Abstract
Subarachnoid hemorrhage due to rupture of a pre-existing intracranial aneurysm has quite a poor outcome in spite of intensive medical care. Hemodynamic stress loaded on intracranial arterial walls is considered as a trigger and a regulator of formation and progression of the disease, but how intracranial arterial walls or intracranial aneurysm walls behave under hemodynamic stress loading remains unclear. The purpose of this study was to visualize and analyze the wall motion of intracranial aneurysms to detect a pathological flow condition. We subjected a transgenic rat line, in which endothelial cells are specifically visualized by expression of a green fluorescent protein, to an intracranial aneurysm model and observed a real-time motion of intracranial arterial walls or intracranial aneurysm walls by a multiphoton laser confocal microscopy. The anterior cerebral artery–olfactory artery bifurcation was surgically exposed for the monitoring. First, we observed the proper flow-dependent physiological dilatation of a contralateral intracranial artery in response to increase of blood flow by one side of carotid ligation. Next, we observed intracranial aneurysm lesions induced in a rat model and confirmed that a wall motion of the dome was static, whereas that of the neck was more dynamic in response to pulsation of blood flow. We successfully monitored a real-time motion of intracranial aneurysm walls. Findings obtained from such a real-time imaging will provide us many insights especially about the correlation of mechanical force and the pathogenesis of the disease and greatly promote our understanding of the disease.
Keywords: intracranial aneurysm, real-time imaging, bio-imaging, wall motion, rat
Introduction
Subarachnoid hemorrhage (SAH) due to rupture of a pre-existing intracranial aneurysm (IA) has quite a poor outcome.1) Thus, an appropriate treatment intervention to IAs as a preemptive one based on accurate understanding of their underlying pathogenesis is mandatory for social health.2) Recent experimental studies and ones using human data or specimen have accumulated evidence regarding the pathogenesis and defined IAs as a macrophage-mediated chronic inflammatory disease affecting intracranial arterial walls.3–8) In another point of view, hemodynamic stress loaded on intracranial arterial walls is considered as a trigger and a regulator of formation and progression of the disease.9–16) Based on this assumption, it is a central question how hemodynamic stress is loaded on intracranial arterial walls, how it regulates inflammation in situ or what factor among various hemodynamic factors is important for the pathogenesis. However, how intracranial arterial walls or IA walls behave under hemodynamic stress loading remains unclear.
Currently, many believe that a hemodynamic force greatly influences the pathogenesis of an IA and thereby this disease is considered as a hemodynamics-sensitive one.13,15,17) Because such a concept is mainly developed through computational fluid dynamic (CFD) analysis of human cases or lesions induced in animal models, many remain to be elucidated mainly what indeed happens and what kind of molecular machineries drives in situ of an IA lesion loaded by a hemodynamic force. In imaging analysis, because of the limited spatiotemporal resolution of modalities used in a daily medical practice such as magnetic resonance angiography and computed tomography angiography, it is usually difficult to detect such a tiny and a rapid change of IA walls in response to remarkable changes in hemodynamic status due to heart beat. Furthermore, in humans, we can certainly monitor a motion of IA walls during surgery but this procedure needs the extensive resection of arachnoid bundles and dissection of connective tissues surrounding lesions presumably making results of imaging more artificial.
In this manuscript, to know better what happens in IA lesions in situ especially in response to hemodynamic stress loading and how hemodynamic force influences a morphology of IA lesions, we subjected a transgenic rat line, in which endothelial cells are specifically visualized by expression of a green fluorescent protein (GFP) to an IA model and observed real-time motion of IA walls by a multiphoton laser confocal microscopy.
Materials and Methods
Rodent IA models and histological analysis of induced IA
All of the following experiments, including animal care and use, complied with the National Institute of Health’s Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Kyoto University Graduate School of Medicine and also of National Cerebral and Cardiovascular Center.
