Abstract
Background/Objective
This study is a case–control study to explore risk and protective factors, including clinical data and bone mineral density (BMD), affecting vertebral body fragility fracture in elderly men and postmenopausal women. In addition, we investigate the effectiveness of lumbar spine BMD by quantitative computed tomography (QCT) in discriminating vertebral fragility fracture.
Methods
In this case–control study, 52 males and 198 females with vertebral fragility fracture were compared with sex- and age-matched healthy controls to analyse the risk factors that may affect vertebral fragility fracture. The L1–L3 vertebral BMDs were measured by QCT. The difference in risk factors between fracture cases and controls were analysed using student t test and Mann–Whitney U test. The correlation between BMD, age, height and weight were analysed using univariate analysis. Multiple logistic regression analysis was used to study statistically significant indexes. The receiver operating characteristic curve was used to calculate the cut-off values for positive and negative predictive values of BMD for vertebral fracture discrimination.
Results
In males, body weight and BMD were significantly different between the fracture group and the control group, whereas BMD was only weakly correlated with age (r = −0.234). In females, only BMD was significantly different between the fracture and control groups. BMD was weakly correlated with height (r = 0.133) and weight (r = 0.120) and was moderately correlated with age (r = −0.387). There was no correlation between BMD and the remaining variables in this study. In both men and women, the BMD (p = 0.000) was the independent protective factor against vertebral fracture. The cut-off values of vertebral BMD for fractures were 64.16 mg/cm3 for males and 55.58 mg/cm3 for females. QCT-measured BMD has a high positive predictive value and negative predictive value for discriminating vertebral fragility fracture across a range of BMD values.
Conclusion
This study suggests that BMD is closely related to vertebral fragility fracture and that QCT is an effective technique to accurately discriminate vertebral fragility fracture.
The translational potential of this article
The spine BMD measured by QCT is closely related to fracture, which may allow clinicians to more accurately discriminate which individuals are likely to experience vertebral fragility fracture.
Keywords: Bone mineral density, Quantitative computed tomography, Vertebral fragility fracture
Introduction
Osteoporosis is characterized by a decrease in bone strength and an increased risk of fracture. Epidemiological study found that osteoporosis was present in 15.7% of the elderly population in China [1]. Osteoporotic fractures, also known as fragility fractures, develop in the final stages of osteoporosis and are serious medical consequences that result in morbidity, high mortality caused by disability and high medical costs [2], [3]. In postmenopausal women, the risk of osteoporotic fracture is 40%, far higher than that of breast, endometrial and ovarian cancer combined [1]. Osteoporotic fractures of the spine are the most likely to occur among the common fracture sites. Women with osteoporotic vertebral body fractures are four times more likely to refracture than those without vertebral fractures. Men with a history of vertebral fragility fractures are also at higher risk of refracture [2], [4]. These vertebral fragility fractures are a strong risk factor for other fractures in the future. Some studies have shown that patients with vertebral body fractures experience loss of body function, back pain and height loss, as well as difficulties in social interaction [5]. Other research studies have shown that osteoporotic fracture of vertebral body can reduce life expectancy in patients and may worsen the mortality and permanent disability rates of long-term bedridden patients [6].
Despite the prevalence of vertebral fractures, only a small number of people with fractures are found early, especially with atraumatic fractures, the aetiology of which is still relatively unknown, and vertebral fractures are often delayed in treatment compared with limb bone fractures. In a European study, more than one-eighth of the 15,570 individuals, aged between 50 and 79 years, without a history of fractures had vertebral deformation [7]. In developing countries, atraumatic vertebral fractures due to osteoporosis are more likely to be ignored, and osteoporosis is perceived as a disease that occurs only in developed countries and is the inevitable result of ageing, which is neither treatable nor preventable. At the same time, there is not enough attention to bone health. However, the atraumatic fracture of vertebral body can have a greater impact on the quality of life of the elderly, so its prevention and treatment should be paid more attention.
In general, vertebral fracture occurs when external force acts on the vertebral body and exceeds its load. In atraumatic vertebral fracture, decreased bone strength is a primary cause of fracture. Vertebral body bone strength is determined by bone size, shape and density. It is also related to bone microstructure, collagen characteristics and microdamage [8]. A previous study shows that areal bone mineral density (aBMD) can explain 50–70% changes in vertebral compression strength [9].
Accurate measurement of bone mineral density (BMD) is an important basis for the diagnosis of osteoporosis. In recent years, more attention has been paid to quantitative computed tomography (QCT) because of its unique advantages, such as more accuracy than dual-energy X-ray absorptiometry (DXA) to reflect the changes in BMD. In this study, QCT was used to directly measure volume bone mineral density [vBMD (mg/cm3)] of lumbar vertebrae. We explore the correlations between QCT-measured BMD and the characteristics of age, sex, body mass index (BMI) and osteoporotic vertebral fracture and investigate their value in discriminating vertebral fragility fracture.
