Abstract
Background
Physical activity has been shown to mitigate the unwanted psychological and physiological side effects of prostate cancer treatments, but sustainable exercise possibilities are limited.
Objective
Our objective was to examine whether football in a real-world setting (i.e., local football clubs) was safe and feasible in practice and could improve quality of life, mitigate decline in muscle mass and bone density, and increase fat mass in patients with prostate cancer.
Methods
In this pragmatic, multicentre, parallel randomized controlled trial, men diagnosed with prostate cancer were recruited from five Danish urological departments. Men (N = 214) diagnosed with prostate cancer were randomly allocated, using random generated lists (block size 4–8) stratified for center and androgen-deprivation therapy status, to either 1 h of football twice weekly in a local football club or to usual care, which was a 15- to 30-min telephone session covering their options for physical activity or free-of-charge rehabilitation delivered as standard in Denmark. Allocation was concealed from the trial investigator performing the randomization, but—given the nature of the intervention—this was not possible for personnel and participants. Assessments were performed at baseline, 12 weeks, and 6 months. The primary outcome was mean change difference in prostate cancer-specific quality of life at 12 weeks. Secondary outcomes were body composition, 12-Item Short Form Health Survey (SF-12) physical and mental health, and safety—reported as fractures, falls, and serious adverse events.
Results
Attrition was 1 and 3% at 12 weeks, and 5% and 5% at 6 months for the usual care and football groups, respectively. Prostate cancer-specific quality of life was equal between groups at 12 weeks (mean difference + 1.9 points, 95% confidence interval [CI] –1.0–4.8; P = 0.20) and at 6 months (+ 0.5 points, 95% CI –2.8–3.8; P = 0.76). Fractures were equally distributed, with two fractures in the usual care group and one in the football group. Likewise, body composition outcomes were equal. Mental health improved after 6 months of football (mean difference + 2.7 points, 95% CI 0.8–4.6; P = 0.006).
Conclusions
In this trial, community-based football was a feasible exercise strategy for men with prostate cancer. Football did not improve prostate cancer-specific quality of life but did improve mental health; the clinical significance of this is unclear.
Trial registration
ClinicalTrials.gov: NCT02430792.
Electronic supplementary material
The online version of this article (10.1007/s40279-018-1031-0) contains supplementary material, which is available to authorized users.
Key Points
| Systematic reviews of efficacy trials have shown that exercise is efficacious in improving quality of life, fatigue and exercise capacity. The current evidence base is limited regarding the evaluation of effectiveness of exercise strategies that can be sustained in the long term. |
| The results of this trial suggest that football implemented in local clubs for men with prostate cancer is a feasible exercise strategy and yields results comparable to those from being referred to physical activity and rehabilitation. |
| Football was low cost, and a majority of the men continued in the football club after the study period. |
Background
With 5.6 million men living with prostate cancer globally, and 1.4 million new diagnoses annually, the disease is not only the most frequent cancer among men but also a leading cause of preterm mortality in high- to middle-income countries [1, 2]. Treatment options vary according to patient and cancer characteristics, and most treatments induce unwanted psychological and/or physiological side effects [3]. In the case of localized disease, patients are offered curative treatment, including surgery. However, when disease is recurrent and/or advanced at the time of diagnosis, patients are offered androgen-deprivation therapy (ADT), with an estimated one in two patients receiving ADT at one point during their treatment course [4]. ADT has unintended effects, such as weight gain due to increase in fat mass, decrease in bone and lean muscle mass as well as development of metabolic syndrome and loss of masculinity [3, 5–7]. Some of these may be preventable or reversible with exercise [8, 9]. In addition, physical activity has been shown to reduce both overall and prostate cancer-specific mortality by 33% and 35%, respectively, independent of whether patients received ADT [10]. Trials testing the efficacy of exercise for patients with prostate cancer have demonstrated positive results [8, 9], but supporting long-term adherence and incorporating exercise into already existing infrastructures is challenging [11]. No trials fulfilling pragmatic design criteria have previously been identified in the field of exercise in men with prostate cancer [9]. Pragmatic trials are essential to close the gap between practice and science, as they are conducted in a real-world setting, include outcome measures that are relevant to patients, and are targeted at reducing resource use to support subsequent implementation and dissemination of the intervention [12]. We previously conducted a small-scale explanatory randomized controlled trial exploring the physiological effects of football training and demonstrated improved muscle strength and greater lean body mass and hip bone mineral density [13, 14]. These promising findings prompted us to examine the real-world effectiveness of this intervention when delivered in pre-existing infrastructure (i.e., local football clubs) [15]. Sports participation in a real-world setting is often referred to as a usable public health strategy for promoting physical activity [16, 17] but has never been assessed, to our knowledge, in a pragmatic randomized controlled trial that includes a clinical population.
We therefore designed the Football Club (FC) Prostate Community trial to examine the effectiveness and safety of community-based football compared with usual care. We hypothesized that football would be effective in improving quality of life (QoL), mitigating decline in muscle mass and bone density, and increasing fat mass.
Methods
Trial Design
The FC Prostate Community trial was a pragmatic, multicentre, randomized controlled trial (1:1) comparing community-based football with standard (usual) care. The trial was performed in accordance with the Helsinki Declaration and Good Clinical Practice (GCP) principles. The Ethics Committee for the Capital Region of Denmark (H-2-2014-099) and the Danish Data Protection Agency approved all of the centers involved. No changes were made during the conduct of the trial in relation to the original protocol [18] or trial registration.
Setting and Participants
Eligible participants were men diagnosed with prostate cancer, able to complete trial documents in Danish, and willing to sign informed consent. Participants were excluded if their treating physician proscribed participation in football or if they had a T score below the criterion for osteoporosis (i.e., T score < 2.5 for spine or hip). Patients undergoing prostatectomy were not enrolled until 6 weeks after surgery.
The first patient was enrolled in June 2015 and the last in February 2017. Five Danish urological departments recruited patients. Patients were recruited by referral when attending follow-up appointments at their local outpatient urology clinic. Recruitment material was also available at local community centers that provide usual care rehabilitation.
Randomization and Masking
Patients were randomly allocated to either a football group (FG) or a usual care group (UG) according to a computer-generated list of random numbers after all baseline assessments were completed. A statistician not otherwise involved in the trial generated separate lists with a 1:1 ratio and varying block sizes (n = 4–8) stratified for each center and treatment category (receiving ADT or not) using SAS. The allocation was concealed from trial personnel as the statistician received a password-protected email from the trial management system (EasyTrial®) with an upload function for the allocation sequence. After participants were enrolled by trial personnel at each hospital, the trial manager telephoned participants who had provided written informed consent and undergone all baseline measurements. Randomization was done using the web-based trial management system, and the participants were told which group they were allocated to. Given the nature of the intervention, neither participants nor coaches were blinded. Blinding was implemented for objective outcomes, so personnel performing the dual-energy X-ray absorptiometry (DXA) assessments had no information on the group allocation.
Interventions
A detailed description of the intervention and procedures has been published in the trial protocol [18], and the development of the educational programme for the football coaches has been described in a qualitative study [19]. All participants were informed during a telephone session, in which they were also randomized, of the guidelines for physical activity for cancer survivors: to be physically active > 30 min daily and > 10 min of vigorous activity twice a week [20].
