Abstract
Cholesterol is an essential molecule in the membranes of mammalian cells. It is known to be distributed heterogeneously within the cells, between the bilayer leaflets, as well as between lateral domains within the bilayer. However, we do not know exactly how cholesterol is distributed and what forces drive this sorting process because it extremely difficult to study using currently available methods. To further elucidate this distribution, we measured how cholesterol partitions between different phospholipid (PL) environments using different methods based on cholesterol, TopFluor-cholesterol, and cholesta-5,7,9(11)-triene-3-β-ol. Based on the obtained relative partition coefficients, we made predictions regarding how cholesterol would be distributed between lateral domains and between the inner and outer leaflets of the plasma membrane. In addition, using a trans-parinaric acid fluorescence-based method, we tested how cholesterol could influence lateral segregation through its interaction with unsaturated PLs with different headgroups. The results showed that the lower the affinity of cholesterol was for the different unsaturated PLs, the more cholesterol stimulated lateral segregation in a ternary bilayer of unsaturated PL/N-palmitoyl-D-erythro-sphingomyelin and cholesterol. Overall, the results indicate that both the distribution of cholesterol between different lipid environments and the impact of cholesterol on lateral segregation can be predicted relatively accurately from determined relative partition coefficients.
Introduction
Cholesterol is unevenly distributed within mammalian cells, and the cholesterol content in different membrane compartments varies markedly. Further, cholesterol is also distributed unevenly in the bilayer membrane, both laterally and between the inner and outer leaflets of the bilayer (1, 2, 3).
It is still unclear how the distribution of cholesterol between leaflets, lateral domains, and bilayer compartments is controlled, but it can be assumed that it is at least partially controlled by the interactions between cholesterol and other membrane lipids and proteins. It has repeatedly been shown that cholesterol has a particularly strong preference for sphingomyelin (SM) (4, 5, 6, 7), and results from studies on lipid homeostasis suggest that cholesterol and SM are colocalized in the plasma membrane (8). Hence, it seems possible that by measuring the strength of the interactions between cholesterol and different phospholipids (PLs), knowledge about how the sterol is distributed both within and between cellular membranes can be obtained.
Many studies have convincingly demonstrated that cholesterol (and other sterols) have an increased affinity for PLs with saturated acyl chains when compared to PLs with unsaturated acyl chains (9, 10, 11). However, other properties also clearly influence the interactions between cholesterol and PLs, as indicated by the observation that cholesterol interacts more strongly with SM than with phosphatidylcholine, even with a matching acyl-chain order in the bilayers (4). As published data suggest, it can be expected that the PL headgroups would also influence the magnitude of the affinity that cholesterol has for the lipids (6, 8, 11, 12, 13, 14). Because the different PL classes are distributed unevenly between the inner and outer leaflets of the bilayer, the interactions between cholesterol and lipids with different headgroups may be an important factor in determining the interleaflet distribution of cholesterol. In addition, the lateral organization within the bilayers may be influenced by the interactions between cholesterol and different PLs (7).
A reliable way to determine the affinity of cholesterol (and other sterols) for different PL environments is to use an equilibrium partition method that measures the partitioning of sterols between different PL bilayers or between PL bilayers and methyl-β-cyclodextrin (mβCD) in the aqueous phase (14, 15, 16). Here, we have measured the affinity of cholesterol, cholesta-5,7,9(11)-triene-3-β-ol (CTL), and TopFluor (TF)-cholesterol for lipid bilayers with different PL compositions using such approaches. This allowed the comparison of the partitioning behavior of cholesterol to that of the fluorescent analogs. An advantage of using fluorescent probes to analyze the interactions between sterols and PLs is that they allow studies with very low bilayer sterol content, low enough to eliminate the formation of sterol-rich domains or effects of sterols on the bulk membrane properties. This makes the interpretation of the data more straightforward and allows the determination of the affinity of sterols for PLs at low sterol concentration.
One important property of cholesterol is its ability to induce fluid-fluid phase separation, as has been observed in model membranes (7, 17). In cell membranes, actual phase separation may not occur at physiological temperatures, but there is evidence that cholesterol can also induce lateral heterogeneity in cell membranes (18). Recently, it has been further shown that cholesterol-dependent phase separation occurs at subphysiological temperatures in cell-derived giant unilamellar vesicles (19). Therefore, it is clear that cholesterol-induced lateral heterogeneity is important and that several cellular processes are likely to be influenced by it. To determine the degree to which cholesterol facilitates the lateral segregation of lipids and the formation of nanodomains through interactions with the different PLs, we used a time-resolved fluorescence-based approach that is sensitive to the appearance of gel or liquid-ordered (lo)-like domains in the bilayers (7, 20). This was done in ternary lipid mixtures with varying unsaturated PLs, N-palmitoyl-D-erythro-sphingomyelin (PSM), and cholesterol.
The results from the equilibrium partitioning experiments showed that cholesterol (as well as the two fluorescent sterol analogs) had different affinities for saturated and unsaturated PLs with different headgroups. Interestingly, all three sterols showed similar PL preferences, and the affinity of the sterols for the different PLs correlated with the degree to which cholesterol facilitated lateral segregation in the ternary bilayers, including the different PLs. The usefulness of relative partitioning coefficients as a tool for predicting cholesterol distribution within cells, between bilayer leaflets, and between lateral domains will be analyzed and discussed.
Materials and Methods
Materials
1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), N-oleoyl-D-erythro-sphingomyelin (OSM), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE), 1-palmitoyl-2-linoleoyl-sn-glycero-3-phosphoethanolamine (PLPE), 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-L-serine (POPS), 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-(1′-rac-glycerol) (POPG), 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (DPPE), 1,2-dipalmitoyl-sn-glycero-3-phospho-L-serine DPPS), 1,2-dipalmitoyl-sn-glycero-3-phospho-(1′-rac-glycerol) (DPPG), egg SM, and TF-cholesterol were obtained from Avanti Polar Lipids (Alabaster, AL). N-palmitoyl-D-erythro-sphingomyelin (PSM) was purified from egg SM by reverse-phase high performance liquid chromatography (Supelco Discovery C18 column, dimensions 250 × 21.2 mm, 5 μm particle size; Supelco, Bellefonte, PA) using methanol as eluent. The purity and identity of PSM was verified by electrospray ionization mass spectrometry. 1-Palmitoyl-2- diphenylhexatriene-sn-glycero-3-phosphocholine (DPH-PC) was purchased from Molecular Probes (Eugene, OR), and mβCD from Sigma Chemicals (St. Louis, MO). Cholesta-5,7,9(11)-triene-3-β-ol (CTL) was prepared according to published procedures (21, 22). trans-parinaric acid (tPA) was synthesized and purified according to published procedures (23). It was stored at −87°C and contained 0.5 mol% butylated hydroxytoluene to prevent oxidation. Stock solutions of DPH-PC, TF-cholesterol, tPA, and CTL were prepared in ethanol. Fluorophore concentrations were determined based on the molar extinction coefficients of CTL (11,250 cm−1 M−1), DPH-PC (92,000 cm−1 M−1), TF-cholesterol (90,000 cm−1 M−1), and tPA (88,000 cm−1 M−1). The concentration of PLs was determined according to (24), and cholesterol concentration was determined using a surface barostat (25). Solutions were stored in the dark at −20°C and warmed to ambient temperature before use. The water used for all experiments was purified by reverse osmosis, followed by passage through a Millipore UF Plus water-purification system (Millipore, Billerica, MA) to yield a product resistivity of 18.2 mΩcm. All vesicles were prepared in buffer (50 mM Tris, 140 mM NaCl (pH 7.4)).
Equilibrium distribution of CTL between mβCD and LUVs
CTL partitioning between large unilamellar vesicles (LUVs) and mβCD was measured as described in detail in the Supporting Materials and Methods.
Equilibrium distribution of cholesterol between donor and acceptor vesicles
The equilibrium partitioning of cholesterol between large unilamellar donor vesicles (donor LUVs) and large unilamellar acceptor vesicles (acceptor LUVs) was determined using an anisotropy-based assay, described in detail in the Supporting Materials and Methods.
