Abstract
Background
In phenylketonuria (PKU), weaning is considered more challenging when compared to feeding healthy infants. The primary aim of weaning is to gradually replace natural protein from breast milk or standard infant formula with solids containing equivalent phenylalanine (Phe). In addition, a Phe-free second stage L-amino acid supplement is usually recommended from around 6 months to replace Phe-free infant formula. Our aim was to assess different weaning approaches used by health professionals across Europe.
Methods
A cross sectional questionnaire (survey monkey®) composed of 31 multiple and single choice questions was sent to European colleagues caring for inherited metabolic disorders (IMD). Centres were grouped into geographical regions for analysis.
Results
Weaning started at 17–26 weeks in 85% (n = 81/95) of centres, >26 weeks in 12% (n = 11/95) and < 17 weeks in 3% (n = 3/95). Infant's showing an interest in solid foods, and their age, were important determinant factors influencing weaning commencement. 51% (n = 48/95) of centres introduced Phe containing foods at 17–26 weeks and 48% (n = 46/95) at >26 weeks. First solids were mainly low Phe vegetables (59%, n = 56/95) and fruit (34%, n = 32/95).
A Phe exchange system to allocate dietary Phe was used by 52% (n = 49/95) of centres predominantly from Northern and Southern Europe and 48% (n = 46/95) calculated most Phe containing food sources (all centres in Eastern Europe and the majority from Germany and Austria). Some centres used a combination of both methods.
A second stage Phe-free L-amino acid supplement containing a higher protein equivalent was introduced by 41% (n = 39/95) of centres at infant age 26–36 weeks (mainly from Germany, Austria, Northern and Eastern Europe) and 37% (n = 35/95) at infant age > 1y mainly from Southern Europe. 53% (n = 50/95) of centres recommended a second stage Phe-free L-amino acid supplement in a spoonable or semi-solid form.
Conclusions
Weaning strategies vary throughout European PKU centres. There is evidence to suggest that different infant weaning strategies may influence longer term adherence to the PKU diet or acceptance of Phe-free L-amino acid supplements; rendering prospective long-term studies important. It is essential to identify an effective weaning strategy that reduces caregiver burden but is associated with acceptable dietary adherence and optimal infant feeding development.
Keywords: Weaning, Infant, Phenylketonuria, Phenylalanine, Phe-free infant formula
1. Background
Infants with phenylketonuria (PKU) are treated with a low phenylalanine (Phe) diet supplemented with Phe-free L-amino acids (L-AA). In early infancy, either breast milk or a standard infant formula is given to supply Phe requirements. The volume of either milk is titrated to individual tolerance with the remaining nutritional requirements being provided by Phe-free L-AA infant formula [1], until the commencement of solid introduction (weaning).
The World Health Organization (WHO) and UK Scientific Advisory Board on Nutrition (2018) recommends that weaning should commence from 6 months of age (≈26 weeks) [2] as infants are usually developmentally ready to accept solids. Alternatively, the European Society for Pediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) advocates weaning from 17 to 24 weeks [3]. In PKU, the definitive age for the inauguration of weaning remains undefined as infants have already been exposed to complementary feeds in the form of Phe-free L-AA infant formula. In addition, infants have extra stages in their feeding development that require consideration such as the introduction of a second stage protein substitute [4]. The weaning process may be prolonged as each feeding stage has to be directed by a dietitian/doctor before changes can occur in the feeding plan.
In 1999, a multicentre study from the UK and Australia (n = 13 centres) [5] reported that solids were usually introduced early (between 3 and 6 months), with low Phe solids such as fruit and vegetables being given first. Although this timing is different from weaning recommendations, few infants were breast fed in this study (28% of United Kingdom patients and 41% of Australia patients). In PKU, some infants may not be satiated with Phe-free L-AA infant formula and there is less flexibility in adjusting breast or standard infant formula volumes in response to appetite without adversely affecting metabolic control (unless a protein-free formula is also administered). Although in PKU there is no evidence to suggest that Phe-free L-AA infant formula impacts on appetite, non PKU studies have established that whole protein is more satiating than amino acid formula [6,7].
