1. Introduction
Gingivitis is the inflammation of the soft tissue surrounding the tooth that may progress to a more destructive disease called periodontitis (Carranza and Newman, 2012). The onset and progression of gingivitis is dependent on systemic and genetic factors; however, the main etiological factor for gingivitis is the accumulation of plaque and the harmful bacteria found attached to it (Carranza and Newman, 2012). The oral cavity is normally colonized by more than 500 bacterial species most of which are relatively harmless commensal bacteria; however, it can also host potentially pathogenic bacteria (Jakubovics and Palmer, 2013, Suzuki et al., 2005). The accumulation of plaque can increase the predominance of pathogenic bacteria, alter the homeostasis, and induce disease.
The first line of periodontal therapy is mechanical removal of accumulated plaque/calculus through scaling, root planning and polishing accompanied by oral hygiene instruction for the maintenance of oral health (Carranza and Newman, 2012, Jakubovics and Palmer, 2013). Additionally, chemical antimicrobial agents can play a significant role in supporting standard mechanical procedures by temporarily controlling the microbial load. Antimicrobial agents such as chlorhexidine, cetylpyridinium chloride (CPC), essential oils, chlorine dioxide, hydrogen peroxide, and triclosan are the commonly used (Tartaglia et al., 2017). Chlorhexidine is considered the most effective agent used in plaque control and in periodontal therapy; however, side effects such as tooth discoloration, oral mucosal erosion and taste alteration can be observed (da Costa et al., 2017, Justus et al., 2015).
Probiotics are living microorganisms, mainly bacteria, that are safe for human consumption and positively influence health (Gupta, 2011, Haukioja, 2010). Various strains of Lactobacillus and Bifidobacterium are commonly used to promote gastrointestinal health. Recently, it has been suggested that some of these strains, as well as strains of Streptococcus, can colonize the oral cavity and reduce the concentration of pathogenic bacteria responsible for caries, periodontal disease and halitosis (Haukioja, 2010, Hillman et al., 2009, Maekawa and Hajishengallis, 2014). Clinical trials have been performed to assess the effect of probiotics on pathogenic oral bacteria after systemic and topic application of the probiotics with promising results (Karuppaiah et al., 2013, Zahradnik et al., 2009); however, publications that include controlled clinical assessment after treatment with probiotics are not abundant.
Probiotic products specifically formulated for oral health are limited and Probiora 3 is the only one containing three strains of streptococci. The purpose of this study is to assess the effect of Probiora 3 mouthwash used for the treatment of plaque induced gingivitis and produce controlled clinical data to support or reject the use of probiotics for treatment of gongivitis. The study also aims to compare the effect of the probiotic mouthwash relative to chlorhexidine, since it is considered the most effective chemical aid in the management of periodontal disease. The clinical signs of gingivitis were assessed and compared to conventional periodontal treatment protocols.
2. Materials and methods
2.1. Sample
Fifteen patients with moderate to severe gingivitis attending RAK College of Dental Sciences Dental Clinics were included in this study. The study was approved by the RAK Medical and Health Sciences University Research Ethics Committee (RAKMHSU-REC-7-2016-UG-D) and the Ras Al Khaimah Research Ethics Committee (RAK-REC-45-2016-UG-D). The sample was selected based on the following inclusion criteria: both genders; 20–30 years of age; moderate to severe chronic gingivitis; pockets of 2–3 mm. Patients were excluded if they presented any of the following exclusion criteria: active focus of infection other than gingivitis; gingival recession; pockets of more than 3 mm; stage III-IV caries; missing permanent first molars, first premolars and/or incisors; remaining roots; antibiotic treatment within 6 months prior to the experiment; pregnancy; chronic systemic disease or medication. The patients were asked to join the study and the experiment procedures, purpose, risks and benefits were clearly explained to them before signing a consent form.
2.2. Clinical procedures
The 15 patients were randomly divided into three groups of 5 patients each. The negative control group underwent mechanical periodontal treatment and oral health maintenance education alone. The positive control group, chlorhexidine group, included adjuvant treatment with a chlorhexidine mouthwash. The experimental group, probiotic group, included adjuvant treatment with a probiotic mouthwash.
Patients received mechanical treatment for plaque induced gingivitis that consisted of ultrasonic scaling and polishing to remove plaque, as well as, education on oral hygiene and oral health maintenance. The patients were educated on brushing techniques and frequency, and use of oral hygiene auxiliaries like dental floss and mouthwash. They were instructed to keep a log book to follow up on their adherence to the established oral health maintenance plan. The control group was instructed to rinse with 30 ml of placebo mouthwash made of distilled water, after brushing. The chlorhexidine group was instructed to use 30 ml of Parodontax® Extra (GSK, Germany) mouthwash containing 0.2% chlorhexidine gluconate after brushing. The probiotic group was instructed to use 10 ml of BreathActive® (Cleanition®, Switzerland) mouthwash containing 100 mg/10 ml of Probiora3, after brushing. The manufacturer’s recommendations were used to determine the amount of Parodontax and BreathActive mouthwash used.