Equilibrium distribution of TF-cholesterol between donor and acceptor vesicles
The distribution of TF-cholesterol between donor LUVs and acceptor LUVs was studied using a Förster resonance energy transfer (FRET) approach in which the FRET donor was DPH-PC and the FRET acceptor was TF-cholesterol, described in detail in the Supporting Materials and Methods.
Calculation of sterol distribution based on relative partitioning coefficients
To use the determined relative partitioning coefficient to estimate the distribution of cholesterol (and the other sterols) in different lipids systems, an average relative partitioning coefficient was calculated using the following equation:
| (1) |
This is a weighted average value, where PLn is the number of a particular lipid (given, e.g., as the mol%) and KR,n is the relative partitioning coefficient of this particular lipid. This average KR is calculated for a particular lipid composition, such as the outer leaflet of the plasma membrane. Having calculated the average KR for both environments of interest (e.g., the inner and outer leaflets), the distribution of the sterol between them can be calculated simply by dividing the two average KR parameters by each other.
Determination of lipid segregation based on tPA fluorescence
To study how the lipid bilayers segregated into lateral domains (either liquid disordered (ld) and lo or ld and gel), the fluorescence lifetimes of tPA in bilayers were measured as a function of PSM concentration at 23°C. For these experiments, multilamellar vesicles were prepared by hydrating dry lipid films with argon-saturated buffer at 60°C for 30 min. After this, the samples were cooled to room temperature. The final lipid concentration in the samples was 100 μM (containing 1 mol% tPA). The formation of gel or lo domains was detected by measuring the average fluorescence lifetime of tPA in a series of samples in which the molar fraction of PSM was increased in the samples while the cholesterol fraction was kept constant (between 0 and 20 mol%). The formation of gel or lo domains was determined as the concentration of PSM at which the average lifetime function changed direction (because of a dramatically longer longest lifetime component).
Results
CTL partitioning between PL vesicles and mβCD
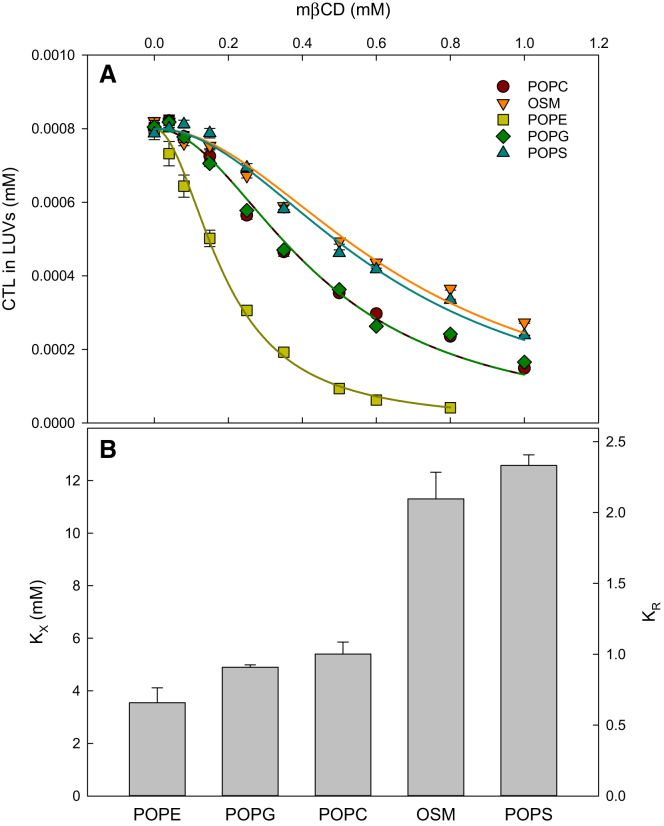
CTL has been shown to be a good fluorescent cholesterol analog (26), and we have previously developed a method for measuring the equilibrium partitioning of CTL between LUVs and mβCD (10). Here, this method was used to determine how CTL interacted with PLs with different polar headgroups. Hence, we measured CTL partitioning between mβCD and LUVs composed of POPC, OSM, POPE, POPS, or POPG at 37°C. The results of these measurements are shown in Fig. 1, and calculated Gibbs free energies are shown in Table S1. The data in the figure show that the addition of an increasing amount of mβCD to the vesicles resulted in a concomitant decrease in the fluorescence anisotropy of CTL in the lipid bilayers (Fig. S1), which then can be translated into the fraction of CTL in the bilayers (Fig. 1 A) and in complex with mβCD. By fitting the data presented in Fig. 1 A with Eq. S2, the molar fraction partitioning coefficients (KX) describing the equilibrium partitioning of CTL between the vesicles and mβCD could be determined. All determined partitioning coefficients are shown in Fig. 1 B.
Figure 1.
Equilibrium partitioning of CTL between LUVs and mβCD. (A) shows the amounts of CTL in the PL vesicles calculated from the anisotropy data shown in Fig. S1 using Eq. S1. The solid lines are the best fits that were obtained to the data with Eq. S2. (B) shows the molar fraction partitioning (KX) and the relative partitioning (KR) coefficients, which were determined at 37°C. Values are averages ± SD with n ≥ 3. To see this figure in color, go online.
The lowest partitioning coefficient was observed for acceptor LUVs composed of POPE, but POPG and POPC LUVs gave rise to only slightly higher coefficients. Markedly higher partitioning coefficients were measured with OSM and POPS LUVs. Based on earlier work with cholesterol, it was expected that CTL would have a high affinity for OSM bilayers (27). The high affinity of the sterol for POPS is also in good agreement with previous observations (11, 14, 28, 29, 30). However, that it would be as high as for OSM was not expected. This result is interesting because it suggests that monounsaturated phosphatidylserines in the inner leaflet could interact favorably with cholesterol and thereby influence both the lateral and trans-bilayer distribution of cholesterol in the bilayer.
TF-cholesterol partitioning between donor and acceptor LUVs
TF-cholesterol is a more recently developed cholesterol analog than CTL. Because of its fluorescence properties, it is more suitable for fluorescence microscopy than CTL. It has been shown to partition into an lo phase (31). In FRET experiments, we observed that TF-cholesterol could not be removed from lipid bilayers with mβCD (Fig. S2). Apparently, the addition of the fluorescent group interferes with the formation of the cyclodextrin-cholesterol complex (32, 33). Hence, partitioning of TF-cholesterol between mβCD and LUVs could not be studied. Instead, partitioning between donor and acceptor LUVs was measured.
To measure how TF-cholesterol was distributed between donor and acceptor LUVs, we used a FRET-based assay, in which the FRET between DPH-PC and TF-cholesterol was measured. Donor LUVs were prepared of POPC with 0.5 mol% DPH-PC and 0.5 mol% TF-cholesterol, as well as acceptor LUVs with varying compositions. Because the FRET efficiency depended linearly on the TF-cholesterol concentration in the donor LUVs (and DPH-PC remains in the donor LUVs), the data could be used to calculate the partitioning coefficient for how the sterol was distributed between the donor and acceptor LUVs (Figs. S3 and S4).
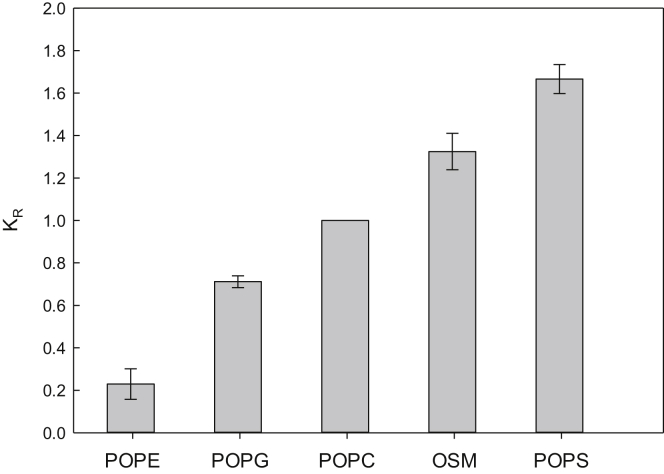
Similarly to CTL, the binding of TF-cholesterol to unsaturated PL bilayers with different headgroups was investigated. For this, POPC donor LUVs were mixed 1:1 with acceptor LUVs composed of pure POPC, OSM, POPE, POPS, or POPG and incubated overnight at 37°C to ensure that equilibrium had been reached (Fig. S5). Subsequently, the FRET efficiencies in the samples were determined by measuring the DPH-PC fluorescence lifetimes. From the FRET efficiencies, we calculated the relative partition coefficients (KR, using Eqs. S4 and S5), which indicated the relative affinity of the sterol for the different PLs in relation to the POPC donor LUVs.