Furthermore, some infants have poor acceptance of Phe-free L-AA infant formula and fail to drink the prescribed amounts, so they may be hungry, leaving little choice but to proceed with the introduction of solid foods to satisfy appetite.
It is almost 20 years since the first paper on multi-centre weaning practices was published in PKU [5]. Differences in the timing of solid food introduction, policies on allocation of dietary Phe, dosage and type of protein substitute prescribed at weaning may impact on future feeding development. Therefore, it is important to re-examine how dietary advice on weaning differs between professionals and compare this with weaning advice for the general population. We report the weaning practices of health professionals working with PKU across Europe.
2. Material and methods
A cross sectional, non-validated, survey monkey® questionnaire was sent to European IMD (Inherited Metabolic Disorders) health professionals (dietitians/nutritionists and medical doctors) who were either members of the ‘Society for the Study of Inborn Errors of Metabolism’ (SSIEM) or who have participated in previous European surveys assessing dietary practices [8,9]. The questionnaire was composed of 31 open, single and multiple-choice questions on infant feeding in PKU with 14 questions focused on weaning. Questions examined: weaning age; first weaning foods; age of introduction of Phe containing foods; method of allocating Phe into the diet; age of introduction of low protein milk, low protein bread and ‘finger foods’; changes to protein substitute prescription during weaning and age of introduction of second stage protein substitute in the weaning period.
The questionnaire was written in English but linguistic support in Portuguese, French, Spanish and Italian was provided by the first author [AP] to clarify any queries. Only one answer per centre was accepted from health professionals and participants were instructed to answer each question according to their general clinical practice rather than for individual patient cases. The questions were devised to encompass all the possible/most common practices across different European countries. A pilot questionnaire was given to 20 dietitians who attended an International meeting. Because of this, changes were made to the questionnaire to improve clarity of questions if there was ambiguity with question structure.
The questionnaire responses were analysed according to the individual centres in addition to the geographical region of responding centres. Results were analysed in two parts: 1) early infant feeding and 2) weaning procedures. The results of the survey about early feeding practices has already been published [10].
3. Results
3.1. Participants
Ninety-five centres from 21 European countries responded to the questionnaire. Centres were grouped into the following geographical regions in order to analyse the results:
-
•
Southern Europe (n = 30 centres): Portugal (n = 7 centres), Italy (n = 6 centres), Spain (n = 9 centres), Greece (n = 2 centres) and Turkey (n = 6 centres);
-
•
Western Europe A (n = 16 centres): Belgium (n = 6 centres), France (n = 5 centres) and The Netherlands (n = 5 centres);
-
•
Western Europe B (n = 15 centres): Germany (n = 13 centres) and Austria (n = 2 centres);
-
•
Northern Europe (n = 24 centres): United Kingdom (n = 17 centres), Sweden (n = 3 centres), Norway (n = 1 centre), Denmark (n = 1 centre), Finland (n = 1 centre) and Ireland (n = 1 centre);
-
•
Eastern Europe (n = 10 centres): Latvia (n = 1 centre), Poland (n = 6 centres), Slovakia (n = 1 centre), Hungary (n = 1 centre) and Estonia (n = 1 centre).
3.2. Weaning commencement
In the participating centres, weaning commenced at age: <17 weeks in 3% (n = 3/95), from 17 to 26 weeks in 85% (n = 81/95) and > 26 weeks of age in 12% (n = 11/95). The main factors influencing solids commencement were infant's age (n = 85/95 centres) and infants showing an interest in food (n = 70/95). The first weaning foods suggested by centres were low Phe vegetables (59%, n = 56/95), low Phe fruits (34%, n = 32/95), low Phe cereal (6%, n = 6/95) and low protein finger foods [1%, n = 1/95].
Centres introduced Phe containing foods such as cereal, potato or yoghurt to replace Phe from breast milk or standard infant formula at infant age: < 17 weeks in 1% (n = 1/95), from 17 to 26 weeks in 51% (n = 48/95) and > 26 weeks in 48% (n = 46/95). Centres expressed a preference for either allocating Phe containing foods by an exchange system (52% of centres, n = 49/95) or calculating the Phe content of individual foods (48%, n = 46/95). Some centres commented how they choose a system according to the preference and ability of an individual family and so use both systems in their centres.