All patients underwent periodontal diagnosis which included gingival index, simplified oral hygiene index and periodontal index before treatment (T0). The gingival index and oral hygiene index was assessed again six days (T1) and 12 days (T2) after treatment. The periodontal index was assessed again 30 days (TP) after treatment. Participants received a booklet with a hygiene log book where they kept record of their daily oral hygiene routine. The patient’s compliance with the oral hygiene recommendations and mouthwash use instructions was monitored throughout the experiment.
2.3. Statistical analysis
Results obtained in the study were summarized and plotted into graphs representing the group means and standard deviations. The data collected from each group at T0, T1 and T2 were compared using analysis of variance (ANOVA) of repeated measures to assess the changes within each group. The difference in index measurements between T0 to T1 and T1 to T2 were analyzed using ANOVA of gained scores to assess improvement between sessions. A Two-Way ANOVA of gained scores was performed to compare the changes in the three groups throughout the experiment. A P-value of less than 0.05 was considered significant for each of these tests.
3. Results
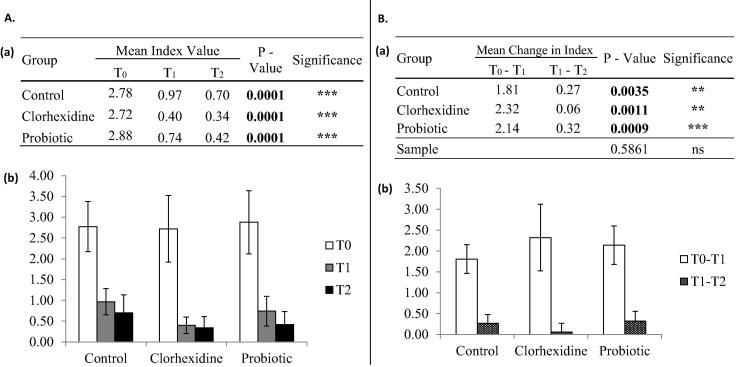
Improvement in the gingival index was observed in all treatment approaches (Fig. 1A). Removal of plaque and calculus accompanied by changes in oral hygiene practices alone can decrease gingival bleeding. However, greater improvement is observed when using adjuvants such as chlorhexidine or probiotic mouthwashes (Fig. 1Aa). The decrease in gingival index is constant when adjuvants are not used, as there is no significant difference between the change in index values between T0–T1 and T1–T2 (Fig. 1Ba). The patients who used chlorhexidine and probiotic mouthwashes experienced improvement of the gingival index that progressively increased with use of the products. The decrease in gingival index was significantly higher between T0–T1 and T1–T2 (Fig. 1Ba). One, two and three patients with a gingival index of zero were observed in the control, chlorhexidine and probiotic groups, respectively. A significant interaction was observed between the treatment length and treatment protocol (p = 0.0205); therefore, our result suggests that the changes in gingival index may depend on both the protocol used and the amount of time the treatment is extended (Fig. 1Ba).
Fig. 1.
Changes in Gingival Index. A – A significant decrease in gingival bleeding was observed in all groups throughout treatment. Higher statistical significance was observed in the chlorhexidine and probiotic groups compared to the control group. Decrease in bleeding was comparable between the chlorhexidine and probiotic groups. B – The decrease in bleeding observed after 6 and 12 days was constant in the control group but was significantly different for the chlorhexidine and probiotic groups. A significant interaction of the treatment used and the length of treatment was observed. NS = no significance; [*] = P < 0.05; [**] = P < 0.01; [***] = P < 0.001.
Improvement in the oral hygiene index was equally observed in all treatment approaches (Fig. 2Aa). The decrease in plaque accumulation was progressively reduced in all groups; however, a greater decrease in plaque accumulation between T0–T1 and T1–T2 was observed in patients who used the probiotic mouthwash as adjuvant to conventional treatment (Fig. 2Ba). An oral hygiene index of zero was observed in only two patients; one in the chlorhexidine mouthwash group and one in the probiotic mouthwash group. A significant interaction was not observed between the treatment length and treatment protocol (p = 0.5861); therefore, our result suggests that the changes in oral hygiene index may depend on the protocol used or the length of treatment, independently (Fig. 2Ba).