Transgenic Wistar rat expressing GFP specifically in endothelial cells (W-Tg(Tek-GFP)1Soh18)) was supplied by the National BioResource Project-Rat at Kyoto University (Stock Number #0604, Kyoto, Japan, http://www.anim.med.kyoto-u.ac.jp/NBR/). Animals were maintained on a light/dark cycle of 14 h/10 h, and had a free access to chow and water. To induce IA, under general anesthesia by intraperitoneal injection of pentobarbital sodium (80 mg/kg), 7-week-old male rats (n = 9) were subjected to ligation of the left carotid artery and systemic hypertension induced by the combination of salt overloading and ligation of the left renal artery.7,19,20) Immediately after above surgical procedures, animals were fed the chow containing 8% sodium chloride and 0.12% 3-aminopropionitrile (Tokyo Chemical Industry, Tokyo, Japan), an inhibitor of lysyl oxidase that catalyzes the cross-linking of collagen and elastin, to exacerbate fragility of arterial walls and facilitate IA progression.7,19,20) At times indicated in the corresponding figure legends or results after aneurysm induction, animals were anesthetized by intraperitoneal injection with a pentobarbital sodium (80 mg/kg) and subjected to a real-time imaging experiment (n = 6) as shown in the next paragraph in detail. In some experiments, some animals were deeply anesthetized by intraperitoneal injection with a lethal dose of pentobarbital sodium, and transcardially perfused with 4% paraformaldehyde. The bifurcation site including the induced IA lesion concerned was then stripped, and serial frozen sections were made for immunohistochemistry.
Real-time imaging of intracranial arteries of rats
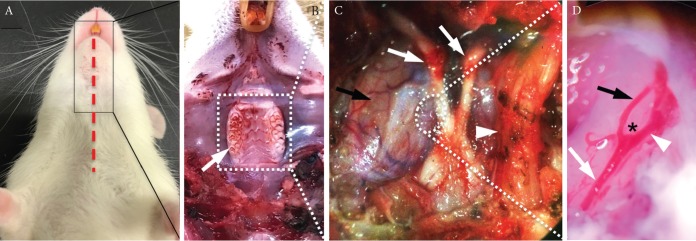
W-Tg(Tek-GFP)1Soh rats were subjected to a tracheotomy and a longitudinal split of mandible under general anesthesia (pentobarbital sodium, 80 mg/kg) (Fig. 1A), and performed a craniectomy of maxilla and skull base (Fig. 1B). An anterior cerebral artery (ACA)–olfactory artery (OA) bifurcation site of the circle of Willis located adjacent to an optic nerve was then exposed (Figs. 1C and 1D). Wall motion of arterial walls at this bifurcation site was visualized by expression of GFP in endothelial cells using a multiphoton-laser confocal microscopy system (A1MP, Nikon Corporation, Tokyo, Japan) and monitored. The frame rate of this imaging method was 30 frames/s. The excitation spectrum is 920 nm.
Fig. 1.
The surgical exposure of an intracranial vasculature. (A–D) The surgical exposure of the anterior cerebral (ACA)–olfactory artery (OA) bifurcation site of the circle of Willis in a W-Tg(Tek-GFP)1Soh rat line. After the skin incision (a red dash line in A) and a longitudinal split of mandible, molars were exposed (a white arrow in B). Molars, maxilla and skull base were resected and a right trigeminal nerve was removed. Structures of skull base including bilateral optic nerves (white arrows), a left trigeminal nerve (a white arrowhead) and a surface of dura matter of right hemisphere (a black arrow) were then exposed (C). After the incision of dura (as shown in a circle in C), the ACA–OA bifurcation site with surrounding arteries was exposed (a white arrow; an internal carotid artery, a white arrowhead; ACA, a black arrow; OA, asterisk; a bifurcation site, D).
Immunohistochemistry
Immunohistochemical analyses were performed as previously described.7,21) Briefly, at the indicated period after aneurysm induction, 5-μm-thick frozen sections were prepared. After blocking with 3% donkey serum (Jackson ImmunoResearch, Baltimore, MD, USA), slices were incubated with primary antibodies followed by incubation with secondary antibodies conjugated with a fluorescence dye (Jackson ImmunoResearch). Finally, fluorescent images were acquired on a confocal fluorescence microscope system (Lsm-710, Carl Zeiss Microscopy GmBH, Gottingen, Germany).
The following primary antibodies were used: anti-actin, α-smooth muscle-Cy3 antibody produced in mouse (#C6198, SIGMA, St. Louis, MO, USA), purified rat anti-mouse CD31 (#550274, BD Pharmingen, Franklin Lakes, NJ, USA).