When centres used exchange systems to allocate Phe, different Phe amounts were used to define one Phe exchange: between 10 and 25 mg of Phe (19% of centres, n = 18/95), 50 mg of Phe (31%, n = 29/95), or an unspecified amount (11%, n = 10/95), with some centres using more than one exchange system, which was determined by the Phe concentration of foods.
Low protein milks (e.g. ProZero®, Sno-Pro® and Dalia®) given as a drink or added to foods such as low protein cereal/desserts were introduced: <6 months of age in 1% (n = 1/95) of centres, 6–8 months in 19% (n = 18/95), 9–12 months in 23% (n = 22/95) and > 12 months in 57% (n = 54/95).
Low protein bread was introduced by centres at: <6 months of age in 3% (n = 3/95), between 6 and 8 months in 36% (n = 34/95), 9–12 months, 44% (n = 42/95) and > 12 months in 17% (n = 16/95).
3.3. Weaning process: L-AA supplement
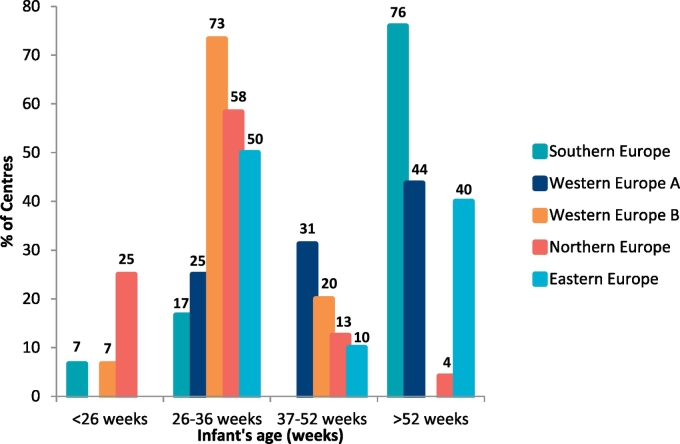
A second stage L-AA supplement containing a higher Phe-free amino acid concentration than Phe-free infant formula was introduced by centres at the following infant ages: <26 weeks, 9% (n = 9/95); 26–36 weeks, 41% (n = 39/95); 37–52 weeks, 13% (n = 12/95); and > 1y, 37% (n = 35/95) [Fig. 1].
Fig. 1.
The age of introduction of a second stage protein substitute in each age category by centres in different geographical regions.
Over half of the centres (53%, n = 50/95) recommended that a second stage L-AA supplement was administered as a semi-solid (paste/gel), 18% (n = 17/95) as a concentrated drink, 19% (n = 18/95) as a non-concentrated drink and 11% (n = 10/95) gave a second stage protein substitute via a different, non-specified method.
4. Discussion
This paper reports European health professionals' infant weaning practices in PKU. The initial approaches to weaning are very similar, with the majority of centres introducing solids between 17 and 26 weeks of age commencing with low Phe fruit and vegetables. There were different systems to allocate calculated Phe containing foods into the diet, and although over half of the centres introduced a more concentrated source of protein substitute from 6 months, centres from Southern Europe and Western Europe A (Portugal, Spain, Greece, Turkey, Belgium, France and The Netherlands) delaying its introduction until after the first year of age.
There is evidence in non-PKU healthy infants that early solid introduction reduces breast milk volume and deferring weaning until 6 months of age is not associated with lower acceptance of solid foods [2]. However, in PKU, the age of weaning may affect the acceptance of a second stage protein substitute and any rejection of protein substitute is associated with worsening of blood Phe control. Although the ideal time to introduce solids in infant’s with PKU is not established, as second stage protein substitute is often added in parallel with low Phe food introduction, later food introduction may alter acceptance of second stage protein substitute [11].