As is clear from the results shown in Fig. 2 (calculated Gibbs free energies are shown in Table S1), TF-cholesterol interacted differently with different PLs. The sterol seems to interact most strongly with POPS bilayers, closely followed by OSM. For POPE bilayers, the sterol showed a very low affinity compared to the other PLs, whereas the affinity for POPC and POPG was intermediate. These results resembled those obtained with CTL, suggesting that despite the structural differences between the two sterols, their relative affinity for the different PLs was similar.
Figure 2.
Relative partitioning of TF-cholesterol between different PL vesicles. The relative partitioning (KR) between POPC and the other PLs was measured at 37°C using the FRET assay described in the methods section. Values are averages + SD with n ≥ 3.
Cholesterol partitioning between donor and acceptor vesicles
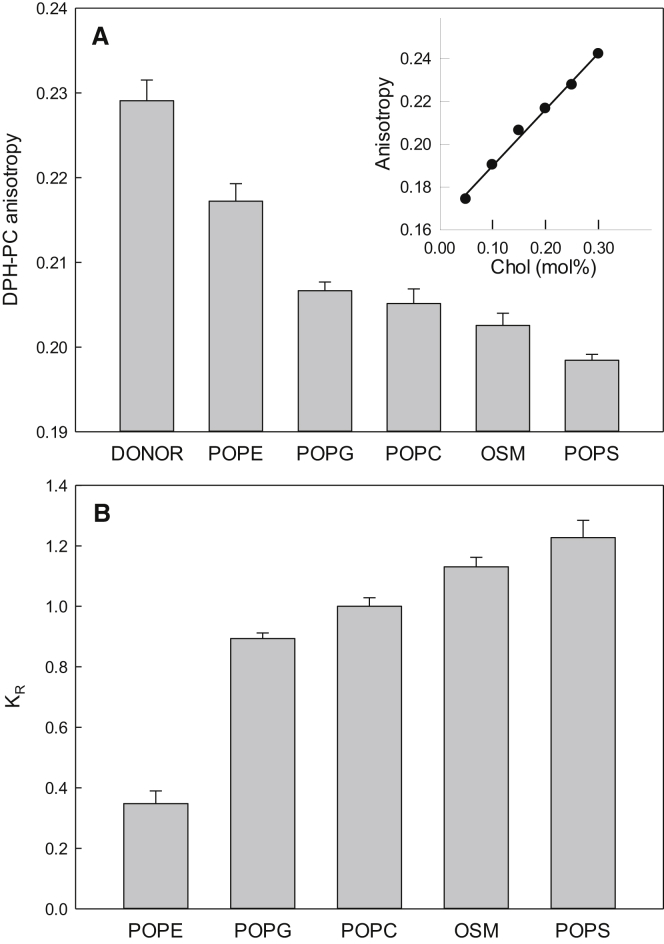
To measure the partitioning of cholesterol between different PL bilayers, a setup based on donor and acceptor LUVs was chosen. In these experiments, the donor LUVs were composed of POPC with 25 mol% cholesterol and 1 mol% DPH-PC. The acceptor LUVs were composed of pure unsaturated PLs (POPC, OSM, POPE, POPS, and POPG). The donor and acceptor LUVs were mixed 1:1 and incubated overnight at 37°C, after which the fluorescence anisotropy of DPH-PC was measured at the same temperature. The measured anisotropies of DPH-PC indicated that the amount of cholesterol in the donor LUVs varied with the lipid composition in the acceptor LUVs (Fig. 3 A). To determine the amount of cholesterol in the donor LUVs, a standard curve for the DPH-PC in POPC bilayers with 0–35 mol% cholesterol was used (inset in Fig. 3 A). Using the standard curve, the amount of cholesterol in the acceptor and donor LUVs was calculated and the relative partitioning coefficient was determined (Fig. 3 B), and calculated Gibbs free energies are shown in Table S1. From the results, it is clear that cholesterol interacts most unfavorably with POPE, as can be seen from the low KR. The other lipids interact more similarly with cholesterol, but the affinity of the sterol seems to be highest for OSM and POPS. Compared with the other two sterols, the cholesterol assay showed smaller differences in KR between the different lipids (with the exception of POPE). We think this possibly could be due to the significantly higher sterol content in these experiments. With this much cholesterol in the bilayer, the bilayer as whole is affected by the sterol, whereas with less than ≤2 mol% sterol (as with CTL and TF-cholesterol methods), only local regions of the bulk bilayer are affected by the sterols. Indeed, it has been reported that the amount of cholesterol in the bilayer affects its partitioning behavior (15, 34). However, even though the observed difference in the affinity of cholesterol for the different PLs was not as large as with the other sterols, the relative affinity of cholesterol for the PLs was again similar to data obtained with the fluorescence sterol analogs.
Figure 3.
Equilibrium partitioning of cholesterol between different PL vesicles. The equilibrium partitioning of cholesterol between donor and acceptor LUVs was measured by monitoring the anisotropy of DPH-PC in the donor POPC LUVs initially containing 25 mol% cholesterol (A). The distribution of cholesterol between the LUVs was determined using a standard curve (inset in A). (B) shows the resulting relative partitioning coefficients (KR) relative to POPC-POPC partitioning. Values are average + SD with n ≥ 3.
Sterol interactions with saturated PLs
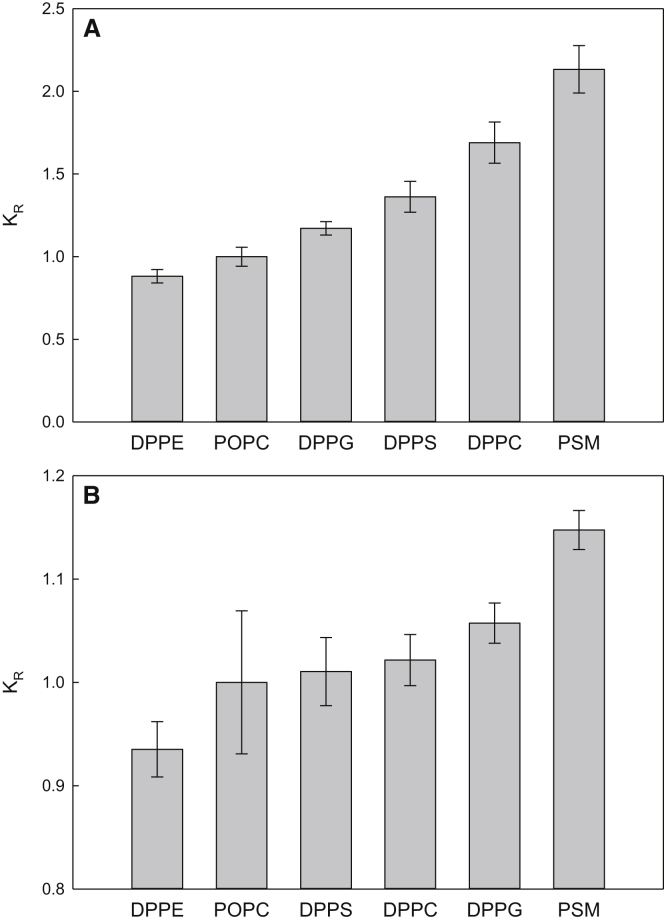
As the interactions between PLs with saturated acyl chains and sterols are also of interest, we included such lipids in this study. First, the affinity of CTL for saturated PLs was investigated by measuring the equilibrium partitioning of CTL between mβCD and LUVs composed of 80% POPC and 20% DPPC, PSM, DPPE, DPPS, or DPPG at 37°C. With these compositions at this temperature, no gel phase formation occurred at the experimental temperature (data not shown), nor is it expected that lo domains form (7, 35). To have the same reference scale as in the experiments with the unsaturated lipids, a pure POPC sample was also included. The results from these experiments are summarized in Fig. 4 A, and calculated Gibbs free energies are shown in Table S1. The inclusion of all saturated lipids, except DPPE, led to increased membrane partitioning of CTL compared to pure POPC. This indicates that CTL favored all saturated PLs (except DPPE) over POPC. Overall, the results with the saturated lipids were similar to those with the pure unsaturated acceptor LUVs, with the exception that the affinity of CTL for LUVs containing DPPS was lower than for those containing DPPC or PSM, whereas the opposite was observed with unsaturated lipids. This indicates that the affinity of CTL for a PL bilayer is affected both by the acyl chains and the headgroup of the lipids and by the combination of these.