Football Group
FG participants were invited to 6 months of recreational football for 1 h twice weekly at a local football club. A start-up day was decided, and participants were given the local football coach’s contact information and told that all communication on adherence, injuries, and other football matters was to be handled by their coach. After the 6-month period, participants had the opportunity to continue the intervention by joining the football club on the local club’s terms, including paying the membership fee. Football sessions were scheduled to last 1 h and comprised 20 min of warm-up based on the Fédération Internationale de Football Association (FIFA) 11 + program [21] with modified upper-body exercises and 20 min each of drills and match play. The football coaches were recruited by the local club and underwent an 8- to 10-h educational program on prostate cancer treatment, patient symptoms, football-specific functional tests, and the intervention manual. The intervention was developed to work in everyday life, as this is the natural setting for sports participation. The rationale also included aligning the intervention with traditional masculine ideals because the participants can be at risk of a loss of masculinity [5].
Usual Care Group
UG participants were told that they would not be offered football and received a 15- to 30-min telephone session covering their options for physical activity and free-of-charge rehabilitation delivered by the municipalities, which is standard practice in Denmark; the rehabilitation services offered varies across municipalities [22]. They subsequently received an email containing the same information.
Outcomes
The primary outcome was mean change in total prostate cancer-specific QoL measured with the Functional Assessment of Cancer Therapy-Prostate (FACT-P) questionnaire [23] (Likert scoring 0–4; range 0–156; the highest score indicates the best QoL) assessed 12 weeks after randomization. The primary time point was chosen as 12 weeks post baseline, based on the assumption that placing the primary assessment of change in QoL earlier (i.e., 3 months post baseline) rather than later (i.e., 6 months post baseline) would limit the risk of response shift [24].
Objectively measured secondary outcomes assessed by DXA were mean change in lean body mass, fat mass, and hip, femoral neck, spine, and whole-body bone mineral density after 6 months (for information on scanners at each center, see the Electronic Supplementary Material [ESM]). Self-reported outcomes were physical activity measured with the short International Physical Activity Questionnaire (IPAQ) and general physical and mental health measured with the 12-Item Short Form Health Survey (SF-12). All participants, irrespective of allocation group, were followed-up using the same procedures. Safety outcomes were fractures and falls requiring medical assessment and any serious adverse events (SAEs) defined according to GCP [25]. To enable unbiased assessment between groups, the two safety outcomes were evaluated using a self-completed case report form administered via the web-based data capture system. The cost of delivering the program was calculated using the actual price paid to the football clubs to cover expenses for purchasing equipment and reimbursement of coaches. We report the fraction of FG participants who continued to play football after 6 months; continuers were defined as attending more than five training sessions in the period from 6 months to 1 year. The coaches used a tablet-based app to submit data on adherence to the intervention, injuries, and fidelity in delivering the intervention after each session.
Statistical Analysis
Sample Size
The sample size calculation was based on detection of a minimal clinically important difference (MCID) of 6 points on the FACT-P questionnaire [26] at 12 weeks. Based on results from a pivotal exercise trial for men with prostate cancer [27], which reported a standard deviation (SD) of 10 FACT-P points, an SD of 15 FACT-P points was chosen for the calculation as we expected the participants to be more heterogeneous than those in the study by Segal et al. [27]. With a two-sided significance level of 5% and a power of 80%, we required a minimum of 100 participants in each group to detect an MCID.
Analysis of Primary and Secondary Outcomes
The main analyses of the primary, secondary, and safety outcomes were performed according to the intention-to-treat (ITT) principle. We assessed distributions of continuous variables for normality, and residuals were inspected to validate the models used.
If any single item was missing in FACT-P, the official scoring guidelines were applied, removing this item by multiplying the sum of the subscale with the number of the items in the subscale and then dividing by the number of items answered. No imputations of data from missing participants were performed as missing data occurred in < 3% of cases for the primary outcome and < 6% for any other outcomes. In the analysis of the primary outcome, we compared change score of disease-specific QoL score (total score of FACT-P) at 12 weeks between allocation groups using analysis of covariance, where we included the allocation group and adjusted for baseline value, age, and the stratification factor ADT status in the model. Changes are presented as marginal mean differences between allocation groups with 95% confidence intervals and p-values. For the secondary outcomes (lean body mass, fat mass, whole-body and regional bone measures) with two timepoints, we used the same approach as for the primary outcome. All figures delineate results from unadjusted models.
We used Fischer’s exact test to compare differences in proportions of the safety outcomes (fractures, falls, and SAEs).
We performed subgroup analyses defined a priori on participants undergoing ADT. We also performed post hoc subgroup analyses stratifying participants according to physical activity categories proposed in the official IPAQ scoring protocol [28]. These analyses were performed as described for the ITT population. To determine the effect of playing football, and not just being assigned to football, we performed per-protocol analyses on a priori defined criteria of 50% adherence. To address any confounding when comparing all UG participants with a subgroup that cannot be stratified for, we adjusted for lifestyle, demographics, and disease prognostic risk factors known at baseline as suggested by Hernán et al. [29]. For the same reason, we used a logistic regression incorporating the same risk factor variable to analyze the comparative safety outcomes of fractures, falls, and admissions.
Post hoc sensitivity analyses that included disease stage, treatment, and Gleason score were performed to examine whether heterogeneity influenced effect estimates. All analyses were conducted with STATA 15, version 1. The plan for the statistical analyses was published before any outcome data were collected on the trials registry site.
Patient and Public Involvement
Two patient partners were involved in the trial’s steering group and contributed actively in all stages of the trial, including seeking funding, developing research questions, trial conduct, and discussing trial results and the manuscript draft. Specifically, patient partners in the steering group helped guide strategies to recruit patients, which also included help/advice from the national prostate cancer patient organization, which agreed to tell its members about the trial and to provide details about participation through newsletters.
The intervention manual was developed based on expert interviews and focus groups with patients and other stakeholders, e.g., volunteer football coaches and urology nurses, as described elsewhere [19].
Published reports of trial results will be emailed to participants and disseminated to patient advocates through the national prostate cancer organization and relevant scientific meetings and conferences.
Results
Study Sample
Between 15 June 2015 and 28 February 2017, we screened 459 patients, 238 (51%) of whom were assessed for eligibility, and 214 (90%) were randomized (Fig. 1). Geographic distance/travel time and poor physical condition were the two most prevailing reasons for declining participation in the trial. Of the 459 patients, 37 (8%) declined to participate, explicitly stating they had no interest in football. Table 1 presents the demographics and clinical characteristics (n = 214).
Fig. 1.