Fig. 2.
Changes in Oral Hygiene Index. A – A significant decrease in plaque accumulation was observed throughout treatment in the control, chlorhexidine and probiotic groups, equally. B – The decrease in bleeding observed after 6 and 12 days was significantly different in all groups but higher statistical significance was observed in the probiotic group. [**] = P < 0.01; [***] = P < 0.001.
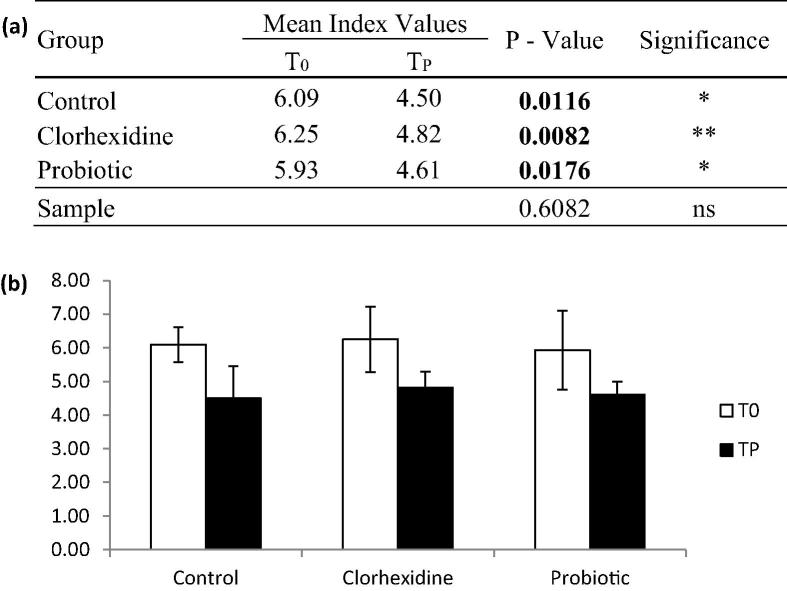
Improvement in the periodontal index was observed in all treatment approaches (Fig. 3). Greater improvement in pocket depth was observed in the patients using chlorhexidine mouthwash as adjuvant to mechanical treatment (Fig. 3a). A significant interaction was not observed between the treatment length and treatment protocol (p = 0.6082); therefore, our result suggests that the changes in the periodontal index may depend on the protocol used or the length of treatment, independently (Fig. 3a).
Fig. 3.
Changes in Periodontal Index. A significant decrease in pocket depth was observed in all groups 30 days after initiation of treatment. Higher statistical significance was observed in the chlorhexidine group. [*] = P < 0.05; [**] = P < 0.01.
4. Discussion
Our data shows that clinical improvement is observed in all of the treatment protocols included in this study. However, the use of mouthwashes as treatment aids increases the degree of improvement of all parameters observed. Chlorhexidine and probiotic mouthwashes have comparable effects on gingival bleeding. The probiotic mouthwash demonstrated greater effects on plaque accumulation while the chlorhexidine mouthwash showed greater effects on pocket depth.
Chlorhexidine has been proven to significantly reduce gingival bleeding (Gupta et al., 2014, Jose et al., 2015). This may be an effect of the elimination of pathogenic and non-pathogenic bacteria which in turn decreases inflammation (James et al., 2017, Jose et al., 2015). The high surface substantivity of chlorhexidine enables it to remove high concentrations of microorganisms. Probiotic mouthwashes have shown significant decrease in pathogenic bacteria which are replaced by strains of non-pathogenic, naturally occurring bacteria (Maekawa and Hajishengallis, 2014, Zahradnik et al., 2009). Additionally, hydrogen peroxide (H2O2) produced by the bacterial strains in the probiotic mouthwash can reduce inflammation and reduce gingival redness (Hasturk et al., 2012, Hossainian et al., 2011, Okahashi et al., 2013). The depletion of the oral microflora can affect the non-pathogenic bacteria that constitute the first line of defense against infection in the oral cavity (Jakubovics and Palmer, 2013, Silva et al., 2015). Probiotics have the potential to protect and reinforce this innate immune response (Haukioja, 2010, Hillman and Socransky, 1989).
Improvement of the oral hygiene index is expected with or without the use of mouthwashes as observed in the current study where a mechanical removal of plaque and calculus followed by appropriate oral hygiene were enough to cause significant improvement of the patients’ plaque accumulation. Chlorhexidine has been shown to disrupt supragingival plaque (Gupta et al., 2014, James et al., 2017). Previous studies have not found consistent decrease of dental plaque accumulation by hydrogen peroxide (Hoenderdos et al., 2009, Hossainian et al., 2011); however, probiotics can affect the concentration of collagenolytic molecules in saliva which may contribute to the reduction of plaque accumulation (Jäsberg et al., 2018). This may partially explain the highly significant decrease in plaque accumulation observed in our sample using the probiotic mouthwash.