Figure 4.
The preference of sterols for saturated PLs. CTL partitioning between LUVs composed of POPC/saturated PL (80:20) and mβCD was measured at 37°C. The resulting KX are presented as KR relative to pure POPC LUVs in (A). The relative partitioning of cholesterol between POPC donor LUVs and acceptor LUVs composed of POPC/saturated PL (80:20) at 37°C is shown in (B). Note the scale on the y axis in (B). Values are averages + SD with n ≥ 3.
Similar experiments were also conducted with cholesterol (results in Fig. 4 B; Table S1). In these experiments, the interactions between cholesterol and saturated PLs were studied using acceptor LUVs consisting of 80 mol% POPC and 20 mol% saturated lipids (DPPC, PSM, DPPE, DPPS, or DPPG). As with the unsaturated lipids, the donor LUVs were composed of POPC with 25 mol% cholesterol and 1 mol% DPH-PC. Because of the high cholesterol/saturated PL ratio in these experiments, it is obvious that the results would show only very small differences between the different PLs (Fig. 4 B). Based on the results shown in Fig. 4, cholesterol partitioned similarly into the DPPG-, DPPS-, and DPPC-containing bilayers because the addition of these lipids to POPC did not seem to influence the affinity of cholesterol for the acceptor LUVs significantly. Meanwhile, the inclusion of PSM increased the affinity of the sterol for the donor vesicles, as seen from a small but significant increase in the partitioning coefficient. Gibbs free enegy for the transfer of cholesterol from POPC to POPC/PSM bilayers was calculated to be 0.35 kcal/mol; this is similar to what was observed in a previous study (34). The lowest preference for the acceptor LUVs was achieved with POPC/DPPE vesicles. This suggests that cholesterol interacts poorly with phosphatidylethanolamine (PE) molecules, likely because of the relatively small headgroup found in these molecules.
Lateral segregation in the lipid bilayers with and without cholesterol
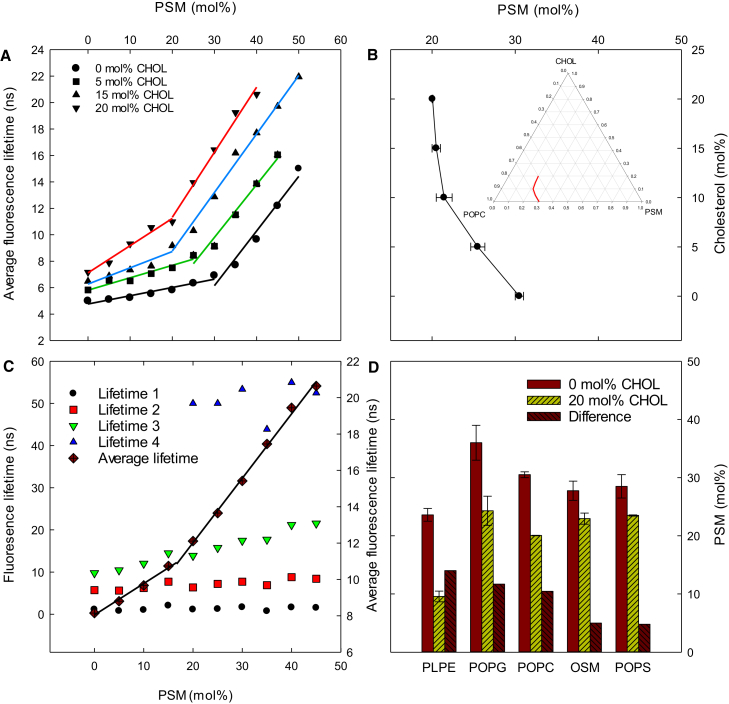
It has previously been observed that the relative affinity of cholesterol for the different PLs in a bilayer correlates with both how prone the system is to form an lo domain as well as with the properties of the formed lo domains (7). Hence, it was of interest to test how the unsaturated lipids studied within this project could influence the transition from pure ld bilayers to bilayers in which ld and lo domains coexist through their different interactions with cholesterol. By measuring the fluorescence lifetimes of tPA in lipid bilayers as a function of the molar fraction of PSM, the critical PSM concentration required for the formation of lo domains could be determined. Fig. 5 A shows the results from measurements done with POPC bilayers having a constant cholesterol content (0, 10, 15, or 20 mol%) and an increasing PSM concentration (0–50 mol%) at 23°C with 1 mol% tPA. As can be seen in the figure, the average fluorescence lifetime of tPA increased with the PSM content, and at a critical mol% PSM, a more dramatic increase is observed, resulting in a clear change in slope. As a result, the data is better fitted with two linear functions, and the point at which these cross each other is the concentration of PSM at which lateral domains with a higher degree of order (gel or lo) start to form (20). In the series without cholesterol, gel domains start to form at ∼30 mol% PSM, and the addition of cholesterol lowers the amount of PSM that is needed to form gel/lo domains (quantified in Fig. 5 B). At ∼10 mol% cholesterol, the boundary between ld and the ordered phase (gel or lo) changes direction. This change of direction is interpreted to be due to a change in the nature of the ordered phase (gel below and lo above ∼10 mol% cholesterol).
Figure 5.
Influence of cholesterol and PL headgroup on the lateral segregation. (A) shows the tPA fluorescence lifetimes (representative data) as a function of PSM content at different cholesterol concentrations together with POPC at 23°C. The PSM concentration at which these trendlines change slope defines the PSM content at which the lateral segregation was initiated. For the POPC-based bilayers, these are summarized in (B) (values are averages + SD with n ≥ 3.). (C) shows representative lifetime components observed in different ternary mixtures of PLPE, PSM, and 5 mol% cholesterol. The impact of different headgroups in the unsaturated lipids on the formation of the gel and lo phases (and the difference between these two) by PSM at 23°C is quantified in (D). These values are averages + SD with n ≥ 3. To see this figure in color, go online.
In Fig. 5 C, the individual lifetime components of a typical fluorescence decay of tPA are shown (PLPE/PSM/cholesterol with 5 mol% cholesterol). Commonly, three to four lifetime components were needed to achieve a good fit to the experimental data. As is clear from Fig. 5 C, a fourth lifetime component needed to be added to samples with ≥20 mol% PSM, that is, above the discontinuity in the lifetime function. Because this fourth lifetime component is longer than the other ones, it indicates that at this PSM concentration, a new, more ordered lipid environment was formed.
When the mol% PSM needed to form gel and lo domains in bilayers containing different unsaturated PLs was compared, we observed that the headgroup structure affected both ld-gel and ld-lo transitions (Fig. 5 D). Because the primary interest was to establish the impact of cholesterol on lateral segregation, the fraction of PSM needed to start the transition from the ld to gel phases was compared with that needed to form lo at 20 mol% cholesterol. The 20 mol% cholesterol trajectory was chosen because the largest shift of the boundary was observed with this cholesterol concentration in all PL samples studied. Because the experiments were performed at 23°C, we could not use POPE as a low-Tm lipid because of its ld-gel transition at ∼25°C. Instead, PLPE was used as the low-Tm phosphatidylethanolamine. With PLPE/PSM bilayers, we were not able to determine the transition from ld to lo at 20 mol% cholesterol. However, with up to 15 mol% cholesterol, the lo boundary could be determined from the clear discontinuity in the lifetime function (Fig. S6). We assumed that this was the largest shift of the boundary (caused by cholesterol inclusion) because the boundary was at ∼10 mol% PSM from 10 to 15 mol% cholesterol (Fig. S6). However, we also performed additional FRET experiments in which it was confirmed that lo domains started to form above 10 mol% PSM in the PLPE/PSM/cholesterol bilayers (Fig. S7). Of the compared PLs, the largest effect of cholesterol inclusion was observed with PLPE-containing bilayers, in which lateral segregation occurred at ∼15 mol% lower PSM concentration with cholesterol than without. In POPG- and POPC-based bilayers, the impact of cholesterol on lateral segregation was similar but smaller than observed with PLPE. In bilayers in which POPS and OSM were the low-Tm lipids, the effect of cholesterol was small, and the phase transition was lowered only ∼5 mol% PSM when cholesterol was included.
