Abstract
Considering the synthetic value of introducing active alcoholic hydroxyl group, developing C–H functionalization of alcohols is of significance. Herein, we present a photochemical method that under visible light irradiation, selectfluor can effectively promote the oxidative cross-coupling between alcohols and heteroarenes without the external photocatalysis, achieving the selective α sp3 C–H arylation of alcohol, even in the presence of ether. The N-F activation of selectfluor under blue LEDs irradiation is evidenced by electron paramagnetic resonance (EPR) study, which is the key process for the oxidative activation of α sp3 C–H alcohols. The observed reactivity may have significant implications for chemical transformations.
Alcohols are very useful building blocks in organic synthesis, however C–H functionaliztion in presence of free –OH groups is highly challenging. Here, the authors report a selective visible light-promoted α-functionalization of free alcohols with heteroarenes mediated by Selectofluor.
Introduction
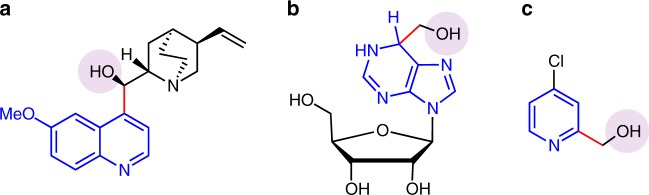
Alcohols as one of the most common raw chemical materials, are indispensable to organic chemistry and chemical engineering. The presence of hydroxyl group enables them to play diverse roles such as good solvents, competent nucleophiles1–5, suitable directing group6–8, and frequently used proton source for a long time9–11. The strategies, sp3 C–H functionalization of alcohols including α sp3 C–H functionalization and remote sp3 C–H functionalization12–15, which can transform alcohols into value-added chemicals, are significant for the organic synthesis and bio-pharmaceuticals (Fig. 1)16,17.
Fig. 1.
Important molecules containing alcoholic hydroxyl groups. a Anti-malarial natural product quinine. b Inhibitor of adenosine deaminase. c Inhibitor of gastric acid secretion
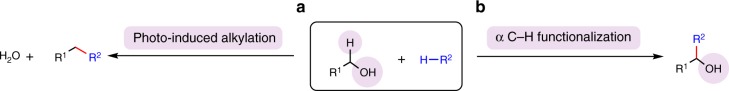
During the past decade, photoredox catalysis featured by the conversion of light energy into chemical energy and single electron transfer (SET) events, has facilitated the discovery of numerous elegant and challenging chemical transformations18–26. Particularly, impressive studies on employing the alcohols as alkylating reagent with the loss of alcoholic hydroxyl groups—achieved photochemical alkylation of electron-deficient heteroarenes (Fig. 2a)27–30. Considering the multiple functionality of alcoholic hydroxyl group in bioactive molecules and the frequency of its use as synthetic handles, selective functionalization of α sp3 C–H of alcohols via a photochemical process is undeniably attractive for its applications in synthetic organic chemistry (Fig. 2b)31.
Fig. 2.
The transformation of alcohols. a Photo-induced alkylation of heteroarenes by using alcohols as alkylating reagent. b α sp3 C–H functionalization of alcohols
To enable selective functionalization of α sp3 C–H of alcohols to introduce the alcoholic hydroxyl group, enormous efforts have been made by chemists. For example, remarkable works on the cross-coupling between alcohols with unsaturated bonds such as alkenes, allenes and alkynes have been extensively reported32–40, providing effective routes for α sp3 C–H activation and functionalization of alcohols. Furthermore, oxidation-induced C–H functionalization as a powerful tool41–48, is successfully applied in the α sp3 C–H functionalization of alcohols49–58. Direct oxidative α sp3 C–H arylation by C–H/C–H cross-coupling to acquire the modified alcohols is undoubtedly the most step- and atom-economical method. It is worth noting that peroxide-mediated oxidative arylation of alcohols with different heterocycles predominates this topic59,60. Herein, we describe an oxidative α sp3 C–H arylation of alcohols with heterocycles promoted by selectfluor under visible light irradiation, which is selective for the α sp3 C–H of alcohols, even in the presence of ethers. The N–F activation of selectfluor by blue light emitting diodes (LEDs) irradiation is evidenced by EPR studies. The observed reactivity may have important implications for sp3 C–H functionalization.
