activity as monotherapy in many types of cancer. The performance plateau of immune checkpoint blockade is now being pushed upward by applying combinatorial strategies (e.g., ICI + chemotherapy or ICI + ICI). It can be expected that combinatorial approaches that include ICIs will be the major development that will unlock the full potential of cancer vaccines. Indeed, a robust activation of T-cells (as potentially achieved by a powerful vaccine) will induce expression of counterregulatory checkpoints such as CTLA-4 and PD-1. CTLA-4 can “steal the steam” of signaling through the B7-CD28 costimulatory axis, hereby shutting down T-cell activation by the APC. PD-1, when engaging PD-L1 which is abundantly expressed on cancer cells and intratumoral myeloid cells by exposure to IFN-γ and/or hypoxia, results in paralysis of T-cell effectors at the tumor front. As a clinical indication for this obstacle to vaccine efficacy, in the trial evaluating the TG4010 Muc-1 vaccine in lung cancer only patients whose tumor expressed low levels of PD-L1 had a marked benefit in progression-free survival (56).
Mechanistically, ICIs can potentiate vaccine responses in two main ways. Anti-CTLA-4 checkpoint inhibition will mainly act by boosting the amplitude of the priming phase, by broadening the repertoire of the T-cell response (92) and also by removing the suppressive activity of T-regs in the TME, as noted earlier. PD-1 or PD-L1 blockade will ensure that vaccine-elicited anti-tumoral T-cells can exert their function unhampered once inside the tumor micro-environment. Conversely, vaccination may be an additional combination partner to improve the performance of checkpoint inhibition, whose response rate as monotherapy across all tumors plateaus around 20% in biomarker-unselected patients.
The benefits of combining vaccines with ICIs have been demonstrated in numerous preclinical tumor models (93–96), and these proof-of-concepts have already led to the design of several clinical trials (summarized in Table 1D). Initial results in humans were not encouraging though, when a pivotal trial showed no benefit at all of combining an adjuvanted gp100 peptide vaccine with anti-CTLA4, compared with anti-CTLA4 alone (44). However, more advanced vaccine platforms may still benefit from combination with ICI, as illustrated by a more recent phase 2 trial exploring the combination of a DC vaccine plus ipilimumab: objective response rates and survival were markedly superior than historical data with ipilimumab as monotherapy (97).
The relative timing of vaccination and immune checkpoint blockade could be very critical for optimal anti-tumor effect. CTLA-4 blockade was found to synergize optimally with a prostate cancer GVAX vaccine when administered after vaccination (98). Likewise, responses to TG4010 (Muc-1-targeted MVA vaccine) were enhanced when PD-1 blockade was administered several days after the vaccine (99). By contrast McNeel et al. observed that responses to a PSA-targeted DNA vaccine against prostate cancer were only observed with concurrent rather than sequential PD-1 checkpoint blockade, both in murine models as well as in a small clinical trial (100). The sequencing could be different when it comes to PD-L1 blockade: PD-L1 upregulation is a physiological phenomenon upon DC activation which may serve to protect the DC from elimination during cognate interaction with the CD8+ T-cell. Hence, PD-L1 blockade at the time of vaccination/DC activation may result in abortive T-cell priming due to shortened APC survival and limit effector T-cell polarization and expansion.
Additional checkpoint molecules are currently being explored as clinical targets. Lymphocyte-activation gene 3 (LAG3) is the third immune checkpoint to have been targeted in humans after CTLA4 and the PD-1/PD-L1 axis. LAG-3 is expressed by “exhausted” TILs and T-regs. It shares high structural homology to CD4 and binds MHC class II on APCs. Besides keeping the T-cell itself in an inactive state, LAG3 can reverse-signal to the APC and maintain the latter in an immature/pro-tolerogenic state with impaired upregulation of costimulatory molecules and IL-12 secretion (101). LAG3 blockade as such shows limited effects, but it can roughly double the response rate to PD-1 blockade when used in combination, an added benefit that is clearly enhanced in LAG3-expressing tumor beds (NCT01968109, P. Ascierto et al presented at ESMO 2017). Interestingly, a soluble dimeric recombinant protein consisting of four LAG3 extracellular domains fused to the Fc portion of human IgG1 (LAG3-Ig) has been shown to act as an “APC activator” (102). A possible concern however is that it also stimulates release of the chemokines CCL17 and CCL22, which are known to preferentially attract Th2 lymphocytes and T-regs. The clinical compound, IMP321, is now being evaluated in patients in combination with cancer vaccines in different tumor settings (Table 1D).
Besides an abundance in negative checkpoint molecules, the tumor milieu also fosters immune tolerance through a lack in costimulatory molecules. Agonists of T-cell costimulatory pathways are in clinical development, notably monoclonal antibodies that bind to TNF-superfamily receptors such as OX40 and 4-1BB. Preclinical experiments indicate that costimulation agonists can synergize with vaccination to break tolerance toward poorly immunogenic tumors (103, 104), with several clinical trials now underway (Table 1E).
Dealing With the Immunosuppressive Metabolic Tumor Environment
Next to defined molecular axes, the global metabolic climate within solid tumors provides a hostile environment for proper effector T-cell function as well. An important counterregulatory mechanism in response to an IFN-γ-dominated T-cell attack is the upregulation of IDO (indoleamine-2,3-dioxygenase). Also, activation of DCs results in IDO expression in these cells and promotes paradoxical induction of T-regs (105). Prostaglandin E2, generated by COX2-expressing TAMs, is also an inducer of IDO (106, 107). Originally identified as a major contributor to immune tolerance at the maternofoetal interface (108), IDO enzymatic activity is now recognized as one of the “metabolic checkpoints” in tumors such as melanoma and lung cancer: IDO catabolises tryptophan, which is also a “fuel” for proper T-cell activation and proliferation, into kynurenines that act as T-cell toxic metabolites. Tryptophan depletion will also favor the induction of T-regs (109). IDO inhibitors have demonstrated positive effects in many preclinical models of cancer immunotherapy (109). Clinical development of IDO inhibitors took a hit recently with negative phase 3 results in combination with ICI in melanoma, despite promising phase 2 data (NCT02752074, results presented at ASCO 2018). Nevertheless, results in other tumors are still pending, and combining IDO-inhibition with a vaccine may still be an effective strategy (110) (Table 1F). Arginase activity is also increased in tumors in proportion to myeloid cell infiltration and induces T-cell paralysis by depleting arginine (as described above). Arginase inhibitors are currently in early clinical development [NCT02903914 (111)], with preclinical data showing clear synergism with anti-PD-L1 checkpoint inhibition (112). No clinical trials combining arginase inhibitors with a cancer vaccine have been reported to date.
