Abstract
The status anxiety hypothesis proposes that systematic inflammation as a consequence of chronic psycho-social stress is a possible pathway linking socio-economic position (SEP) to premature ageing and is a possible explanation for cross-national variation in patterns of health and well-being. Harmonised data from the LIFEPATH consortium on 18,349 individuals aged 50 to 75 and 30,632 observations are used to measure variation in the association between inflammation measured as C-reactive protein and SEP across four countries (Britain, Ireland, Portugal and Switzerland) and five studies (ELSA, Whitehall II, TILDA, EPIPorto and SKIPOGH). Adjusting for population composition, mean concentrations of CRP are highest in Portugal, the country with the highest income inequality and lowest in Switzerland, a lower income inequality country. Across all of the studies, lower SEP groups have higher mean concentrations of CRP and, as predicted by the theory, absolute differentials between SEP groups reflect the pattern of societal income inequality. Adjustment for lifestyle indicators reduces SEP differentials by between 45% and 52% but cannot account for country variation in mean inflammation.
Introduction
The ‘inverse health gradient’ between socioeconomic position (henceforce SEP), health and life expectancy is now a well-established fact across all societies where evidence has been collected1. Irrespective of the measure of SEP used (income, education, social class or status), socially and economically disadvantaged individuals have a shorter life expectancy and worse health during life2. The gradient in health emerges early in childhood and gets steeper with age3,4.
SEP determines the level of resources available to individuals and households, influencing biological function via the material environment, e.g. the location and quality of housing and exposure to pollutants, damp and microbial load5 as well as health behaviours6 but it is becoming increasingly clear that SEP may also shape biological health via neurological and hormonal pathways7. Under this paradigm, chronic psycho-social stress caused by economic strain8, uncertainty and lack of control9 or threats to social status and social threat10–12 are purported to cause SEP inequalities in health either directly or indirectly via adverse health behaviours. The direct pathway occurs through activation of glucocorticoid and adrenergic signalling pathways13,14 that, amongst other consequences, result in a chronic inflammatory response. There are now a number of studies which provide evidence of an association between SEP and systematic inflammation in adulthood15–19. A possible physiological process mediating the association between chronic psycho-stress and inflammation has been shown by Tawakol et al.20. Raised amygdala activation increases haemopoietic activity and arterial inflammation leading to an elevated risk of cardiovascular events. The same underlying process has been shown to contribute to the risk of other diseases such as type 2 diabetes21 and cancer22.
The emerging link between psycho-social stress and disease has also been put forward as an explanation for the pattern of cross-national variation in health and well-being23–26. Proponents of this hypothesis, known as the status anxiety hypothesis, argue that, irrespective of national wealth, all developed societies exhibit a gradient in levels of morbidity and mortality by SEP rather than a step-function between advantaged and disadvantaged. Absolute variation in morbidity and mortality across societies is more strongly correlated with level of income inequality than to gross domestic product per capita; among rich developed countries, differences in income and wealth matter less than how they are distributed across the population. Proponents of the status anxiety hypothesis put forward three linked propositions. First, that human social psychology was shaped by evolution which occurred almost entirely within hunter-gather societies with limited inequalities in material standard of living and social status27. Second, that income inequality is one measure of a society’s hierarchy of social status and third, that more inequality in the distribution of income and other scarce resources28, leads to increased psycho-social stress at the individual level at all points in the SEP distribution with consequences for health and well-being. If true, this hypothesis would imply that the differential between low and high SEP in status anxiety, psycho-social stress and inflammation will be positive with income inequality. Another implication is that everyone experiences higher psycho-social stress and inflammatory response in more unequal societies, not just those in the lowest SEP.
The extent of perceived status anxiety has been shown to vary significantly across European countries by SEP28,29 and more unequal countries (as measured by income inequality) have larger differentials in perceived status anxiety by SEP29,30 and worse mental health30. Moreover, more unequal countries have higher status anxiety overall. The highest SEP groups in more unequal societies have similar levels of status anxiety to the lowest SEP groups in more equal societies29. However, to date, there have been no studies comparing SEP differentials in inflammation across countries which vary by level of income inequality. If the status anxiety hypothesis is correct, higher income inequality should be associated with larger social differentials in inflammation between SEP and a higher mean level of inflammation. Alternative hypotheses for the association between income inequality and health have been proposed which would not implicate psycho-social stress and inflammation30–32 but evidence of social structuring of inflammation by SEP and country inequality would be supportive of the status anxiety hypothesis.
However, average levels of inflammation could also vary within and between societies as a function of health behaviours. Chronic psycho-social stress has been shown to promote behaviours with adverse effects on health such as smoking, poor diet and excess alcohol consumption9 and these variables have been shown to be a mediator between SEP and inflammation16,17,19,33.
In this paper, we examine the prediction of the status anxiety hypothesis that countries with higher income inequality will have larger differentials in inflammation by SEP as well as higher average levels of inflammation in the population overall. Ideally, we would use individual level data with information on SEP, perceived status anxiety, inflammatory markers and lifestyle indicators for a large number of countries which differ in terms of income inequality, preferably measured with repeated observations over time. Unfortunately, such data are not available at present; instead, we draw on harmonised data on SEP (European Socio-economic Classification - EsEC), inflammation (C-reactive protein concentration – henceforth CRP) and lifestyle factors (smoking, body-mass index, diabetes & hypertension) from the LIFEPATH Project, an EC Horizon 2020 consortium, to carry out a more limited examination of the hypothesis in four European countries: Britain, Ireland, Portugal and Switzerland. Figure 1 shows that these countries have consistently had different levels of income inequality with Portugal having the highest average income inequality (measured using the GINI coefficient) at 34.5, followed by the United Kingdom at 33.2, Ireland at 30 and Switzerland at 29.6. These country differences in levels of income inequality are relatively muted in comparison to other nations. For example, the USA has a GINI coefficient of 45 compared to Sweden at 24.9. This would imply that our test of the status anxiety hypothesis is relatively conservative and more likely to produce a null-finding compared a test using countries which vary more in their levels of income inequality.
Figure 1.
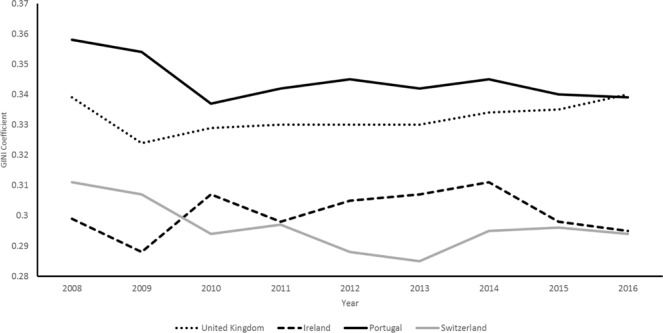
GINI Coefficient (Income Inequality) by Year and Country.
Data from The English Longitudinal Study of Ageing34 (ELSA) and the Whitehall II Study35 are used for the UK, The Irish Longitudinal Study of Ageing36 (TILDA) is used for Ireland, The Swiss Kidney Project on Genes and Hypertension37 (SKIPOGH) for Switzerland and the EPIPorto Study38 for Portugal.