The use of chlorhexidine has shown to decrease pocket depth and relatively improve the clinical attachment level (da Costa et al., 2017). The use of probiotics has also shown to improve conditions for wound healing by its effects on the oral microbiota and through host intrinsic mechanisms that reduce the production of inflammatory cytokines (Maekawa and Hajishengallis, 2014). The hydrogen peroxide produced by the probiotic strains may also contribute to the improvement of pocket depth, as low levels of these reactive oxygen species may stimulate wound healing (Schäfer and Werner, 2008).
Although chlorhexidine has proven through time to be effective in the treatment of periodontal disease from gingivitis to periodontitis, it has been associated with side effects such as taste alterations, tooth discoloration and mucosal erosion (Carranza and Newman, 2012, da Costa et al., 2017, James et al., 2017, Justus et al., 2015). Until date, clinical studies of probiotic products used for periodontal health have not reported such side effects and are considered safe for long term use (Hillman et al., 2009, Jäsberg et al., 2018, Karuppaiah et al., 2013, Maekawa and Hajishengallis, 2014, Zahradnik et al., 2009).
The results presented in this study may serve as an initial proof of the effects of probiotics containing streptococci strains in the improvement of periodontal health. The impact of these results is limited to the small sample size; however, the homogeneity of the sample based on strict selection criteria may also increase its significance. Future studies presenting microbiological and molecular comparisons between the groups are necessary to confirm the mechanisms of action that have been suggested in the discussion.
5. Conclusions
The observations recorded from the control group confirm that mechanical removal of plaque and proper oral hygiene habits are effective in the treatment of gingivitis and improvement of gingival inflammation, plaque accumulation and subgingival tissue healing. The use of mouthwashes as aids to mechanical treatment can increase the speed and degree of recovery, as suggested by the observations from the chlorhexidine and probiotic groups. Probiotics demonstrated better treatment results when compared to mechanical treatment alone and where comparable to those of chlorhexidine.
Conflict of interest
The authors declare that there are no conflicts of interest.
Acknowledgements
The authors would like to thank RAK College of Dental Sciences for funding this project and Dr. Yaco Smith from The Dental Studio (Dubai, UAE) for supplying the probiotic oral healthcare products used in this study.
Footnotes
Peer review under responsibility of King Saud University.
References
- Carranza F.A., Newman M.G. Elsevier Saunders; St. Louis, MO: 2012. Carranza's Clinical Periodontology. [Google Scholar]
- da Costa L.F.N.P., Amaral C.D.S.F., Barbirato D.D.S., Leão A.T.T., Fogacci M.F. Chlorhexidine mouthwash as an adjunct to mechanical therapy in chronic periodontitis: a meta-analysis. J Am Dent Assoc. 2017;148(5):308–318. doi: 10.1016/j.adaj.2017.01.021. [DOI] [PubMed] [Google Scholar]
- Gupta D., Bhaskar D., Gupta R., Karim B., Jain A., Singh R., Karim W. A randomized controlled clinical trial of ocimum sanctum and chlorhexidine mouthwash on dental plaque and gingival inflammation. J. Ayurveda Integr. Med. 2014;5(2):109–116. doi: 10.4103/0975-9476.131727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta G. Probiotics and periodontal health. J. Med. Life. 2011;4(4):387–394. [PMC free article] [PubMed] [Google Scholar]
- Hasturk H., Kantarci A., Dyke T.E. Oral inflammatory diseases and systemic inflammation: role of the macrophage. Front. Immunol. 2012;16(3):118. doi: 10.3389/fimmu.2012.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haukioja A. Probiotics and oral health. Eur. J. Dent. 2010;4(3):348–355. [PMC free article] [PubMed] [Google Scholar]
- Hillman J.D., Socransky S.S. The theory and application of bacterial interference to oral disease. In: Myers H.M., editor. New biotechnology in oral research. S. Krager; Basel, Switzerland: 1989. [Google Scholar]