Results
Exploration of reaction pathways
Selectfluor 1 is well-known as a powerful fluorination reagent and oxidant, frequently combined with a metal catalyst or photocatalyst in the organic synthesis61–68. The N–F breakage of selectfluor resorts to the immigration of external electron from a reductant. We questioned whether the visible light irradiation could induce the N–F activation of selectfluor to directly yield the corresponding N radical cation 2 and F radical 3 (Fig. 3a). The generated N radical cation 2 is responsible for the abstraction of α sp3 C–H of alcohol 4 to the hydroxyalkyl radical 6 (Fig. 3b). Afterward, the electron-deficient heteroarenes 7 protonated by acid can capture the relatively nucleophilic radical and deliver the corresponding radical adducts 8 (Fig. 3c). The oxidation and deprotonation of this radical adduct 8 by another selectfluor would then afford the α-arylated product 9 (Fig. 3d). The key difference between this oxidative α sp3 C–H arylation of alcohols and those reported photochemical alkylation of heteroarenes28–30, is the oxidation condition. Under the designed oxidation condition, the spin center shift process of the intermediate 8 can be avoided and the alcoholic hydroxyl group is unaffected, achieving the oxidative α sp3 C–H arylation of alcohols with heteroarenes.
Fig. 3.
The designed reaction pathways. a The N–F activation of selectfluor under blue LEDs irradiation. b The hydrogen-atom transfer (HAT) between generated N radical cation with alcohols to yield hydroxyalkyl radical. c The nucleophilic addition of hydroxyalkyl radical to electron-deficient heteroarenes. d The oxidative aromatization of the radical adducts to the final product
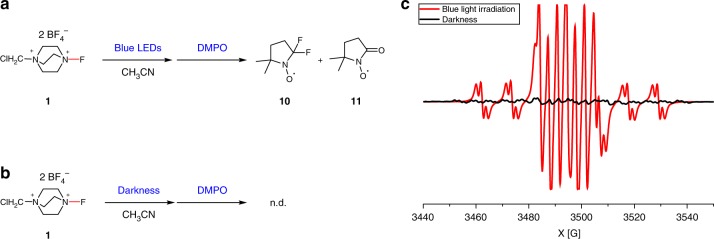
This assumption that the N–F activation of selectfluor could be achieved by blue light irradiation was evidenced by EPR experiments (see Supplementary Methods). Two kinds of radical signals were observed, when selectfluor in acetonitrile was irradiated by blue LEDs and 5,5-dimethyl-1-pyrroline N-oxide (DMPO) was employed as a radical scavenger. Fitting the EPR spectra on the basis of electron spin resonance parameters of spin adducts69, one of the radical 10 was confirmed as the radical adduct between two fluorine radical and DMPO, while the other one 11 was resulted from the oxidation of DMPO, whose ratio is 3:8 (Fig. 4a, c). In contrast, we did not detect the radical adduct between two fluorine radical and DMPO under the darkness (Fig. 4b, c).
Fig. 4.
The electron paramagnetic resonance (EPR) experiments. a The EPR experiment of selectfluor under blue light irradiation. b The EPR experiment of selectfluor under darkness. c The EPR spectra of selectfluor under blue light irradiation and darkness
Investigation of reaction conditions
With the mechanistic evidence in hand, we started our investigations with isoquinoline 12 and ethanol as the model substrates. We identified that using selectfluor as a visible light-activated oxidant irradiated by blue LEDs, in the presence of 1.5 equiv trifluoroacetic acid (TFA), the desired oxidative α sp3 C–H arylation product can be afforded in 87% yield (Table 1, entry 1). It is noteworthy that upon treatment of this reaction with green light irradiation or darkness, no product was detected (Table 1, entries 2 and 3). Even if the reaction system was heated to 80 °C under darkness, the conversion was still not promoted (Table 1, entry 4). These results might reveal that only the shorter wavelength visible light, which possesses the higher energy could achieve the N–F activation of selectfluor and then promote the oxidative α sp3 C–H arylation of alcohol, while the longer wavelength visible light and heating failed. In addition, the contemporary fluorination reagents N-fluorobenzenesulfonimide was also examined under the same condition with blue light irradiation. However, a poor reactivity was observed (Table 1, entry 5), probably resulted from the higher bond dissociation energy of N–F compared with selectfluor70. Although the common-used oxidants such as t-butylhydroperoxide (TBHP), potassium persulfate (K2S2O8), and (diacetoxyiodo)benzene (PhI(OAc)2) show efficient capacity for oxidative activation of sp3 C–H, the reaction still could not be promoted by utilizing these oxidants at 80 °C (Table 1, entries 6–8), implying the uniqueness of selectfluor under blue light irradiation for the oxidative α sp3 C–H arylation of alcohols.
Table 1.