Results
Of the 34262 individuals who participated across the five studies, varying proportions contributed blood samples for the collection of CRP. This proportion varied from 39% in EPIPorto to 77% in ELSA. To provide comparative samples across studies, individuals aged between 50 and 75 were selected, reducing the sample to 19,018 individuals overall with at least one CRP sample. Given our focus on chronic inflammation, participants with CRP levels higher than 10 mg/L were excluded from the analysis sample as this could indicate the presence of an acute illness, reducing the sample to 18,349 individuals. Inclusion of multiple observations per person for some samples (Whitehall, SKIPOGH, ELSA) provides 30,632 observations overall. Table 1 provides the distribution of individual characteristics for the sample of individuals by study. The distribution of SEP indicators across countries has important implications for the distribution of lifestyle factors and the patterning of CRP. Three of the samples for analysis (SKIPOGH, TILDA and ELSA) have approximately equal proportions of men and women whereas the EPIPorto sample is 64% female and Whitehall II 69% male. EPIPorto has a higher proportion of the lowest social class (56%) and education groups (72%) compared to the other studies which may lead to higher overall levels of inflammation. The Whitehall II study population is more advantaged in its socio-economic profile. The distribution of lifestyle indicators vary widely across countries: 33% of the TILDA sample are measured as obese compared to just over 18% in Whitehall II and 17% in SKIPOGH. To ensure that the samples are compared on a common basis, sample weights are used to create equal sample distributions for each of the studies by sex, age group and SEP. Distributions of individuals by sex, age and SEP are thus identical across samples in analyses.
Table 1.
Final Sample Characteristics by Study (of individuals).
| EPIPorto | Whitehall | SKIPOGH | TILDA | ELSA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N Individuals | 817 | 7,175 | 487 | 4,570 | 5,300 | |||||
| N Observations | 817 | 13,733 | 813 | 4,570 | 10,699 | |||||
| N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | |
| Socioeconomic Position | ||||||||||
| Most Recent Social Class | ||||||||||
| High Class | 146 | 17.9 | 3,832 | 53.4 | 112 | 23.0 | 1,549 | 33.9 | 1,341 | 25.3 |
| Med. Class | 211 | 25.8 | 2,191 | 30.5 | 169 | 34.7 | 1,105 | 24.2 | 2,259 | 42.6 |
| Low Class | 460 | 56.3 | 1,152 | 16.1 | 206 | 42.3 | 1,916 | 41.9 | 1,700 | 32.1 |
| Highest Education | ||||||||||
| High Education | 139 | 17.0 | 2,449 | 34.3 | 87 | 17.9 | 785 | 17.2 | 798 | 15.3 |
| Med. Education | 91 | 11.1 | 1,919 | 26.9 | 145 | 29.8 | 1,676 | 36.7 | 1,180 | 22.6 |
| Low Education | 587 | 71.9 | 2,764 | 38.8 | 255 | 52.4 | 2,109 | 46.2 | 3,243 | 62.1 |
| Demographics | ||||||||||
| Sex | ||||||||||
| Men | 295 | 36.1 | 4,974 | 69.3 | 221 | 45.4 | 2,148 | 47.0 | 2,445 | 46.1 |
| Women | 522 | 63.9 | 2,201 | 30.7 | 266 | 54.6 | 2,422 | 53.0 | 2,855 | 53.9 |
| Age Groups | ||||||||||
| Age 50–54 | 204 | 25.0 | 2,714 | 37.8 | 135 | 27.7 | 1,071 | 23.4 | 669 | 12.6 |
| Age 55–59 | 185 | 22.6 | 2,409 | 33.6 | 102 | 20.9 | 1,124 | 24.6 | 1,493 | 28.2 |
| Age 60–64 | 162 | 19.8 | 1,213 | 16.9 | 78 | 16.0 | 949 | 20.8 | 1,100 | 20.8 |
| Age 65–69 | 142 | 17.4 | 662 | 9.2 | 95 | 19.5 | 789 | 17.3 | 1,076 | 20.3 |
| Age 70–74 | 124 | 15.2 | 177 | 2.5 | 77 | 15.8 | 637 | 13.9 | 962 | 18.2 |
| Lifestyle Indicators | ||||||||||
| Body Mass Index | ||||||||||
| Healthy Weight | 227 | 27.9 | 2,463 | 35.8 | 211 | 43.3 | 1,006 | 22.1 | 1,309 | 26.3 |
| Overweight | 380 | 46.7 | 3,193 | 46.4 | 195 | 40.0 | 2,049 | 44.9 | 2,258 | 45.3 |
| Obese | 207 | 25.4 | 1,220 | 17.7 | 81 | 16.6 | 1,507 | 33.0 | 1,414 | 28.4 |
| Diabetes | ||||||||||
| No | 723 | 88.5 | 6,761 | 95.3 | 471 | 96.7 | 4,284 | 93.7 | 4,313 | 94.2 |
| Yes | 94 | 11.5 | 337 | 4.8 | 16 | 3.3 | 286 | 6.3 | 268 | 5.9 |
| Tobacco Consumption | ||||||||||
| Never Smoker | 531 | 65.2 | 3,237 | 47.7 | 209 | 42.9 | 2,081 | 45.5 | 2,208 | 41.7 |
| Past Smoker | 186 | 22.9 | 2,638 | 38.9 | 194 | 39.8 | 1,747 | 38.2 | 2,296 | 43.3 |
| Current Smoker | 97 | 11.9 | 913 | 13.5 | 84 | 17.3 | 742 | 16.2 | 794 | 15.0 |
| Hypertension | ||||||||||
| No | 340 | 41.6 | 5,625 | 78.5 | 404 | 83.0 | 4,262 | 93.3 | 3,196 | 62.8 |
| Yes | 477 | 58.4 | 1,538 | 21.5 | 83 | 17.0 | 308 | 6.7 | 1,891 | 37.2 |
Descriptive Differentials in CRP Concentration
Using weights to adjust for sample composition across studies, mean observed CRP concentration is significantly higher for low SEP compared to high SEP in all studies and across the age range, although the pattern is less distinct for SKIPOGH (Table 2), for the contrast between medium SEP and high and when education is the SEP measure. Being overweight and particularly being defined as obese, is associated with a significantly higher CRP than among those who are healthy weight. The absolute increase in CRP with obesity is higher than when the individual is overweight. The pattern of increases in CRP with indications for diabetes and hypertension are more mixed with consistent, significant differences in Whitehall II and ELSA but not in the other studies. Being a current daily smoker is associated with a significantly higher mean CRP, although not in EPIPorto. Women have higher mean CRP at all ages and across studies.
Table 2.