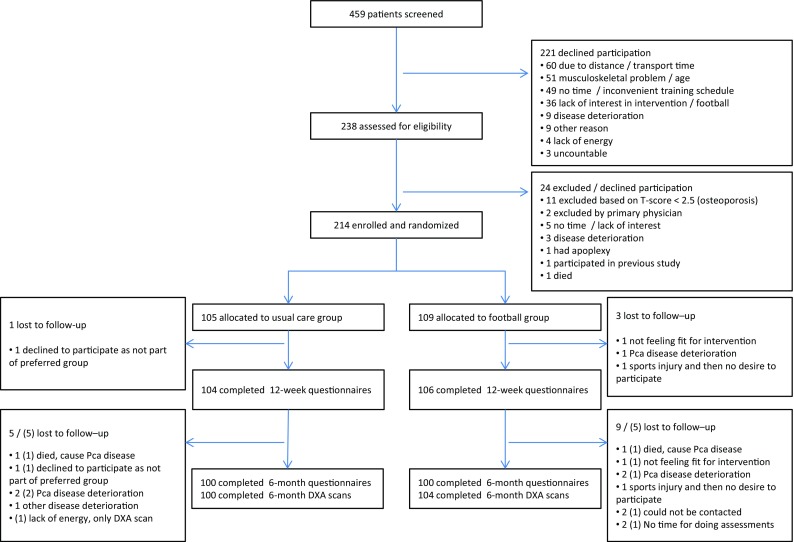
Flow of participants. DXA Dual-energy X-ray absorptiometry, Pca prostate cancer, () DXA lost to follow up
Table 1.
Baseline characteristics of patients according to allocation group
| Characteristic | FG (n = 109) | UG (n = 105) | Total (n = 214) |
|---|---|---|---|
| Age (years) | 67.8 ± 6.2 | 69.0 ± 6.2 | 68.4 ± 6.2 |
| Employment status | |||
| Paid work | 26 (24) | 26 (25) | 52 (24) |
| Unemployed | 2 (2) | 0 (0) | 2 (1) |
| Sick leave | 1 (1) | 2 (2) | 3 (1) |
| Retired | 80 (73) | 77 (73) | 157 (73) |
| Education | |||
| No education | 7 (6) | 5 (5) | 12 (6) |
| Primary education (9th/10th grade) | 4 (4) | 5 (5) | 9 (4) |
| Vocational education | 33 (30) | 28 (27) | 61 (29) |
| Secondary education (12th grade) | 10 (9) | 15 (14) | 25 (12) |
| Completed college or higher | 55 (50) | 52 (50) | 107 (50) |
| Marital status | |||
| Married or living with partner | 92 (84) | 93 (89) | 185 (86) |
| Other (single, divorced, or widowed) | 17 (16) | 12 (11) | 29 (14) |
| Smoking status | |||
| Yes | 17 (16) | 11 (10) | 28 (13) |
| No, stopped | 49 (45) | 51 (49) | 100 (47) |
| No, never | 43 (39) | 43 (41) | 86 (40) |
| Alcohol consumption (drinks per week) | 9.1 ± 7.2 | 8.5 ± 7.0 | 8.8 ± 7.1 |
| Time since diagnosis, years | 3.0 ± 2.7 | 3.8 ± 3.9 | 3.4 ± 3.4 |
| Risk group | |||
| Localized, prostatectomized | 16 (15) | 15 (14) | 31 (14) |
| Localized, not prostatectomized | 27 (25) | 28 (27) | 55 (26) |
| Locally advanced | 39 (36) | 42 (40) | 81 (38) |
| Metastatic | 26 (24) | 19 (18) | 45 (21) |
| Unknown | 1 (1) | 1 (1) | 2 (1) |
| ISUP Gleason Grading | |||
| Group 1 (Gleason score 2–6) | 15 (14) | 13 (12) | 28 (13) |
| Group 2 (Gleason score 3 + 4) | 29 (27) | 36 (34) | 65 (30) |
| Group 3 (Gleason score 4 + 3) | 18 (17) | 13 (12) | 31 (14) |
| Group 4 (Gleason score 8) | 18 (17) | 13 (12) | 31 (14) |
| Group 5 (Gleason score 9–10) | 28 (26) | 24 (23) | 52 (24) |
| Unknown | 1 (1) | 6 (6) | 7 (3) |
| Number of men with bone metastasis | 22 (20) | 19 (18) | 41 (19) |
| Current treatment at baseline | |||
| No treatmenta | 46 (42) | 42 (40) | 88 (41) |
| Anti-androgen monotherapy | 15 (14) | 21 (20) | 36 (17) |
| Castration (surgical or pharmacological) | 46 (42) | 41 (39) | 87 (41) |
| Unknown | 2 (2) | 1 (1) | 3 (1) |
| Previous treatment at baseline | |||
| Prostatectomy | 27 (25) | 39 (37) | 66 (31) |
| Radiation | 37 (34) | 29 (28) | 66 (31) |
| ADT and radiation with curative intent | 21 (19) | 16 (15) | 37 (17) |
| Chemotherapy (docetaxel) | 9 (8) | 10 (10) | 19 (9) |
| No prior or current treatment | 21 (20) | 24 (22) | 45 (21) |
| Time on ADT, median days (n = 88) | 512.5 (208–881) | 580 (235–1089) | 520 (213–982) |
| Number of comorbidities | |||
| Zero | 35 (32) | 29 (28) | 64 (30) |
| One | 45 (41) | 46 (44) | 91 (43) |
| Two | 16 (15) | 18 (17) | 34 (16) |
| Three or more | 13 (12) | 12 (11) | 25 (12) |
| Baseline values on outcomes | |||
| Prostate cancer-specific QoL (FACT-P, points) | 123.7 ± 17.3 | 124.6 ± 16.6 | 124.1 ± 16.9 |
| Lean body mass (kg) | 56.6 ± 6.3 | 57.5 ± 7.1 | 57.0 ± 6.7 |
| Fat mass (kg) | 27.5 ± 8.0 | 28.3 ± 8.9 | 27.9 ± 8.4 |
| Whole-body BMC (kg) | 3.1 ± 0.5 | 3.1 ± 0.6 | 3.1 ± 0.5 |
| Whole-body BMD (g/cm2) | 1.215 ± 0.116 | 1.219 ± 0.125 | 1.217 ± 0.120 |
| Femoral neck BMD (g/cm2) | 0.877 ± 0.155 | 0.877 ± 0.147 | 0.877 ± 0.151 |
| Total hip BMD (g/cm2) | 1.015 ± 0.132 | 1.025 ± 0.138 | 1.020 ± 0.134 |
| Lumbar BMD (g/cm2) | 1.188 ± 0.226 | 1.189 ± 0.223 | 1.188 ± 0.224 |
| Weekly self-reported PA (median MET)b | 3649 (1824–6693) | 4098 (2394–7732) | 4046 (2010–6845) |
Data are presented as mean ± standard deviation, n (%) or median (interquartile range)
ADT androgen-deprivation therapy, BMC bone mineral content, BMD bone mineral density, FACT-P Functional Assessment of Cancer Therapy-Prostate questionnaire, FG football group, ISUP International Society of Urological Pathology, MET metabolic equivalent task, PA physical activity, QoL quality of life, UG usual care group
aWatchful waiting, active surveillance, previous prostatectomy, or radiation
b102 patients in the FG and 96 patients in the UG
In total, 105 patients were randomly allocated to the UG and 109 to the FG. Retention in the study was 98% at 12 weeks and 95% at 6 months. For the FG, median attendance from baseline to 12 weeks was 16 [interquartile ratio (IQR) 10] trainings (64%), and from baseline to 6 months it was 28 (IQR 19) trainings (59%). From baseline to 12 weeks, and from baseline to 6 months, 82 (75%) and 71 (65%) participants, respectively, had an attendance to the intervention of ≥ 50% defining the per-protocol population.