While dietary Phe (to replace Phe from breast milk or infant formula) was added either in the form of an exchange system or by calculating Phe intake of most foods, there was suggestion that dietary practices were changing and were more flexible. Some centres introduced lower Phe containing fruits and vegetables at weaning without calculation which should benefit infants by improving infant exposure to a wider range of tastes and textures. Over recent years the system of allocating dietary Phe has provoked much discussion but increasing evidence is supporting a ‘simple’ diet in PKU [[12], [13], [14]] and there is no data to suggest that this is associated with loss of blood Phe control. The European PKU Guidelines [15] supported a system where fruits and vegetables containing Phe ≤75 mg/100 g are considered as ‘exchange free’ foods and thereby permitted without measurement [[16], [17], [18]]. There is also evidence suggesting that there may be some over restriction of prescribed dietary Phe below individual tolerance [19]. European countries using Phe exchange systems do not seem to have worse metabolic control than centres that estimate all Phe intake [20]. In addition, Phe food analysis is associated with inaccuracy and published figures of the Phe content of food are inconsistent [21]. Furthermore, different definitions for the amount of Phe in one exchange were used in our survey and future PKU guidelines should aim to harmonize practice. It is essential patients receive standard information from health professionals, especially when so much information is accessible through the internet and social media.
There is already a high burden for caregivers of children with PKU with the extra time associated with managing the disorder [22,23], which may lead to caregivers reducing working hours or even leaving their jobs [23]. It has been reported in a UK survey that a median of almost 2 h per week was spent weighing foods [23] using an exchange system. It is likely that even more time will be necessary if Phe intake from almost all food is measured, resulting in additional anxiety, although it has been documented that many families do not sustain the practice of weighing foods beyond early childhood [24].
This survey indicated low use of low protein special foods during weaning. Generally, Northern European centres introduced these foods earlier than other regions, which may be influenced by low protein food availability and national prescription systems. Introduction of low protein foods in the first year might be associated with better acceptance, but if caregivers must pay for them, they may consider that high waste (common during weaning) does not justify their expense. In addition, caregivers are at an early stage in attaining their low protein cooking skills, which may also limit infant exposure to low protein special products. Delayed introduction and lack of exposure to their textures or taste may lead to rejection but this needs further exploration in PKU.
Second stage protein substitutes were recommended in various forms according to geographical region. This could be related to availability of protein substitutes in different countries. Almost two thirds of centres introduced a second, more concentrated source of Phe-free L-AA supplement by the time infants were aged one year. The majority of centres from Southern Europe delayed its introduction until after the first year of age but overall there was a change in practice from an earlier multicentre study [5], which was associated with an even later introduction of a concentrated protein substitute. A study examining feeding problems in PKU, showed that high volumes of Phe-free liquid L-AA supplement (required if a concentrated protein substitute is not introduced during weaning) was associated with crying, screaming, gagging and protein substitute rejection [25,26]. Practice may be to add second stage protein substitute to a feeding bottle, and although this may help to conceal its bitter taste and distinct smell, it does not support good dental health or overall feeding development.
In a retrospective, longitudinal review over 15 years, Evans et al. [11] showed that a semi-solid/spoonable L-AA supplement given from 20 weeks was well tolerated, and any feeding problems encountered were comparable to the problems observed in children without PKU. In this study, there was a trend for children weaned before 17 weeks of age and those commencing weaning protein substitute late (>26 weeks of age) to refuse a second stage, spoonable L-AA supplement at some point. In our survey, only 9% of centres commenced a second stage weaning L-AA supplement before 26 weeks and 37% started only after the first year of age. Prospective longitudinal studies are required to compare the effect of earlier and late introduction of second stage protein substitute with longer-term acceptance of L-AA supplements in PKU.
This study had several limitations. It was a cross-sectional study rather than prospective. The questionnaire was non-validated although a pilot questionnaire was tested. Data was collected about general health professional practice rather than individual prescriptions. Therefore, this survey did not collect data about individual Phe tolerance or blood Phe control. Only one questionnaire was completed by each PKU centre and it is possible that different professionals may have had different approaches within the same centre.