Observed (Unweighted) Mean Deviation in CRP Concentration (mg/L) by Characteristic, Study and Age Group.
| Characteristic | Study | Age Group | ||||
|---|---|---|---|---|---|---|
| 50–54 | 55–59 | 60–64 | 65–69 | 70–75 | ||
| Social Class Low v Social Class High |
EPIPorto | −0.01 | 0.54 | 0.70 | 0.96 | 0.63 |
| Whitehall | 0.58 | 0.62 | 0.71 | 0.60 | 1.02 | |
| SKIPOGH | 0.02 | 0.75 | −0.14 | 0.55 | −0.18 | |
| TILDA | 0.33 | 0.45 | 0.28 | 0.30 | 0.55 | |
| ELSA | 0.42 | 0.59 | 0.63 | 0.53 | 0.28 | |
| Social Class Medium v Social Class High |
EPIPorto | −0.24 | 0.88 | −0.14 | 0.05 | 0.54 |
| Whitehall | 0.29 | 0.30 | 0.36 | 0.60 | 0.66 | |
| SKIPOGH | 0.24 | 0.37 | −0.20 | 0.77 | 0.11 | |
| TILDA | 0.22 | 0.14 | 0.12 | −0.05 | 0.69 | |
| ELSA | −0.16 | 0.31 | 0.16 | 0.32 | 0.09 | |
| Education Low v Highest Education High |
EPIPorto | −0.03 | 0.36 | 1.00 | 1.54 | 0.68 |
| Whitehall | 0.26 | 0.33 | 0.36 | 0.38 | 0.50 | |
| SKIPOGH | 0.92 | 0.76 | 0.29 | 0.37 | 0.01 | |
| TILDA | 0.27 | 0.71 | 0.51 | 0.08 | 0.68 | |
| ELSA | 0.63 | 0.56 | 0.79 | 0.69 | 0.42 | |
| Highest Education Medium v Highest Education High |
EPIPorto | −0.83 | 0.79 | 0.67 | 0.60 | 1.01 |
| Whitehall | 0.12 | 0.11 | 0.15 | 0.23 | 0.00 | |
| SKIPOGH | 0.44 | −0.08 | 0.25 | 0.38 | 0.34 | |
| TILDA | 0.25 | 0.21 | 0.42 | −0.10 | 0.39 | |
| ELSA | 0.41 | 0.24 | 0.56 | 0.48 | 0.23 | |
| BMI Overweight v Healthy Weight |
EPIPorto | 0.49 | 0.56 | −0.03 | 0.07 | 0.38 |
| Whitehall | 0.56 | 0.65 | 0.66 | 0.66 | 0.63 | |
| SKIPOGH | 0.50 | 0.66 | 0.39 | 0.36 | 0.99 | |
| TILDA | 0.49 | 0.54 | 0.15 | 0.47 | 0.05 | |
| ELSA | 0.58 | 0.61 | 0.63 | 0.39 | 0.27 | |
| BMI Obese v Healthy Weight |
EPIPorto | 1.12 | 1.83 | 0.64 | 0.59 | 0.59 |
| Whitehall | 1.64 | 1.62 | 1.63 | 1.63 | 1.56 | |
| SKIPOGH | 1.69 | 2.24 | 1.99 | 1.88 | 1.53 | |
| TILDA | 1.49 | 1.47 | 1.08 | 1.32 | 0.76 | |
| ELSA | 1.68 | 1.53 | 1.57 | 1.52 | 1.11 | |
| Diabetes Indication v No Diabetes Ind. |
EPIPorto | 1.07 | 0.61 | −0.24 | −0.03 | 0.14 |
| Whitehall | 1.13 | 1.01 | 0.79 | 0.39 | 0.98 | |
| SKIPOGH | 1.29 | 0.62 | 0.88 | 1.78 | 0.02 | |
| TILDA | 1.06 | 0.34 | 0.40 | 0.06 | 0.04 | |
| ELSA | 0.84 | 0.35 | 1.23 | −0.07 | −0.16 | |
| Hypertension Indicated v No Hypertension Indicated |
EPIPorto | 0.69 | 0.84 | 0.59 | −0.20 | 0.22 |
| Whitehall | 0.37 | 0.44 | 0.38 | 0.35 | 0.43 | |
| SKIPOGH | 0.22 | −0.10 | 0.18 | 0.12 | 0.16 | |
| TILDA | 0.61 | 0.16 | −0.14 | −0.10 | −0.29 | |
| ELSA | 0.09 | 0.36 | 0.50 | 0.20 | 0.32 | |
| Smoked but Quit | EPIPorto | −0.54 | −0.62 | −0.49 | −1.30 | −0.73 |
| Whitehall | 0.07 | 0.04 | 0.09 | −0.04 | 0.05 | |
| SKIPOGH | −0.29 | 0.06 | −0.25 | 0.44 | 0.11 | |
| TILDA | 0.23 | −0.03 | 0.10 | 0.38 | 0.05 | |
| ELSA | −0.12 | −0.04 | 0.04 | 0.10 | 0.08 | |
| Daily Smoker | EPIPorto | 0.18 | 0.87 | 0.41 | −0.48 | 1.06 |
| Whitehall | 0.76 | 0.67 | 1.01 | 0.87 | 1.78 | |
| SKIPOGH | −0.22 | −0.54 | 0.15 | 0.77 | 0.06 | |
| TILDA | 0.61 | 0.53 | 0.75 | 0.67 | 0.21 | |
| ELSA EPIPorto |
0.69 | 0.60 | 0.85 | 0.84 | 0.49 | |
| Female v Male | 0.62 | 0.46 | 0.39 | 0.89 | 1.04 | |
| Whitehall | 0.35 | 0.42 | 0.54 | 0.56 | 0.99 | |
| SKIPOGH | 0.56 | −0.09 | 0.15 | 0.10 | 0.34 | |
| TILDA | 0.35 | 0.25 | 0.12 | −0.09 | 0.33 | |
| ELSA | 0.27 | 0.18 | 0.24 | 0.20 | 0.33 | |
Key: Bold Alone - P < 0.05; Italic Alone - P < 0.01; Bold & Italic: P < 0.001.
Predicted Country Differentials in CRP Concentration by Study
Using weights to adjust for sample composition across studies (Fig. 2), predicted mean concentrations of CRP are highest in EPIPorto (2.42 mg/L: 95% CI 2.24–2.59) and ELSA (2.42 mg/L: 95% CI 2.37–2.47) and lowest in SKIPOGH (1.87 mg/L: 95% CI 1.71–2.04) with the lower mean concentrations for Whitehall II and SKIPOGH significantly lower than those for EPIPorto and ELSA (P < 0.001). Between study variations in absolute mean CRP concentration falls with age and all mean differences in CRP between studies become insignificant by age 70. Confidence intervals for mean CRP concentration for EPIPorto, ELSA and TILDA overlap, but the pattern of study differences in mean CRP is broadly in line with the hypothesis that the country with the highest overall income inequality (Portugal/EPIPorto) will have higher average levels of inflammation compared to the country with the lowest (Switzerland/SKIPOGH).
Figure 2.
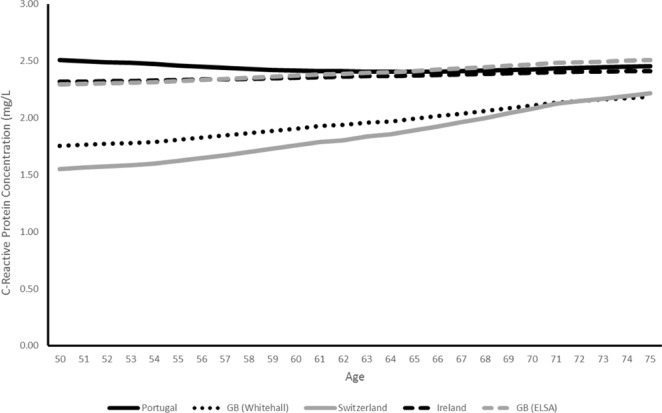
Predicted Overall CRP Concentrations by Country and Age Trajectory, (mg/L).