Investigation of the reaction conditions.*
|
| ||||
|---|---|---|---|---|
| Entry | Oxidant | Light source | T (°C) | Yield (%)† |
| 1 | Selectfluor | Blue LEDs | 25 | 87 |
| 2 | Selectfluor | Green LEDs | 25 | N.D. |
| 3 | Selectfluor | Darkness | 25 | N.D |
| 4 | Selectfluor | Darkness | 80 | Trace |
| 5 | NFSI | Blue LEDs | 25 | Trace |
| 6 | TBHP | Darkness | 80 | N.D. |
| 7 | K2S2O8 | Darkness | 80 | N.D. |
| 8 | PhI(OAc)2 | Darkness | 80 | N.D. |
*Conditions: 12 (0.3 mmol), ethanol (1.5 mL), selectfluor (0.6 mmol), TFA (0.45 mmol), in CH3CN (2.0 mL) under a nitrogen atmosphere, irradiated with 3 W blue LEDs at 25 °C for 24 h; N.D. not detected
†Isolated yield
Substrate scope
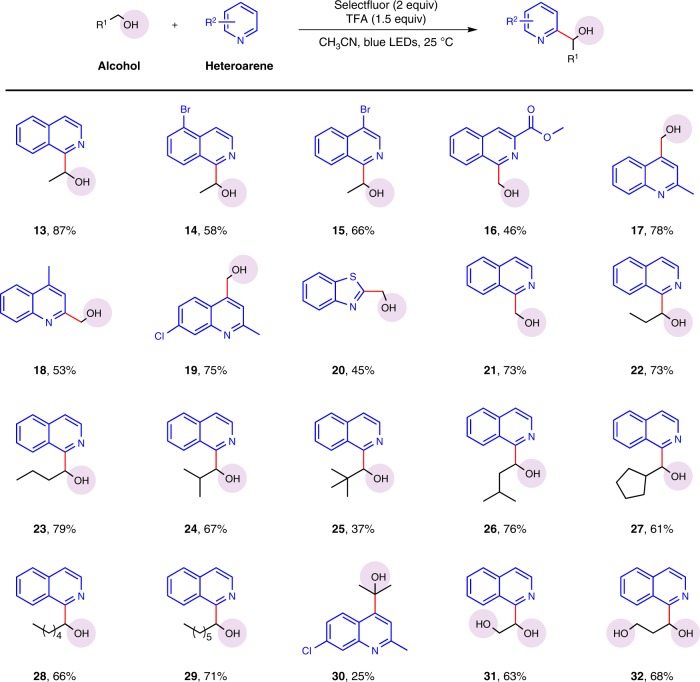
With the optimized conditions established, we hoped this method could be applied to other noble heteroaromatics (Fig. 5). Isoquinolines with halides and esters substituents are competent functionalization partners, successfully delivering the desired oxidative α sp3 C–H arylation products (14–16). It was found that the quinoline derivatives such as methyl and halides substituted quinolines performed good reactivities (17–19). Importantly, the addition of methanol to benzothiazole could be smoothly proceeded under the standard condition (20). Unfortunately, the reactivity of pyridine and pyrazine was poor under the same catalytic system. Subsequently, a variability of alcohols were examined in details. Methanol, n-propanol and n-butyl alcohol were effectively oxidized to corresponding nucleophilic radicals and reacted with isoquinoline in good to high yields under the photochemical condition (21–23). When the ethanols containing isopropyl, tert-butyl, isobutyl and cyclopentyl were tested, we still isolated the oxidative arylation products with moderate yields, in spite of the steric hindrance proximal to the α sp3 C–H of alcohols (24–27). It is worth noting that long-chain alkyl alcohols like n-hexanol and n-heptanol are also suitable for this protocol (28 and 29). Isopropanol could also be tolerated, even though a low yield was obtained (30). Notably, dioles were successfully tolerated, delivering the modified monoarylation dioles (31 and 32).
Fig. 5.
Substrate scope for the α sp3 C–H arylation of alcohols with heteroarenes. Reaction conditions: heteroarene (0.3 mmol), alcohol (see Supplementary Methods for details), selectfluor (0.6 mmol), TFA (0.45 mmol), in CH3CN (2.0 mL) (additional 0.75 mL DCE was added for 25) under a nitrogen atmosphere, irradiated with 3 W blue LEDs at 25 °C for 24 h
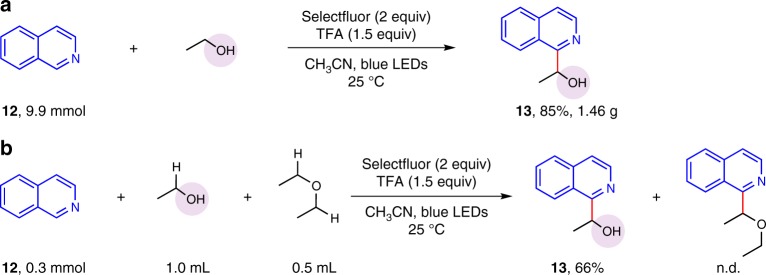
Additionally, the gram-scale synthesis experiment was carried out (see Supplementary Methods). A comparable yield 85% was obtained when the model reaction was performed in nearly 10 mmol scale, providing promising application in preparative synthesis (Fig. 6a). Then, an intermolecular competition experiment was carried out to explore the selectivity of this oxidative α sp3 C–H arylation of alcohols with heteroarenes (see Supplementary Methods). It is significant that the single selectivity and good yield for the oxidative α sp3 C–H arylation of alcohols in the presence of ether sp3 C–H were observed (Fig. 6b).