More difficult to correct through therapeutic intervention are the consequences of aberrant energy metabolism in tumors, where cancer cells out-compete TILs for glucose availability and establish a high lactate/low-pH milieu that blocks T-cell proliferation and IFN-γ release (113). These conditions are further exacerbated by the poor quality of the tumor vasculature which prevents proper clearance of toxic metabolites and exacerbates intratumoral hypoxia. The latter induces upregulation of glucose transporters on tumor cells, further decreasing extracellular glucose availability for effector T-cells.
Metformin, better known as a therapy for insulin-resistant diabetes, also inhibits cancer cell oxygen consumption. This has been shown to decrease tumoral hypoxia, hereby augmenting intratumoral CD8+ T-cell activation and unlocking synergistic effects with checkpoint blockade in otherwise immunotherapy-resistant tumors (114).
Hypoxia also increases expression of ectonucleotidases on the cell membrane of cancer cells and myeloid cells, resulting in degradation of ATP to adenosine. Adenosine triggers A2AR, the most predominant adenosine receptor on immune cells, leading to an increase in intracellular cAMP levels which mediates a plethora of immunosuppressive effects: inhibition T-cell and NK-cell functions, suppression of DC maturation and IL-12 secretion, increase in IL-10 production, induction of T-regs (115).
A2AR antagonists have been developed, with preclinical studies showing promising activity. In a phase I trial the A2AR antagonist CPI-444 produced marked CD8 T-cell infiltration when comparing pre- vs. post-treatment biopsies (116). Preliminary clinical data suggests synergism with PD-L1 blockade, however it is clear from their biological effect that adenosine receptor or ectonucleotidase inhibitors could be attractive add-ons in a therapeutic vaccine setting.
Improving Tumor Visibility to the Immune System
For vaccine-induced T-cells to fulfil their final role, in addition to intratumoral penetration and surmounting suppressive mechanisms, tumor cells must expose sufficient levels of relevant antigen on their surface. This cannot be taken for granted as cancer cells can reduce expression of tumor-associated antigens or downregulate critical components of the antigen-processing and MHC presentation machinery. Interestingly, this loss of “visibility” to the immune system seems to be mediated by epigenetic mechanisms, i.e., DNA hypermethylation and histone deacetylation, which opens up opportunity for therapeutic modulation (117). Expression of cancer-testis antigens is in particular regulated through epigenetic mechanisms, and treatment with DNA methyl transferase (DNMT) inhibitors can increase cancer-testis antigen (CTAG) expression levels on cancer cells. Components of the antigen-processing machinery (APM) such as TAP-1, TAP-2, LMP-2 and Tapasin can be increased by treatment of cancer cells with histone deacetylase (HDAC) inhibitors, which ends up increasing surface expression of MHC class I molecules as well (118, 119).
In addition, epigenetic drugs can help create a more favorite immunological climate within tumors. HDAC inhibitors have been shown to induce Th1, CD8 and NK-cell-attracting chemokines and boost response to anti-PD1 immune checkpoint blockade (120). The combination of DNMT and HDAC-inhibition can also potentiate ICI efficacy by reducing granulocytic MDSC levels (121). Another fascinating discovery is the fact that DNMT-inhibitors can awaken expression of endogenous retroviral vectors (also known as long terminal repeat retro-transposons), thus generating intracellular dsRNAs that can be sensed by the MAD5/MAVS cytosolic sensor and trigger type 1 interferon responses (122).
A large number of clinical trials are now combining checkpoint inhibitors with epigenetic modulators, however only 1 trial exploring the combination a DNMT-inhibitor with a DC-based cancer vaccines in pediatric sarcoma has been completed: remarkably 1 patient of the 10 included experienced a complete response (123). A few other trials combining vaccination with epigenetic modulation are active at the time of this writing (Table 1G).
Conclusion
Given the daunting complexity of tumor-associated immune suppressive networks, it comes as no surprise that vaccination in a therapeutic setting has delivered so little benefits to cancer patients so far. Still, the overwhelming amount of preclinical data supports the notion that vaccination can control or even eradicate tumors, just as preclinical work showed the value of immune checkpoint blockade many years ago. Given the multiple obstacles to T-cell mediated cancer cell destruction, it is clear that the success of a vaccine will depend on our capacity to accurately map the dominant immunosuppressive pathway for each individual patient. An essential aspect when it comes to therapeutic modulation of these pathways is to delineate the hierarchy of obstacles to effective immune responses. For instance, combining a vaccine with immune checkpoint blockade is an effort in vain when a large part of the tumor has acquired defects in MHC class I presentation. An important challenge will be to develop technologies that can deliver comprehensive tumor “immunomics” in a timely and cost-effective fashion. The aim is to provide clinicians with robust biomarkers to guide therapeutic decision making especially when it comes to the wide repertoire of possible combination therapies. An additional challenge is to take into account both the spatial and the temporal heterogeneity of a tumor for a given patient, i.e., are different metastatic sites sensitive/resistant to immunotherapy to the same extent, and how does this evolve over time during the course of specific treatments? As the field of cancer immunology further evolves, several additional questions are raised: what is the role of CD4+ T-cells in vaccine-induced anti-tumor responses? Which could be the optimal chemotherapy or radiotherapy regimen in combination with a cancer vaccine? Does the gut microbiome impact on cancer vaccine efficacy the same way as it influences responses to checkpoint inhibitors? As difficult as these challenges may be, the reward is considerable given the excellent tolerability of vaccines and the promise of long term protective immunological memory, which may transform disease control into cure.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Glossary
Abbreviations
- ASC
Apoptosis-Associated Speck-Like Protein Containing CARD
- CCL
CC chemokine ligand
- cGAS
Cyclic GMP-AMP synthase
- CSF-1R
Colony-stimulating factor receptor-1
- CTLA-4
Cytotoxic T-Lymphocyte Associated Protein 4
- IFN
Interferon
- IKK
IκB kinase
- IL
Interleukin
- IRF3
Interferon regulatory factor 3
- ISCOM
Immune stimulating complexes
- LMP-2
Epstein–Barr virus (EBV) latent membrane protein 2
- NFκB
Nuclear Factor kappa-light-chain-enhancer of activated B cells
- TAA
Tumor-associated antigen
- TAP-1, Transporter 1
ATP Binding Cassette Subfamily B Member
- TBK1
TANK Binding Kinase 1
- TCR
T-cell receptor
- TGF-β
Transforming growth factor beta
- TRAIL
TNF-related apoptosis-inducing ligand.