Results
Endothelial cell-specific expression of GFP in a W-Tg(Tek-GFP)1Soh rat line
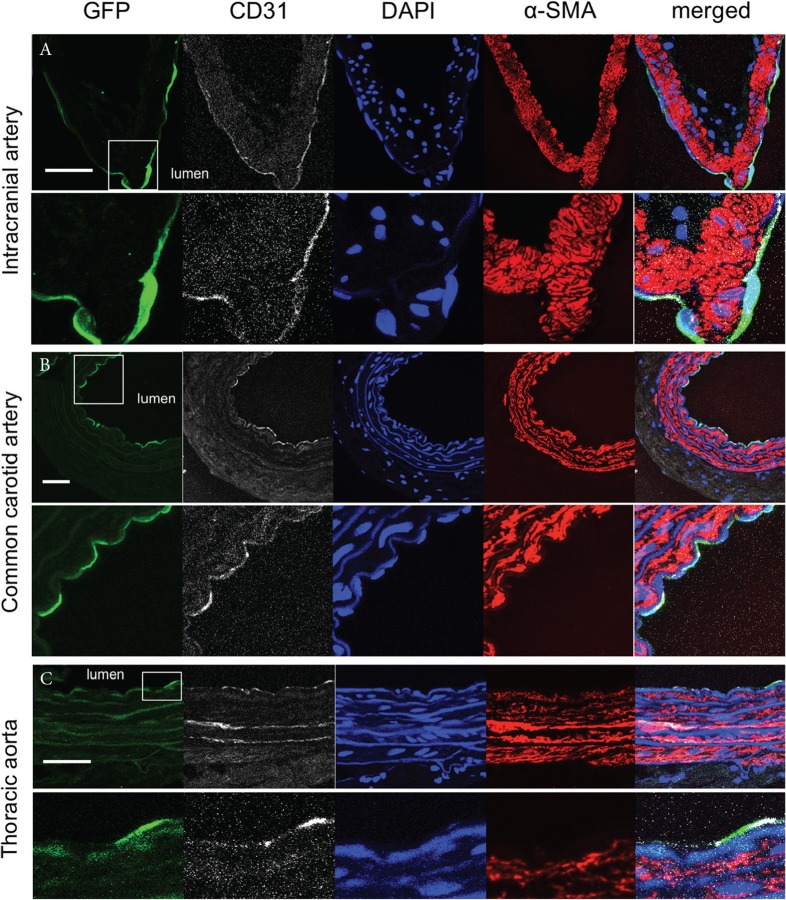
First, we examined expression of GFP in a W-Tg(Tek-GFP)1Soh rat line (Tie2-GFP transgenic rat line) to confirm a restricted expression in endothelial cells by immunohistochemistry. We harvested an intracranial artery as a sample and immunostained for GFP and, in some experiments, also for CD31 [Platelet endothelial cell adhesion molecule-1 (PECAM-1)], a marker for endothelial cells. We then found that GFP-positive cells were present in cells located at most lumen side of intracranial arteries (Fig. 2A) and these cells were positive also for CD31 (Fig. 2A), confirming endothelial cell-specific expression of GFP in a W-Tg(Tek-GFP)1Soh rat line. Furthermore, in common carotid artery and thoracic aorta of this rat line, we validated that GFP-positive cells were also positive for CD31 and thus GFP expression was restricted in endothelial cells as well (Figs. 2B and 2C).
Fig. 2.
Endothelial cell-specific expression of GFP in a W-Tg(Tek-GFP)1Soh (Tie2-GFP) rat line. Representative images of immunostaining of specimens from a cerebral artery (A), a common carotid artery (B) or a thoracic aorta (C) for GFP (green), CD31 (white), a marker of endothelial cells, α-smooth muscle actin (α-SMA, red), a marker of vascular smooth muscle cells, nuclear staining by DAPI (blue) and merged images are shown. In lower panels, magnified images corresponding to a white square in upper panels are shown. Bar: 20 μm.