Fig. 6.
Investigation and application of this protocol. a Gram-scale synthesis experiment. b Intermolecular competition experiment
Discussion
To further understand this visible light-induced protocol, we conducted several mechanistic experiments (Fig. 7). While 2,2,6,6-tetramethylpiperidinooxy (TEMPO) as radical-trapping reagent was subjected to the standard reaction condition (see Supplementary Methods), the oxidative α sp3 C–H arylation of alcohols was totally suppressed, thus revealing a radical pathway might be involved (Fig. 7a). Whereafter, the intermolecular kinetic isotope effect (KIE) experiment was undertaken (see Supplementary Methods and Supplementary Fig. 41 for details). A KIE value of 2.2 indicated the cleavage of α sp3 C–H is the rate-determining step for this protocol (Fig. 7b). Importantly, the highly reactive N-oxide is not the reaction intermediate59, because no product was afforded while isoquinoline N-oxide 34 was employed (Fig. 7c).
Fig. 7.
Mechanistic studies. a Radical inhibition experiment. b Intermolecular kinetic isotope effect experiment. c The intermediates experiment
In summary, we have developed a visible light-induced oxidative α sp3 C–H arylation of alcohols with heteroarenes, which is promoted by selectfluor under the blue LEDs irradiation. What is essential for this protocol is the N–F activation of selectfluor achieved by blue light irradiation. The EPR study provided important evidence for the visible light-induced N–F activation of selectfluor. The selective oxidative α sp3 C–H arylation of alcohols with heteroarenes in the presence of ethers is demonstrated. The development of related oxidative C(sp3)–H functionalization is underway in our laboratory.
Methods
General procedure (13)
A solution of isoquinoline 12 (0.3 mmol, 1.0 equiv, 38.7 mg), 1.5 mL ethanol, selectfluor (0.6 mmol, 2.0 equiv, 212.5 mg) and TFA (0.45 mmol, 1.5 equiv, 51.3 mg) in degassed dry CH3CN (2.0 mL) were stirred under nitrogen atmosphere and irradiated by 3 W blue LEDs at 25 °C for 24 h. Afterwards, the reaction system was quenched by saturated NaHCO3 aqueous solution. The aqueous solution was extracted with ethyl acetate (3 × 10 mL) and the combined extracts were dried with anhydrous Na2SO4. The solvents were removed under reduced pressure by rotary evaporation. Then, the pure product was obtained by flash column chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 5:1), directly giving the desired product 13 in 87% yield as a pale yellow liquid. For 1H NMR and 13C NMR spectra of compounds 13–32 see Supplementary Figs. 1–40. Full experimental details can be found in the Supplementary Methods.
Supplementary information
Acknowledgments
This work was supported by the National Natural Science Foundation of China (21520102003) and the Hubei Province Natural Science Foundation of China (2017CFA010). The Program of Introducing Talents of Discipline to Universities of China (111 Program) is also appreciated.
Author contributions
L.N., J.L., X.-A.L. and S.W. performed and analyzed experiments. A.L. and L.N. conceived the project and designed the experiments. A.L. and L.N. wrote the manuscript. All the authors discussed the results and commented on the manuscript.
Data availability
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary information files.
Competing interests
The authors declare no competing interests.
Footnotes
Journal peer review information: Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at 10.1038/s41467-019-08413-9.