- Hillman J.D., McDonell E., Cramm T., Hillman C.H., Zahradnik R.T. A spontaneous lactate dehydrogenase deficient mutant of Streptococcus rattus for use as a probiotic in the prevention of dental caries. J. Appl. Microbiol. 2009;107(5):1551–1558. doi: 10.1111/j.1365-2672.2009.04333.x. [DOI] [PubMed] [Google Scholar]
- Hoenderdos N.L., Rosema N.A., Slot D.E., Timmerman M.F., van der Velden U., van der Weijden G.A. The influence of a hydrogen peroxide and glycerol containing mouthrinse on plaque accumulation: a 3-day non-brushing model. Int. J. Dent. Hyg. 2009;7(4):294–298. doi: 10.1111/j.1601-5037.2009.00367.x. [DOI] [PubMed] [Google Scholar]
- Hossainian N., Slot D.E., Afennich F., Van der Weijden G.A. The effects of hydrogen peroxide mouthwashes on the prevention of plaque and gingival inflammation: a systematic review. Int. J. Dent. Hyg. 2011;9(3):171–181. doi: 10.1111/j.1601-5037.2010.00492.x. [DOI] [PubMed] [Google Scholar]
- Jakubovics N.S., Palmer R.J. Caister Academic Press; Norfolk: 2013. Oral Microbial Ecology: Current Research and New Perspectives. [Google Scholar]
- James P., Worthington H.V., Parnell C., Harding M., Lamont T., Cheung A., Whelton H., Riley P. Chlorhexidine mouthrinse as an adjunctive treatment for gingival health. Cochrane Database Syst. Rev. 2017;31;3:CD008676. doi: 10.1002/14651858.CD008676.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäsberg H., Tervahartiala T., Sorsa T., Söderling E., Haukioja A. Probiotic intervention influences the salivary levels of Matrix Metalloproteinase (MMP)-9 and Tissue Inhibitor of metalloproteinases (TIMP)-1 in healthy adults. Arch. Oral Biol. 2018;85:58–63. doi: 10.1016/j.archoralbio.2017.10.003. [DOI] [PubMed] [Google Scholar]
- Jose A., Butler A., Payne D., Maclure R., Rimmer P., Bosma M.L. A randomised clinical study to evaluate the efficacy of alcohol-free or alcohol-containing mouthrinses with chlorhexidine on gingival bleeding. Br. Dent. J. 2015;219(3):125–130. doi: 10.1038/sj.bdj.2015.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justus B., Sirajuddin S., Gundapaneni V., Biswas S., Mn K., Mp R. Iatrogenic damage to the periodontium by chemicals and dental materials. Open Dent. J. 2015;26(9):223–227. doi: 10.2174/1874210601509010223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karuppaiah R.M., Shankar S., Raj S.K., Ramesh K., Prakash R., Kruthika M. Evaluation of the efficacy of probiotics in plaque reduction and gingival health maintenance among school children – a randomized control trial. J. Int. Oral Health. 2013;5(5):33–37. [PMC free article] [PubMed] [Google Scholar]
- Maekawa T., Hajishengallis G. Topical treatment with probiotic Lactobacillus brevis CD2 inhibits experimental periodontal inflammation and bone loss. J. Periodontal Res. 2014;49(6):785–791. doi: 10.1111/jre.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okahashi N., Nakata M., Sumitomo T., Terao Y., Kawabata S. Hydrogen peroxide produced by oral streptococci induces macrophage cell death. Plos ONE. 2013;8(5):e62563. doi: 10.1371/journal.pone.0062563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer M., Werner S. Oxidative stress in normal and impaired wound repair. Pharmacol. Res. 2008;58(2):165–171. doi: 10.1016/j.phrs.2008.06.004. Epub 2008 Jun 19. [DOI] [PubMed] [Google Scholar]
- Silva N., Abusleme L., Bravo D., Dutzan N., Garcia-Sesnich J., Vernal R., Hernández M., Gamonal J. Host response mechanisms in periodontal diseases. J. Appl. Oral Sci. 2015;23(3):329–355. doi: 10.1590/1678-775720140259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N., Yoshida A., Nakano Y. Quantitative analysis of multi-species oral biofilms by TaqMan real-time PCR. Clin. Med. Res. 2005;3(3):176–185. doi: 10.3121/cmr.3.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia G.M., Kumar S., Fornari C.D., Corti E., Connelly S.T. Mouthwashes in the 21st century: a narrative review about active molecules and effectiveness on the periodontal outcomes. Expert Opin. Drug Deliv. 2017;14(8):973–982. doi: 10.1080/17425247.2017.1260118. Epub 2016 Nov 20. [DOI] [PubMed] [Google Scholar]
- Zahradnik R.T., Magnusson I., Walker C., McDonell E., Hillman C.H., Hillman J.D. Preliminary assessment of safety and effectiveness in humans of ProBiora3, a probiotic mouthwash. J. Appl. Microbiol. 2009;107(2):682–690. doi: 10.1111/j.1365-2672.2009.04243.x. [DOI] [PubMed] [Google Scholar]