Footnotes
Funding. KV is supported by an FWO Senior Clinical Investigator Grant.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2019.00008/full#supplementary-material
References
- 1.Andreu P, Johansson M, Affara NI, Pucci F, Tan T, Junankar S, et al. FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell (2010) 17:121–34. 10.1016/j.ccr.2009.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schioppa T, Moore R, Thompson RG, Rosser EC, Kulbe H, Nedospasov S, et al. B regulatory cells and the tumor-promoting actions of TNF-α during squamous carcinogenesis. Proc Natl Acad Sci USA. (2011) 108:10662–7. 10.1073/pnas.1100994108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiang CL-L, Coukos G, Kandalaft LE. Whole tumor antigen vaccines: where are we? Vaccines (2015) 3:344–72. 10.3390/vaccines3020344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keenan BP, Jaffee EM. Whole cell vaccines–past progress and future strategies. Semin Oncol. (2012) 39:276–86. 10.1053/j.seminoncol.2012.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melief CJM, van Hall T, Arens R, Ossendorp F, van der Burg SH. Therapeutic cancer vaccines. J Clin Invest. (2015) 125:3401–12. 10.1172/JCI80009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gulley JL, Madan RA, Pachynski R, Mulders P, Shiek NA, Trager J, et al. Role of antigen spread and distinctive characteristics of immunotherapy in cancer treatment. J Natl Cancer Inst. (2017) 109:djw261. 10.1093/jnci/djw261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science (2015) 348:124–8. 10.1126/science.aaa1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vermaelen K, Waeytens A, Kholmanskikh O, Van den Bulcke M, Van Valckenborgh E. Perspectives on the integration of immuno-oncology biomarkers and drugs in a health care setting. Semin Cancer Biol. (2017). 52:166–77. 10.1016/j.semcancer.2017.11.011 [DOI] [PubMed] [Google Scholar]
- 9.McGranahan N, Furness AJS, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science (2016) 351:1463–9. 10.1126/science.aaf1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turajlic S, Litchfield K, Xu H, Rosenthal R, McGranahan N, Reading JL, et al. Insertion-and-deletion-derived tumour-specific neoantigens and the immunogenic phenotype: a pan-cancer analysis. Lancet Oncol. (2017) 18:1009–21. 10.1016/S1470-2045(17)30516-8 [DOI] [PubMed] [Google Scholar]
- 11.Kreiter S, Castle JC, Türeci O, Sahin U. Targeting the tumor mutanome for personalized vaccination therapy. Oncoimmunology (2012) 1:768–9. 10.4161/onci.19727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sahin U, Derhovanessian E, Miller M, Kloke B-P, Simon P, Löwer M, et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature (2017) 547:222–6. 10.1038/nature23003 [DOI] [PubMed] [Google Scholar]
- 13.Rogers FB, Maloney RJ. Gaston ramon, 1886–1963. Arch Environ Health (1963) 7:723–5. [DOI] [PubMed] [Google Scholar]
- 14.Qureshi N, Takayama K, Ribi E. Purification and structural determination of nontoxic lipid A obtained from the lipopolysaccharide of Salmonella typhimurium. J Biol Chem. (1982) 257:11808–15. [PubMed] [Google Scholar]
- 15.Tefit JN, Serra V. Outlining novel cellular adjuvant products for therapeutic vaccines against cancer. Expert Rev Vaccines (2011) 10:1207–20. 10.1586/erv.11.84 [DOI] [PubMed] [Google Scholar]
- 16.Mitchell MS, Abrams J, Thompson JA, Kashani-Sabet M, DeConti RC, Hwu W-J, et al. Randomized trial of an allogeneic melanoma lysate vaccine with low-dose interferon Alfa-2b compared with high-dose interferon Alfa-2b for Resected stage III cutaneous melanoma. J Clin Oncol. (2007) 25:2078–85. 10.1200/JCO.2006.10.1709 [DOI] [PubMed] [Google Scholar]
- 17.Gérard C, Baudson N, Ory T, Louahed J. Tumor mouse model confirms MAGE-A3 cancer immunotherapeutic as an efficient inducer of long-lasting anti-tumoral responses. PLoS ONE (2014) 9:e94883. 10.1371/journal.pone.0094883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coler RN, Bertholet S, Moutaftsi M, Guderian JA, Windish HP, Baldwin SL, et al. Development and characterization of synthetic glucopyranosyl lipid adjuvant system as a vaccine adjuvant. PLoS ONE (2011) 6:e16333. 10.1371/journal.pone.0016333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson BS, Chilton PM, Ward JR, Evans JT, Mitchell TC. The low-toxicity versions of LPS, MPL adjuvant and RC529, are efficient adjuvants for CD4+ T cells. J Leukoc Biol. (2005) 78:1273–80. 10.1189/jlb.0305172 [DOI] [PubMed] [Google Scholar]
- 20.Steinhagen F, Kinjo T, Bode C, Klinman DM. TLR-based immune adjuvants. Vaccine (2011) 29:3341–55. 10.1016/j.vaccine.2010.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu H. TLR Agonists for cancer immunotherapy: tipping the balance between the immune stimulatory and inhibitory effects. Front Immunol. (2014) 5:83. 10.3389/fimmu.2014.00083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pulko V, Liu X, Krco CJ, Harris KJ, Frigola X, Kwon ED, et al. TLR3-stimulated dendritic cells up-regulate B7-H1 expression and influence the magnitude of CD8 T cell responses to tumor vaccination. J Immunol. (2009) 183:3634–41. 10.4049/jimmunol.0900974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Volpi C, Fallarino F, Bianchi R, Orabona C, De Luca A, Vacca C, et al. A GpC-rich oligonucleotide acts on plasmacytoid dendritic cells to promote immune suppression. J Immunol. (2012) 189:2283–9. 10.4049/jimmunol.1200497 [DOI] [PubMed] [Google Scholar]