Predicted Absolute Differentials in CRP Concentration
Table 3, Panel A provides the estimated CRP concentrations (mg/L) by SEP obtained from the weighted generalized linear models with terms for age, sex, study and interactions of study, SEP and sex with polynomials of age. These confirm that across the studies, the low and middle SEP have significantly higher concentrations of CRP compared to the high SEP in all studies. Absolute differentials between low SEP and high SEP are largest in EPIPorto (0.52 mg/L: 95% CI 0.37–0.68) and lowest in SKIPOGH (0.27 mg/L: 95% CI 0.23–0.3).
Table 3.
Predicted C-reactive (mg/L) levels by study and SEP.
| A: Unadjusted | B: Adjusted | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | P Value | % Increase | Mean | 95% CI | P Value | % Increase | |||
| EPIPorto | ||||||||||
| SEP High | 2.10 | 1.72 | 2.47 | Ref. | 1.43 | 1.18 | 1.67 | Ref. | ||
| SEP Medium | 2.44 | 2.14 | 2.74 | <0.001 | 16% | 1.59 | 1.38 | 1.79 | <0.001 | 11% |
| SEP Low | 2.62 | 2.40 | 2.84 | <0.001 | 25% | 1.63 | 1.47 | 1.79 | <0.001 | 14% |
| Whitehall II | ||||||||||
| SEP High | 1.72 | 1.58 | 1.86 | Ref. | 1.24 | 1.15 | 1.34 | Ref. | ||
| SEP Medium | 2.11 | 2.01 | 2.22 | <0.001 | 23% | 1.42 | 1.33 | 1.50 | 0.055 | 14% |
| SEP Low | 2.24 | 2.09 | 2.38 | <0.001 | 30% | 1.46 | 1.33 | 1.59 | 0.004 | 17% |
| SKIPOGH | ||||||||||
| SEP High | 1.64 | 1.37 | 1.90 | Ref. | 1.24 | 1.03 | 1.45 | Ref. | ||
| SEP Medium | 1.91 | 1.63 | 2.18 | <0.001 | 17% | 1.37 | 1.19 | 1.56 | <0.001 | 11% |
| SEP Low | 1.90 | 1.67 | 2.14 | <0.001 | 16% | 1.29 | 1.13 | 1.45 | <0.001 | 4% |
| TILDA | ||||||||||
| SEP High | 2.16 | 2.06 | 2.25 | Ref. | 1.40 | 1.32 | 1.48 | Ref. | ||
| SEP Medium | 2.34 | 2.23 | 2.45 | <0.001 | 9% | 1.51 | 1.41 | 1.60 | <0.001 | 8% |
| SEP Low | 2.57 | 2.48 | 2.67 | <0.001 | 19% | 1.54 | 1.46 | 1.63 | <0.001 | 10% |
| ELSA | ||||||||||
| SEP High | 2.17 | 2.07 | 2.27 | Ref. | 1.41 | 1.33 | 1.50 | Ref. | ||
| SEP Medium | 2.32 | 2.24 | 2.39 | <0.001 | 7% | 1.49 | 1.41 | 1.56 | <0.001 | 5% |
| SEP Low | 2.62 | 2.52 | 2.71 | <0.001 | 21% | 1.59 | 1.50 | 1.68 | <0.001 | 12% |
Predicted values adjust for sex, age, age2 using study specific generalized linear models. Panel B adjusts additionally for smoking, indications of hypertension, BMI and indications of diabetes.
Predicted SEP differentials by age vary across studies (Fig. 3a,b). In all studies except EPIPorto, absolute differentials between low and high SEP groups fall with age and lowest in TILDA then SKIPOGH from age 60. The low-high SEP differential for EPIPorto on the other hand increases steeply with age from 0.14 mg/L at 50 (95% CI: 0 to 1.39) to 1 mg/L at 75 (95% CI: 0 to 2.76) although wide confidence intervals for the SEP differential in EPIPorto mean that the pattern could logically also be decreasing rather than increasing.
Figure 3.
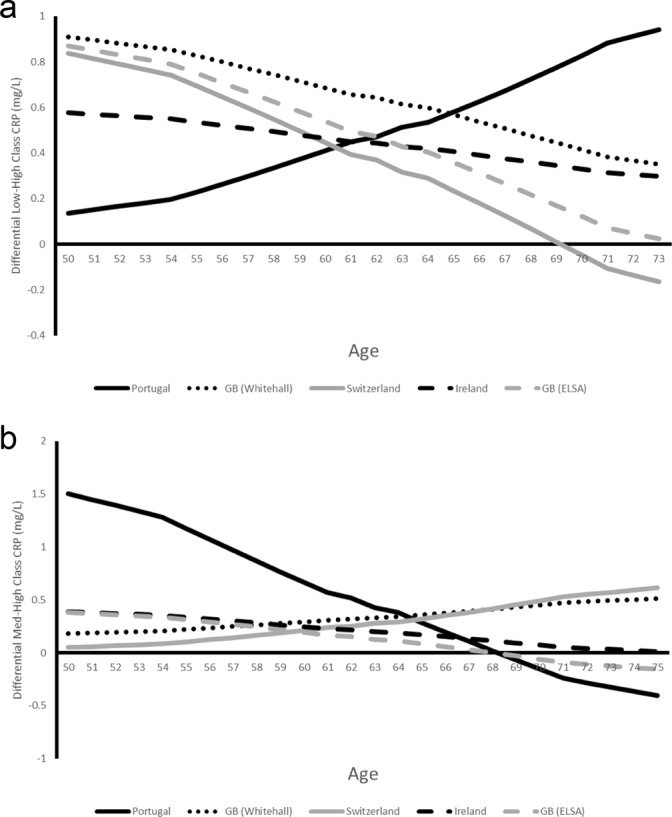
(a) Predicted Absolute Differentials (Low SEP – High SEP) in CRP Concentration (mg/L) by Country and Age. (b) Predicted Absolute Differentials (Middle SEP – High SEP) in CRP Concentration (mg/L) by Country and Age.
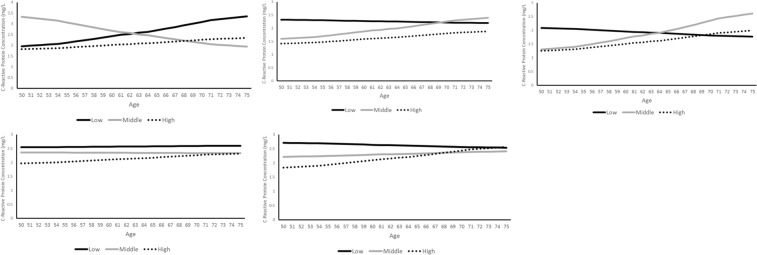
Figure 4a–e show the predicted age trajectories of CRP by SEP for the five studies and illustrate why the SEP differential with age is different for EPIPorto. In that study, CRP rises for both low and high SEP, but the increase is steeper for the low SEP group.