Flow-dependent dilatation of intracranial arteries in response to unilateral ligation of the common carotid artery
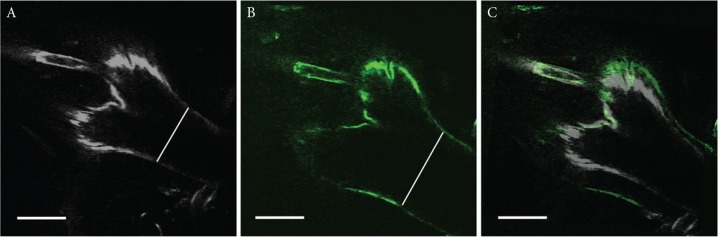
It is well known that a vasculature dilates (increases radius) in response to increase of blood flow to maintain a wall shear stress (WSS) loaded within a certain range based on Poiseuille equation. Thereby, to verify that our observation obtained from a real-time imaging technique of intracranial arteries in this study indeed reflects a normal physiological response, we ligated a unilateral cervical common carotid artery (left) and monitored a change in a diameter of an arterial wall of a contralateral (right) internal carotid artery. The diameter of a right intracranial internal carotid artery before the ligation of a left cervical common carotid artery was 135 μm (Fig. 3A) whereas the diameter after ligation was 177 μm (Fig. 3B). Therefore, as expected, we could detect the physiological dilatation of an internal carotid artery in response to an increase of blood flow (Fig. 3C).
Fig. 3.
A physiological flow-dependent dilatation of intracranial arteries in response to ligation of the contralateral cervical common carotid artery. Freeze-frames of a right ACA–OA bifurcation site and surrounding arterial walls obtained from a real-time imaging before (A) or 4 h after (B) the ligation of left cervical common carotid artery. The overlaid image of A and B is shown in C. Noted the dilatation of right intracranial internal carotid artery after the ligation of a left common carotid artery (as shown by a white line in each image, 135 μm in A, 177 μm in B). Bar: 100 μm.
Real-time imaging of IA walls in a rat model
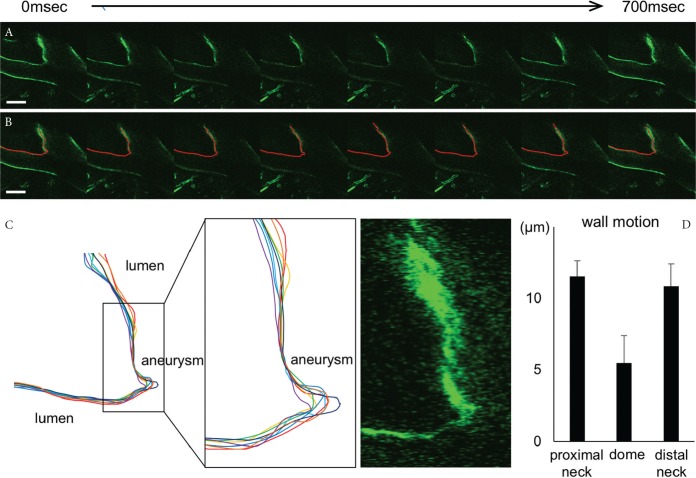
Rats were subjected to aneurysm induction and an IA lesion at the ACA–OA bifurcation was surgically exposed at 8 weeks after induction. Three rats among six rats surgically manipulated developed IAs at the ACA–OA bifurcation. We then monitored a spatiotemporal movement of the IA lesion with surrounding arterial walls at the ACA–OA bifurcation site (30 frames/s, Fig. 4A). We traced out the wall of the ACA–OA bifurcation including the IA wall induced there through referencing expression of GFP in endothelial cells on sequentially acquired images (Fig. 4B). By comparing the traced line at each time point, we found that a motion of the neck was more dynamic than that of the dome (Figs. 4C and 4D).
Fig. 4.
Real-time imaging of IA lesions in a rat model. (A and B), Serial freeze-frame images from a real-time imaging analysis by a multiphoton-laser confocal microscopy system. The IA lesion induced at the ACA–OA bifurcation site of W-Tg(Tek-GFP)1Soh (Tie2-GFP) rat at 8 weeks after aneurysm induction was monitored. Serial freeze-frame images at about every 100 ms were obtained (A) and traced out by a red line (B). Representative images from one out of 3 rats were investigated. (C) The overlaid images of each traced line at each time point. Magnified images corresponding to the square in the left panel and a fluorescent image of GFP expressed in endothelial cells obtained from a real-time imaging are also shown. (D) Maximum displacement of the dome, the proximal neck and the distal neck. All bars represent mean ± SEM (n = 3).