Materials and methods
Fracture group and control group
Fifty-two males aged 60 years or above and 198 postmenopausal women with atraumatic spine fractures diagnosed using X-ray, computed tomography (CT) or magnetic resonance imaging were recruited into the study from February 2010 to October 2012 in Beijing Jishuitan Hospital. All participants underwent QCT examination of lumbar vertebrae. Sex- and age-matched controls were retrieved from the database of Jishuitan hospital.
All participants were required to meet the following criteria for inclusion in the study:
-
1.
Elderly men (≥60 years) or postmenopausal women.
-
2.
Absence of abnormal bone metabolism disease (e.g., diabetes, thyroid hyperfunction, hyperparathyroidism, etc.) and no history of taking drugs that affect bone metabolism (e.g., corticosteroids, calcitonin, vitamin D, diphosphonates, etc.)
Collection of general information (e.g., age, sex, ethnicity, place of residence, height, weight, menstruation, etc.) and a history of diseases and drug use that may affect bone metabolism was performed. The field epidemiological surveys of all participants in both the fracture group and the control group were conducted by unified trained investigators. The clinical study was approved by the Ethics Committee of the Beijing Jishuitan Hospital, and written informed consents were obtained from all participants.
CT scan acquisition and data measurement
All participants underwent CT scans using a multidetector CT scanner (Aquilion, 16-slice; Toshiba, Tokyo, Japan) following a standard protocol from the L1 to the L5. Scanning parameters were as follows: 120 kVp, 125 mAs, slice thickness of 1 mm and field of view of 40 cm. A lateral scout view of T4 to L4 vertebrae is obtained for localization. The scan table height was set at 90 cm using Mindways quality control phantom (Mindways, Austin, TX, USA). When scanning with the calibration model, the patient was placed in the supine position, and his or her head was raised with both hands while the phantom was placed beneath the patient's body.
Image processing
vBMD of the lumbar spine L1–L3 was measured using the commercial software QCT Pro (Mindways Software Inc., Austin, TX, USA). When fracture occurred in any of the L1–L3 vertebrae, the BMD of the L4 or L5 vertebra was measured and used as a replacement measurement for the abandoned fractured vertebrae. In either case, BMD was calculated using the average value of three intact vertebrae (mg/cm3).
Diagnosis of vertebral fracture
The fracture patients were all atraumatic and admitted to hospital for operation; all patients underwent complete X-ray, CT and/or magnetic resonance imaging of the spine for diagnosis of fractures.
Statistical analysis
The normal distribution data were presented as mean ± standard deviation and compared using independent Student t test. The other data with skewed distribution were expressed as median (quartile difference) and compared using Mann–Whitney U test. The correlation between BMD and clinical characteristics (age, height, weight and BMI) was analysed by univariate analysis (correlation coefficient bounds from 0–0.09 to 0.1–0.3 are weakly correlated; from 0.3 to 0.5 are moderately correlated and from 0.5 to 1.0 are strongly correlated). According to the data of the univariate analysis, the statistically significant indexes were added to the multivariate logistic regression analysis. Multivariate-corrected odds ratio value of discrimination factors was calculated. Furthermore, the area under the receiver operating characteristic (ROC) curve was used as the standard to calculate the cut-off values for positive predictive value and negative predictive value of BMD in the diagnosis of vertebral fracture. All statistical analyses were performed using SPSS 19.0 (SPSS Inc., Chicago, IL, USA) and MedCalc (MedCalc Software, Mariakerke, Belgium). A p value < 0.05 was considered statistically significant.
Results
Difference of general characteristics between the fracture group and control group
A total of 250 cases (52 males and 198 females) and 250 controls, matched for sex and age, were included in this study. The information (age, height, weight, BMI and BMD) of all participants is shown in Table 1.
Table 1.