Figure 4.
(a) Predicted CRP Concentration by Class and Age Trajectory, (mg/L) Portugal. (b) Predicted CRP Concentration by Class and Age Trajectory, (mg/L) GB (Whitehall II). (c) Predicted CRP Concentration by Class and Age Trajectory, (mg/L) Switzerland. (d) Predicted CRP Concentration by Class and Age Trajectory, (mg/L) Ireland. (e) Predicted CRP Concentration by Class and Age Trajectory, (mg/L) GB (ELSA).
Relative SEP Differentials in CRP Concentration
Relative SEP differentials (low/high SEP - Table 3, Panel A) are lowest in SKIPOGH (116%: 95% CI 111–118%) and highest in Whitehall II (130%: 95% CI 122–124%) and EPIPorto (125%: 95% CI 113–128%). The pattern of relative SEP differentials by age largely mirrors the pattern of absolute differentials by age (Fig. 5a,b) with decreases across all studies except EPIPorto, although, wide confidence intervals make interpretation problematic.
Figure 5.
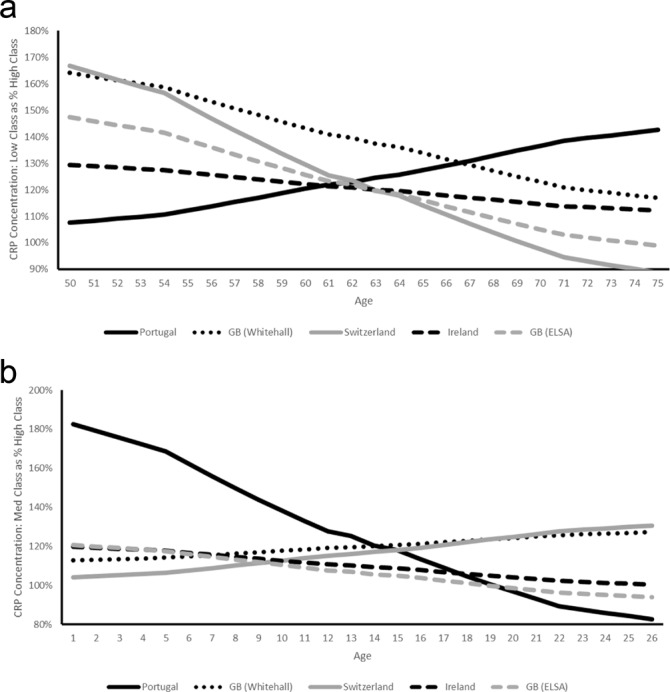
(a) Predicted Relative Differentials (Low SEP - High SEP) in CRP Concentration (mg/L) by Country and Age. (b) Predicted Relative Differentials (Middle SEP - High SEP) in CRP Concentration (mg/L) by Country and Age.
SEP Differentials in CRP Concentration Adjusting for Lifestyle Factors
To examine the extent to which study and SEP differentials can be accounted for by differences in lifestyle behaviours across samples and groups, generalised linear models were estimated adjusting for the base model and lifestyle factors (Table 3, Panel B). With adjustment, both absolute and relative differentials in CRP between low, middle and high SEP groups fall but the differentials between low/middle and high SEP groups remains significant. The relative differential between low and high SEP groups after adjustment for lifestyle factors is lowest in SKIPOGH (104%: 95% CI 0% to 109%) and highest in Whitehall II (117%: 95% CI 114% to 116%).
Figure 6 gives the reduction in relative differential between low and high SEP groups by the addition of lifestyle variables for each study. Single adjustment for BMI produces the largest reduction in differential (between 31% and 37% across studies). Adjusting for all of the lifestyle factors simultaneously decreases the low – high SEP differential by between 45% (EPIPorto) and 52% (SKIPOGH).
Figure 6.
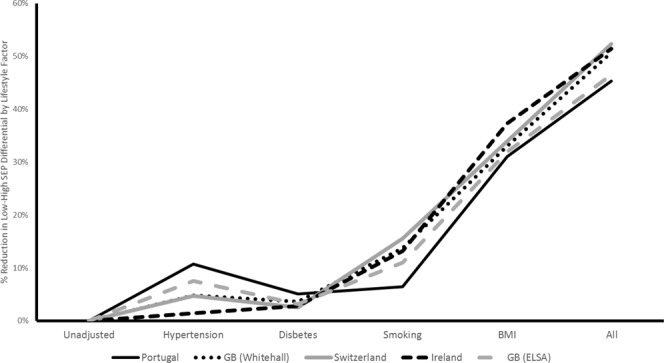
Predicted Reduction in Low/High SEP Differential by Adjustment of Lifestyle Factors by Country.
Discussion
This paper provides new insight into the validity of the status anxiety hypothesis using biological data on inflammation measured as C-reactive protein. The hypothesis proposes that variation in income inequality between societies explains cross-national variation in differentials in health between SEP groups as well as at least some cross-national variation in population health. According to the hypothesis, societies which have larger social status differentials or which put more emphasis on social status will induce higher average levels of psycho-social stress with damaging biological consequences through activation of glucocorticoid and adrenergic signalling pathways13,14 that result in a chronic inflammatory response. Using data from five large cohort studies from four European countries we tested whether countries with higher income inequality will have higher average levels of inflammation and greater differentials in inflammation by SEP.
Overall, our findings support the predictions of the hypothesis: after constraining the samples to have the same age, sex and SEP distributions, mean concentrations of CRP are highest in Portugal, the country with the highest income inequality and lowest in Switzerland, the lower income inequality country, although country differences reduce with age and are not significant over the age of 70. Across all of the studies, lower SEP groups have a higher mean concentrations of CRP and, as predicted, absolute differentials between low SEP and high SEP are largest in Portugal (EPIPorto) and lowest in Switzerland (SKIPOGH).
Across all studies except EPIPorto, low SEP – high SEP differentials in inflammation are highest at age 50 and fall thereafter. The finding of decreasing differentials with age mirrors those of other studies internationally and support an ‘age as leveller’ process with the health status of surviving members of lower SEP groups converging with that of higher SEP groups. US and Finnish studies have previously found significant positive absolute differentials in younger age groups39–41 but diminishing or no differential for older age groups. Results from a recent British study are broadly consistent with this pattern although, differentials in CRP by income and education increased until age 60 before decreasing thereafter19.
Adjusting for the prevalence of different indicators of health behaviour reduces the country variation in relative SEP differentials in inflammation but does not fully explain the differential between low and high SEP groups. As found in previous studies15,16,19,33, adjustment for BMI across studies reduced differentials the most followed by adjustment for current and past smoking. Adjustment for all the lifestyle factors together reduced the low/high SEP differential by between 45% and 52%. The importance of BMI as a risk factor for raised levels of inflammation has been observed in numerous studies15,16,19,33 and reflects the active role of adipose tissue in endocrine function. Excess adipose tissue promotes inflammation due to its role as a store for macrophages42 and the pro-inflammatory effects of endogenous lipids43,44. A recent meta-analysis found that health behaviours mediated between 20% and 26% of the SEP differential in all-cause mortality, between 16 and 33% of the differential for cardiovascular disorders and between 17 and 29% of the differential for metabolic disorders6. Our results suggest a greater role for health behaviours in the development of inflammation, although it could be argued that our measures of health behaviours could be caused, at least in part, by the status anxiety process that we have been studying. If so, this would imply that we are statistically over-adjusting to a certain extent with these measures.