- 24.Ragupathi G, Gardner JR, Livingston PO, Gin DY. Natural and synthetic saponin adjuvant QS-21 for vaccines against cancer. Expert Rev Vaccines (2011) 10:463–70. 10.1586/erv.11.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marty-Roix R, Vladimer GI, Pouliot K, Weng D, Buglione-Corbett R, West K, et al. Identification of QS-21 as an inflammasome-activating molecular component of saponin adjuvants. J Biol Chem. (2016) 291:1123–36. 10.1074/jbc.M115.683011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dubensky TW, Kanne DB, Leong ML. Rationale, progress and development of vaccines utilizing STING-activating cyclic dinucleotide adjuvants. Ther Adv Vaccines (2013) 1:131–43. 10.1177/2051013613501988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chandra D, Quispe-Tintaya W, Jahangir A, Asafu-Adjei D, Ramos I, Sintim HO, et al. STING ligand c-di-GMP improves cancer vaccination against metastatic breast cancer. Cancer Immunol Res. (2014) 2:901–10. 10.1158/2326-6066.CIR-13-0123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woo S-R, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MYK, et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity (2014) 41:830–42. 10.1016/j.immuni.2014.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gulen MF, Koch U, Haag SM, Schuler F, Apetoh L, Villunger A, et al. Signalling strength determines proapoptotic functions of STING. Nat Commun. (2017) 8:427. 10.1038/s41467-017-00573-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanson MC, Crespo MP, Abraham W, Moynihan KD, Szeto GL, Chen SH, et al. Nanoparticulate STING agonists are potent lymph node-targeted vaccine adjuvants. J Clin Invest. (2015) 125:2532–46. 10.1172/JCI79915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conlon J, Burdette DL, Sharma S, Bhat N, Thompson M, Jiang Z, et al. Mouse, but not human STING, binds and signals in response to the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid. J Immunol. (2013) 190:5216–25. 10.4049/jimmunol.1300097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dranoff G. GM-CSF-based cancer vaccines. Immunol Rev. (2002) 188:147–54. 10.1034/j.1600-065X.2002.18813.x [DOI] [PubMed] [Google Scholar]
- 33.Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. (2004) 64:6337–43. 10.1158/0008-5472.CAN-04-0757 [DOI] [PubMed] [Google Scholar]
- 34.Slingluff CL, Petroni GR, Olson WC, Smolkin ME, Ross MI, Haas NB, et al. Effect of granulocyte/macrophage colony-stimulating factor on circulating CD8+ and CD4+ T-cell responses to a multipeptide melanoma vaccine: outcome of a multicenter randomized trial. Clin Cancer Res. (2009) 15:7036–44. 10.1158/1078-0432.CCR-09-1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suto R, Srivastava PK. A mechanism for the specific immunogenicity of heat shock protein-chaperoned peptides. Science (1995) 269:1585–8. [DOI] [PubMed] [Google Scholar]
- 36.Li Z, Menoret A, Srivastava P. Roles of heat-shock proteins in antigen presentation and cross-presentation. Curr Opin Immunol. (2002) 14:45–51. 10.1016/S0952-7915(01)00297-7 [DOI] [PubMed] [Google Scholar]
- 37.Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol. (2000) 12:1539–46. 10.1093/intimm/12.11.1539 [DOI] [PubMed] [Google Scholar]
- 38.Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol. (2002) 20:395–425. 10.1146/annurev.immunol.20.100301.064801 [DOI] [PubMed] [Google Scholar]
- 39.Testori A, Richards J, Whitman E, Mann GB, Lutzky J, Camacho L, et al. Phase III comparison of vitespen, an autologous tumor-derived heat shock protein gp96 peptide complex vaccine, with physician's choice of treatment for stage IV melanoma: the C-100-21 Study Group. J Clin Oncol. (2008) 26:955–62. 10.1200/JCO.2007.11.9941 [DOI] [PubMed] [Google Scholar]
- 40.Wood C, Srivastava P, Bukowski R, Lacombe L, Gorelov AI, Gorelov S, et al. An adjuvant autologous therapeutic vaccine (HSPPC-96; vitespen) versus observation alone for patients at high risk of recurrence after nephrectomy for renal cell carcinoma: a multicentre, open-label, randomised phase III trial. Lancet (2008) 372:145–54. 10.1016/S0140-6736(08)60697-2 [DOI] [PubMed] [Google Scholar]
- 41.Hamid O, Solomon JC, Scotland R, Garcia M, Sian S, Ye W, et al. Alum with interleukin-12 augments immunity to a melanoma peptide vaccine: correlation with time to relapse in patients with resected high-risk disease. Clin Cancer Res. (2007) 13:215–22. 10.1158/1078-0432.CCR-06-1450 [DOI] [PubMed] [Google Scholar]
- 42.Didierlaurent AM, Morel S, Lockman L, Giannini SL, Bisteau M, Carlsen H, et al. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J Immunol. (2009) 183:6186–97. 10.4049/jimmunol.0901474 [DOI] [PubMed] [Google Scholar]
- 43.Temizoz B, Kuroda E, Ishii KJ. Vaccine adjuvants as potential cancer immunotherapeutics. Int Immunol. (2016) 28:329–38. 10.1093/intimm/dxw015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. (2010) 363:711–23. 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kersten GFA, Crommelin DJA. Liposomes and ISCOMs. Vaccine (2003) 21:915–20. 10.1016/S0264-410X(02)00540-6 [DOI] [PubMed] [Google Scholar]