General characteristics between the fracture group and control group.
| Characteristic | Male |
Female |
||||
|---|---|---|---|---|---|---|
| Fracture group | Control group | p | Fracture group | Control group | p | |
| Age (yrs) | 77.50 (69.25–80.00) | 77.50 (69.25–80.00) | 0.948 | 68.00 (61.00–75.00) | 68.00 (61.00–75.00) | 0.937 |
| Height (cm) | 169.29 ± 5.99 | 169.77 ± 5.37 | 0.667 | 158.00 (155.00–162.00) | 158.00 (154.00–162.00) | 0.564 |
| Weight (kg) | 65.79 ± 11.03 | 69.75 ± 9.14 | 0.049 | 60.00 (54.00–65.00) | 60.00 (55.00–67.00) | 0.352 |
| BMI (kg/m2) | 22.95 ± 3.61 | 24.20 ± 2.96 | 0.057 | 23.80 (22.05–25.78) | 24.03 (22.37–26.37) | 0.155 |
| BMD (mg/cm3) | 57.22 ± 21.66 | 99.29 ± 30.02 | 0.000 | 39.37 (28.48–55.62) | 89.91 (69.92–114.60) | 0.000 |
BMI = body mass index; BMD = average bone mineral density measured by QCT; QCT = quantitative computed tomography.
In the males, weight and BMD were significantly different between the fracture group and the control group, whereas in female participants, only BMD was significantly different between the fracture and control groups.
History of trauma and clinical presentation of the fracture group
With regards to injuries, in the fracture group, although all patients had not suffered violent trauma, most patients still had varying degrees of history of injury. Of these, 87.6% (219 cases) had minor daily life injuries, and 12.4% (31 cases) had osteoporotic vertebral compression fractures without any history of trauma. Of the patients with a history of trauma, 70.32% (154 cases) were caused by minor injuries caused by flat falls, and 26.94% (59 cases) were caused by sprains caused by bending over to lift objects or shifting positions in their daily lives. Cough, epilepsy and other muscle twitching in a very small number of patients (2.74% 6 cases) can lead to vertebral compression fractures. Under normal circumstances, these injuries do not lead to fracture of the vertebral body.
Lumbar back pain is a typical symptom of osteoporotic vertebral fracture. Most of the patients (243 cases, 97.2%) had moderate or mild pain, but a small number had no obvious pain. The pain site of most patients was consistent with the site of fracture (224 cases, 89.6%), but a small number of patients (19 cases, 7.6%) complained of pain outside the fracture site (including diffused pain with vague location of the pain site). A small number of patients presented with significant kyphosis deformity and decreased height. Constipation is also a common symptom (in more than 30% of patients).
Correlation between BMD and age, height, weight and BMI
In males, BMD was only weakly correlated with age (r = −0.234). In females, BMD was weakly correlated with height (r = 0.133) and weight (r = 0.120) and was moderately correlated with age (r = −0.387). The correlation between BMD and the remaining variables in this study was not significant (Table 2, Table 3).
Table 2.
Correlation analysis between variables in men.
| Parameters | Height | Weight | BMI | BMD |
|---|---|---|---|---|
| Age | −0.033 | −0.039 | 0.020 | −0.234* |
| Height | — | 0.351* | −0.096 | −0.035 |
| Weight | — | — | 0.897* | 0.147 |
| BMI | — | — | — | 0.151 |
*p < 0.05; BMI = body mass index; BMD = average bone mineral density measured by QCT; QCT = quantitative computed tomography.
Table 3.
Correlation analysis between variables in women.
| Parameters | Height | Weight | BMI | BMD |
|---|---|---|---|---|
| Age | −0.256* | −0.197* | −0.091 | −0.387* |
| Height | — | 0.502* | 0.550 | 0.133* |
| Weight | — | — | 0.864* | 0.120* |
| BMI | — | — | — | 0.060 |
*p < 0.05; BMI = body mass index; BMD = average bone mineral density measured by QCT; QCT = quantitative computed tomography.
Multivariate logistic regression analysis of fracture discrimination
According to the results of univariate analysis, weight, BMI and BMD for both men and women were included in the multivariate logistic regression model. Because weight and BMI are in a collinear relationship, we choose the forward/backward: linear regression (LR) method (stepwise regression) to exclude its effects when we construct a multivariate logistic regression model.
In males, the results showed that BMD (p = 0.000) was an independent protective factor for vertebral fracture in men (p = 0.000). This suggests that, in men, the higher the BMD, the lower the discrimination of fracture. The logistic regression equation for elderly males is modelled: logit (p) = ((−0.069*BMD) + 5.189) (Table 4).
Table 4.
Multivariate logistic regression analysis of discrimination factors for vertebral fragility fracture.
| Sex | Discrimination factors | B | Standard error | Wald | p | Odds ratios | Odds ratio value 95% confidence interval |
|
|---|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | |||||||
| Male | BMD | −0.069 | 0.013 | 26.678 | 0.000 | 0.934 | 0.910 | 0.958 |
| (Constant) | 5.189 | |||||||
| Female | BMD | −0.068 | 0.007 | 105.027 | 0.000 | 0.934 | 0.922 | 0.946 |
| (Constant) | 4.523 | |||||||
BMD = average bone mineral density measured by QCT; QCT = quantitative computed tomography.