When the observed effects of cholesterol on lateral segregation are compared to the affinity of sterols for the different unsaturated PLs, it is clear that the higher the affinity the sterol showed toward the lipid, the smaller the impact cholesterol had on lateral segregation in the bilayer.
Discussion
The composition of the membranes in mammalian cells is complex, and it has proven extremely difficult to obtain detailed information about the arrangement of these components from direct studies of cells and their membranes. However, by studying how the different components interact and organize themselves in model membranes, we obtain data that help us predict the architecture of the cellular membranes and deepen our understanding of how they function. In this work, we measured the partitioning of sterols between bilayers and investigated how the observed PL interactions influenced lateral organization within the bilayer, finding a clear correlation between these events.
Sterol affinity for different PLs
Cholesterol is an essential component in mammalian membranes, and it is known to be involved in many cellular functions (2, 36). Through its interactions with the other lipid components in the membranes, cholesterol can promote lipid segregation and the formation of lateral domains, often called lipid rafts (7, 37, 38, 39). The intermolecular interactions between cholesterol and different PLs control this process, and by understanding these interactions we come closer to understanding the formation of lateral domains and the role they may play in cells.
Several different approaches have been used to study cholesterol partitioning between vesicles or vesicles and cyclodextrin (11, 14, 15, 27, 40). In this work, we used three different approaches, two of which use fluorescent analogs of cholesterol. A clear advantage of using a fluorescent sterol analog is that very low sterol concentrations can be used. This makes the interpretation of the data more straightforward because the bulk effects of sterols on the overall membrane properties and phase separation can be avoided. It also allows us to compare how the sterol partitions in lipid environments with lower sterol content, such as ld phases. The results with the methods based on fluorescent analogs were also very similar to those using the cholesterol-based approach (Fig. S8). Using expensive lipids, it is also an advantage that very small amounts of lipids are needed for each assay.
When the interactions of the three sterols with the different unsaturated PLs were compared, it was clear that the relative affinities for the PLs was similar for all three sterols (POPE < POPG < POPC < OSM < POPS) (Figs. 1, 2, and 3; Table S1). These results were in agreement with previous observations (6, 11, 12, 14), although published results from DSC measurements have suggested that cholesterol interacted more strongly with SM than with PS (12). However, the magnitude of the difference in the affinity for different lipids depended on the sterol that was used (Fig. S8). Clearly, the smallest difference between the different PLs was observed with cholesterol. This may be partially due to the low sensitivity of the method, but more likely it was due to the higher sterol content used in these experiments. The lower concentration of sterol used with the fluorescent sterols (more than 10 times lower) eliminates the bulk ordering effect of the sterols on the lipids and should thus be more dependent on the intermolecular interactions. The effect of the high cholesterol concentration was even more evident in the experiments with POPC/saturated PL (80:20), in which only the bilayers containing PSM or DPPE clearly differed from the rest (Fig. 4). Significantly clearer differences between the different lipids were observed in the CTL partitioning experiments. Interestingly, with saturated lipids, cholesterol did not interact as favorably with PS as with the unsaturated lipids, which suggests that the combination of an acyl chain and headgroup can also determine how sterols interact with a PL. It is also noteworthy that the sterols had a lower affinity for DPPE than for POPC (Fig. 4), although DPPE seems to partition significantly into lo domains, at least when fluorescent probes are linked to the headgroup (41, 42).
Cholesterol is known to favor saturated over unsaturated acyl chains, and at least for CTL, the affinity for PL bilayers seem to correlate with the acyl-chain order (10, 27). However, the high affinity is not due to acyl-chain order alone because other molecular features also influence the interactions between sterols and PLs (4). In this study, it was observed that both cholesterol and CTL preferred POPC over DPPE, and TF-cholesterol had about as high an affinity for POPS and OSM as has been reported for DPPC (7). These findings support the role of other molecular features besides acyl chains in the determination of the affinity of sterols for PLs.
Comparing how CTL, TF-cholesterol, and cholesterol partition between POPC and fluid PSM (Fig. S9), it is obvious that the sterol structure influences the degree to which a sterol prefers saturated over unsaturated chains. This, of course, was expected because it is well known that sterols with different structures interact differently with PLs (26, 43, 44, 45). However, it is a good reminder that when using fluorescent analogs to gain insight into cholesterol distribution, for example, within cells, the analogs may behave differently than cholesterol. Still, both CTL and TF-cholesterol showed similar relative partition behavior as cholesterol, supporting their usefulness.
By obtaining the relative partitioning coefficient describing the distribution of cholesterol between different PL bilayers, we hoped to gain insight into how cholesterol is distributed within cells and how its presence can shape the membrane. One can ask how well relative partitioning describes the behavior of the sterol in complex bilayers. Compared with the way in which cholesterol partitions between the ld and lo domains calculated based on phase diagrams for ternary lipid mixtures, it is clear the partitioning between lateral domains is not the same as that between pure PL bilayers (5, 46, 47), although there are similarities. For instance, cholesterol partitions more into lo domains the more cis double bonds there are in the unsaturated lipids. Further, cholesterol partitions more into the lo domains when the saturated lipid is SM rather than PC, which is exactly what would be predicted based on our findings (7). In POPC/brain-SM/cholesterol bilayers, the KR (lo/ld) for cholesterol is ∼4, which is much lower than what has been reported for partitions between POPC and PSM bilayers (∼12) (5, 34). However, because SM is present in both the lo and ld phases, this should be taken into account in the calculations. When we correct for this and use the partition coefficients for bilayer-bilayer partition (using Eq. 1), we obtain an estimated KR (lo/ld) of 2.85, which is closer to the KR (lo/ld) obtained from the phase diagrams. Likely, other factors such as the cholesterol content in the two phases should still be taken into account to obtain a more accurate prediction. However, we think that it is clear that by measuring how cholesterol partitions between bilayers of different lipids, one can roughly estimate how cholesterol is distributed between lateral domains in membranes.
Lateral segregation
We have previously shown that the relative affinity of cholesterol for the unsaturated and saturated PLs in a ternary system affects the tendency of the system to segregate into lateral domains with different lipid compositions (7). From the cited study, it was clear that when the number of double bonds in the unsaturated PL was increased, the affinity of cholesterol for the lipid decreased and the tendency to form lateral domains increased in ternary lipid mixtures. Based on molecular dynamics simulations, it has been suggested that cholesterol drives the lateral segregation of unsaturated and saturated lipids because of enthalpy gain from more favorable van der Waals interactions between saturated acyl chains and cholesterol, and that unfavorable entropic contributions are working against the lo domain formation (24). In this study, we observed that changes in sterol affinity, resulting from headgroup structure, also affected the membrane’s tendency to segregate laterally in a similar way (Fig. 5). Commonly, the thermal stability of lateral domains is used as a measure of how prone a lipid mixture is to form domains (41, 48, 49, 50, 51). This parameter is mostly dependent on the lipid composition of the domains, and the higher the fraction of sterol and saturated PL is, the higher the melting temperature becomes. The approach we have used here is to determine the critical PSM concentration at which lateral domains appear at a constant temperature. This indicates the solubility limit of PSM in the fluid phase at this temperature. Because we do not have phase diagrams describing the phase behavior of all the studied lipid mixtures, we chose to follow sample trajectories along a constant cholesterol concentration. The concentration 20 mol% was chosen because the available phase diagrams suggest that we should observe the formation of lo domains and not gel domains at this cholesterol concentration, at least at low PSM concentrations (5, 35, 52, 53). Because it is expected that the different unsaturated PLs will mix differently with PSM, we cannot determine the effect of cholesterol on lateral segregation in the different systems without understanding the cholesterol-free systems. Therefore, we used the same tPA-fluorescence-based approach to determine the PSM concentration at which PSM-rich gel phases start to form in binary bilayers composed of the different unsaturated lipids and increasing amounts of PSM. This PSM concentration is the highest fraction of PSM that is soluble in the ld phase at this temperature. In all the studied systems, the addition of cholesterol lowered the solubility of PSM in the ld phase in a concentration-dependent manner (Fig. 5, A and B), that is, cholesterol facilitated the formation of both gel (below ∼10 mol% cholesterol) and lo (above ∼10 mol% cholesterol) domains. This effect of cholesterol may be expected because cholesterol, according to determined phase diagrams, does partition favorably into both gel and lo phase as compared to the ld phase (5, 54). In fact, it would be surprising if cholesterol did not influence the formation of gel and lo domains.