- 46.Wilson NS, Duewell P, Yang B, Li Y, Marsters S, Koernig S, et al. Inflammasome-dependent and -independent IL-18 production mediates immunity to the ISCOMATRIX adjuvant. J Immunol. (2014) 192:3259–68. 10.4049/jimmunol.1302011 [DOI] [PubMed] [Google Scholar]
- 47.Davis ID, Chen W, Jackson H, Parente P, Shackleton M, Hopkins W, et al. Recombinant NY-ESO-1 protein with ISCOMATRIX adjuvant induces broad integrated antibody and CD4(+) and CD8(+) T cell responses in humans. Proc Natl Acad Sci USA. (2004) 101:10697–702. 10.1073/pnas.0403572101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Geest BG. Engineering the immune system with particles, step-by-step. Mol Immunol. (2018) 98:25–7. 10.1016/j.molimm.2018.02.015 [DOI] [PubMed] [Google Scholar]
- 49.Vansteenkiste JF, Cho B-C, Vanakesa T, De Pas T, Zielinski M, Kim MS, et al. Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non-small-cell lung cancer (MAGRIT): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. (2016) 17:822–35. 10.1016/S1470-2045(16)00099-1 [DOI] [PubMed] [Google Scholar]
- 50.Atanackovic D, Altorki NK, Stockert E, Williamson B, Jungbluth AA, Ritter E, et al. Vaccine-induced CD4+ T cell responses to MAGE-3 protein in lung cancer patients. J Immunol. (2004) 172:3289–96. 10.4049/jimmunol.172.5.3289 [DOI] [PubMed] [Google Scholar]
- 51.Pujol J-L, De Pas T, Rittmeyer A, Vallières E, Kubisa B, Levchenko E, et al. Safety and immunogenicity of the PRAME cancer immunotherapeutic in patients with resected non-small cell lung cancer: a phase I dose escalation study. J Thorac Oncol. (2016) 11:2208–17. 10.1016/j.jtho.2016.08.120 [DOI] [PubMed] [Google Scholar]
- 52.Mitchell P, Thatcher N, Socinski MA, Wasilewska-Tesluk E, Horwood K, Szczesna A, et al. Tecemotide in unresectable stage III non-small-cell lung cancer in the phase III START study: updated overall survival and biomarker analyses. Ann Oncol. (2015) 26:1134–42. 10.1093/annonc/mdv104 [DOI] [PubMed] [Google Scholar]
- 53.Katakami N, Hida T, Nokihara H, Imamura F, Sakai H, Atagi S, et al. Phase I/II study of tecemotide as immunotherapy in Japanese patients with unresectable stage III non-small cell lung cancer. Lung Cancer (2017) 105:23–30. 10.1016/j.lungcan.2017.01.007 [DOI] [PubMed] [Google Scholar]
- 54.Ramírez JC, Gherardi MM, Esteban M. Biology of attenuated modified vaccinia virus Ankara recombinant vector in mice: virus fate and activation of B- and T-cell immune responses in comparison with the Western Reserve strain and advantages as a vaccine. J Virol. (2000) 74:923–33. 10.1128/JVI.74.2.923-933.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Quoix E, Ramlau R, Westeel V, Papai Z, Madroszyk A, Riviere A, et al. Therapeutic vaccination with TG4010 and first-line chemotherapy in advanced non-small-cell lung cancer: a controlled phase 2B trial. Lancet Oncol. (2011) 12:1125–33. 10.1016/S1470-2045(11)70259-5 [DOI] [PubMed] [Google Scholar]
- 56.Quoix E, Lena H, Losonczy G, Forget F, Chouaid C, Papai Z, et al. TG4010 immunotherapy and first-line chemotherapy for advanced non-small-cell lung cancer (TIME): results from the phase 2b part of a randomised, double-blind, placebo-controlled, phase 2b/3 trial. Lancet Oncol. (2016) 17:212–23. 10.1016/S1470-2045(15)00483-0 [DOI] [PubMed] [Google Scholar]
- 57.Tzai TS, Shiau AL, Liu LL, Wu CL. Immunization with TGF-beta antisense oligonucleotide-modified autologous tumor vaccine enhances the antitumor immunity of MBT-2 tumor-bearing mice through upregulation of MHC class I and Fas expressions. Anticancer Res. (2000) 20:1557–62. [PubMed] [Google Scholar]
- 58.Nemunaitis J, Dillman RO, Schwarzenberger PO, Senzer N, Cunningham C, Cutler J, et al. Phase II study of belagenpumatucel-L, a transforming growth factor beta-2 antisense gene-modified allogeneic tumor cell vaccine in non-small-cell lung cancer. J Clin Oncol. (2006) 24:4721–30. 10.1200/JCO.2005.05.5335 [DOI] [PubMed] [Google Scholar]
- 59.Giaccone G, Bazhenova LA, Nemunaitis J, Tan M, Juhász E, Ramlau R, et al. A phase III study of belagenpumatucel-L, an allogeneic tumour cell vaccine, as maintenance therapy for non-small cell lung cancer. Eur J Cancer (2015) 51:2321–9. 10.1016/j.ejca.2015.07.035 [DOI] [PubMed] [Google Scholar]
- 60.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity (2013) 39:1–10. 10.1016/j.immuni.2013.07.012 [DOI] [PubMed] [Google Scholar]
- 61.Lanitis E, Irving M, Coukos G. Targeting the tumor vasculature to enhance T cell activity. Curr Opin Immunol. (2015) 33:55–63. 10.1016/j.coi.2015.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dirkx AEM, oude Egbrink MGA, Castermans K, van der Schaft DWJ, Thijssen VLJL, Dings RPM, et al. Anti-angiogenesis therapy can overcome endothelial cell anergy and promote leukocyte-endothelium interactions and infiltration in tumors. Faseb J. (2006) 20:621–30. 10.1096/fj.05-4493com [DOI] [PubMed] [Google Scholar]
- 63.Buckanovich RJ, Facciabene A, Kim S, Benencia F, Sasaroli D, Balint K, et al. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat Med (2008) 14:28–36. 10.1038/nm1699 [DOI] [PubMed] [Google Scholar]