In females, the results showed that BMD (p = 0.000) was also an independent protective factor for vertebral fracture (p = 0.000). The logistic regression equation for postmenopausal females is modelled: logit (p) = ((−0.068*BMD) + 4.523) (Table 4).
ROC curve
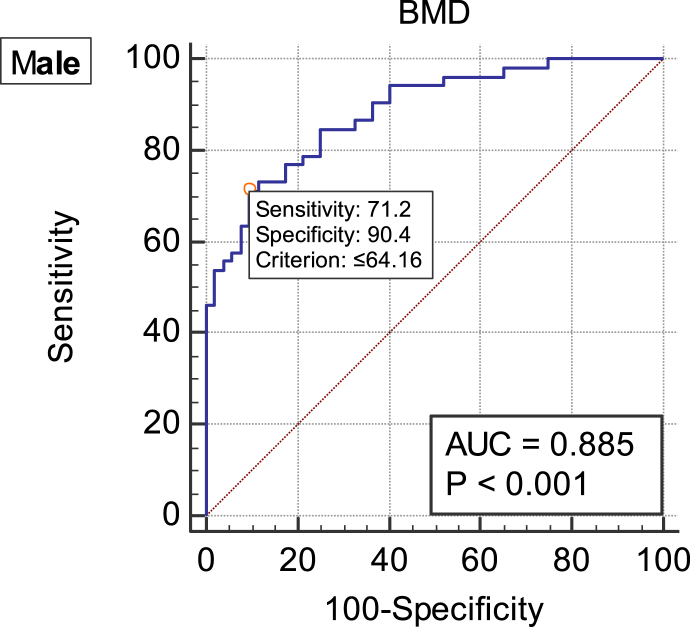
In males, the area under the ROC curve of BMD in the diagnosis of vertebral fracture was 0.885 (p < 0.05), and the cut-off value was 64.16 mg/cm3 (Figure 1, Table 5).
Figure 1.
ROC curve of vertebral fragility fracture diagnosed by BMD in males. AUC = 0.885; cut-off value = 64.16 mg/cm3. AUC = area under the ROC curve; BMD = average bone mineral density measured by QCT; QCT = quantitative computed tomography; ROC = receiver operative characteristic.
Table 5.
Area under the ROC curve in diagnosis of vertebral fracture by BMD.
| Parameter | Sex | AUC | Standard error | p | 95% CI |
|
|---|---|---|---|---|---|---|
| Lower limit | Upper limit | |||||
| BMD | Male | 0.885 | 0.0315 | 0.000 | 0.807 | 0.939 |
| Female | 0.906 | 0.0146 | 0.000 | 0.873 | 0.933 | |
AUC = area under the ROC curve; BMD = average bone mineral density measured by QCT; CI = confidence interval; QCT = quantitative computed tomography; ROC = receiver operative characteristic.
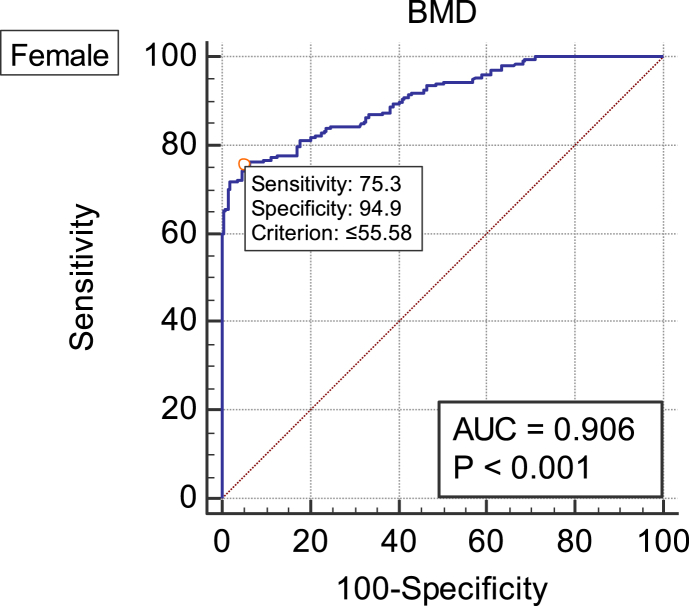
In females, the area under the ROC curve of BMD in the diagnosis of vertebral fracture was 0.906 (p < 0.05), and the cut-off value was 55.58 mg/cm3 (Figure 2, Table 5).
Figure 2.
ROC curve of vertebral fragility vertebral fracture diagnosed by BMD in females. AUC = 0.906; cut-off value = 55.58 mg/cm3. AUC = area under ROC the curve; BMD = average bone mineral density measured by QCT; QCT = quantitative computed tomography; ROC = receiver operative characteristic.