Intervention
Fidelity to the intervention manual by football coaches was 3.8 (3.7–3.9) points on a 5-point Likert scale, where 1 was non-adherence and 5 was perfect adherence to the intervention manual. During the trial, 616 football sessions were conducted. On average, sessions lasted 58.8 (58.4–59.3) minutes, with 20.4 (20.0–20.8) minutes spent on warm-up, 16.1 (15.5–16.6) minutes on drills, and 22.3 (21.8–22.9) on match play.
Outcomes
At 12 weeks, adjusted mean change on the FACT-P score was 1.9 points (95% CI − 1.0–4.8; P = 0.20) higher in the FG than in the UG. The FACT-P adjusted mean change value at 6 months was 0.5 points (95% CI − 2.8–3.8; P = 0.76) (Fig. 2).
Fig. 2.
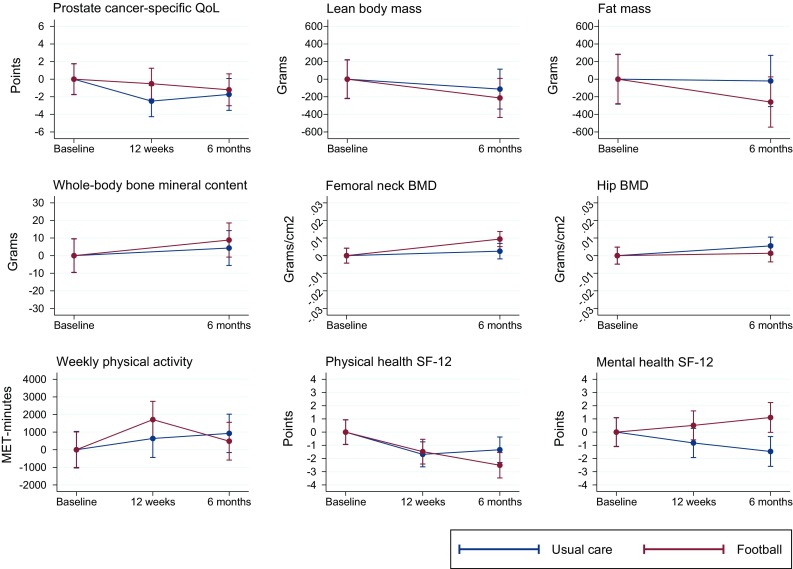
Mean change in outcomes, according to allocation group and time points. QoL quality of life, BMD bone mineral density
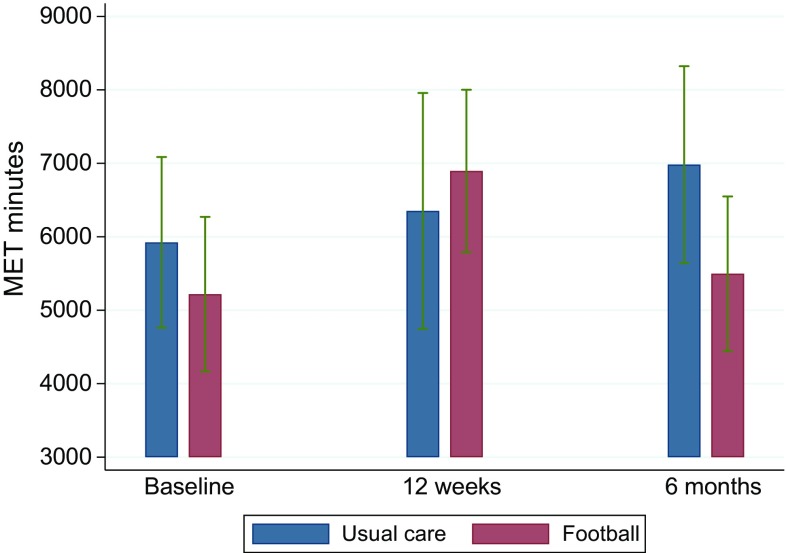
At both 12 weeks and 6 months, the level of self-reported physical activity was similar between the FG and the UG (P = 0.46 and 0.34) (Table 2). A significant within-group increase in the FG was seen from baseline to 12 weeks, with an increase of 115 min in the vigorous activity category. This increase was completely reversed at 6 months (see Fig. 3).
Table 2.
Outcomes for the intention-to-treat population according to group allocation
| FG (n = 109) | UG (n = 105) | Covariance analysis, difference between groups | Unadjusted analyses, t test, difference between groups | |||||
|---|---|---|---|---|---|---|---|---|
| N | Mean (95% CI) | N | Mean (95% CI) | Adjusted for ADT, age, and baseline score | P value | Unadjusted | P value | |
| Change in prostate cancer-specific quality of life (points; higher is better) | ||||||||
| 12 weeks | 106 | − 0.5 (− 2.6 to 1.5) | 104 | − 2.5 (− 4.6 to − 0.4) | 1.9 (− 1.0 to 4.8) | 0.20 | 2.0 (− 0.9 to 4.9) | 0.18 |
| 6 months | 100 | − 1.2 (− 3.6 to 1.2) | 100 | − 1.7 (− 4.1 to 0.6) | 0.5 (− 2.8 to 3.8) | 0.76 | 0.5 (− 2.8 to 3.9) | 0.76 |
| Change in lean body mass (kg) | ||||||||
| 6 months | 104 | − 0.2 (− 0.5 to 0.1) | 100 | − 0.1 (− 0.4 to 0.2) | − 0.1 (− 0.6 to 0.3) | 0.60 | − 0.1 (− 0.6 to 0.4) | 0.66 |
| Change in fat mass (kg) | ||||||||
| 6 months | 104 | − 0.3 (− 0.7 to 0.2) | 100 | 0.0 (− 0.4 to 0.4) | − 0.3 (− 0.9 to 0.3) | 0.28 | − 0.2 (− 0.8 to 0.3) | 0.42 |
| Change in whole-body bone mineral content (g) | ||||||||
| 6 months | 104 | 8.9 (− 5.1 to 22.8) | 100 | 4.3 (− 9.9 to 18.5) | 3.2 (− 16.9 to 23.3) | 0.75 | 4.5 (− 15.3 to 24.4) | 0.65 |
| Change in whole-body BMD (g/cm2) | ||||||||
| 6 months | 104 | 0.002 (− 0.002 to 0.007) | 100 | 0.001 (− 0.003 to 0.006) | 0.001 (− 0.006 to 0.007) | 0.87 | 0.001 (− 0.006 to 0.007) | 0.75 |
| Femoral neck BMD (g/cm2) | ||||||||
| 6 months | 104 | 0.009 (0.003 to 0.016) | 100 | 0.003 (− 0.003 to 0.009) | 0.007 (− 0.002 to 0.016) | 0.12 | 0.007 (− 0.002 to 0.016) | 0.13 |
| Change in total hip BMD (g/cm2) | ||||||||
| 6 months | 104 | 0.001 (− 0.006 to 0.008) | 100 | 0.006 (− 0.002 to 0.013) | − 0.005 (− 0.015 to 0.005) | 0.34 | − 0.004 (− 0.014 to 0.006) | 0.41 |
| Change in lumbar spine L1–L4 BMD (g/cm2) | ||||||||
| 6 months | 104 | 0.014 (0.003 to 0.025) | 99 | 0.011 (0.000 to 0.022) | 0.003 (− 0.013 to 0.019) | 0.72 | 0.003 (− 0.013 to 0.018) | 0.74 |
| Change in weekly physical activity (MET minutes) | ||||||||
| 12 weeks | 96 | 1710 (328 to 3092) | 89 | 638 (− 797 to 2073) | 698 (1158 to 2555) | 0.46 | 1072 (− 920 to 3064) | 0.29 |
| 6 months | 90 | 485 (− 759 to 1729) | 87 | 928 (− 337 to 2193) | − 754 (− 2316 to 808) | 0.34 | − 443 (− 2217 to 1332) | 0.62 |
| Change in general physical health (SF-12) | ||||||||
| 12 weeks | 106 | − 1.5 (− 2.6 to − 0.4) | 104 | − 1.7 (− 2.8 to − 0.6) | 0.1 (− 1.4 to 1.6) | 0.92 | 0.2 (− 1.3 to 1.8) | 0.79 |
| 6 months | 100 | − 2.5 (− 3.8 to − 1.2) | 100 | − 1.3 (− 2.6 to − 0.1) | − 1.1 (− 2.9 to 0.6) | 0.21 | − 1.2 (− 3.0 to 0.6) | 0.20 |
| Change in general mental health (SF-12) | ||||||||
| 12 weeks | 106 | 0.5 (− 0.8 to 1.8) | 104 | − 0.8 (− 2.1 to 0.5) | 1.5 (− 0.2 to 3.3) | 0.08 | 1.3 (− 0.5 to 3.2) | 0.15 |
| 6 months | 100 | 1.1 (− 0.4 to 2.6) | 100 | − 1.5 (− 2.9 to 0.0) | 2.7 (0.8 to 4.6) | 0.01 | 2.6 (0.5 to 4.7) | 0.02 |
Data are presented as n or mean (95% confidence interval) unless otherwise indicated
ADT androgen-deprivation therapy, BMD bone mineral density, CI confidence interval, FG football group, MET metabolic equivalent, SF-12 12-Item Short Form Health Survey, UG usual care group
Fig. 3.