- 64.Li B, Lalani AS, Harding TC, Luan B, Koprivnikar K, Huan Tu G, et al. Vascular endothelial growth factor blockade reduces intratumoral regulatory T cells and enhances the efficacy of a GM-CSF-secreting cancer immunotherapy. Clin Cancer Res. (2006) 12:6808–16. 10.1158/1078-0432.CCR-06-1558 [DOI] [PubMed] [Google Scholar]
- 65.Ohm JE, Gabrilovich DI, Sempowski GD, Kisseleva E, Parman KS, Nadaf S, et al. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood (2003) 101:4878–86. 10.1182/blood-2002-07-1956 [DOI] [PubMed] [Google Scholar]
- 66.Gabrilovich DI, Ishida T, Nadaf S, Ohm JE, Carbone DP. Antibodies to vascular endothelial growth factor enhance the efficacy of cancer immunotherapy by improving endogenous dendritic cell function. Clin Cancer Res (1999) 5:2963–70. [PubMed] [Google Scholar]
- 67.Nicholaou T, Ebert LM, Davis ID, McArthur GA, Jackson H, Dimopoulos N, et al. Regulatory T-cell-mediated attenuation of T-cell responses to the NY-ESO-1 ISCOMATRIX vaccine in patients with advanced malignant melanoma. Clin Cancer Res. (2009) 15:2166–73. 10.1158/1078-0432.CCR-08-2484 [DOI] [PubMed] [Google Scholar]
- 68.Jacobs C, Duewell P, Heckelsmiller K, Wei J, Bauernfeind F, Ellermeier J, Kisser U, et al. An ISCOM vaccine combined with a TLR9 agonist breaks immune evasion mediated by regulatory T cells in an orthotopic model of pancreatic carcinoma. Int J Cancer (2011) 128:897–907. 10.1002/ijc.25399 [DOI] [PubMed] [Google Scholar]
- 69.Pitt JM, Marabelle A, Eggermont A, Soria JC, Kroemer G, Zitvogel L. Targeting the tumor microenvironment: removing obstruction to anticancer immune responses and immunotherapy. Ann Oncol. (2016) 27:1482–92. 10.1093/annonc/mdw168 [DOI] [PubMed] [Google Scholar]
- 70.Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. (2007) 56:641–8. 10.1007/s00262-006-0225-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tang F, Du X, Liu M, Zheng P, Liu Y. Anti-CTLA-4 antibodies in cancer immunotherapy: selective depletion of intratumoral regulatory T cells or checkpoint blockade? Cell Biosci. (2018) 8:30 10.1186/s13578-018-0229-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mahne AE, Mauze S, Joyce-Shaikh B, Xia J, Bowman EP, Beebe AM, et al. Dual roles for regulatory T-cell depletion and costimulatory signaling in agonistic GITR targeting for tumor immunotherapy. Cancer Res. (2017) 77:1108–18. 10.1158/0008-5472.CAN-16-0797 [DOI] [PubMed] [Google Scholar]
- 73.Morillon YM, Hammond SA, Durham NM, Schlom J, Greiner JW. Enhanced immunotherapy by combining a vaccine with a novel murine GITR ligand fusion protein. Oncotarget (2017) 8:73469–82. 10.18632/oncotarget.20703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Villarreal DO, Chin D, Smith MA, Luistro LL, Snyder LA. Combination GITR targeting/PD-1 blockade with vaccination drives robust antigen-specific antitumor immunity. Oncotarget (2017) 8:39117–30. 10.18632/oncotarget.16605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, et al. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. (2007) 13:828–35. 10.1038/nm1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Raber P, Ochoa AC, Rodriguez PC. Metabolism of L-arginine by myeloid-derived suppressor cells in cancer: mechanisms of T cell suppression and therapeutic perspectives. Immunol Invest. (2012) 41:614–34. 10.3109/08820139.2012.680634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. (2012) 12:253–68. 10.1038/nri3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Anani W, Shurin MR. Targeting myeloid-derived suppressor cells in cancer. Adv Exp Med Biol. (2017) 1036:105–28. 10.1007/978-3-319-67577-0_8 [DOI] [PubMed] [Google Scholar]
- 79.Kodumudi KN, Woan K, Gilvary DL, Sahakian E, Wei S, Djeu JY. A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin Cancer Res. (2010) 16:4583–94. 10.1158/1078-0432.CCR-10-0733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 70:3052–61. 10.1158/0008-5472.CAN-09-3690 [DOI] [PubMed] [Google Scholar]
- 81.Suzuki E, Kim S, Cheung HK, Corbley MJ, Zhang X, Sun L, et al. A novel small-molecule inhibitor of transforming growth factor beta type I receptor kinase (SM16) inhibits murine mesothelioma tumor growth in vivo and prevents tumor recurrence after surgical resection. Cancer Res. (2007) 67:2351–9. 10.1158/0008-5472.CAN-06-2389 [DOI] [PubMed] [Google Scholar]
- 82.Welters MJ, van der Sluis TC, van Meir H, Loof NM, van Ham VJ, van Duikeren S, et al. Vaccination during myeloid cell depletion by cancer chemotherapy fosters robust T cell responses. Sci Transl Med. (2016) 8:334ra52. 10.1126/scitranslmed.aad8307 [DOI] [PubMed] [Google Scholar]
- 83.Bialkowski L, van Weijnen A, Van der Jeught K, Renmans D, Daszkiewicz L, Heirman C, et al. Intralymphatic mRNA vaccine induces CD8 T-cell responses that inhibit the growth of mucosally located tumours. Sci Rep. (2016) 6:22509. 10.1038/srep22509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kusmartsev S, Cheng F, Yu B, Nefedova Y, Sotomayor E, Lush R, et al. All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res. (2003) 63:4441–9. [PubMed] [Google Scholar]