The positive predictive values and negative predictive values for the diagnosis of vertebral fracture between men and women at different BMD values are shown in Table 6.
Table 6.
Positive and negative predictive values for the diagnosis of vertebral fractures with different BMD values.
| BMD (mg/cm3) | Male |
Female |
||
|---|---|---|---|---|
| PPV | NPV | PPV | NPV | |
| 45 | 100 | — | 100 | 71.48 |
| 50 | 100 | 65.00 | 97.88 | 76.77 |
| 60 | 89.19 | 71.64 | 86.03 | 79.72 |
| 70 | 81.63 | 78.18 | 77.31 | 82.77 |
| 80 | 77.19 | 82.98 | 69.84 | 84.72 |
| 90 | 70.00 | 91.18 | 65.15 | 89.90 |
| 100 | 64.94 | 92.59 | 61.34 | 92.77 |
| 110 | 56.67 | 92.87 | 58.41 | 100 |
| 120 | 53.76 | 100 | 56.37 | 100 |
BMD = average bone mineral density measured by QCT; NPV = negative predictive value; PPV = positive predictive value; QCT = quantitative computed tomography.
Discussion
In this study, we investigated the discrimination factors of vertebral fragility fracture in elderly men and postmenopausal women. Based on the vBMD measured by QCT, a model was established to predict the discrimination of vertebral fracture in elderly men and postmenopausal women. The cut-off value of BMD and the positive predictive value and negative predictive value in the diagnosis of vertebral fracture were obtained.
Vertebral fractures, the most prominent sign of osteoporosis, usually occur in the middle of the thoracic vertebra and the thoracolumbar vertebrae [10] and are the most common fragility fractures observed in the elderly population. Most patients have only minor injuries, such as falls, pick up, and even muscle twitching caused by coughing. Even many patients do not have a clear trauma. Lumbar back pain is a typical symptom of osteoporotic vertebral fracture. Physical examination showed limited spinal movement and vertebral body tenderness and percussion pain. The pain is characterized by aggravation after activity and relief at rest. The degree of pain in patients with fractures can range from mild-to-severe twitch-like pain, typically moderate pain. Mild pain is easy to be ignored. For patients who do not heal for a long time, especially patients with obvious kyphosis, vertebral compression fracture is often possible. These fractures have been associated with weight loss, spinal deformation, chronic pain and decreased quality of life. The usefulness of vertebral body fractures as a strong predictor of future fractures independent of BMD [11], [12] is of great clinical significance. Considering the sizable prevalence of vertebral fractures in the population, it is alarming that the aetiology of this fragility fracture still remains unclear. The reason for this may be in part because only a small percentage of this fracture-bearing population obtains imaging evidence of vertebral deformities. It may also be that formal medical treatment of vertebral fractures is often much more delayed than treatment of limb fractures. Both scenarios would mask the condition from medical attention at a population level, obscuring better understanding.
The fracture of the vertebral body is determined by the ratio of the vertebral body's damage load to external force applied to the spine. Fracture occurs when the strength of the bone is less than the stress applied. Fragile bone structure or great external stress can be high risk factors for fracture. The changes in compressive strength of the vertebral body are mostly determined by the size of the vertebral body, or bone mass, and BMD. Laboratory studies have shown that aBMD can explain the 50–70% change in the compression strength of the vertebral body [8].
The load-bearing capacity of the vertebral body is determined by the structural capacity of the vertebral body and the state of daily activity and trauma load. Fracture can be regarded as a mechanical event when the load exceeds the strength of the bone. The damage load of the vertebral body is related not only to bone density and structure of cancellous bone in the vertebral body but also to the biomechanical properties of the vertebral body, the spine and neurophysiology [10]. The maximum strength of cancellous bone is determined by the maximum pressure on the overall structure of the vertebra. Therefore, the trivial injury mechanism or type of microdamage affects the fragility fracture. When bone strength is less than physiological or traumatic stress, fracture occurs. From the mechanical point of view, the compression force applied to the spine is transmitted from the intervertebral disc to the endplate and then distributed over the cancellous bone and the thin cortical bone that make up the vertebral body. Axial force is mainly carried by cancellous bone. External axial force is applied to the vertebral body, which produces stress and strain on the center of the cancellous bone. In extreme cases, when bone strength is weak to a certain extent, muscle forces can also lead to fracture. So, “injury” and bone strength are very important mechanical characteristic of fracture risk [13].