References
- 1.Liu C, et al. Palladium-catalyzed aerobic oxidative direct esterification of alcohols. Angew. Chem. Int. Ed. 2011;50:5144–5148. doi: 10.1002/anie.201008073. [DOI] [PubMed] [Google Scholar]
- 2.Wu XF. A general and efficient zinc-catalyzed oxidation of benzyl alcohols to aldehydes and esters. Chem. Eur. J. 2012;18:8912–8915. doi: 10.1002/chem.201201105. [DOI] [PubMed] [Google Scholar]
- 3.Jagadeesh RV, et al. Selective oxidation of alcohols to esters using heterogeneous Co3O4-N@C catalysts under mild conditions. J. Am. Chem. Soc. 2013;135:10776–10782. doi: 10.1021/ja403615c. [DOI] [PubMed] [Google Scholar]
- 4.Hie L, et al. Nickel-catalyzed esterification of aliphatic amides. Angew. Chem. Int. Ed. 2016;55:15129–15132. doi: 10.1002/anie.201607856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yi H, et al. Photocatalytic dehydrogenative cross-coupling of alkenes with alcohols or azoles without external oxidant. Angew. Chem. Int. Ed. 2017;56:1120–1124. doi: 10.1002/anie.201609274. [DOI] [PubMed] [Google Scholar]
- 6.Adam W, Prein M. Diastereoselective [4+2] cycloaddition of singlet oxygen in the photooxygenation of chiral naphthyl alcohols: evidence for a hydroxy group-directing effect. J. Am. Chem. Soc. 1993;115:3766–3767. doi: 10.1021/ja00062a049. [DOI] [Google Scholar]
- 7.Rodriguez-Berrios RR, Torres G, Prieto JA. Stereoselective VO(acac)2 catalyzed epoxidation of acyclic homoallylic diols. complementary preparation of C2-syn-3,4-epoxy alcohols. Tetrahedron. 2011;67:830–836. doi: 10.1016/j.tet.2010.11.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun J, et al. Copper-catalyzed hydroxyl-directed aminoarylation of alkynes. ACS Catal. 2016;6:3674–3678. doi: 10.1021/acscatal.6b00759. [DOI] [Google Scholar]
- 9.Asensio G, Aleman P, Gil J, Domingo LR, Medio-Simon M. Stereoselection parameters and theoretical model in the enantioselective protonation of enolates with α-sulfinyl alcohols. J. Org. Chem. 1998;63:9342–9347. doi: 10.1021/jo981294y. [DOI] [Google Scholar]
- 10.Lam K, Marko IE. Chemoselective chemical and electrochemical deprotections of aromatic esters. Org. Lett. 2009;11:2752–2755. doi: 10.1021/ol900828x. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Nakajima M, Martin R. Ni-catalyzed regioselective hydrocarboxylation of alkynes with CO2 by using simple alcohols as proton sources. J. Am. Chem. Soc. 2015;137:8924–8927. doi: 10.1021/jacs.5b05513. [DOI] [PubMed] [Google Scholar]
- 12.Wu. X, et al. Tertiary-alcohol-directed functionalization of remote C(sp3)-H bonds by sequential hydrogen atom and heteroaryl migrations. Angew. Angew. Chem. Int. Ed. 2018;57:1640–1644. doi: 10.1002/anie.201709025. [DOI] [PubMed] [Google Scholar]
- 13.Wu. X, et al. Metal-free alcohol-directed regioselective heteroarylation of remote unactivated C(sp3)-H bonds. Nat. Commun. 2018;9:3343. doi: 10.1038/s41467-018-05522-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu A, et al. δ-Selective functionalization of alkanols enabled by visible-light-induced ligand-to-metal charge transfer. J. Am. Chem. Soc. 2018;140:1612–1616. doi: 10.1021/jacs.7b13131. [DOI] [PubMed] [Google Scholar]
- 15.Zhu Y, et al. Silver-catalyzed remote Csp3-H functionalization of aliphatic alcohols. Nat. Commun. 2018;9:2625. doi: 10.1038/s41467-018-05014-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duncton MAJ. Minisci reactions: versatile CH-functionalizations for medicinal chemists. Med. Chem. Commun. 2011;2:1135. doi: 10.1039/c1md00134e. [DOI] [Google Scholar]
- 17.Wang FX, et al. Total synthesis of lycopodium alkaloids palhinine A and palhinine D. J. Am. Chem. Soc. 2017;139:4282–4285. doi: 10.1021/jacs.6b13401. [DOI] [PubMed] [Google Scholar]
- 18.Yoon TP, Ischay MA, Du JN. Visible light photocatalysis as a greener approach to photochemical synthesis. Nat. Chem. 2010;2:527–532. doi: 10.1038/nchem.687. [DOI] [PubMed] [Google Scholar]