- 85.Nagaraj S, Youn JI, Weber H, Iclozan C, Lu L, Cotter MJ, et al. Anti-inflammatory triterpenoid blocks immune suppressive function of MDSCs and improves immune response in cancer. Clin Cancer Res. 16:1812–23. 10.1158/1078-0432.CCR-09-3272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sumida K, Wakita D, Narita Y, Masuko K, Terada S, Watanabe K, et al. Anti-IL-6 receptor mAb eliminates myeloid-derived suppressor cells and inhibits tumor growth by enhancing T-cell responses. Eur J Immunol. (2012) 42:2060–72. 10.1002/eji.201142335 [DOI] [PubMed] [Google Scholar]
- 87.Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. (2006) 203:2691–702. 10.1084/jem.20061104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Melani C, Sangaletti S, Barazzetta FM, Werb Z, Colombo MP. Amino-biphosphonate-mediated MMP-9 inhibition breaks the tumor-bone marrow axis responsible for myeloid-derived suppressor cell expansion and macrophage infiltration in tumor stroma. Cancer Res. (2007) 67:11438–46. 10.1158/0008-5472.CAN-07-1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kerkar SP, Goldszmid RS, Muranski P, Chinnasamy D, Yu Z, Reger RN, et al. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J Clin Invest. (2011) 121:4746–57. 10.1172/JCI58814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Priceman SJ, Sung JL, Shaposhnik Z, Burton JB, Torres-Collado AX, Moughon DL, et al. Targeting distinct tumor-infiltrating myeloid cells by inhibiting CSF-1 receptor: combating tumor evasion of antiangiogenic therapy. Blood (2010) 115:1461–71. 10.1182/blood-2009-08-237412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pienta KJ, Machiels J-P, Schrijvers D, Alekseev B, Shkolnik M, Crabb SJ, et al. Phase 2 study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 (CCL2), in metastatic castration-resistant prostate cancer. Invest New Drugs (2013) 31:760–8. 10.1007/s10637-012-9869-8 [DOI] [PubMed] [Google Scholar]
- 92.Kvistborg P, Philips D, Kelderman S, Hageman L, Ottensmeier C, Joseph-Pietras D, et al. Anti-CTLA-4 therapy broadens the melanoma-reactive CD8+ T cell response. Sci Transl Med. (2014) 6:254ra128. 10.1126/scitranslmed.3008918 [DOI] [PubMed] [Google Scholar]
- 93.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. (2006) 116:1935–45. 10.1172/JCI27745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Karyampudi L, Lamichhane P, Scheid AD, Kalli KR, Shreeder B, Krempski JW, et al. Accumulation of memory precursor CD8 T cells in regressing tumors following combination therapy with vaccine and anti-PD-1 antibody. Cancer Re.s (2014) 74:2974–85. 10.1158/0008-5472.CAN-13-2564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Soares KC, Rucki AA, Wu AA, Olino K, Xiao Q, Chai Y, et al. PD-1/PD-L1 blockade together with vaccine therapy facilitates effector T-cell infiltration into pancreatic tumors. J Immunother. (2015) 38:1–11. 10.1097/CJI.0000000000000062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Carreno BM, Magrini V, Becker-Hapak M, Kaabinejadian S, Hundal J, Petti AA, et al. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science (2015) 348:803–8. 10.1126/science.aaa3828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wilgenhof S, Corthals J, Heirman C, van Baren N, Lucas S, Kvistborg P, et al. Phase II study of autologous monocyte-derived mRNA electroporated dendritic cells (TriMixDC-MEL) plus ipilimumab in patients with pretreated advanced melanoma. J Clin Oncol. (2016) 34:1330–8. 10.1200/JCO.2015.63.4121 [DOI] [PubMed] [Google Scholar]
- 98.Wada S, Jackson CM, Yoshimura K, Yen H-R, Getnet D, Harris TJ, et al. Sequencing CTLA-4 blockade with cell-based immunotherapy for prostate cancer. J Transl Med. (2013) 11:89. 10.1186/1479-5876-11-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Remy-Ziller C, Thioudellet C, Hortelano J, Gantzer M, Nourtier V, Claudepierre M-C, et al. Sequential administration of MVA-based vaccines and PD-1/PD-L1-blocking antibodies confers measurable benefits on tumor growth and survival: Preclinical studies with MVA-βGal and MVA-MUC1 (TG4010) in a murine tumor model. Hum Vaccin Immunother. (2018) 14:140–5. 10.1080/21645515.2017.1373921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McNeel DG, Eickhoff JC, Wargowski E, Zahm C, Staab MJ, Straus J, et al. Concurrent, but not sequential, PD-1 blockade with a DNA vaccine elicits anti-tumor responses in patients with metastatic, castration-resistant prostate cancer. Oncotarget (2018) 9:25586–96. 10.18632/oncotarget.25387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Andrews LP, Marciscano AE, Drake CG, Vignali DAA. LAG3 (CD223) as a cancer immunotherapy target. Immunol Rev. (2017) 276:80–96. 10.1111/imr.12519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Andreae S, Piras F, Burdin N, Triebel F. Maturation and activation of dendritic cells induced by lymphocyte activation gene-3 (CD223). J Immunol. (2002) 168:3874–80. 10.4049/jimmunol.168.8.3874 [DOI] [PubMed] [Google Scholar]
- 103.Cuadros C, Dominguez AL, Lollini P-L, Croft M, Mittler RS, Borgström P, et al. Vaccination with dendritic cells pulsed with apoptotic tumors in combination with anti-OX40 and anti-4-1BB monoclonal antibodies induces T cell-mediated protective immunity in Her-2/neu transgenic mice. Int J Cancer (2005) 116:934–43. 10.1002/ijc.21098 [DOI] [PubMed] [Google Scholar]