Discussion
The real-time imaging demonstrated here revealed the heterogeneous wall motion in IA lesions induced at ACA–OA bifurcation of a rat model, indicating that the wall motion can be mechanical stimuli to cellular components in each part of arterial walls and may influence a biological process in microenvironment there leading to IAs. The major mechanical force loaded on arterial walls by blood flow and its pulsation are tensile stress and WSS.22) Our observation from a real-time live imaging of IAs induced in a rat model that at the neck portion wall motion is dynamic during cardia cycle but almost a kinetic at the dome may be consistent with previous studies by a numerical simulation. In a numerical simulation, both tensile stress and WSS are highest at the neck portion but are remarkable low at the dome except for blebs.9,10,12,13) Here, strength of tensile stress is tens of times higher than that of WSS.23) Furthermore, it is of note that, in many CFD analyses, wall properties are assumed to be an isotropic non-linear hyperelastic or linear elastic material, and wall thickness to be uniform of 30–300 μm.23,24) It thus remains unclear whether WSS can indeed distort arterial walls. Meanwhile, in addition of a direct mechanical impact, non-physiological WSS loaded on prospective site of IA formation or IA walls may influence the wall motion by affecting a wall property (i.e. elasticity). For example, in a rabbit model of IAs, high WSS and positive WSS gradient correlates with the histological changes resembling IAs, i.e. degeneration and thinning of arterial walls,25,26) presumably through induction of matrix metalloproteinase-9 (MMP-9)27) and reduction in the stiffness. Also, decreased expression of endothelial nitric oxide synthase is observed resulting in the maladaptation to increase of WSS.28) Furthermore, in in vitro experiment, non-physiological excessive low WSS observed in the dome induces expression of chemoattractants for macrophages like monocyte chemoattractant protein-1 in cultured endothelial cells and presumably facilitate degeneration of arterial walls in situ through macrophage-producing MMP-9.9)
In the present study, we used an already-established transgenic rat line18) supplied by the National BioResource Project-Rat at Kyoto University. This rat line has been established in Wistar rat but unfortunately in this line the size of aneurysms induced at ACA–OA bifurcation is remarkably smaller than that in SD rat which we have usually used.3,7,27) We have thus been trying to establish a similar rat line in which a fluorescent protein is expressed specifically in endothelial cell to make a real-time imaging more precisely reflecting human IAs. In addition to transgenic rat lines, recently, establishment of a gene-deficient rat line or other type of a gene-modified line becomes much easier thanks to development of the CRISPR/Cas9 system29) or some other genome-editing techniques. Thereby, we can establish various rat lines necessary for examinations to clarify machineries regulating IA pathogenesis, i.e. for visualization of a certain cell type, of expression of some objective factors or of activation of signaling molecules. We and others have clarified the involvement of chronic inflammatory responses in intracranial arterial walls presumably triggered by high WSS in the pathogenesis of IAs7,15,17,19,30,31) but the precise causative relationship between hemodynamic force and induction of pro-inflammatory factors remains to be elucidated because of the lack of methods to simultaneously examine mechanical force and molecular events. A real-time monitoring of intracranial arteries in a gene-modified rat line during IA development will thus greatly promote our understanding of the pathogenesis.
One of the major limitations in this experiment is a relatively low frame rate, 30 frames/s. Because a heart rate of a rat is several times higher than that in human, about 300–400 beats/min (over 5 beats/s), 30 frames/s is not adequate to monitor many important events in situ such as a streamline and a flow-dependent motion of monocytes. Equipment of high-speed CCD camera will thus be certain to provide us many important insights about flow-dependent events in situ.