Weekly MET minutes, according to allocation group. MET metabolic Equivalent
No significant differences in change between the two groups were seen in lean body mass, fat mass, and hip, femoral neck, spine, and whole-body bone mineral density (Table 2). For subgroup analyses prespecified for the ADT population, no difference from the ITT population was observed (see Table 2 in the ESM). Per-protocol analyses likewise yielded no difference from the results of the ITT population (see Table 4 in the ESM). To examine whether heterogeneity of participants affected results, sensitivity analyses were conducted that included disease stage, treatment, and Gleason score, but they did not change the results, e.g., the adjusted mean change on 12-week FACT-P score was 2.3 points (95% CI − 0.6–5.1; P = 0.12) higher in the FG than in the UG.
In analyses where participants were stratified according to baseline physical activity level, participants who were categorised as being low or moderate physically active had a non-significant increase in lean body mass and a significant decrease in fat mass of 0.5 kg (95% CI − 0.9 to − 0.0; P = 0.04). The opposite pattern was seen for highly active participants, i.e., non-significant decrease in lean body mass and non-significant increase in fat mass (see Fig. 4).
Fig. 4.
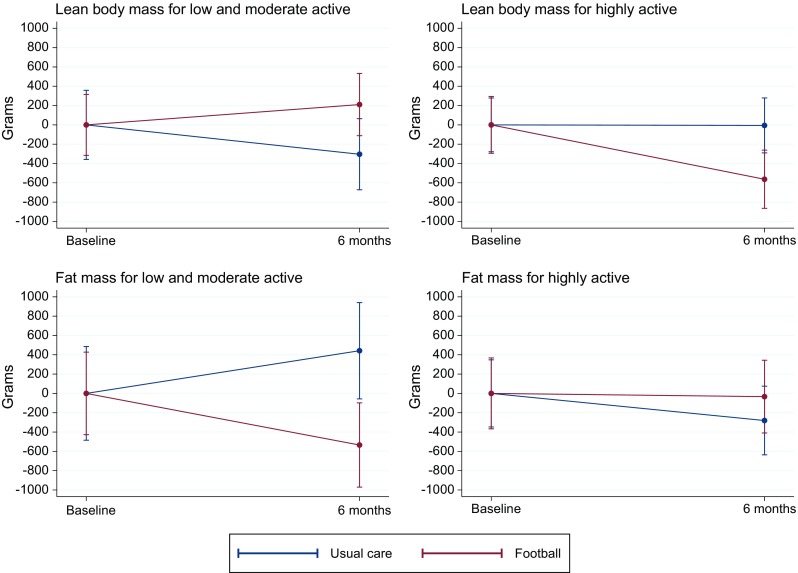
Mean changes in body composition based on baseline physical activity
For physical health (SF-12) at 12 weeks, the adjusted mean change was 0.1 points (95% CI − 1.4–1.6; P = 0.92) higher in the FG than in the UG. The score at 6 months was 1.1 points (95% CI − 2.9–0.6; P = 0.21) lower in the FG than in the UG. For mental health (SF-12) at 12 weeks, the adjusted mean change was 1.5 points (95% CI − 0.2–3.3; P = 0.08) higher in the FG than in the UG. The score at 6 months was 2.7 points (95% CI 0.8–4.6; P = 0.006) higher in the FG than in the UG.
Safety Outcomes, Adverse Events, and Sports Injuries
FG participants reported ten falls compared with six falls in the UG (P = 0.44). Two fractures were reported in the UG and one in the FG (P = 1.00). Overall, 33 hospital admissions (SAEs by GCP definitions) were reported, 22 in the UG and 11 in the FG (P = 0.12) (see Table 1 in the ESM). One SAE related to the intervention was reported in the study and occurred due to an excoriation/scratch sustained when a shin guard scratched the lower leg. This later became infected and was not treated with the adequate antibiotic, resulting in a skin transplant from the thigh to the shin. The participant returned to football play after 8 weeks. Two deaths, one in each group and both due to prostate cancer, were reported. A total of 60 sports injuries (58 minor and two major) resulted in either ending a football session early or missing one or more football session. The most frequent injuries were muscle strain or sprains (n = 40), and the two major injuries were a partially and a fully ruptured Achilles tendon (see Table 6 in the ESM).
Cost and Continuation
The cost of delivering the FG intervention was Danish Krone (DKK) 297,500 ($US46,213 on 17 October 2018). After the 6-month study period, 64 (59%) of the 109 participants allocated to football continued the intervention and joined their local football club.
Discussion
This trial demonstrated it was possible to recruit and retain men with prostate cancer, with and without metastases and comorbidities, to community-based football. We found no differences in changes in prostate cancer-specific QoL or in body composition between the FG and the UG. However, at the 6-month follow-up, we found improvement in the exploratory outcome concerning the mental health domain of the SF-12 in the FG compared with the UG. The comparative safety outcomes demonstrated no differences in incidence rates of fractures, falls, and hospital admissions. FG participants experienced a substantial number of mostly minor sports injuries during the intervention. While these injuries did not lead to an increase in withdrawal from/termination of the intervention, they may have contributed to the decrease in physical activity after 12 weeks in participants recovering from their sports injury. A concurrent qualitative study including FG participants who had sustained an injury indicated that participants viewed injuries as a largely acceptable, intrinsic feature of football that also provided an opportunity to embody masculine ideals (unpublished data). We had expected a general deterioration in QoL and body composition outcomes in the UG because of the natural course of the disease and the side effects of treatments [6, 30, 31]. However, in light of the self-reported physical activity data, which delineated that both groups were highly physically active, the finding on equality is not surprising. Physical activity reported at baseline also shows that our sample of patients with prostate cancer was highly physically active before randomization. Therefore, we performed sensitivity analyses separating highly physically active participants from those who were only low or moderately active. As Fig. 4 shows, different patterns were observed for changes in muscle mass and fat mass in the two populations, indicating that football might only benefit patients who are less physically active. This is in line with other findings in a recently reported exercise trial for men with prostate cancer [32].