- 104.Ito F, Li Q, Shreiner AB, Okuyama R, Jure-Kunkel MN, Teitz-Tennenbaum S, et al. Anti-CD137 monoclonal antibody administration augments the antitumor efficacy of dendritic cell-based vaccines. Cancer Res (2004) 64:8411–9. 10.1158/0008-5472.CAN-04-0590 [DOI] [PubMed] [Google Scholar]
- 105.Wobser M, Voigt H, Houben R, Eggert AO, Freiwald M, Kaemmerer U, et al. Dendritic cell based antitumor vaccination: impact of functional indoleamine 2,3-dioxygenase expression. Cancer Immunol Immunother. (2007) 56:1017–24. 10.1007/s00262-006-0256-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee SY, Choi HK, Lee KJ, Jung JY, Hur GY, Jung KH, et al. The immune tolerance of cancer is mediated by IDO that is inhibited by COX-2 inhibitors through regulatory T cells. J Immunother. (2009) 32:22–8. 10.1097/CJI.0b013e31818ac2f7 [DOI] [PubMed] [Google Scholar]
- 107.Basu GD, Tinder TL, Bradley JM, Tu T, Hattrup CL, Pockaj BA, Mukherjee P. Cyclooxygenase-2 inhibitor enhances the efficacy of a breast cancer vaccine: role of IDO. J Immunol (2006) 177:2391–402. 10.4049/jimmunol.177.4.2391 [DOI] [PubMed] [Google Scholar]
- 108.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science (1998) 281:1191–3. [DOI] [PubMed] [Google Scholar]
- 109.Munn DH, Mellor AL. IDO in the tumor microenvironment: inflammation, counter-regulation, and tolerance. Trends Immunol. (2016) 37:193–207. 10.1016/j.it.2016.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ou X, Cai S, Liu P, Zeng J, He Y, Wu X, et al. Enhancement of dendritic cell-tumor fusion vaccine potency by indoleamine-pyrrole 2,3-dioxygenase inhibitor, 1-MT. J Cancer Res Clin Oncol. (2008) 134:525–33. 10.1007/s00432-007-0315-9 [DOI] [PubMed] [Google Scholar]
- 111.Papadopoulos KP, Tsai FY-C, Bauer TM, Muigai L, Liang Y, Bennett MK, et al. CX-1158-101: a first-in-human phase 1 study of CB-1158, a small molecule inhibitor of arginase, as monotherapy and in combination with an anti-PD-1 checkpoint inhibitor in patients (pts) with solid tumors. J Clin Oncol. (2017) 35:3005 10.1200/JCO.2017.35.15_suppl.3005 [DOI] [Google Scholar]
- 112.Steggerda SM, Bennett MK, Chen J, Emberley E, Huang T, Janes JR, et al. Inhibition of arginase by CB-1158 blocks myeloid cell-mediated immune suppression in the tumor microenvironment. J ImmunoTher Cancer (2017) 5:101. 10.1186/s40425-017-0308-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Scharping NE, Delgoffe GM. Tumor microenvironment metabolism: a new checkpoint for anti-tumor immunity. Vaccines (2016) 4:E46. 10.3390/vaccines4040046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Scharping NE, Menk AV, Whetstone RD, Zeng X, Delgoffe GM. Efficacy of PD-1 Blockade Is Potentiated by Metformin-Induced Reduction of Tumor Hypoxia. Cancer Immunol Res (2017) 5:9–16. 10.1158/2326-6066.CIR-16-0103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ohta A. A metabolic immune checkpoint: adenosine in tumor microenvironment. Front Immunol (2016) 7:109. 10.3389/fimmu.2016.00109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Emens L, Powderly J, Fong L, Brody J, Forde P, Hellmann M, et al. Abstract CT119: CPI-444, an oral adenosine A2a receptor (A2aR) antagonist, demonstrates clinical activity in patients with advanced solid tumors. Cancer Res. (2017) 77:CT119 10.1158/1538-7445.AM2017-CT119 [DOI] [Google Scholar]
- 117.Chiappinelli KB, Zahnow CA, Ahuja N, Baylin SB. Combining epigenetic and immunotherapy to combat cancer. Cancer Res. (2016) 76:1683–9. 10.1158/0008-5472.CAN-15-2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Khan ANH, Gregorie CJ, Tomasi TB. Histone deacetylase inhibitors induce TAP, LMP, Tapasin genes and MHC class I antigen presentation by melanoma cells. Cancer Immunol Immunother. (2008) 57:647–54. 10.1007/s00262-007-0402-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Setiadi AF, David MD, Seipp RP, Hartikainen JA, Gopaul R, Jefferies WA. Epigenetic control of the immune escape mechanisms in malignant carcinomas. Mol Cell Biol. (2007) 27:7886–94. 10.1128/MCB.01547-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zheng H, Zhao W, Yan C, Watson CC, Massengill M, Xie M, et al. HDAC inhibitors enhance T-Cell chemokine expression and augment response to PD-1 immunotherapy in lung adenocarcinoma. Clin Cancer Res. (2016) 22:4119–32. 10.1158/1078-0432.CCR-15-2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim K, Skora AD, Li Z, Liu Q, Tam AJ, Blosser RL, et al. Eradication of metastatic mouse cancers resistant to immune checkpoint blockade by suppression of myeloid-derived cells. Proc Natl Acad Sci USA. (2014) 111:11774–9. 10.1073/pnas.1410626111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chiappinelli KB, Strissel PL, Desrichard A, Li H, Henke C, Akman B, et al. Inhibiting DNA methylation causes an interferon response in cancer via dsrna including endogenous retroviruses. Cell (2017) 169:361. 10.1016/j.cell.2017.03.036 [DOI] [PubMed] [Google Scholar]
- 123.Krishnadas DK, Shusterman S, Bai F, Diller L, Sullivan JE, Cheerva AC, et al. A phase I trial combining decitabine/dendritic cell vaccine targeting MAGE-A1, MAGE-A3 and NY-ESO-1 for children with relapsed or therapy-refractory neuroblastoma and sarcoma. Cancer Immunol Immunother. (2015) 64:1251–60. 10.1007/s00262-015-1731-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.