There are a number of potential limitations to this paper. First, the data available to us have limitations. Although we examine the predictions of the status anxiety hypothesis in terms of the impact of country income inequality on inflammation, we do not directly measure the mediating variable of status anxiety or psycho-social stress. Logically, inflammation at the individual level should vary with perceived status anxiety and the chronic stress response. There are no current data available, as far as we are aware, which contain survey measures of status anxiety alongside bio-markers of chronic stress (e.g cortisol) and inflammation.
A true test of the status anxiety hypothesis would include longitudinal data at the individual level on status anxiety and inflammation (as well as health behaviours and SEP) for a statistical sample of countries which vary widely in their levels of income inequality both within country over time and between countries in each time period. Such data would permit adjustment for country and individual fixed effects and confounding processes at the individual level whilst measuring the change in inflammation associated with a change in country level inequality. Unfortunately, internationally harmonised data on inflammatory markers is still very rare so our analysis is confined to four countries with a relatively narrow range in income inequality.
Second, the age range available for analysis was limited by the age range covered by the cohort studies available. Two of the five studies are older persons’ cohort studies (TILDA & ELSA) which include respondents aged 50+ thus setting the lower age limit. On the other hand, studies which include younger respondents have smaller numbers over the age of 75, limiting our observation period from age 50 to 75. It could be that the pattern of SEP differentials in SEP changes after age 75 although this is unlikely.
Third, this study used ESeC social class as the measure of SEP whereas other studies have used income and education measures. Past research has shown that income, education and social class have independent effects on health and mortality2, although all are correlated. Social class is often regarded as a measure of ‘permanent income’ as it tends to be correlated more highly with income averaged over a longer period of time than at any one point in time2 and so provides a less volatile measure than cross-sectional measures of income. Education, on the other hand, is an important predictor of income but its explanatory value varies widely with employment status, age and age cohort. Although CRP concentration varies over shorter periods of time, it is likely that chronic inflammatory response is a function of extended exposure to poor social and economic conditions thus social class is more highly correlated than income or education. Analyses of our data using highest education level as the SEP measure (available on request) show very similar patterns to the results using social class.
Fourth, this study focuses on CRP alone rather than analysing a range of inflammatory markers. This inevitably narrows the range of conclusions that can be drawn from the study. CRP is an acute phase protein, which is produced in response to innate pro-inflammatory cytokines including IL-6 and IL-145,46. Low level elevations of CRP have been shown to correlate with childhood exposure to parental neglect47 and to be associated with increased risk of cardiovascular disease48,49 and cancer50. Our results are in line with those of other studies which have examined a wider set of inflammatory markers19,39–41. CRP is an endpoint of multiple pro-inflammatory pathways and does not capture anti-inflammatory pathways which are likely to be very important for the development of disease. CRP rises in acute inflammatory states in response to the production of innate immune inflammatory cytokines, but in homeostatic conditions, levels of CRP drop once inflammation is resolved51. Chronic elevation of CRP levels is therefore strongly indicative of a chronic pro-inflammatory state and persistently elevated CRP is a strong predictor of cardiovascular disease development, independent of traditional risk factors.
Fifth, the range of lifestyle indicators available on a harmonised basis was smaller than in other studies where detailed measures of diet and physical activity were available19. This means that we should be tentative about drawing firm conclusions about the extent to which we have adjusted for differences in lifestyles across studies and between social groups. It is likely that a wider or more detailed set of lifestyle indicators would increase the degree of attenuation produced and so reduce the residual differential between SEP groups.
Sixth, whilst our analyses suggest the health behaviours cannot account for the SEP differential in inflammation, this does not imply that the unexplained component should be attributed to solely to direct psycho-social processes. This paper could not exclude the role of a long list of other potential mediators between SEP and inflammation such as differentials in direct environmental exposures such as pollution, poor housing and microbial and parasitic load.
Methods
Data Sources
LIFEPATH is a Horizon 2020 funded project which aims to investigate the biological pathways which explain the differentials in premature ageing across different SEP. A consortium of 15 research teams harmonised data from 18 cohort studies from different countries which contained socio-economic, demographic, clinical and biological information. Detailed information on the LIFEPATH study and the harmonisation protocols used can be found elsewhere52. Five cohort studies (The English Longitudinal Study of Ageing34 (ELSA), The Irish Longitudinal Study of Ageing36 (TILDA), The Whitehall II Study35, The Swiss Kidney Project on Genes and Hypertension37 (SKIPOGH) and the EPIPorto Study38, all of which included data on the required variables: ESeC social class, CRP, age, sex, smoking, indications for diabetes, indications for hypertension and body mass index for individuals aged between 50 and 75. Overall, data on 18,349 individuals and 30,632 observations of CRP were available for analysis. Summary information on study design, years of recruitment and ethics review is available in Supplementary Table 1.
Measures
Inflammatory Markers
Inflammation was measured using CRP in mg/L using a high-sensitivity assay across all of the five studies. Multiple observations per person were available for Whitehall II, SKIPOGH, ELSA. CRP is extensively used as a biomarker clinically and in epidemiologic studies to define systemic inflammation.
Socio-economic Position
Both individual social class and highest educational level were harmonised across the five studies. Social class was harmonised using the European Socio-Economic Classification (ESeC) which measures the individuals employment position within labour markets and production units based on a ten group classification53. For this paper, these ten groups have been collapsed to three groups: professional and managerial class; intermediate class; manual working class referred to as low, medium and high SEP. Educational qualifications across the four countries have been harmonised using the International Standard Classification of Education (ISCED 2011) and collapsed into three groups: lower secondary education or less; upper secondary education; third level education.
Body Mass Index
Data from measured heights and weights was used to calculate body mass index using weight(kg)/height(m2) which was divided into underweight (<18.5), healthy (18.5–24.9), overweight (25–29.9) and obese (30+).
Smoking
Current and past smoking behaviour was divided into three categories: never smoked (1), past smoker (2) and current smoker (3).
Diabetes
Information on reported diagnosis by a clinician, reported diabetes medications and HbA1c measures were combined to produce a measure of diabetes. Respondents with a previous diagnosis, or who were currently prescribed medications of Anatomic Therapeutic Classification (ATC) codes ‘A10A’ for insulin and ‘A10B’ for oral ant-glycaemic medications or with a HbA1c of >=6.5% were defined as diabetic.
Hypertension
Hypertension was defined as having an SBP >=140 mmHg and DBP >=90 mmHg or reported diagnosis by a clinician of high blood pressure or current use of any antihypertensive medication.
Gender and Age
Age and gender are used to adjust for demographic composition. Age is used in categorical form in descriptive tables (50–54, 55–59, 60–64, 65–69, 70–75) and in continuous form in statistical modelling.