Comparison of the effect of cholesterol on the solubility of PSM in bilayers composed of the different unsaturated PLs showed that the solubility in PLPE was most affected by cholesterol, whereas the smallest influence was observed in the OSM and POPS bilayers (Fig. 5 D). The overall trend was that the influence of cholesterol on the PSM solubility in the ld phase increased with decreasing cholesterol affinity for the unsaturated PL. These results agree with our previously published results, showing that the relative affinity of cholesterol for the two PLs in the bilayer determines how much PSM is needed to start the transition from the ld to the lo or gel phases (7). Therefore, one can argue that the results of both of these studies show that the lateral organization of lipids in bilayers is governed by both push and pull mechanisms (55).
The molecular shape of PE can likely explain the observation that cholesterol disliked PE both in partitioning and lateral domain formation experiments. The interactions between phospholipids and cholesterol have also been described in the umbrella model (56), which predicts that the small headgroup of PE makes the interactions between cholesterol and PE more unfavorable that those between, e.g., PC and cholesterol. In addition, it has been shown that cholesterol inclusion in PE bilayers facilitates the formation of the hexagonal phase (45). Hence, it may be expected that colocalization of PE and cholesterol in a bilayer would increase the intrinsic pressure in bilayers formed of these two lipids. Removal of some of the cholesterol from the PE bilayers into cyclodextrin complexes/acceptor LUVs or by lateral segregation of cholesterol and PE may therefore decrease the curvature stress in the bilayer. This suggests that lateral segregation, at least in this case, could be driven by the bilayer’s tendency to strive for the least possible curvature stress. Phosphatidylcholines with multiple double bonds, especially those with double bonds in both acyl chains, can also have a molecular shape that introduces a curvature stress in the bilayers. Hence, the introduction of curvature stress could be the force behind the large impact of cholesterol on the lateral segregation in bilayers containing highly unsaturated PC molecules (7).
Biological implications
Cholesterol is unevenly distributed within the eukaryotic cell, and the concentration in the membranes increases along the secretory pathway, from the endoplasmic reticulum toward the plasma membrane, which is the membrane most enriched in cholesterol (1). It is believed that this cholesterol gradient plays an important role in the sorting of transmembrane proteins within the cell.
It has long been clear that PLs are distributed heterogeneously between the inner and outer leaflets of the plasma membrane (reviewed in (3)). However, the distribution of cholesterol between the two leaflets has remained unclear (3). In recent molecular dynamics simulations, it has been observed that cholesterol distributes evenly (although with a slight preference for the outer leaflet) in membranes designed to mimic cellular plasma membranes (57, 58). Considering the relatively high content of cholesterol in the plasma membrane, these results seem reasonable. If we use the determined partition coefficients and lipid composition used in simulations, we can make a rough estimation of how cholesterol would partition between the two leaflets (disregarding the presence of the lipids for which we do not have a partitioning coefficient). We obtain an average distribution of 62% of the cholesterol in the outer and 38% in the inner leaflets of average cells, and in brain cells, the distribution would be 64 and 36%, respectively (calculated using Eq. 1; see Table S2 for details). In these calculations, we disregarded the presence of polyunsaturated acyl chains. If they were to be included in the calculation, the fraction of cholesterol in the outer leaflet would be even higher. In both cell types, we have a higher fraction of cholesterol in the outer leaflet than suggested by the computer simulations. One reason our calculations overestimate the amount of cholesterol in the outer leaflet could be that the fraction of cholesterol in the cell membranes is higher than that used in our experiments. As the cholesterol/PSM ratio increases, the impact of PSM on the distribution of cholesterol decreases. Similarly, we observed a much smaller impact of adding 20 mol% PSM to POPC bilayers when we measured the partitioning of 25 mol% cholesterol than when we measured the partitioning of 2 mol% CTL (Fig. 4). With a high cholesterol content, an lo phase may also be present, which we know has an impact on the partitioning behavior of sterols (20). Hence, a deeper understanding of cholesterol partitioning with varying cholesterol levels is needed before we can improve these predictions. Likely, many other parameters may also influence how cholesterol is distributed between the leaflets, i.e., the lipid packing density in the leaflets, etc. By gaining deeper insight into the interactions between cholesterol and phospholipids, we may eventually be able to perform calculations that are more exact.
The distribution of cholesterol between the inner and outer leaflets has been estimated using the fluorescent cholesterol analogs dehydroergosterol and CTL (59). It was reported that the majority of the fluorescent sterols were located in the inner leaflet (59). Here we estimated a larger fraction of CTL than of cholesterol in the inner leaflet (Table S2), and we observe differences in partitioning behavior between CLT and cholesterol that could explain the observations made with CTL in cells. For example, the relative affinity for PSM is markedly higher for cholesterol than for CTL, whereas the opposite was observed for the inner-leaflet lipid POPS. Particularly in membranes in which the cholesterol content is approaching 50 mol% of all lipids, the competition of CTL and cholesterol for interactions with the PLs may push CTL more to the inner leaflet while cholesterol may remain evenly distributed or even located foremost in the outer leaflet.
The role of the lipids of the inner leaflet in the formation of lateral domains in cell membranes has been addressed in several studies (60, 61, 62). We observed that both the high affinity of cholesterol for unsaturated PS and the low affinity of the sterol for unsaturated PE affected the formation of lo domains (Figs. 4 and 5). It is plausible that the preferential interactions of cholesterol with PS over PE could be a driving force that shapes the lateral structure of the inner leaflet in combination with the unfavorable interactions between polyunsaturated acyl chains and cholesterol (63). Such cholesterol-dependent lateral segregation of two unsaturated PLs has been reported previously (7).
Conclusions
In this study, we determined the equilibrium partitioning of CTL, cholesterol, and TF-cholesterol between different PL bilayers and observed that the PL headgroup had a marked effect on the sterol affinity for the lipids. In experiments examining the effect of cholesterol on lateral segregation in bilayers composed of PSM, cholesterol, and different unsaturated PLs, we observed that the affinity of cholesterol for the unsaturated lipids correlated with the degree to which cholesterol facilitated the segregation. Based on analysis of the data, we can conclude that knowledge of how cholesterol partitions between different PL bilayers is useful when attempting to make qualitative predictions of how the sterol is distributed within cells, between bilayer leaflets, and between lateral membrane domains.
Author Contributions
T.K.M.N., O.E., S.J., and J.P.S. planned the research. S.J., V.H., and T.K.M.N. performed the experiments. All authors analyzed the data. T.K.M.N. wrote the article, with contributions from O.E., S.J., and J.P.S.
Acknowledgments
This study was supported by the Academy of Finland, the Sigrid Juselius Foundation, Medicinska Understödsföreningen Liv och Hälsa R.F., and Magnus Ehrnrooth’s Foundation.
Editor: Tommy Nylander.
Footnotes
Supporting Materials and Methods, nine figures, and two tables are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(18)34457-6.
Supporting Citations
References (64, 65) appear in the Supporting Material.