Conclusion
In this study, we demonstrated a real-time imaging of IA lesions induced in a rat model and found that a wall motion is not uniform but rather remarkably different even within one IA lesion. Because hemodynamic force greatly influences the pathogenesis of IAs, the precise understanding of the pathological hemodynamic condition is essential to get a whole picture of IA development. In addition, a causative relationship between such a hemodynamic force and molecular events leading to IA development should be clarified to identify the pathological hemodynamic condition and responsible biological process. In a rat model, we have indeed examined molecular events happening in situ during IA development and revealed some important machineries, like a macrophage-mediated chronic inflammation, contributing to the pathogenesis. Establishment of a real-time imaging technique described in this manuscript will thus greatly help our understanding of the pathogenesis and contribute to develop a novel diagnostic strategy such as a detection of a pathological hemodynamic force as a marker of rupture-prone lesions and a novel therapeutic drug to prevent rupture of IAs.
Acknowledgments
We thank all of our technical staffs and secretaries for their kind assistance. We also thank the National BioResource Project-Rat at Kyoto University for preparing the transgenic rat line. T. A. was supported by the Coordination Fund from the Japanese Ministry for Education, Culture, Sports, Science and Technology (MEXT) and Astellas Pharma Inc. to Kyoto University until 31st/March/2017. S. N. is also supported by the Coordination Fund from MEXT and Astellas Pharma to Kyoto University and is a scientific advisor to Astellas Pharma. No potential conflicts of interest were disclosed by the other authors.
Footnotes
Conflicts of Interest Disclosure
This work was supported in part by Core Research for Evolutional Science and Technology (CREST) on Mechanobiology from the Japan Agency for Medical Research and Development (AMED) (JP18gm0810006, T.A.). S. N. is a scientific adviser to Astellas Pharma Consultant to Toray, and received a milestone payment from Astellas Pharma. The remaining authors have no conflict of interest respectively.
References
- 1).van Gijn J, Kerr RS, Rinkel GJ: Subarachnoid haemorrhage. Lancet 369: 306–318, 2007 [DOI] [PubMed] [Google Scholar]
- 2).Aoki T, Nozaki K: Preemptive medicine for cerebral aneurysms. Neurol Med Chir (Tokyo) 56: 552–568, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Aoki T, Kataoka H, Ishibashi R, Nozaki K, Egashira K, Hashimoto N: Impact of monocyte chemoattractant protein-1 deficiency on cerebral aneurysm formation. Stroke 40: 942–951, 2009 [DOI] [PubMed] [Google Scholar]
- 4).Kanematsu Y, Kanematsu M, Kurihara C, et al. : Critical roles of macrophages in the formation of intracranial aneurysm. Stroke 42: 173–178, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Frösen J, Tulamo R, Paetau A, et al. : Saccular intracranial aneurysm: pathology and mechanisms. Acta Neuropathol 123: 773–786, 2012 [DOI] [PubMed] [Google Scholar]
- 6).Aoki T, Narumiya S: Prostaglandins and chronic inflammation. Trends Pharmacol Sci 33: 304–311, 2012 [DOI] [PubMed] [Google Scholar]
- 7).Aoki T, Frȍsen J, Fukuda M, et al. : Prostaglandin E2-EP2-NF-κB signaling in macrophages as a potential therapeutic target for intracranial aneurysms. Sci Signal 10: 465, eaah6037 2017 [DOI] [PubMed] [Google Scholar]
- 8).Aoki T, Yamamoto R, Narumiya S: Targeting macrophages to treat intracranial aneurysm. Oncotarget 8: 104704–104705, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Aoki T, Yamamoto K, Fukuda M, Shimogonya Y, Fukuda S, Narumiya S: Sustained expression of MCP-1 by low wall shear stress loading concomitant with turbulent flow on endothelial cells of intracranial aneurysm. Acta Neuropathol Commun 4: 48, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Takeuchi S, Karino T: Flow patterns and distributions of fluid velocity and wall shear stress in the human internal carotid and middle cerebral arteries. World Neurosurg 73: 174–185; discussion e27, 2010 [DOI] [PubMed] [Google Scholar]