BMD measurement is the relatively simple standardized biomechanical indicator in bone research and is considered the most important quantitative index of bone strength [10]. Several studies on the relationship between BMD and fractures [14], [15], [16], [17], [18] confirmed that BMD was the primary indicator of osteoporotic fractures [19]. Studies have shown that BMD of lumbar vertebrae and hip in patients with fragile fracture is 20–30% lower on average than that in healthy controls [20]. For every 1–standard deviation decrease in BMD in American women, the risk of vertebral body fracture increased by 1.5–1.8 times [21] and that in Chinese women increased by 2.5–3.2 times [22]. Zhang et al provided normative BMD data of cervical vertebrae in an age- and sex-stratified population [23], which is beneficial in designing a more comprehensive preoperative surgical plan.
In this study, we demonstrate that BMD is an independent protective factor of osteoporotic vertebral fracture in both males and females by univariate analysis, correlation analysis, multivariate logistic regression, and ROC curve. The result is consistent with that of previous studies.
In recent years, the quantitative measurement of BMD by DXA and QCT has been widely reported. We used QCT instead of DXA to measure vertebral body BMD. Although the World Health Organization has set DXA as the standard for the evaluation of BMD and for the diagnosis of osteoporosis in clinical work [25], the two-dimensional images acquired by DXA cannot estimate bone mass to the same degree of geometric detail as is possible with QCT's more sensitive cross-sectional three-dimensional images, and limitations such as plane projection inconsistency result in a false-positive increase of the measurement results. On the other hand, QCT can accurately obtain the true three-dimensional data and vBMD of cortical and cancellous bone in the vertebrae [4] without being impacted by severe spinal degeneration and hyperplasia, vascular calcification, oral contrast agent, posture, etc. The International Society for Clinical Densitometry Official Positions [26] assert that QCT is more sensitive than DXA. In addition to the superior imaging sensitivity of QCT over DXA, the raw data obtained by QCT can be used to analyse and study the structural changes and characteristics of bone through complex image processing.
In previous studies, Yi et al showed preoperative QCT evaluation of bone loss in femoral head and clinical application [27]. Su et al predicted hip fracture type of elderly Asian patients with low-energy fall by vBMD and femoral morphology from QCT [28]. Ma et al measured the age-related changes of bone mass in the population of East China by QCT and concluded that QCT vBMD was positively correlated with aBMD [29]. Compared with hip computed tomography X-ray absorptiometry aBMD, spinal vBMD was more sensitive to the detection of osteoporosis. Amstrup et al research data suggest that the various techniques (DXA, high resolution (HR)-pQCT and QCT) measure different characteristics of bone, and in clinical practice, they can supplement well [30].
A limitation of this study is that prospective follow-up results were not provided. Another limitation is that the sample size of male cases observed was relatively small. In addition, other factors known to affect the incidence of fracture such as paravertebral muscle traits, fat distribution, etc. were not included in this study [31], [32], [33], [34], [35], [36]. Further research is needed.
Conclusions
In summary, this study suggests that vertebral fragility fracture is closely related to BMD. BMD was an independent protective factor in both elderly men and postmenopausal women. The cut-off values of BMD in the diagnosis of vertebral fracture were 64.16 mg/cm3 (male) and 55.58 mg/cm3 (female). BMD measured by QCT demonstrates high specificity and sensitivity in the diagnosis of vertebral fracture, suggesting its important value in predicting vertebral fracture.
Conflicts of interest
All authors declare that they have no conflict of interest.
Acknowledgement
This study was partially supported by the fund “Beijing Natural Science Foundation project: 17L20188”. It was also funded by the “Beijing Municipal Administration of Hospitals Clinical medicine Development of special funding support (code: XMLX201843)”, and by the grants from the “Beijing Bureau of 215 Program (No. 2013-3-033; 2009-02-03)”.
Contributor Information
Xiao-Lin Shi, Email: xlshi-2002@163.com.
Xiao-Guang Cheng, Email: xiao65@263.net.