5. Conclusions
Weaning strategies vary widely according to different European regions. There is very little data about the definitive weaning process in PKU and this survey gives useful information on how different health professionals in treatment centres across Europe manage PKU. There is also suggestion that weaning practices have changed compared with an earlier survey. No reports are available assessing how different strategies may influence adherence to the PKU diet and acceptance of Phe-free L-AA supplement during childhood and later in life, so prospective long-term studies are important. It is essential to define the most acceptable weaning strategy for infants associated with the least caregiver burden and the best long term outcome in PKU.
Author's contributions
AP and AM developed the questionnaire, interpreted data, analysed data and wrote the first draft of the manuscript. All the remaining authors were involved in data collection, interpretation of data and critical revision of the paper for important intellectual content and final approval of the version before publication.
Funding
Funding was not needed to develop this study.
Conflicts of interest
Alex Pinto has received an educational grant from Cambrooke Therapeutics and grants from Vitaflo, Nutricia, Merck Serono and Biomarin to attend scientific meetings. Anne Daly has undertaken evaluation work for the nutritional companies – Vitaflo Ltd., Nutricia Ltd. and Metax. Sharon Evans is a research dietitian funded by Nutricia; financial support from Nutricia and Vitaflo to attend study days and conferences. Júlio César Rocha is member of the European Nutrition Expert Panel (Biomarin), member of an Advisory Board for Applied Pharma Research and has received fees from Merck Serono, Vitaflo, Nutricia and Cambrooke. Anita MacDonald has received research funding and honoraria from Nutricia, Vitaflo International and Merck Serono. She is a member of the European Nutrition Expert Panel (Biomarin), member of Sapropterin Advisory Board (Biomarin), member of the Advisory Board entitled ELEMENT (Danone-Nutricia), and member of an Advisory Board for Arla and Applied Pharma Research. Rita Carvalho received grants to attend scientific meetings from Biomarin and Jaba Recordati. Liesbeth van der Ploeg received grants from Nutricia and from Vitaflo to attend scientific meetings on the field of metabolic diseases. Agnieszka Chrobot declares grants to attend scientific meetings from Nutricia, Vitaflo and Mead Johnson. Kamilla Straczek received honoraria fromNutricia Metabolics, Vitaflo and Mead-Johnson. Maria Giżewska received honoraria and was a consultant for: Nutricia International/Danone, Merck-Serono, Mead Johnson, BioMarin and Vitaflo. Alice Rossi has received funding from Biomarin, Nutricia, Piam Farmaceutici and Vitaflo to attend scientific meetings and courses. She is also a member of the European Nutritionist Expert Panel in PKU (Biomarin). Katharina Dokoupil has received honoraria from Nutricia, Vitaflo, Merck Serono and Dr. Schär and grants to attend the SSIEM Annual Symposium from Nutricia Metabolics. She is also a member of the European Nutrition Expert Panel (Biomarin), member of a Nutricia Advisory Board and member of a Nestlé Advisory Board. Consuelo Pedrón-Giner has received support from Vitaflo to attend SSIEM meetings. Ulrike Och declares grants to attend scientific meetings from Biomarin and Dr. Schär. Barbara Cochrane has received research funding and honoria from Nutricia and Vitaflo International. Katarzyna Chyż received honoraria from Mead Johnson, Nutricia and Vitaflo, and grants from Biomarin and Vitaflo to attend scientific meetings. Joanna Żółkowska received honoraria from Mead Johnson, Nutricia and Vitaflo, to attend scientific meetings. Regina Hensler received grants to attend scientific meetings from Nutricia and Dr. Schär and honoraria from Dr. Schär and DDG (Deutsche Diabetes-Gesellschaft). Karen van Wyk is a member of a Nutricia Advisory Board and has received honoraria from Nutricia. The remaining authors declare no competing interests.
Acknowledgments
We thank Vitaflo for supporting the publication cost of this paper.