- 11).Boussel L, Rayz V, McCulloch C, et al. : Aneurysm growth occurs at region of low wall shear stress: patient-specific correlation of hemodynamics and growth in a longitudinal study. Stroke 39: 2997–3002, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Tanoue T, Tateshima S, Villablanca JP, Viñuela F, Tanishita K: Wall shear stress distribution inside growing cerebral aneurysm. AJNR Am J Neuroradiol 32: 1732–1737, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Dolan JM, Kolega J, Meng H: High wall shear stress and spatial gradients in vascular pathology: a review. Ann Biomed Eng 41: 1411–1427, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Shojima M, Oshima M, Takagi K, et al. : Magnitude and role of wall shear stress on cerebral aneurysm: computational fluid dynamic study of 20 middle cerebral artery aneurysms. Stroke 35: 2500–2505, 2004 [DOI] [PubMed] [Google Scholar]
- 15).Turjman AS, Turjman F, Edelman ER: Role of fluid dynamics and inflammation in intracranial aneurysm formation. Circulation 129: 373–382, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Can A, Du R: Association of hemodynamic factors with intracranial aneurysm formation and rupture: systematic review and meta-analysis. Neurosurgery 78: 510–520, 2016 [DOI] [PubMed] [Google Scholar]
- 17).Fukuda M, Aoki T: Molecular basis for intracranial aneurysm formation. Acta Neurochir Suppl 120: 13–15, 2015 [DOI] [PubMed] [Google Scholar]
- 18).Ohtsuki S, Kamiya N, Hori S, Terasaki T: Vascular endothelium-selective gene induction by Tie2 promoter/enhancer in the brain and retina of a transgenic rat. Pharm Res 22: 852–857, 2005 [DOI] [PubMed] [Google Scholar]
- 19).Aoki T, Nishimura M, Matsuoka T, et al. : PGE(2) -EP(2) signalling in endothelium is activated by haemodynamic stress and induces cerebral aneurysm through an amplifying loop via Nf-κB. Br J Pharmacol 163: 1237–1249, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Hashimoto N, Handa H, Hazama F: Experimentally induced cerebral aneurysms in rats. Surg Neurol 10: 3–8, 1978 [PubMed] [Google Scholar]
- 21).Miyata H, Koseki H, Takizawa K, et al. : T cell function is dispensable for intracranial aneurysm formation and progression. PloS ONE 12: e0175421, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Ando J, Yamamoto K: Effects of shear stress and stretch on endothelial function. Antioxid Redox Signal 15: 1389–1403, 2011 [DOI] [PubMed] [Google Scholar]
- 23).Torii R, Oshima M, Kobayashi T, Takagi K, Tezduyar TE: Fluid–structure interaction modeling of aneurysmal conditions with high and normal blood pressures. Comput Mech 38: 482–490, 2006 [Google Scholar]
- 24).Isaksen JG, Bazilevs Y, Kvamsdal T, et al. : Determination of wall tension in cerebral artery aneurysms by numerical simulation. Stroke 39: 3172–3178, 2008 [DOI] [PubMed] [Google Scholar]
- 25).Metaxa E, Tremmel M, Natarajan SK, et al. : Characterization of critical hemodynamics contributing to aneurysmal remodeling at the basilar terminus in a rabbit model. Stroke 41: 1774–1782, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Meng H, Wang Z, Hoi Y, et al. : Complex hemodynamics at the apex of an arterial bifurcation induces vascular remodeling resembling cerebral aneurysm initiation. Stroke 38: 1924–1931, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Aoki T, Kataoka H, Morimoto M, Nozaki K, Hashimoto N: Macrophage-derived matrix metalloproteinase-2 and -9 promote the progression of cerebral aneurysms in rats. Stroke 38: 162–169, 2007 [DOI] [PubMed] [Google Scholar]
- 28).Wang Z, Kolega J, Hoi Y, et al. : Molecular alterations associated with aneurysmal remodeling are localized in the high hemodynamic stress region of a created carotid bifurcation. Neurosurgery 65: 169–177; discussion 177–178, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Doudna JA, Charpentier E: Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 346: 1258096, 2014 [DOI] [PubMed] [Google Scholar]
- 30).Aoki T, Nishimura M: Targeting chronic inflammation in cerebral aneurysms: focusing on NF-kappaB as a putative target of medical therapy. Expert Opin Ther Targets 14: 265–273, 2010 [DOI] [PubMed] [Google Scholar]
- 31).Aoki T, Kataoka H, Shimamura M, et al. : NF-kappaB is a key mediator of cerebral aneurysm formation. Circulation 116: 2830–2840, 2007 [DOI] [PubMed] [Google Scholar]