A previous study by Uth et al. [13] that was optimized to explore the causal effects of football, i.e., comparing football (delivered in an ideal setting) with no football, showed an improvement in lean body mass of 700 g favoring football. The hypothesis for the current pragmatic trial was that football would be a more potent intervention than the standard usual care (rehabilitation offered by local authorities), but the present findings did not confirm this. The discrepancy between the findings in the two trials might be due to differences in the interventions in the comparison groups. It can be argued that control group participants perhaps are less likely to find physical activity interventions by themselves knowing that they will be offered an intervention after 12 weeks, which was the case in the previous explanatory trial. In the current pragmatic trial, control group participants knew they would not be offered the football intervention for a whole year. With regard to attendance and workload during football training, our data show results similar to those from the previous explanatory trial [33]. In the explanatory trial, where participants were coached by a professional sport physiologist, participants ran 905 ± SD 297 m at speeds > 6 km/h and 2646 ± 705 m per session. In comparison, activity profiles sampled conveniently (n = 67) in the current pragmatic trial with local coaches showed that participants ran 973 ± 582 at running speeds > 6 km/h and a total of 2684 ± 918 m per session.
Several recently published systematic reviews on exercise for men with prostate cancer have outlined that exercise improves QoL, fatigue, fitness, and functional outcomes [8, 9, 34–36]. The studies generally focused on delivering exercise in healthcare clinics. Recent studies have focused on safety, feasibility, and initial efficacy of physical exercise for patients with prostate cancer with skeletal metastases [37, 38]. Even though the current trial was not designed to explore the issue of safety for this subpopulation, we enrolled 41 patients with skeletal metastases. We tested whether men with metastases had fewer training sessions than those without metastases and found this not to be the case (data not shown). This suggests that future studies powered and designed to test whether vigorous exercise is safe for men with bone metastases are possible.
We conducted a qualitative study, which indicated that men with prostate cancer undergoing ADT viewed football as an opportunity to take back control and responsibility for their own health without being in the role of patient, and that football enabled peer-to-peer caring behavior in a male setting [39]. This can be interpreted as being in alignment with the improvement in mental health, which at the same time should be interpreted with caution, as this was an exploratory outcome chosen primarily for the purpose of conducting health economic analysis. Only 4% (data not shown) of participants played football before entering the study and 57% joined their local club and continued play after the study period.
The financial cost of delivering the football intervention was DKK2600 ($US404) per participant. In comparison, a report by the Danish National Centre for Social Research estimated the average cost of rehabilitation delivered by two different local authorities in Denmark to be DKK29,211 ($US4538) for 12.9 weeks of rehabilitation and DKK32,478 ($US5045) for 10.8 weeks per patient [40].
Collectively, this information supports the suggestion that sports participation is appealing and feasible as a public health strategy for clinical populations [16, 17].
The trial had various methodological strengths. First, assessments and DXA scans were performed by blinded assessors, and the self-reported questionnaires were automated via a web-based system, helping to minimize errors and limit the influence of trial staff on participants. Safety outcomes and SAEs were followed-up using the same method for all participants; at the same time, all injuries related to the intervention were collected prospectively. Second, attrition bias was minimal as retention was 98 and 95%. Third, the trial followed GCP principles, ensuring homogeneity between study sites.
Limitations include the obvious inability to blind participants and coaches delivering the intervention. While this can induce bias on the self-reported outcomes, these measures yielded similar results to the objectively measured and assessor-blinded outcomes. When claiming equality between groups, several methodological features need to be considered: the sample size should be large because CIs should not exceed a certain marginal size, which preferably should have been set in advance, though this was not the case in this trial. In the interpretation of the trial results, we used the delta values stated a priori from our original sample size calculations, i.e., the MCID of 6 points for FACT-P [26] and a 700 g change in lean body mass from Uth et al. [13]. We found that 95% CIs did not exceed these margins. We observed lesser variations than expected in outcomes, which is why the trial had more power than anticipated. Last, a trial can wrongfully claim equivalence in safety if a sufficient proportion of participants are lost to follow-up or if participants not receiving the intervention are diluting the estimates of the ITT analysis. This trial had a negligible withdrawal rate, and we performed per-protocol analysis as sensitivity analyses to address any dilution (see the ESM). In regard to the per-protocol population defined as adherence of 50%, a limitation is that only two sessions were scheduled per week and higher attendance could have incurred possible benefits, especially for the physiological outcomes. Regarding generalizability, the pragmatic multicentre design is valuable as it ensures that the estimated results reflect a real-world implementation of community-based football compared with relevant alternatives. One issue concerning the pragmatic design is whether the heterogeneity of participants potentially reduced the ability to infer validly on the measured outcomes. For this reason, we performed post hoc sensitivity analyses with any baseline covariate that might be a prognostic factor to test whether this would change the results. However, it only changed the results minimally and did not lead to any difference in interpretations. We also report subgroup analyses for the ADT population, which, as the width of CIs shows, was adequately powered with a total of 87 participants.
Conclusion
In the current trial, football implemented in local football clubs under the supervision of lay coaches was a feasible exercise strategy for patients with prostate cancer. It improved the exploratory outcome mental health but offered no additional benefits with respect to prostate cancer-specific QoL or body composition after 6 months compared with encouragement/referral to physical activity and rehabilitation. As the risk of sports injuries seems to be an embedded aspect of participating in football, patients wishing to partake must accept this. Future research on sport, exercise, and physical activity should be conducted in real-world structures to enable low-cost upscaling.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the participants, football coaches, and nurses (Helene Holm Pedersen, Lisa Gruschy, Birgit K. Bach, Bodil Petersen, Johanne Vind Hansen, Stine Thim, Karina Klovgaard, Sara Thor, Gitte Honoré, Anne-Kirsten Bech, and Maj-Britt Jensen) for making this trial possible. We thank Morten Zacho for technical help with data collection web applications. We thank trial steering group members (Professor Tine Tjørnhøj-Thomsen and Kenneth Grønlund Rasmussen from the Danish Football Association [DBU]), patient partners (Michael Nyberg, Jens Ingwersen, and Axel Petersen), and research colleagues (Nanna Maria Hammer, Ditte Marie Bruun, Mette Leth, Malene Kronborg, Mette Rørth, Morten Randers, Jacob Uth, Cecilie Køllner Olsen, and Phillip Bredahl Mogensen) for their valuable input.