References
- 1.Willson T., Nelson S.D., Newbold J., Nelson R.E., Lafleur J. The clinical epidemiology of male osteoporosis: a review of the recent literature. Clin Epidemiol. 2015;7(default):65–76. doi: 10.2147/CLEP.S40966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Åkesson K., Marsh D., Mitchell P.J., McLellan A.R., Stenmark J., Pierroz D.D. Capture the Fracture: a Best Practice Framework and global campaign to break the fragility fracture cycle. Osteoporosis Int. 2013;24(8):2135. doi: 10.1007/s00198-013-2348-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cosman F., Beur S.J.D., LeBoff M.S., Lewiecki E.M., Tanner B., Randall S. The clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359–2381. doi: 10.1007/s00198-014-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoo J.H., Moon S.H., Ha Y.C., Dong Y.L., Gong H.S., Si Y.P. Osteoporotic fracture: 2015 position statement of the Korean society for bone and mineral research. J Bone Metab. 2015;22(4):175–181. doi: 10.11005/jbm.2015.22.4.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reginster J.Y., Burlet N. Osteoporosis: a still increasing prevalence. Bone. 2006;38(2):4–9. doi: 10.1016/j.bone.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 6.Sale J.E., Beaton D., Bogoch E. Secondary prevention after an osteoporosis-related fracture: an overview. Clin Geriatr Med. 2014;30(2):317–332. doi: 10.1016/j.cger.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 7.O'Neill T.W., Felsenberg D., Varlow J., Cooper C., Kanis J.A., Silman A.J. The prevalence of vertebral deformity in European men and women: the European vertebral osteoporosis study. J Bone Miner Res. 2010;11(7):1010–1018. doi: 10.1002/jbmr.5650110719. [DOI] [PubMed] [Google Scholar]
- 8.Christiansen B.A., Bouxsein M.L. Biomechanics of vertebral fractures and the vertebral fracture cascade. Curr Osteoporos Rep. 2010;8(4):198–204. doi: 10.1007/s11914-010-0031-2. [DOI] [PubMed] [Google Scholar]
- 9.Lochmuller E.M., Burklein D., Kuhn V., Glaser C., Muller R., Gluer C.C. Mechanical strength of the thoracolumbar spine in the elderly: prediction from in situ dual-energy X-ray absorptiometry, quantitative computed tomography (QCT), upper and lower limb peripheral QCT, and quantitative ultrasound. Bone. 2002;31(1):77–84. doi: 10.1016/s8756-3282(02)00792-5. [DOI] [PubMed] [Google Scholar]
- 10.Briggs A.M., Greig A.M., Wark J.D. The vertebral fracture cascade in osteoporosis: a review of aetiopathogenesis. Osteoporos Int. 2007;18(5):575–584. doi: 10.1007/s00198-006-0304-x. [DOI] [PubMed] [Google Scholar]
- 11.Klotzbuecher C.M., Ross P.D., Landsman P.B., Iii T.A.A., Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2000;15(4):721–739. doi: 10.1359/jbmr.2000.15.4.721. [DOI] [PubMed] [Google Scholar]
- 12.Delmas P.D., Genant H.K., Crans G.G., Stock J.L., Wong M., Siris E. Severity of prevalent vertebral fractures and the risk of subsequent vertebral and nonvertebral fractures: results from the MORE trial. Bone. 2003;33(4):522. doi: 10.1016/s8756-3282(03)00241-2. [DOI] [PubMed] [Google Scholar]
- 13.Myers E.R., Wilson S.E. Biomechanics of osteoporosis and vertebral fracture. Spine. 1997;22(24 Suppl.):25S. doi: 10.1097/00007632-199712151-00005. [DOI] [PubMed] [Google Scholar]
- 14.Siris E.S., Genant H.K., Laster A.J., Chen P., Misurski D.A., Krege J.H. Enhanced prediction of fracture risk combining vertebral fracture status and BMD. Osteoporos Int. 2007;18(6):761–770. doi: 10.1007/s00198-006-0306-8. [DOI] [PubMed] [Google Scholar]
- 15.Fujiwara S., Kasagi F., Masunari N., Naito K., Suzuki G., Fukunaga M. Fracture prediction from bone mineral density in Japanese men and women. J Bone Miner Res. 2003;18(8):1547–1553. doi: 10.1359/jbmr.2003.18.8.1547. [DOI] [PubMed] [Google Scholar]
- 16.Siris E.S., Miller P.D., Barrettconnor E., Faulkner K.G., Wehren L.E., Abbott T.A. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. JAMA. 2001;286(22):2815. doi: 10.1001/jama.286.22.2815. [DOI] [PubMed] [Google Scholar]
- 17.Marshall D., Johnell O., Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;25(7041):1254. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paul D.M.M.D., Siris E.S., Barrett-Connor E., Faulkner K.G., Wehren L.E., Abbott T.A. Prediction of fracture risk in postmenopausal white women with peripheral bone densitometry: evidence from the national osteoporosis risk assessment. J Bone Miner Res. 2002;17(12):2222–2230. doi: 10.1359/jbmr.2002.17.12.2222. [DOI] [PubMed] [Google Scholar]