References
- 1.Rocha J.C., MacDonald A. Dietary intervention in the management of phenylketonuria: current perspectives. Pediatric Health Med. Therap. 2016;7:155–163. doi: 10.2147/PHMT.S49329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.T.S.A.C.o.N. (SACN) Public Health; England: 2018. Feeding in the First Year of Life. [Google Scholar]
- 3.Agostoni C., Decsi T., Fewtrell M., Goulet O., Kolacek S., Koletzko B., Michaelsen K.F., Moreno L., Puntis J., Rigo J., Shamir R., Szajewska H., Turck D., van Goudoever J. Complementary feeding: a commentary by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2008;46:99–110. doi: 10.1097/01.mpg.0000304464.60788.bd. [DOI] [PubMed] [Google Scholar]
- 4.MacDonald A., Evans S., Cochrane B., Wildgoose J. Weaning infants with phenylketonuria: a review Journal of human nutrition and dietetics : the official journal of the. Br. Dietetic Assoc. 2012;25:103–110. doi: 10.1111/j.1365-277X.2011.01199.x. [DOI] [PubMed] [Google Scholar]
- 5.Portnoi P., MacDonald A., Watling R., Clarke B., Barnes J., Robertson L., White F., Jarvis C., Laing S., Weetch E. A survey of feeding practices in infants with phenylketonuria. J. Hum. Nutr. Diet. 1999;12:287–292. [Google Scholar]
- 6.MacLeod E.L., Clayton M.K., van Calcar S.C., Ney D.M. Breakfast with glycomacropeptide compared with amino acids suppresses plasma ghrelin levels in individuals with phenylketonuria. Mol. Genet. Metab. 2010;100:303–308. doi: 10.1016/j.ymgme.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weigel C., Rauh M., Kiener C., Rascher W., Knerr I. Effects of various dietary amino acid preparations for phenylketonuric patients on the metabolic profiles along with postprandial insulin and ghrelin responses. Ann. Nutr. Metabol. 2007;51:352–358. doi: 10.1159/000107678. [DOI] [PubMed] [Google Scholar]
- 8.Daly A., Pinto A., Evans S., Almeida M.F., Assoun M., Belanger-Quintana A., Bernabei S.M., Bollhalder S., Cassiman D., Champion H., Chan H., Dalmau J., de Boer F., de Laet C., de Meyer A., Desloovere A., Dianin A., Dixon M., Dokoupil K., Dubois S., Eyskens F., Faria A., Fasan I., Favre E., Feillet F., Fekete A., Gallo G., Gingell C., Gribben J., Kaalund Hansen K., Ter Horst N.M., Jankowski C., Janssen-Regelink R., Jones I., Jouault C., Kahrs G.E., Kok I.L., Kowalik A., Laguerre C., Le Verge S., Lilje R., Maddalon C., Mayr D., Meyer U., Micciche A., Och U., Robert M., Rocha J.C., Rogozinski H., Rohde C., Ross K., Saruggia I., Schlune A., Singleton K., Sjoqvist E., Skeath R., Stolen L.H., Terry A., Timmer C., Tomlinson L., Tooke A., Vande Kerckhove K., van Dam E., van den Hurk T., van der Ploeg L., van Driessche M., van Rijn M., van Wegberg A., Vasconcelos C., Vestergaard H., Vitoria I., Webster D., White F.J., White L., Zweers H., MacDonald A. Dietary practices in propionic acidemia: a European survey. Mol. Genet. Metabol. Rep. 2017;13:83–89. doi: 10.1016/j.ymgmr.