Abbreviations
- ADT
Androgen deprivation therapy
- GCP
Good Clinical Practice
- FG
Football Group
- UG
Usual care Group
- DXA
Dual-energy X-ray Absorptiometry
- QoL
Quality of Life
- FACT-P
Functional Assessment of Cancer Therapy-Prostate
- IPAQ
International Physical Activity Questionnaire
- SF-12
12-Item Short Form Health Survey
- SAEs
Serious Adverse Events
- MCID
Minimal clinical important difference
- SD
Standard Deviation
- ITT
Intention-to-treat
- IQR
Interquartile range
- CI
Confidence Interval
Funding
TrygFonden sponsored the trial. The Danish Cancer Society supplied funding for DXA scans. The funders and sponsors of the study played no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Author Contributions
EDB contributed to the design of the study, the background literature search, funding applications, and patient recruitment; was responsible for data collection, data analysis, and data interpretation; and wrote the first draft of the manuscript. JM contributed to the design of the study and to funding applications, oversaw the data collection and data interpretation, and critically revised the manuscript. KB contributed to the design of the study, funding applications, patient recruitment, data interpretation, and critical revision of manuscript. ABJ and THP contributed to patient recruitment, data collection, data interpretation, and critical revision of manuscript. AT contributed to data analysis and critical revision of manuscript. ARE and JFC contributed to data collection, data interpretation, and critical revision of the manuscript. MB, MHP, PBØ, and SSM contributed to patient recruitment, data interpretation, and critical revision of the manuscript. CJ, PK, and MR contributed to the design of the study, funding applications, data interpretation, and critical revision of manuscript. EDB and JM are guarantors. EDB, KB, and JM had access to all the data. All authors commented on drafts and approved the final report. The corresponding author had final responsibility for the decision to submit for publication.
Ethical Approval
The study was approved by the Ethics Committee for the Capital Region of Denmark (H-2-2014-099) and the Danish Data Protection Agency. All participants provided informed consent before participating.
Conflicts of Interest
All authors have completed the Unified Competing Interest form (available on request from the corresponding author) and received no support from any organization for the submitted work. Eik Dybboe Bjerre, Klaus Brasso, Anders Bojer Jørgensen, Thomas Hindborg Petersen, Alexandra Röthlin Eriksen, Anders Tolver, Jesper Frank Christensen, Mads Hvid Poulsen, Søren Sørensen Madsen, Peter Busch Østergren, Michael Borre, Christoffer Johansen, Mikael Rørth, and Julie Midtgaard have had no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years. Peter Krustrup has received funding for other research activities from the DBU, Union of European Football Associations (UEFA) and FIFA in the previous 3 years. No other relationships or activities could have influenced the submitted work.
Availability of Data and Materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Contributor Information
Eik Dybboe Bjerre, Phone: +45 22749076, Email: eb@ucsf.dk.
Klaus Brasso, Email: klaus.brasso@regionh.dk.
Anders Bojer Jørgensen, Email: anders.b.joergensen@hotmail.com.
Thomas Hindborg Petersen, Email: thomas.hindborg@gmail.com.
Alexandra Röthlin Eriksen, Email: alexandraeriksen@hotmail.com.
Anders Tolver, Email: tolver@math.ku.dk.
Jesper Frank Christensen, Email: jesper.frank.christensen@regionh.dk.
Mads Hvid Poulsen, Email: mads.poulsen@rsyd.dk.
Søren Sørensen Madsen, Email: soeren.soerensen.madsen@rsyd.dk.
Peter Busch Østergren, Email: peter.busch.oestergren@regionh.dk.
Michael Borre, Email: borre@clin.au.dk.
Peter Krustrup, Email: pkrustrup@health.sdu.dk.
Christoffer Johansen, Email: christof@cancer.dk.
Mikael Rørth, Email: mikael.roerth@regionh.dk.
Julie Midtgaard, Email: julie@ucsf.dk.
References
- 1.Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, Abdulkader RS, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abajobir AA, Abbafati C, Abbas KM, Abd-Allah F, Abera SF, Aboyans V, et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1151–1210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skolarus TA, Wolf AMD, Erb NL, Brooks DD, Rivers BM, Underwood W, et al. American Cancer Society prostate cancer survivorship care guidelines. CA Cancer J Clin. 2014;64(4):225–249. doi: 10.3322/caac.21234. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert SM, Kuo YF, Shahinian VB. Prevalent and incident use of androgen deprivation therapy among men with prostate cancer in the United States. Urol Oncol. 2011;29(6):647–653. doi: 10.1016/j.urolonc.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donovan KA, Walker LM, Wassersug RJ, Thompson LM, Robinson JW. Psychological effects of androgen-deprivation therapy on men with prostate cancer and their partners. Cancer. 2015;121(24):4286–4299. doi: 10.1002/cncr.29672. [DOI] [PubMed] [Google Scholar]
- 6.Alibhai SM, Breunis H, Timilshina N, Naglie G, Tannock I, Krahn M, et al. Long-term impact of androgen-deprivation therapy on physical function and quality of life. Cancer. 2015;121(14):2350–2357. doi: 10.1002/cncr.29355. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen PL, Alibhai SM, Basaria S, D’Amico AV, Kantoff PW, Keating NL, et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol. 2015;67(5):825–836. doi: 10.1016/j.eururo.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Gardner JR, Livingston PM, Fraser SF. Effects of exercise on treatment-related adverse effects for patients with prostate cancer receiving androgen-deprivation therapy: a systematic review. J Clin Oncol. 2014;32(4):335–346. doi: 10.1200/JCO.2013.49.5523. [DOI] [PubMed] [Google Scholar]
- 9.Bourke L, Smith D, Steed L, Hooper R, Carter A, Catto J, et al. Exercise for men with prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;69(4):693–703. doi: 10.1016/j.eururo.2015.10.047. [DOI] [PubMed] [Google Scholar]
- 10.Kenfield SA, Stampfer MJ, Giovannucci E, Chan JM. Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J Clin Oncol. 2011;29(6):726–732. doi: 10.1200/JCO.2010.31.5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parsons JK. Prostate cancer and the therapeutic benefits of structured exercise. J Clin Oncol. 2014;32(4):271–272. doi: 10.1200/JCO.2013.53.4289. [DOI] [PubMed] [Google Scholar]
- 12.Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ. 2015;350:h2147. doi: 10.1136/bmj.h2147. [DOI] [PubMed] [Google Scholar]
- 13.Uth J, Hornstrup T, Schmidt JF, Christensen JF, Frandsen C, Christensen KB, et al. Football training improves lean body mass in men with prostate cancer undergoing androgen deprivation therapy. Scand J Med Sci Sports. 2014;24(Suppl 1):105–112. doi: 10.1111/sms.12260. [DOI] [PubMed] [Google Scholar]
- 14.Uth J, Hornstrup T, Christensen JF, Christensen KB, Jorgensen NR, Schmidt JF, et al. Efficacy of recreational football on bone health, body composition, and physical functioning in men with prostate cancer undergoing androgen deprivation therapy: 32-week follow-up of the FC prostate randomised controlled trial. Osteopor Int. 2016;27(4):1507–1518. doi: 10.1007/s00198-015-3399-0. [DOI] [PubMed] [Google Scholar]
- 15.Bruun DM, Bjerre E, Krustrup P, Brasso K, Johansen C, Rorth M, et al. Community-based recreational football: a novel approach to promote physical activity and quality of life in prostate cancer survivors. Int J Environ Res Pub Health. 2014;11(6):5567–5585. doi: 10.3390/ijerph110605567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan KM, Thompson AM, Blair SN, Sallis JF, Powell KE, Bull FC, et al. Sport and exercise as contributors to the health of nations. Lancet (London, England). 2012;380(9836):59–64. doi: 10.1016/S0140-6736(12)60865-4. [DOI] [PubMed] [Google Scholar]
- 17.Reis RS, Salvo D, Ogilvie D, Lambert EV, Goenka S, Brownson RC. Scaling up physical activity interventions worldwide: stepping up to larger and smarter approaches to get people moving. Lancet (London, England). 2016;388(10051):1337–1348. doi: 10.1016/S0140-6736(16)30728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bjerre E, Bruun DM, Tolver A, Brasso K, Krustrup P, Johansen C, et al. Effectiveness of community-based football compared to usual care in men with prostate cancer: protocol for a randomised, controlled, parallel group, multicenter superiority trial (The FC Prostate Community Trial) BMC Cancer. 2016;16(1):767. doi: 10.1186/s12885-016-2805-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bjerre ED, Leth M, Hammer NM, Midtgaard J. Development of an educational program for non-professional soccer coaches in charge of community-based soccer in men with prostate cancer: a qualitative study. Sports Med Open. 2018;4(1):31. doi: 10.1186/s40798-018-0147-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sundhedsstyrelsen. Fysisk aktivitet—håndbog om forebyggelse og behandling. 3.1 edn. Rosendahls-Schultz Grafisk A/S; 2011.