- 19.3Rd M.L., Atkinson E.J., O'Fallon W.M., Wahner H.W., Riggs B.L. Long-term fracture prediction by bone mineral assessed at different skeletal sites. J Bone Miner Res. 1993;8(10):1227. doi: 10.1002/jbmr.5650081010. [DOI] [PubMed] [Google Scholar]
- 20.Ensrud K.E., Schousboe J.T. Clinical practice. Vertebral fractures. N Engl J Med. 2011;364(17):1634. doi: 10.1056/NEJMcp1009697. [DOI] [PubMed] [Google Scholar]
- 21.Cauley J.A., Palermo L., Vogt M., Ensrud K.E., Ewing S., Hochberg M. Prevalent vertebral fractures in black women and white women. J Bone Miner Res. 2008;23(9):1458–1467. doi: 10.1359/JBMR.080411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwok A.W., Gong J.S., Wang Y.X., Leung J.C., Kwok T., Griffith J.F. Prevalence and risk factors of radiographic vertebral fractures in elderly Chinese men and women: results of Mr. OS (Hong Kong) and Ms. OS (Hong Kong) studies. Osteoporos Int. 2013;24(3):877–885. doi: 10.1007/s00198-012-2040-8. [DOI] [PubMed] [Google Scholar]
- 23.Yong Z., Zhuang Z., Wu C.A., Zhao D., Chao W., Cheng X. Population-stratified analysis of bone mineral density distribution in cervical and lumbar vertebrae of Chinese from quantitative computed tomography. Korean J Radiol. 2016;17(5):581–589. doi: 10.3348/kjr.2016.17.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanis J.A., Mccloskey E.V., Johansson H., Cooper C., Rizzoli R., Reginster J.Y. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2012;24(1):23–57. doi: 10.1007/s00198-012-2074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silva B.C., Broy S.B., Boutroy S., Schousboe J.T., Shepherd J.A., Leslie W.D. Fracture risk prediction by non-BMD DXA measures: the 2015 ISCD official positions part 2: trabecular bone score. J Clin Densitom. 2015;18(3):309–330. doi: 10.1016/j.jocd.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 27.Yi C., Wang M., Wei J., Wang J., Wang L., Cheng X. Preoperative QCT assessment of femoral head for assessment of femoral head bone loss. Exp Ther Med. 2017;13(4):1470–1474. doi: 10.3892/etm.2017.4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su Y.B., Cheng X.G., Wang L., Ma Y.M. 2016. Predicting hip fracture type of elderly Asian patients with low-energy fall by volumetric BMD and femoral morphology from QCT. [Google Scholar]
- 29.Ma X.H., Zhang W., Wang Y., Xue P., Li Y.K. Comparison of the spine and hip BMD assessments derived from quantitative computed tomography. Int J Endocrinol. 2015;2015(5):675340. doi: 10.1155/2015/675340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amstrup A.K., Jakobsen N.F., Moser E., Sikjaer T., Mosekilde L., Rejnmark L. Association between bone indices assessed by DXA, HR-pQCT and QCT scans in post-menopausal women. J Bone Miner Metabol. 2016;34(6):1–8. doi: 10.1007/s00774-015-0708-9. [DOI] [PubMed] [Google Scholar]
- 31.Andreoli A., Bazzocchi A., Celi M., Lauro D., Sorge R., Tarantino U. Relationship between body composition, body mass index and bone mineral density in a large population of normal, osteopenic and osteoporotic women. La Radiol Med. 2011;116(7):1115–1123. doi: 10.1007/s11547-011-0689-2. [DOI] [PubMed] [Google Scholar]
- 32.Schiessl H., Frost H.M., Jee W.S. Estrogen and bone-muscle strength and mass relationships. Bone. 1998;22(1):1–6. doi: 10.1016/s8756-3282(97)00223-8. [DOI] [PubMed] [Google Scholar]
- 33.Ma H.T., Griffith J.F., Xu L., Leung P.C. The functional muscle-bone unit in subjects of varying BMD. Osteoporos Int. 2013;25(3):999–1004. doi: 10.1007/s00198-013-2482-7. [DOI] [PubMed] [Google Scholar]
- 34.Macdonald J.H., Evans S.F., Davie M.W., Sharp C.A. Muscle mass deficits are associated with bone mineral density in men with idiopathic vertebral fracture. Osteoporos Int. 2007;18(10):1371–1378. doi: 10.1007/s00198-006-0223-x. [DOI] [PubMed] [Google Scholar]
- 35.Ling W., Wei W., Li X., Cheng X., Ma Y., Dan L. Relation of visceral and subcutaneous adipose tissue to bone mineral density in Chinese women. Int J Endocrinol. 2013;2013(2):378632. doi: 10.1155/2013/378632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Popp K.L., Hughes J.M., Martinezbetancourt A., Scott M., Turkington V., Caksa S. Bone mass, microarchitecture and strength are influenced by race/ethnicity in young adult men and women. Bone. 2017;103:200–208. doi: 10.1016/j.bone.2017.07.014. [DOI] [PubMed] [Google Scholar]