Statistical analyses
Observed differentials in mean CRP by SEP, demographic, socio-economic and lifestyle indicator by age group and study were computed and statistical differences identified. Given previous research, overall mean concentrations of CRP by study and SEP will be determined by the sex, age and SEP distribution of the data and Table 1 provides evidence that these vary widely across the samples. To ensure that results do not reflect the composition of the samples, sample weights were designed which force each sample to replicate the sample composition of the ELSA sample by sex, age group and social class group. Sample composition is therefore identical across studies so variation between samples in CRP concentration cannot reflect variation in the sex, age or SEP composition of the sample.
CRP has a strong positive skew in all studies which makes it unsuitable for analysis using ordinary least squares regression. Instead, generalised linear models were employed which allow the conditional variance and link functions to be chosen which provide the best fit for the data. The modified Park test was used to select the former and Hosmer-Lemeshow test used to select the latter. Tests showed that the CRP distributions across all five studies was best modelled using Poisson GLM with log link. Individuals within the sample had a minimum of one and a maximum of three observations of CRP. The pooled sample across five studies contains at least one CRP observation on 19,018 individuals. Only individuals with at least one non-missing observation of CRP were retained for analysis but across study waves 30,632 observations were available for analysis as some individuals have multiple observations. Robust standard errors are used to adjust for the within individual correlation with observations. Missing data on all predictor variables was imputed using chained-equations implemented in STATA 15.
‘Base Models’ were estimated with terms for sex, age, study and SEP (measured as ESeC social class). To allow for flexible associations of age with CRP, different age polynomials were tested with quadratic and cubic age polynomial terms providing the best statistical fit based on reduction in log-likelihood. To allow for variation in the association between age and CRP across studies, interactions between study and both age, age2 and age3 were estimated. A second model tested for interactions between study, SEP, age and age polynomials to establish whether the SEP gradient varied across studies. In a third step, models were estimated to test whether SEP gradients in CRP could be explained by lifestyle variables. Measures for smoking, BMI, diabetes and hypertension were added separately and together to establish their individual and joint attenuating effects on the SEP/CRP association. Pooled models including both sexes were estimated but Interactions between gender and age were included to account for gender variation in the association between age and CRP.
Ethical and regulatory approval
All of the data used in this paper were collected with ethical approval from the research ethics body of the institution involved. Details of the ethics body for each study are provided in Supplementary Table 1. Ethics review in each instance included scrutiny of the protocols and procedures followed for the collection of the biological samples and physical measures used in each study. The authors confirm that each study was carried out with the full informed consent of all subjects, that only adults aged 18 plus were included and that all human tissue was collected in accordance with relevant guidelines and regulations.
Supplementary information
Acknowledgements
This study was supported by the European Commission Horizon 2020 grant number 633666 to Paolo Vineis.
Author Contributions
R.L. suggested the design of the paper, implemented the statistical analysis and provided the first draft of the manuscript. C.M.C., N.B., A.I.R., S.S. and C.M. helped with drafting and revision of the paper. P.V. and M.K. critically reviewed drafts of the study for intellectual content. All authors approved the final version of the paper.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-37440-7.
References
- 1.Stringhini S, et al. Socioeconomic status and the 25 × 25 risk factors as determinants of premature mortality: a multicohort study and meta-analysis of 1 × 7 million men and women. The Lancet. 2017;388:9. doi: 10.1016/S0140-6736(16)32380-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torssander J, Erikson R. Marital partner and mortality: the effects of the social positions of both spouses. Journal of Epidemiology and Community Health. 2009;63:6. doi: 10.1136/jech.2009.089623. [DOI] [PubMed] [Google Scholar]
- 3.McCrory C, et al. Socioeconomic differences in children’s growth trajectories from infancy to early adulthood: evidence from four European countries. Journal of Epidemiology and Community Health. 2017;71:8. doi: 10.1136/jech-2016-208556. [DOI] [PubMed] [Google Scholar]
- 4.Chen E, Matthews KA, Boyce WT. Socioeconomic differences in children’s health: how and why do these relationships change with age? Psychological Bulletin. 2002;128:5. doi: 10.1037/0033-2909.128.2.295. [DOI] [PubMed] [Google Scholar]
- 5.Blane D, Bartley M, Mitchell R. The inverse housing law and respiratory health. Journal of Epidemiology and Community Health. 2000;54:5. doi: 10.1136/jech.54.10.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petrovic D, et al. The contribution of health behaviors to socioeconomic inequalities in health: A systematic review. Preventive Medicine. 2018;113:16. doi: 10.1016/j.ypmed.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Blane D, Kelly-Irving M, d’Errico A, Bartley M, Montgomery S. Social-biological transitions: how does the social become biological? Longitudinal and Life Course Studies. 2013;4:10. [Google Scholar]
- 8.Nordenmark M, Strandh M, Layte R. The Impact of Unemployment Benefit System on the Mental Wellbeing of the Unemployed in Sweden, Ireland and Great Britain. European Societies. 2006;8:83–110. doi: 10.1080/14616690500491415. [DOI] [Google Scholar]
- 9.Chandola T, et al. Work stress and coronary heart disease: what are the mechanisms? European Heart Journal. 2008;29:9. doi: 10.1093/eurheartj/ehm584. [DOI] [PubMed] [Google Scholar]
- 10.Bjorkqvist K. Social Defeat as a Stressor in Humans. Physiology and Behaviour. 2001;73:8. doi: 10.1016/S0031-9384(01)00490-5. [DOI] [PubMed] [Google Scholar]
- 11.Dickerson SS, Kemeny ME. Acute Stressors and Cortisol Responses: A Theoretical Integration and Synthesis of Laboratory Research. Psychological Bulletin. 2004;130:36. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- 12.Dickerson SS, Gable SL, Irwin MR, Aziz N. & Kemeny, M. E. Social-Evaluative Threat and Proinflammatory Cytokine Regulation. Psychological Science. 2009;20:8. doi: 10.1111/j.1467-9280.2009.02437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herman J, Cullinan W. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends in Neurosciences. 1997;20:6. doi: 10.1016/S0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- 14.Lupien S, McEwen B, Gunnar M, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10:11. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 15.Alley DE, et al. Socioeconomic status and C-reactive protein levels in the US population: NHANES IV. Brain, Behavior, and Immunity. 2006;20:7. doi: 10.1016/j.bbi.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Brummett BH, et al. Socioeconomic indices as independent correlates of C-reactive protein in the National Longitudinal Study of Adolescent Health. Psycho-Somatic Medicine. 2013;75:12. doi: 10.1097/PSY.0000000000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tabassum F, et al. Effects of socioeconomic position on inflammatory and hemostatic markers: a life-course analysis in the 1958 British birth cohort. American Journal of Epidemiology. 2008;167:10. doi: 10.1093/aje/kwn055. [DOI] [PubMed] [Google Scholar]
- 18.Gruenewald TL, Cohen S, Matthews KA, Tracy R, Seeman TE. Association of socioeconomic status with inflammation markers in black and white men and women in the Coronary Artery Risk Development in Young Adults (CARDIA) study. 2009;69:9. doi: 10.1016/j.socscimed.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davillas A, Benzeval M, Kumari M. Socio-economic inequalities in C-reactive protein and fibrinogen across the adult age span: Findings from UnderstandingSociety. Scientific Reports. 2017;7:13. doi: 10.1038/s41598-017-02888-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tawakol A, et al. Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. Lancet. 2017;389:11. doi: 10.1016/S0140-6736(16)31714-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stringhini SG, et al. Association of lifecourse socioeconomic status with chronic inflammation and type 2 diabetes risk: the Whitehall II prospective cohort study. PLoS Medicine. 2013;10:15. doi: 10.1371/journal.pmed.1001479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powell ND, Tarr AJ, Sheridan JF. Psychosocial stress and inflammation in cancer. Brain, Behavior, and Immunity. 2013;30:6. doi: 10.1016/j.bbi.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 23.Pickett K, Wilkinson RG. Income inequality and health: A causal review. Social Science & Medicine. 2015;128:10. doi: 10.1016/j.socscimed.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 24.Wilkinson RG, Pickett K. Income Inequality and Population Health: A Review and Explanation of the Evidence. Social Science and Medicine. 2006;62:1768–1784. doi: 10.1016/j.socscimed.2005.08.036. [DOI] [PubMed] [Google Scholar]
- 25.Wilkinson RG, Pickett K. Income Inequality and Social Dysfunction. Annual Review of Sociology. 2009;35:493–511. doi: 10.1146/annurev-soc-070308-115926. [DOI] [Google Scholar]
- 26.Marmot, M. G. & Wilkinson, R. G. Social Determinants of Health. (Oxford University Press, 2006).