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinto A., Alfadhel M., Akroyd R., Atik Altinok Y., Bernabei S.M., Bernstein L., Bruni G., Caine G., Cameron E., Carruthers R., Cochrane B., Daly A., de Boer F., Delaunay S., Dianin A., Dixon M., Drogari E., Dubois S., Evans S., Gribben J., Gugelmo G., Heidenborg C., Hunjan I., Kok I.L., Kumru B., Liguori A., Mayr D., Megdad E., Meyer U., Oliveira R.B., Pal A., Pozzoli A., Pretese R., Rocha J.C., Rosenbaum-Fabian S., Serrano-Nieto J., Sjoqvist E., Timmer C., White L., van den Hurk T., van Rijn M., Zweers H., Ziadlou M., MacDonald A. International practices in the dietary management of fructose 1-6 biphosphatase deficiency. Orphanet J. Rare Dis. 2018;13:21. doi: 10.1186/s13023-018-0760-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinto A., Adams S., Ahring K., Allen H., Almeida M.F., Garcia-Arenas D., Arslan N., Assoun M., Atik Altinok Y., Barrio-Carreras D., Belanger Quintana A., Bernabei S.M., Bontemps C., Boyle F., Bruni G., Bueno-Delgado M., Caine G., Carvalho R., Chrobot A., Chyz K., Cochrane B., Correia C., Corthouts K., Daly A., De Leo S., Desloovere A., De Meyer A., De Theux A., Didycz B., Dijsselhof M.E., Dokoupil K., Drabik J., Dunlop C., Eberle-Pelloth W., Eftring K., Ekengren J., Errekalde I., Evans S., Foucart A., Fokkema L., Francois L., French M., Forssell E., Gingell C., Goncalves C., Gokmen Ozel H., Grimsley A., Gugelmo G., Gyure E., Heller C., Hensler R., Jardim I., Joost C., Jorg-Streller M., Jouault C., Jung A., Kanthe M., Koc N., Kok I.L., Kozanoglu T., Kumru B., Lang F., Lang K., Liegeois I., Liguori A., Lilje R., Lubina O., Manta-Vogli P., Mayr D., Meneses C., Newby C., Meyer U., Mexia S., Nicol C., Och U., Olivas S.M., Pedron-Giner C., Pereira R., Plutowska-Hoffmann K., Purves J., Re Dionigi A., Reinson K., Robert M., Robertson L., Rocha J.C., Rohde C., Rosenbaum-Fabian S., Rossi A., Ruiz M., Saligova J., Gutierrez-Sanchez A., Schlune A., Schulpis K., Serrano-Nieto J., Skarpalezou A., Skeath R., Slabbert A., Straczek K., Gizewska M., Terry A., Thom R., Tooke A., Tuokkola J., van Dam E., van den Hurk T.A.M., van der Ploeg E.M.C., Vande Kerckhove K., Van Driessche M., van Wegberg A.M.J., van Wyk K., Vasconcelos C., Velez Garcia V., Wildgoose J., Winkler T., Zolkowska J., Zuvadelli J., MacDonald A. Early feeding practices in infants with phenylketonuria across Europe. Mol. Gen. Metabol. Rep. 2018;16:82–89. doi: 10.1016/j.ymgmr.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans S., Daly A., MacDonald J., Pinto A., MacDonald A. Vol. 31. 2018. Fifteen Years of Using a Second Stage Protein Substitute for Weaning in Phenylketonuria: A Retrospective Study; pp. 349–356. [DOI] [PubMed] [Google Scholar]
- 12.Bernstein L., Burns C., Sailer-Hammons M., Kurtz A., Rohr F. Multiclinic observations on the simplified din PKU. J. Nutr. Metabol. 2017;2017 doi: 10.1155/2017/4083293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rohde C., Thiele A.G., Och U., Schonherr K., Meyer U., Rosenbaum-Fabian S., Maddalon C., Matzken S., Blessing H., Lang F., Jorg-Streller M., Beblo S. Effect of dietary regime on metabolic control in phenylketonuria: is exact calculation of phenylalanine intake really necessary? Mol. Genet. Metabol. Rep. 2015;5:36–41. doi: 10.1016/j.ymgmr.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zimmermann M., Jacobs P., Fingerhut R., Torresani T., Thony B., Blau N., Baumgartner M.R., Rohrbach M. Positive effect of a simplified diet on blood phenylalanine control in different phenylketonuria variants, characterized by newborn BH4 loading test and PAH analysis. Mol. Genet. Metab. 2012;106:264–268. doi: 10.1016/j.ymgme.