- 19.Narayanam JM, Stephenson CR. Visible light photoredox catalysis: applications in organic synthesis. Chem. Soc. Rev. 2011;40:102–113. doi: 10.1039/B913880N. [DOI] [PubMed] [Google Scholar]
- 20.Xuan J, Xiao WJ. Visible-light photoredox catalysis. Angew. Chem. Int. Ed. 2012;51:6828–6838. doi: 10.1002/anie.201200223. [DOI] [PubMed] [Google Scholar]
- 21.Hari DP, König B. The photocatalyzed Meerwein arylation: classic reaction of aryl diazonium salts in a new light. Angew. Chem. Int. Ed. 2013;52:4734-–44743. doi: 10.1002/anie.201210276. [DOI] [PubMed] [Google Scholar]
- 22.Ohkubo K, Fujimoto A, Fukuzumi S. Visible-light-induced oxygenation of benzene by the triplet excited state of 2,3-dichloro-5,6-dicyano-p-benzoquinone. J. Am. Chem. Soc. 2013;135:5368–5371. doi: 10.1021/ja402303k. [DOI] [PubMed] [Google Scholar]
- 23.Prier CK, Rankic DA, MacMillan DW. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 2013;113:5322–5363. doi: 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin J, MacMillan DWC. Direct α-arylation of ethers through the combination of photoredox-mediated C–H functionalization and the Minisci reaction. Angew. Chem. Int. Ed. 2015;54:1565–1569. doi: 10.1002/anie.201410432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ravelli D, Protti S, Fagnoni M. Carbon-carbon bond forming reactions via photogenerated intermediates. Chem. Rev. 2016;116:9850–9913. doi: 10.1021/acs.chemrev.5b00662. [DOI] [PubMed] [Google Scholar]
- 26.Romero NA, Nicewicz DA. Organic photoredox catalysis. Chem. Rev. 2016;116:10075–10166. doi: 10.1021/acs.chemrev.6b00057. [DOI] [PubMed] [Google Scholar]
- 27.Jeffrey JL, Terrett JA, MacMillan DW. O–H bonding promotes H-atom transfer from α C–H bonds for C-alkylation of alcohols. Science. 2015;349:1532–1536. doi: 10.1126/science.aac8555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin J, MacMillan DWC. Alcohols as alkylating agents in heteroarene C–H functionalization. Nature. 2015;525:87–90. doi: 10.1038/nature14885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu WB, Yang XB, Zhou ZZ, Li CJ. Simple and clean photo-induced methylation of heteroarenes with MeOH. Chemistry. 2017;2:688–702. doi: 10.1016/j.chempr.2017.03.009. [DOI] [Google Scholar]
- 30.McCallum T, Pitre SP, Morin M, Scaiano JC, Barriault L. The photochemical alkylation and reduction of heteroarenes. Chem. Sci. 2017;8:7412–7418. doi: 10.1039/C7SC03768F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Twilton J, et al. Selective hydrogen atom abstraction through induced bond polarization: direct α-arylation of alcohols through photoredox, HAT, and nickel catalysis. Angew. Chem. Int. Ed. 2018;57:5369–5373. doi: 10.1002/anie.201800749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urry WH, Stacey FW, Huyser ES, Juveland OO. The peroxide- and light-induced additions of alcohols to olefins. J. Am. Chem. Soc. 1954;76:450–455. doi: 10.1021/ja01631a037. [DOI] [Google Scholar]
- 33.LaZerte JD, Koshar RJ. The free-radical catalyzed addition of alcohols and aldehydes to perfluoroölefins. J. Am. Chem. Soc. 1955;77:910–914. doi: 10.1021/ja01609a033. [DOI] [Google Scholar]
- 34.Shi L, et al. A reaction for sp3–sp3 C–C bond formation via cooperation of Lewis acid-promoted/Rh-catalyzed C–H bond activation. J. Am. Chem. Soc. 2005;127:10836–10837. doi: 10.1021/ja0528331. [DOI] [PubMed] [Google Scholar]
- 35.Kamitanaka T, et al. Direct addition of supercritical alcohols, acetone or acetonitrile to the alkenes without catalysts. Tetrahedron Lett. 2007;48:8460–8463. doi: 10.1016/j.tetlet.2007.09.159. [DOI] [Google Scholar]
- 36.Obora Y, Hatanaka S, Ishii Y. Iridium-catalyzed coupling reaction of primary alcohols with 1-aryl-1-propynes leading to secondary homoallylic alcohols. Org. Lett. 2009;11:3510–3513. doi: 10.1021/ol901366q. [DOI] [PubMed] [Google Scholar]