Supporting Material
References
- 1.van Meer G., Voelker D.R., Feigenson G.W. Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maxfield F.R., van Meer G. Cholesterol, the central lipid of mammalian cells. Curr. Opin. Cell Biol. 2010;22:422–429. doi: 10.1016/j.ceb.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steck T.L., Lange Y. Transverse distribution of plasma membrane bilayer cholesterol: picking sides. Traffic. 2018;19:750–760. doi: 10.1111/tra.12586. [DOI] [PubMed] [Google Scholar]
- 4.Lönnfors M., Doux J.P., Slotte J.P. Sterols have higher affinity for sphingomyelin than for phosphatidylcholine bilayers even at equal acyl-chain order. Biophys. J. 2011;100:2633–2641. doi: 10.1016/j.bpj.2011.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petruzielo R.S., Heberle F.A., Feigenson G.W. Phase behavior and domain size in sphingomyelin-containing lipid bilayers. Biochim. Biophys. Acta. 2013;1828:1302–1313. doi: 10.1016/j.bbamem.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demel R.A., Jansen J.W., van Deenen L.L. The preferential interaction of cholesterol with different classes of phospholipids. Biochim. Biophys. Acta. 1977;465:1–10. doi: 10.1016/0005-2736(77)90350-9. [DOI] [PubMed] [Google Scholar]
- 7.Engberg O., Hautala V., Nyholm T.K.M. The affinity of cholesterol for different phospholipids affects lateral segregation in bilayers. Biophys. J. 2016;111:546–556. doi: 10.1016/j.bpj.2016.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohvo-Rekilä H., Ramstedt B., Slotte J.P. Cholesterol interactions with phospholipids in membranes. Prog. Lipid Res. 2002;41:66–97. doi: 10.1016/s0163-7827(01)00020-0. [DOI] [PubMed] [Google Scholar]
- 9.Yasuda T., Tsuchikawa H., Matsumori N. Deuterium NMR of raft model membranes reveals domain-specific order profiles and compositional distribution. Biophys. J. 2015;108:2502–2506. doi: 10.1016/j.bpj.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nyström J.H., Lönnfors M., Nyholm T.K. Transmembrane peptides influence the affinity of sterols for phospholipid bilayers. Biophys. J. 2010;99:526–533. doi: 10.1016/j.bpj.2010.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niu S.L., Litman B.J. Determination of membrane cholesterol partition coefficient using a lipid vesicle-cyclodextrin binary system: effect of phospholipid acyl chain unsaturation and headgroup composition. Biophys. J. 2002;83:3408–3415. doi: 10.1016/S0006-3495(02)75340-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Dijck P.W. Negatively charged phospholipids and their position in the cholesterol affinity sequence. Biochim. Biophys. Acta. 1979;555:89–101. doi: 10.1016/0005-2736(79)90074-9. [DOI] [PubMed] [Google Scholar]
- 13.Van Dijck P.W., De Kruijff B., Demel R.A. The preference of cholesterol for phosphatidylcholine in mixed phosphatidylcholine-phosphatidylethanolamine bilayers. Biochim. Biophys. Acta. 1976;455:576–587. doi: 10.1016/0005-2736(76)90326-6. [DOI] [PubMed] [Google Scholar]
- 14.Leventis R., Silvius J.R. Use of cyclodextrins to monitor transbilayer movement and differential lipid affinities of cholesterol. Biophys. J. 2001;81:2257–2267. doi: 10.1016/S0006-3495(01)75873-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsamaloukas A., Szadkowska H., Heerklotz H. Interactions of cholesterol with lipid membranes and cyclodextrin characterized by calorimetry. Biophys. J. 2005;89:1109–1119. doi: 10.1529/biophysj.105.061846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bar L.K., Barenholz Y., Thompson T.E. Dependence on phospholipid composition of the fraction of cholesterol undergoing spontaneous exchange between small unilamellar vesicles. Biochemistry. 1987;26:5460–5465. doi: 10.1021/bi00391a037. [DOI] [PubMed] [Google Scholar]
- 17.Veatch S.L., Keller S.L. Separation of liquid phases in giant vesicles of ternary mixtures of phospholipids and cholesterol. Biophys. J. 2003;85:3074–3083. doi: 10.1016/S0006-3495(03)74726-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eggeling C., Ringemann C., Hell S.W. Direct observation of the nanoscale dynamics of membrane lipids in a living cell. Nature. 2009;457:1159–1162. doi: 10.1038/nature07596. [DOI] [PubMed] [Google Scholar]
- 19.Levental I., Byfield F.J., Janmey P.A. Cholesterol-dependent phase separation in cell-derived giant plasma-membrane vesicles. Biochem. J. 2009;424:163–167. doi: 10.1042/BJ20091283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nyholm T.K., Lindroos D., Slotte J.P. Construction of a DOPC/PSM/cholesterol phase diagram based on the fluorescence properties of trans-parinaric acid. Langmuir. 2011;27:8339–8350. doi: 10.1021/la201427w. [DOI] [PubMed] [Google Scholar]
- 21.Fischer R.T., Stephenson F.A., Schroeder F. Delta 5,7,9(11)-Cholestatrien-3 beta-ol: a fluorescent cholesterol analogue. Chem. Phys. Lipids. 1984;36:1–14. doi: 10.1016/0009-3084(84)90086-0. [DOI] [PubMed] [Google Scholar]
- 22.Ohvo-Rekilä H., Akerlund B., Slotte J.P. Cyclodextrin-catalyzed extraction of fluorescent sterols from monolayer membranes and small unilamellar vesicles. Chem. Phys. Lipids. 2000;105:167–178. doi: 10.1016/s0009-3084(00)00122-5. [DOI] [PubMed] [Google Scholar]
- 23.Kuklev D.V., Smith W.L. Synthesis of four isomers of parinaric acid. Chem. Phys. Lipids. 2004;131:215–222. doi: 10.1016/j.chemphyslip.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Rouser G., Fkeischer S., Yamamoto A. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970;5:494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- 25.Jungner M., Ohvo H., Slotte J.P. Interfacial regulation of bacterial sphingomyelinase activity. Biochim. Biophys. Acta. 1997;1344:230–240. doi: 10.1016/s0005-2760(96)00147-6. [DOI] [PubMed] [Google Scholar]
- 26.Scheidt H.A., Muller P., Huster D. The potential of fluorescent and spin-labeled steroid analogs to mimic natural cholesterol. J. Biol. Chem. 2003;278:45563–45569. doi: 10.1074/jbc.M303567200. [DOI] [PubMed] [Google Scholar]
- 27.Halling K.K., Ramstedt B., Nyholm T.K. Cholesterol interactions with fluid-phase phospholipids: effect on the lateral organization of the bilayer. Biophys. J. 2008;95:3861–3871. doi: 10.1529/biophysj.108.133744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radhakrishnan A., Anderson T.G., McConnell H.M. Condensed complexes, rafts, and the chemical activity of cholesterol in membranes. Proc. Natl. Acad. Sci. USA. 2000;97:12422–12427. doi: 10.1073/pnas.220418097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huster D., Arnold K., Gawrisch K. Influence of docosahexaenoic acid and cholesterol on lateral lipid organization in phospholipid mixtures. Biochemistry. 1998;37:17299–17308. doi: 10.1021/bi980078g. [DOI] [PubMed] [Google Scholar]
- 30.Garg S., Tang J.X., Naumann C.A. Actin-induced perturbation of PS lipid-cholesterol interaction: a possible mechanism of cytoskeleton-based regulation of membrane organization. J. Struct. Biol. 2009;168:11–20. doi: 10.1016/j.jsb.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 31.Ariola F.S., Li Z., Heikal A.A. Membrane fluidity and lipid order in ternary giant unilamellar vesicles using a new bodipy-cholesterol derivative. Biophys. J. 2009;96:2696–2708. doi: 10.1016/j.bpj.2008.12.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.López C.A., de Vries A.H., Marrink S.J. Computational microscopy of cyclodextrin mediated cholesterol extraction from lipid model membranes. Sci. Rep. 2013;3:2071. doi: 10.1038/srep02071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.López C.A., de Vries A.H., Marrink S.J. Molecular mechanism of cyclodextrin mediated cholesterol extraction. PLoS Comput. Biol. 2011;7:e1002020. doi: 10.1371/journal.pcbi.1002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsamaloukas A., Szadkowska H., Heerklotz H. Thermodynamic comparison of the interactions of cholesterol with unsaturated phospholipid and sphingomyelins. Biophys. J. 2006;90:4479–4487. doi: 10.1529/biophysj.105.080127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Veatch S.L., Keller S.L. Miscibility phase diagrams of giant vesicles containing sphingomyelin. Phys. Rev. Lett. 2005;94:148101. doi: 10.1103/PhysRevLett.94.148101. [DOI] [PubMed] [Google Scholar]