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 15.van Wegberg A.M.J., MacDonald A., Ahring K., Belanger-Quintana A., Blau N., Bosch A.M., Burlina A., Campistol J., Feillet F., Gizewska M., Huijbregts S.C., Kearney S., Leuzzi V., Maillot F., Muntau A.C., van Rijn M., Trefz F., Walter J.H., van Spronsen F.J. The complete European guidelines on phenylketonuria: diagnosis and treatment. Orphanet J. Rare Dis. 2017;12:162. doi: 10.1186/s13023-017-0685-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacDonald A., Rylance G., Davies P., Asplin D., Hall S.K., Booth I.W. Free use of fruits and vegetables in phenylketonuria. J. Inherit. Metab. Dis. 2003;26:327–338. doi: 10.1023/a:1025150901439. [DOI] [PubMed] [Google Scholar]
- 17.Rohde C., Mutze U., Schulz S., Thiele A.G., Ceglarek U., Thiery J., Mueller A.S., Kiess W., Beblo S. Unrestricted fruits and vegetables in the PKU diet: a 1-year follow-up. Eur. J. Clin. Nutr. 2014;68:401–403. doi: 10.1038/ejcn.2013.272. [DOI] [PubMed] [Google Scholar]
- 18.Rohde C., Mutze U., Weigel J.F., Ceglarek U., Thiery J., Kiess W., Beblo S. Unrestricted consumption of fruits and vegetables in phenylketonuria: no major impact on metabolic control. Eur. J. Clin. Nutr. 2012;66:633–638. doi: 10.1038/ejcn.2011.205. [DOI] [PubMed] [Google Scholar]
- 19.van Rijn M., Hoeksma M., Sauer P.J., Modderman P., Reijngoud D.J., van Spronsen F.J. Adult patients with well-controlled phenylketonuria tolerate incidental additional intake of phenylalanine. Ann. Nutr. Metabol. 2011;58:94–100. doi: 10.1159/000324924. [DOI] [PubMed] [Google Scholar]
- 20.Ahring K., Belanger-Quintana A., Dokoupil K., Gokmen-Ozel H., Lammardo A.M., MacDonald A., Motzfeldt K., Nowacka M., Robert M., van Rijn M. Blood phenylalanine control in phenylketonuria: a survey of 10 European centres. Eur. J. Clin. Nutr. 2011;65:275–278. doi: 10.1038/ejcn.2010.258. [DOI] [PubMed] [Google Scholar]
- 21.Weetch E., MacDonald A. The determination of phenylalanine content of foods suitable for phenylketonuria Journal of human nutrition and dietetics : the official journal of the. Br. Dietetic Assoc. 2006;19:229–236. doi: 10.1111/j.1365-277X.2006.00696.x. [DOI] [PubMed] [Google Scholar]
- 22.Eijgelshoven I., Demirdas S., Smith T.A., van Loon J.M., Latour S., Bosch A.M. The time consuming nature of phenylketonuria: a cross-sectional study investigating time burden and costs of phenylketonuria in the Netherlands. Mol. Genet. Metab. 2013;109:237–242. doi: 10.1016/j.ymgme.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 23.MacDonald A., Smith T.A., de Silva S., Alam V., van Loon J.M. Vol. 9. 2016. The Personal Burden for Caregivers of Children with Phenylketonuria: A Cross-Sectional Study Investigating Time Burden and Costs in the UK Molecular Genetics and Metabolism Reports; pp. 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacDonald A., Davies P., Daly A., Hopkins V., Hall S.K., Asplin D., Hendriksz C., Chakrapani A. Does maternal knowledge and parent education affect blood phenylalanine control in phenylketonuria. J. Hum. Nutr. Diet. 2008;21:351–358. doi: 10.1111/j.1365-277X.2008.00891.x. [DOI] [PubMed] [Google Scholar]
- 25.MacDonald A., Harris G., Rylance G., Asplin D., Booth I.W. Abnormal feeding behaviours in phenylketonuria. J. Hum. Nutr. Diet. 1997;10:163–170. [Google Scholar]
- 26.MacDonald A., Rylance G.W., Asplin D.A., Hall K., Harris G., Booth I.W. Feeding problems in young PKU children. Acta Paediatrica. 1994;407:73–74. doi: 10.1111/j.1651-2227.1994.tb13457.x. [DOI] [PubMed] [Google Scholar]