- 37.Zhang SY, Tu YQ, Fan CA, Zhang FM, Shi L. Iron-catalyzed C(sp3)–C(sp3) bond formation through C(sp3)–H functionalization: a cross-coupling reaction of alcohols with alkenes. Angew. Chem. Int. Ed. 2009;48:8761–8765. doi: 10.1002/anie.200903960. [DOI] [PubMed] [Google Scholar]
- 38.Liu ZQ, et al. Free-radical-initiated coupling reaction of alcohols and alkynes: not C–O but C–C bond formation. Org. Lett. 2009;11:1437–1439. doi: 10.1021/ol900145u. [DOI] [PubMed] [Google Scholar]
- 39.Zhang SY, Zhang FM, Tu YQ. Direct sp3 α-C–H activation and functionalization of alcohol and ether. Chem. Soc. Rev. 2011;40:1937–1949. doi: 10.1039/c0cs00063a. [DOI] [PubMed] [Google Scholar]
- 40.Kim SW, Zhang W, Krische MJ. Catalytic enantioselective carbonyl allylation and propargylation via alcohol-mediated hydrogen transfer: merging the chemistry of grignard and sabatier. Acc. Chem. Res. 2017;50:2371–2380. doi: 10.1021/acs.accounts.7b00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu C, Liu D, Lei A. Recent advances of transition-metal catalyzed radical oxidative cross-couplings. Acc. Chem. Res. 2014;47:3459–3470. doi: 10.1021/ar5002044. [DOI] [PubMed] [Google Scholar]
- 42.Jie X, Shang Y, Zhang X, Su W. Cu-catalyzed sequential dehydrogenation-conjugate addition for β-functionalization of saturated ketones: scope and mechanism. J. Am. Chem. Soc. 2016;138:5623–5633. doi: 10.1021/jacs.6b01337. [DOI] [PubMed] [Google Scholar]
- 43.Quint V, et al. Metal-free, visible light-photocatalyzed synthesis of benzo[b]phosphole oxides: synthetic and mechanistic investigations. J. Am. Chem. Soc. 2016;138:7436–7441. doi: 10.1021/jacs.6b04069. [DOI] [PubMed] [Google Scholar]
- 44.Singh KS, Sawant SG, Dixneuf PH. Ruthenium(II)-catalyzed synthesis of pyrrole- and indole-fused isocoumarins by C–H bond activation in DMF and water. ChemCatChem. 2016;8:1046–1050. doi: 10.1002/cctc.201501261. [DOI] [Google Scholar]
- 45.McCallum T, Jouanno LA, Cannillo A, Barriault L. Persulfate-enabled direct C–H alkylation of heteroarenes with unactivated ethers. Synlett. 2016;27:1282–1286. doi: 10.1055/s-0035-1561338. [DOI] [Google Scholar]
- 46.Fu N, Sauer GS, Lin S. Electrocatalytic radical dichlorination of alkenes with nucleophilic chlorine sources. J. Am. Chem. Soc. 2017;139:15548–15553. doi: 10.1021/jacs.7b09388. [DOI] [PubMed] [Google Scholar]
- 47.Li Y, et al. Copper-catalyzed alkoxycarbonylation of alkanes with alcohols. ChemSusChem. 2017;10:1341–1345. doi: 10.1002/cssc.201601587. [DOI] [PubMed] [Google Scholar]
- 48.Yi H, et al. Recent advances in radical C–H activation/radical cross-coupling. Chem. Rev. 2017;117:9016–9085. doi: 10.1021/acs.chemrev.6b00620. [DOI] [PubMed] [Google Scholar]
- 49.Cui Z, Shang X, Shao XF, Liu ZQ. Copper-catalyzed decarboxylative alkenylation of sp3 C–H bonds with cinnamic acids via a radical process. Chem. Sci. 2012;3:2853. doi: 10.1039/c2sc20712e. [DOI] [Google Scholar]
- 50.Liu Y, Jiang B, Zhang W, Xu Z. Multifold bond cleavage and formation between MeOH and quinoxalines (or benzothiazoles): synthesis of carbaldehyde dimethyl acetals. J. Org. Chem. 2013;78:966–980. doi: 10.1021/jo302450f. [DOI] [PubMed] [Google Scholar]
- 51.Meng Y, Guo LN, Wang H, Duan XH. Metal-free oxidative hydroxyalkylarylation of activated alkenes by direct sp3 C–H functionalization of alcohols. Chem. Commun. 2013;49:7540–7542. doi: 10.1039/c3cc44055a. [DOI] [PubMed] [Google Scholar]
- 52.Bohman B, Berntsson B, Dixon RC, Stewart CD, Barrow RA. Alkylations and hydroxymethylations of pyrazines via green Minisci-type reactions. Org. Lett. 2014;16:2787–2789. doi: 10.1021/ol500776j. [DOI] [PubMed] [Google Scholar]
- 53.Li Z, Fan F, Yang J, Liu ZQ. A free radical cascade cyclization of isocyanides with simple alkanes and alcohols. Org. Lett. 2014;16:3396–3399. doi: 10.1021/ol501461u. [DOI] [PubMed] [Google Scholar]