- 21.Soligard T, Myklebust G, Steffen K, Holme I, Silvers H, Bizzini M, et al. Comprehensive warm-up programme to prevent injuries in young female footballers: cluster randomised controlled trial. BMJ. 2008;337:a2469. doi: 10.1136/bmj.a2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kristiansen M, Adamsen L, Piil K, Halvorsen I, Nyholm N, Hendriksen C. A three-year national follow-up study on the development of community-level cancer rehabilitation in Denmark. Scand J Pub Health. 2017. 10.1177/1403494817746535. [DOI] [PubMed]
- 23.Esper P, Mo F, Chodak G, Sinner M, Cella D, Pienta KJ. Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy-prostate instrument. Urology. 1997;50(6):920–928. doi: 10.1016/S0090-4295(97)00459-7. [DOI] [PubMed] [Google Scholar]
- 24.Sprangers MA. Response-shift bias: a challenge to the assessment of patients’ quality of life in cancer clinical trials. Cancer Trea Rev. 1996;22(Suppl A):55–62. doi: 10.1016/S0305-7372(96)90064-X. [DOI] [PubMed] [Google Scholar]
- 25.Group IEW. ICH Harmonised Tripartite Guideline. Clinical safety data management: definitions and standards for expedited reporting. Step 4 version. 1994.
- 26.Cella D, Nichol MB, Eton D, Nelson JB, Mulani P. Estimating clinically meaningful changes for the Functional Assessment of Cancer Therapy–Prostate: results from a clinical trial of patients with metastatic hormone-refractory prostate cancer. Value Health. 2009;12(1):124–129. doi: 10.1111/j.1524-4733.2008.00409.x. [DOI] [PubMed] [Google Scholar]
- 27.Segal RJ, Reid RD, Courneya KS, Malone SC, Parliament MB, Scott CG, et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol. 2003;21(9):1653–1659. doi: 10.1200/JCO.2003.09.534. [DOI] [PubMed] [Google Scholar]
- 28.IPAQ. Guidelines for data processing and analysis of the international physical activity questionnaire (IPAQ)—short and long forms, revised on November 2005. http://www.ipaq.ki.se/scoring.pdf Accessed 10 Nov 2015.
- 29.Hernán MA, Hernandez-Diaz S, Robins JM. Randomized trials analyzed as observational studies. Ann Intern Med. 2013;159(8):560–562. doi: 10.7326/0003-4819-159-8-201310150-00709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith MR, Finkelstein JS, McGovern FJ, Zietman AL, Fallon MA, Schoenfeld DA, et al. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab. 2002;87(2):599–603. doi: 10.1210/jcem.87.2.8299. [DOI] [PubMed] [Google Scholar]
- 31.Herr HW, O’Sullivan M. Quality of life of asymptomatic men with nonmetastatic prostate cancer on androgen deprivation therapy. J Urol. 2000;163(6):1743–1746. doi: 10.1016/S0022-5347(05)67533-7. [DOI] [PubMed] [Google Scholar]
- 32.Taaffe DR, Newton RU, Spry N, Joseph D, Chambers SK, Gardiner RA, et al. Effects of different exercise modalities on fatigue in prostate cancer patients undergoing androgen deprivation therapy: a year-long randomised controlled trial. Eur Urol. 2017;72(2):293–299. doi: 10.1016/j.eururo.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 33.Uth J, Hornstrup T, Christensen JF, Christensen KB, Jorgensen NR, Helge EW, et al. Football training in men with prostate cancer undergoing androgen deprivation therapy: activity profile and short-term skeletal and postural balance adaptations. Eur J Appl Physiol. 2016;116(3):471–480. doi: 10.1007/s00421-015-3301-y. [DOI] [PubMed] [Google Scholar]
- 34.Yunfeng G, Weiyang H, Xueyang H, Yilong H, Xin G. Exercise overcome adverse effects among prostate cancer patients receiving androgen deprivation therapy: an update meta-analysis. Medicine. 2017;96(27):e7368. doi: 10.1097/MD.0000000000007368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vashistha V, Singh B, Kaur S, Prokop LJ, Kaushik D. The effects of exercise on fatigue, quality of life, and psychological function for men with prostate cancer: systematic review and meta-analyses. Eur Urol Focus. 2016;2(3):284–295. doi: 10.1016/j.euf.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 36.Teleni L, Chan RJ, Chan A, Isenring EA, Vela I, Inder WJ, et al. Exercise improves quality of life in androgen deprivation therapy-treated prostate cancer: systematic review of randomised controlled trials. Endocr Relat Cancer. 2016;23(2):101–112. doi: 10.1530/ERC-15-0456. [DOI] [PubMed] [Google Scholar]
- 37.Cormie P, Newton RU, Spry N, Joseph D, Taaffe DR, Galvao DA. Safety and efficacy of resistance exercise in prostate cancer patients with bone metastases. Prostate Cancer Prostatic Dis. 2013;16(4):328–335. doi: 10.1038/pcan.2013.22. [DOI] [PubMed] [Google Scholar]
- 38.Galvão DA, Taaffe DR, Spry N, Cormie P, Joseph D, Chambers SK, et al. Exercise preserves physical function in prostate cancer patients with bone metastases. Med Sci Sports Exerc. 2018;50(3):393–399. doi: 10.1249/MSS.0000000000001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bruun DM, Krustrup P, Hornstrup T, Uth J, Brasso K, Rorth M, et al. “All boys and men can play football”: a qualitative investigation of recreational football in prostate cancer patients. Scand J Med Sci Sports. 2014;24(Suppl 1):113–121. doi: 10.1111/sms.12193. [DOI] [PubMed] [Google Scholar]
- 40.Heidi Hesselberg HL, Maj Bjerre, Lea Graff, Tine Rostgaard, Freya Casier, Torben Fridberg. Rehabilitering PÅ Ældreområdet. Afprøvning Af En model for rehabiliterinsforløb I to kommuner. København: SFI—Det Nationale Forskningscenter for Velfærd; 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.