- 36.Yeagle P.L. Cholesterol and the cell membrane. Biochim. Biophys. Acta. 1985;822:267–287. doi: 10.1016/0304-4157(85)90011-5. [DOI] [PubMed] [Google Scholar]
- 37.Bennett W.F.D., Shea J.E., Tieleman D.P. Phospholipid chain interactions with cholesterol drive domain formation in lipid membranes. Biophys. J. 2018;114:2595–2605. doi: 10.1016/j.bpj.2018.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marsh D. Cholesterol-induced fluid membrane domains: a compendium of lipid-raft ternary phase diagrams. Biochim. Biophys. Acta. 2009;1788:2114–2123. doi: 10.1016/j.bbamem.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 39.Lingwood D., Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 40.Williams J.A., Wassall C.D., Wassall S.R. An electron paramagnetic resonance method for measuring the affinity of a spin-labeled analog of cholesterol for phospholipids. J. Membr. Biol. 2013;246:689–696. doi: 10.1007/s00232-013-9586-z. [DOI] [PubMed] [Google Scholar]
- 41.Pathak P., London E. The effect of membrane lipid composition on the formation of lipid ultrananodomains. Biophys. J. 2015;109:1630–1638. doi: 10.1016/j.bpj.2015.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baumgart T., Hunt G., Feigenson G.W. Fluorescence probe partitioning between Lo/Ld phases in lipid membranes. Biochim. Biophys. Acta. 2007;1768:2182–2194. doi: 10.1016/j.bbamem.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scheidt H.A., Meyer T., Huster D. Cholesterol’s aliphatic side chain modulates membrane properties. Angew. Chem. Int.Engl. 2013;52:12848–12851. doi: 10.1002/anie.201306753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Halling K.K., Slotte J.P. Membrane properties of plant sterols in phospholipid bilayers as determined by differential scanning calorimetry, resonance energy transfer and detergent-induced solubilization. Biochim. Biophys. Acta. 2004;1664:161–171. doi: 10.1016/j.bbamem.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 45.Leppimäki P., Mattinen J., Slotte J.P. Sterol-induced upregulation of phosphatidylcholine synthesis in cultured fibroblasts is affected by the double-bond position in the sterol tetracyclic ring structure. Eur. J. Biochem. 2000;267:6385–6394. doi: 10.1046/j.1432-1327.2000.01726.x. [DOI] [PubMed] [Google Scholar]
- 46.Konyakhina T.M., Feigenson G.W. Phase diagram of a polyunsaturated lipid mixture: brain sphingomyelin/1-stearoyl-2-docosahexaenoyl-sn-glycero-3-phosphocholine/cholesterol. Biochim. Biophys. Acta. 2016;1858:153–161. doi: 10.1016/j.bbamem.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Konyakhina T.M., Wu J., Feigenson G.W. Phase diagram of a 4-component lipid mixture: DSPC/DOPC/POPC/chol. Biochim. Biophys. Acta. 2013;1828:2204–2214. doi: 10.1016/j.bbamem.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cornell C.E., McCarthy N.L.C., Keller S.L. n-Alcohol length governs shift in Lo-Ld mixing temperatures in synthetic and cell-derived membranes. Biophys. J. 2017;113:1200–1211. doi: 10.1016/j.bpj.2017.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bleecker J.V., Cox P.A., Keller S.L. Mixing temperatures of bilayers not simply related to thickness differences between Lo and Ld phases. Biophys. J. 2016;110:2305–2308. doi: 10.1016/j.bpj.2016.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Björkqvist Y.J., Nyholm T.K., Ramstedt B. Domain formation and stability in complex lipid bilayers as reported by cholestatrienol. Biophys. J. 2005;88:4054–4063. doi: 10.1529/biophysj.104.054718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cui J., Lethu S., Murata M. Phosphatidylcholine bearing 6,6-dideuterated oleic acid: a useful solid-state (2)H NMR probe for investigating membrane properties. Bioorg. Med. Chem. Lett. 2015;25:203–206. doi: 10.1016/j.bmcl.2014.11.072. [DOI] [PubMed] [Google Scholar]
- 52.Veatch S.L., Polozov I.V., Keller S.L. Liquid domains in vesicles investigated by NMR and fluorescence microscopy. Biophys. J. 2004;86:2910–2922. doi: 10.1016/S0006-3495(04)74342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ionova I.V., Livshits V.A., Marsh D. Phase diagram of ternary cholesterol/palmitoylsphingomyelin/palmitoyloleoyl-phosphatidylcholine mixtures: spin-label EPR study of lipid-raft formation. Biophys. J. 2012;102:1856–1865. doi: 10.1016/j.bpj.2012.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao J., Wu J., Feigenson G.W. Phase studies of model biomembranes: complex behavior of DSPC/DOPC/cholesterol. Biochim. Biophys. Acta. 2007;1768:2764–2776. doi: 10.1016/j.bbamem.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang C., Krause M.R., Regen S.L. Push and pull forces in lipid raft formation: the push can be as important as the pull. J. Am. Chem. Soc. 2015;137:664–666. doi: 10.1021/ja5115437. [DOI] [PubMed] [Google Scholar]
- 56.Huang J., Buboltz J.T., Feigenson G.W. Maximum solubility of cholesterol in phosphatidylcholine and phosphatidylethanolamine bilayers. Biochim. Biophys. Acta. 1999;1417:89–100. doi: 10.1016/s0005-2736(98)00260-0. [DOI] [PubMed] [Google Scholar]
- 57.Ingólfsson H.I., Carpenter T.S., Lightstone F.C. Computational lipidomics of the neuronal plasma membrane. Biophys. J. 2017;113:2271–2280. doi: 10.1016/j.bpj.2017.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ingólfsson H.I., Melo M.N., Marrink S.J. Lipid organization of the plasma membrane. J. Am. Chem. Soc. 2014;136:14554–14559. doi: 10.1021/ja507832e. [DOI] [PubMed] [Google Scholar]
- 59.Mondal M., Mesmin B., Maxfield F.R. Sterols are mainly in the cytoplasmic leaflet of the plasma membrane and the endocytic recycling compartment in CHO cells. Mol. Biol. Cell. 2009;20:581–588. doi: 10.1091/mbc.E08-07-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bakht O., Pathak P., London E. Effect of the structure of lipids favoring disordered domain formation on the stability of cholesterol-containing ordered domains (lipid rafts): identification of multiple raft-stabilization mechanisms. Biophys. J. 2007;93:4307–4318. doi: 10.1529/biophysj.107.114967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Engberg O., Yasuda T., Slotte J.P. Lipid interactions and organization in complex bilayer membranes. Biophys. J. 2016;110:1563–1573. doi: 10.1016/j.bpj.2015.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang T.Y., Silvius J.R. Cholesterol does not induce segregation of liquid-ordered domains in bilayers modeling the inner leaflet of the plasma membrane. Biophys. J. 2001;81:2762–2773. doi: 10.1016/S0006-3495(01)75919-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wassall S.R., Stillwell W. Polyunsaturated fatty acid-cholesterol interactions: domain formation in membranes. Biochim. Biophys. Acta. 2009;1788:24–32. doi: 10.1016/j.bbamem.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 64.Nyholm T.K., Grandell P.M., Slotte J.P. Sterol affinity for bilayer membranes is affected by their ceramide content and the ceramide chain length. Biochim. Biophys. Acta. 2010;1798:1008–1013. doi: 10.1016/j.bbamem.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 65.Yasuda T., Al Sazzad M.A., Slotte J.P. The influence of hydrogen bonding on sphingomyelin/colipid interactions in bilayer membranes. Biophys. J. 2016;110:431–440. doi: 10.1016/j.bpj.2015.11.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.