- 27.Boehm, C. Hierarchy in the Forest: The Evolution of Egalitarian Behaviour. (Harvard University Press, 2001).
- 28.Wilkinson, R. & Pickett, K. The Spirit Level: Why Equality is Better for Everyone. (Penguin, 2010).
- 29.Layte R, Whelan CT. Who Feels Inferior? A Test of the Status Anxiety Hypothesis of Social Inequalities in Health. European Sociological Review. 2014;30:10. doi: 10.1093/esr/jcu057. [DOI] [Google Scholar]
- 30.Layte R. The Association Between Income Inequality and Mental Health: Testing Status Anxiety, Social Capital, and Neo-Materialist Explanations. European Sociological Review. 2012;28:13. doi: 10.1093/esr/jcr012. [DOI] [Google Scholar]
- 31.Lynch JW, Davey Smith G, Hillemeier M, Raghunathan T, Kaplan GA. Income Inequality, the Psychosocial Environment, and Health: Comparisons of Wealthy Nations. The Lancet. 2001;358:194–200. doi: 10.1016/S0140-6736(01)05407-1. [DOI] [PubMed] [Google Scholar]
- 32.Lynch JW, Davey Smith G, Kaplan GA, House JS. Income inequality and mortality: importance to health of individual income, psychosocial environment, or material conditions. British Medical Journal. 2000;320:5. doi: 10.1136/bmj.320.7243.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonaccio M, et al. Relative contribution of health-related behaviours and chronic diseases to the socioeconomic patterning of lowgrade inflammation. International Journal of Public Health. 2017;62:10. doi: 10.1007/s00038-016-0939-0. [DOI] [PubMed] [Google Scholar]
- 34.Steptoe A, Breeze E, Banks J, Nazroo JY. Cohort Profile: The English Longitudinal Study of Ageing. International Journal of Epidemiology. 2013;42:8. doi: 10.1093/ije/dys168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marmot M, Brunner E. Cohort Profile: The Whitehall II study. International Journal of Epidemiology. 2005;34:5. doi: 10.1093/ije/dyh372. [DOI] [PubMed] [Google Scholar]
- 36.Kearney PM, et al. Cohort Profile: The Irish Longitudinal Study on Ageing. International Journal of Epidemiology. 2011;40:7. doi: 10.1093/ije/dyr116. [DOI] [PubMed] [Google Scholar]
- 37.Pruijm M, et al. Heritability, determinants and reference values of renal length: a family-based population study. European Radiology. 2013;23:6. doi: 10.1007/s00330-013-2900-4. [DOI] [PubMed] [Google Scholar]
- 38.Ramos E, Lopes C, Barros H. Investigating the effect of nonparticipation using a population-based case-control study on myocardial infarction. Annals of Epidemiology. 2004;14:1. doi: 10.1016/j.annepidem.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 39.Jousilahti P, Salomaa V, Rasi V, Vahtera E, Palosuo T. Association of markers of systemic inflammation, C reactive protein, serum amyloid A, and fibrinogen, with socioeconomic status. Journal of Epidemiology and Community Health. 2003;57:4. doi: 10.1136/jech.57.9.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muennig P, Sohler N, Mahato B. Socioeconomic status as an independent predictor of physiological biomarkers of cardiovascular disease: evidence from NHANES. Preventative Medicine. 2007;45:5. doi: 10.1016/j.ypmed.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 41.Mitchell UA, Aneshensel CS. Social Inequalities in Inflammation Age Variations in Older Persons. Journal of Aging and Health. 2017;29:18. doi: 10.1177/0898264316645546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J. Clin. Invest. 2011;121:2111–2117. doi: 10.1172/Jci57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tynan GA, et al. Endogenous oils derived from human adipocytes are potent adjuvants that promote IL-1α-dependent inflammation. Diabetes. 2014;63:13. doi: 10.2337/db13-1476. [DOI] [PubMed] [Google Scholar]
- 44.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. The Journal of Clinical Investigation. 2011;121:8. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Du Clos TW, Mold C. C-reactive protein: an activator of innate immunity and a modulator of adaptive immunity. Immuniological Research. 2004;30:16. doi: 10.1385/IR:30:3:261. [DOI] [PubMed] [Google Scholar]
- 46.Black S, Kushner I, Samols D. C-reactive Protein. Journal of Biological Chemistry. 2004;279:4. doi: 10.1074/jbc.R400025200. [DOI] [PubMed] [Google Scholar]
- 47.Baumeister D, Akhtar R, Ciufolini S, Pariante CM, Mondelli V. Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Molecular Psychiatry. 2015;21:7. doi: 10.1038/mp.2015.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lagrand WK, et al. C-reactive protein as a cardiovascular risk factor: more than an epiphenomenon? Circulation. 1999;100:6. doi: 10.1161/01.CIR.100.1.96. [DOI] [PubMed] [Google Scholar]
- 49.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. New England Journal of Medicine. 2000;342:7. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 50.Bittoni MA, Focht BC, Clinton SK, Buckworth J, Harris RE. Prospective evaluation of C-reactive protein, smoking and lung cancer death in the Third National Health and Nutrition Examination Survey. International Journal of Oncology. 2015;47:7. doi: 10.3892/ijo.2015.3141. [DOI] [PubMed] [Google Scholar]
- 51.Sproston NR, Ashworth JJ. Role of C-Reactive Protein at Sites of Inflammation and Infection. Frontiers In Immunology. 2018;13:12. doi: 10.3389/fimmu.2018.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vineis P, et al. The biology of inequalities in health: the LIFEPATH project. Longitudinal and Life Course Studies. 2017;8:5. doi: 10.14301/llcs.v8i4.448. [DOI] [Google Scholar]
- 53.Rose D, Harrison E. The European Socio-economic Classification: A New Social Class Schema for European Research. European Societies. 2007;9:459–490. doi: 10.1080/14616690701336518. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.