- 54.Zhou W, et al. Metal-free oxidative functionalization of C(sp3)–H bond adjacent to oxygen and radical addition to olefins. Org. Lett. 2015;17:1160–1163. doi: 10.1021/acs.orglett.5b00088. [DOI] [PubMed] [Google Scholar]
- 55.Neubert TD, Schmidt Y, Conroy E, Stamos D. Radical mediated C–H functionalization of 3,6-dichloropyridazine: efficient access to novel tetrahydropyridopyridazines. Org. Lett. 2015;17:2362–2365. doi: 10.1021/acs.orglett.5b00861. [DOI] [PubMed] [Google Scholar]
- 56.Cheng JK, Loh TP. Copper- and cobalt-catalyzed direct coupling of sp3 α-carbon of alcohols with alkenes and hydroperoxides. J. Am. Chem. Soc. 2015;137:42–45. doi: 10.1021/ja510635k. [DOI] [PubMed] [Google Scholar]
- 57.Xu Z, Hang Z, Liu ZQ. Free-radical triggered ordered domino reaction: an approach to C–C bond formation via selective functionalization of α-hydroxyl-(sp3)C–H in fluorinated alcohols. Org. Lett. 2016;18:4470–4473. doi: 10.1021/acs.orglett.6b01946. [DOI] [PubMed] [Google Scholar]
- 58.Guo Sr, Kumar PS, Yang M. Recent advances of oxidative radical cross-coupling reactions: direct α-C(sp3)–H bond functionalization of ethers and alcohols. Adv. Synth. Catal. 2017;359:2–25. doi: 10.1002/adsc.201600467. [DOI] [Google Scholar]
- 59.Correia CA, Yang L, Li CJ. Palladium-catalyzed Minisci reaction with simple alcohols. Org. Lett. 2011;13:4581–4583. doi: 10.1021/ol201774b. [DOI] [PubMed] [Google Scholar]
- 60.He T, Yu L, Zhang L, Wang L, Wang M. Direct C2-alkylation of azoles with alcohols and ethers through dehydrogenative cross-coupling under metal-free conditions. Org. Lett. 2011;13:5016–5019. doi: 10.1021/ol201779n. [DOI] [PubMed] [Google Scholar]
- 61.Yin F, Wang Z, Li Z, Li C. Silver-catalyzed decarboxylative fluorination of aliphatic carboxylic acids in aqueous solution. J. Am. Chem. Soc. 2012;134:10401–10404. doi: 10.1021/ja3048255. [DOI] [PubMed] [Google Scholar]
- 62.Xia JB, Zhu C, Chen C. Visible light-promoted metal-free C–H activation: diarylketone-catalyzed selective benzylic mono- and difluorination. J. Am. Chem. Soc. 2013;135:17494–17500. doi: 10.1021/ja410815u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pitts CR, et al. Direct, catalytic monofluorination of sp3 C–H bonds: a radical-based mechanism with ionic selectivity. J. Am. Chem. Soc. 2014;136:9780–9791. doi: 10.1021/ja505136j. [DOI] [PubMed] [Google Scholar]
- 64.Lin Y, Zhu L, Lan Y, Rao Y. Development of a rhodium(II)-catalyzed chemoselective C(sp3)–H oxygenation. Chem. Eur. J. 2015;21:14937–14942. doi: 10.1002/chem.201502140. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Q, Yin XS, Chen K, Zhang SQ, Shi BF. Stereoselective synthesis of chiral β-fluoro α-amino acids via Pd(II)-catalyzed fluorination of unactivated methylene C(sp3)–H bonds: scope and mechanistic studies. J. Am. Chem. Soc. 2015;137:8219–8226. doi: 10.1021/jacs.5b03989. [DOI] [PubMed] [Google Scholar]
- 66.Bume DD, et al. Ketones as directing groups in photocatalytic sp3 C–H fluorination. Chem. Sci. 2017;8:6918–6923. doi: 10.1039/C7SC02703F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xiang M, Xin ZK, Chen B, Tung CH, Wu LZ. Exploring the reducing ability of organic dye (Acr+-Mes) for fluorination and oxidation of benzylic C(sp3)–H bonds under visible light irradiation. Org. Lett. 2017;19:3009–3012. doi: 10.1021/acs.orglett.7b01270. [DOI] [PubMed] [Google Scholar]
- 68.Xie LY, et al. Selectfluor-mediated regioselective nucleophilic functionalization of N-heterocycles under metal- and base-free conditions. Green Chem. 2018;20:760–764. doi: 10.1039/C7GC03106H. [DOI] [Google Scholar]
- 69.Buettner GR. Spin Trapping: ESR parameters of spin adducts 1474 1528V. Free Radic. Bio. Med. 1987;3:259–303. doi: 10.1016/S0891-5849(87)80033-3. [DOI] [PubMed] [Google Scholar]
- 70.Rueda-Becerril M, et al. Fluorine transfer to alkyl radicals. J. Am. Chem. Soc. 2012;134:4026–4029. doi: 10.1021/ja211679v. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary information files.