Abstract
Triclosan (TCS) is a high-volume chemical used as an antimicrobial ingredient in over 2000 consumer products such as toothpastes, cosmetics, and toys. Due to its widespread use, it causes ubiquitous contamination in the environment and is frequently detected in the human body, raising concerns about its impact on environmental pollution and human health. Our recent study showed that short-time exposure to low-dose TCS causes colonic inflammation, increases severity of colitis, and exacerbates colitis-associated colon tumorigenesis in mice, through gut microbiota- and Toll-like receptor 4 (TLR4)-dependent mechanisms. In addition, we demonstrate that beyond TCS, other antimicrobial chemicals used in consumer products also exaggerate colitis and colon cancer in mice. Together, these results highlight the importance to further evaluate these consumer antimicrobials on gut health, to develop potential further regulatory policies.
Keywords: triclosan, colon inflammation, colon cancer, gut microbiota
Introduction
In the last half century, there has been a dramatic increase in the incidence and prevalence of inflammatory bowel disease (IBD) in the United States and other countries (Molodecky et al., 2012). In 2015, ∼1.3% of U.S. adults (3 million) were estimated to be diagnosed with IBD (Dahlhamer et al., 2016), and this was a large increase from 1999 (0.9% or 2 million adults) (Nguyen et al., 2014). The rapidity of these developments supports that environmental factors, rather than genetic drift, could be primarily responsible for the increased incidences of IBD. Indeed, recent studies show that some common dietary components, such as milk-derived saturated fat and dietary emulsifiers, exaggerate development of IBD by gut microbiome-dependent mechanisms (Devkota et al., 2012; Chassaing et al., 2015). Considering the growing incidences of IBD and potential lethal consequence of IBD-induced colon cancer, it is of critical importance to identify novel risk factors of IBD, to reduce the risks posed by these diseases.
Triclosan (TCS) is a high-volume chemical used as an antimicrobial ingredient in over 2000 consumer products. Due to its widespread use, TCS causes ubiquitous contamination in the environment and is frequently detected in the human body (Halden, 2014). Indeed, it is listed among the top ten pollutants found in U.S. rivers (Halden, 2014), and was detected in ∼75% of the urine samples of individuals tested in the United States (Calafat et al., 2008). In 2016, the U.S. Food and Drug Administration (FDA) removed 19 antimicrobial compounds, including TCS, from over-the-counter handwashing products; however, this ruling only affects soaps and other handwashing products, TCS remains approved by the FDA and U.S. Environmental Protection Agency (EPA) to use in many other consumer products such as toothpastes, cosmetics, and toys (Food and Drug Administration, 2016). Therefore, human exposure to TCS could remain to be a serious and long-lasting problem.
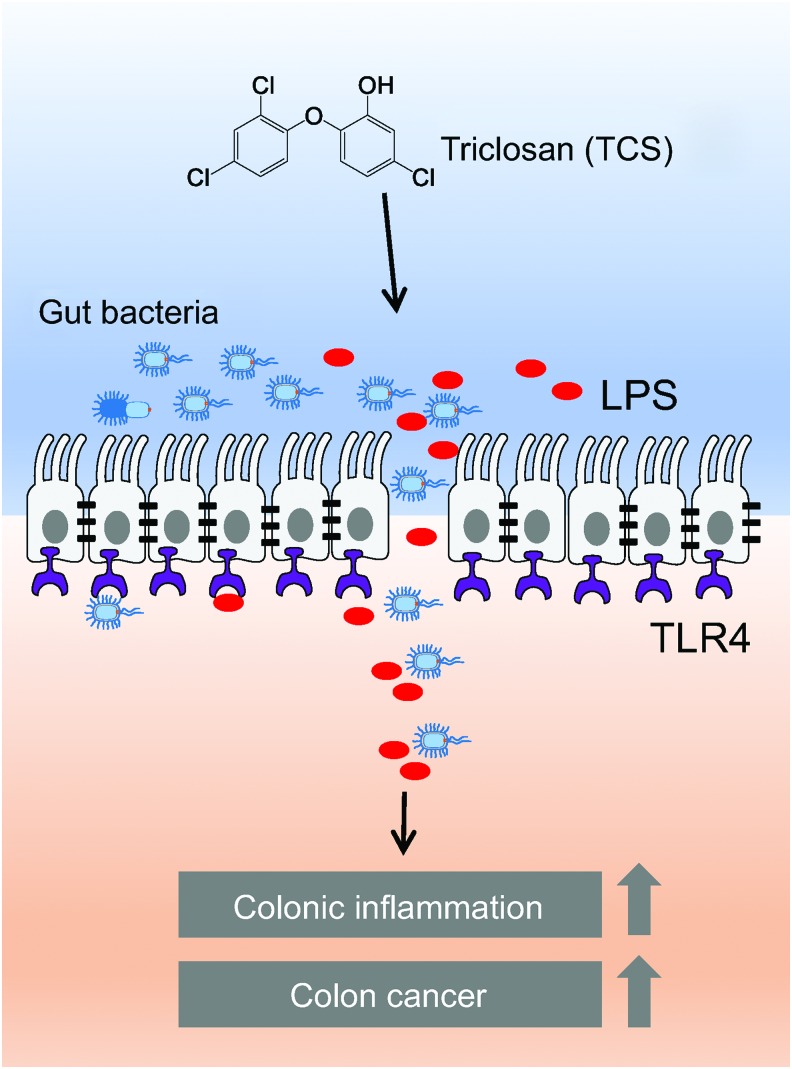
Our recent study shows that exposure to TCS enhances basal colonic inflammation, exacerbates development of IBD, and exacerbates IBD-induced colon tumorigenesis in mice (Fig. 1) (Yang et al., 2018). Notably, we find that in both chemically induced and spontaneous IBD mouse models, exposure to TCS increases infiltration of immune cells, enhances expression of proinflammatory cytokine interleukin 6 (IL-6), and exaggerates crypt damage in the colon tissues, illustrating its potential pro-IBD effects (Yang et al., 2018). In an IBD-induced colon cancer model, exposure to TCS increases tumor number, tumor size, and total tumor burden in mice, demonstrating its potential proneoplastic actions (Yang et al., 2018). About 90% of sporadic colon cancers have activating mutations within the Wnt pathway (Najdi et al., 2011), and we find that TCS exposure increases expressions of Wnt signaling markers, including β-catenin, c-Myc, and Axin2, in colon tumors, suggesting that TCS exposure could deregulate Wnt signaling (Yang et al., 2018). Our finding is largely in agreement with recent studies, which showed that chronic treatment of TCS increases the incidence and size of liver tumor in a diethylnitrosamine-induced hepatocellular carcinoma model in mice (Yueh et al., 2014). Together, these results support that TCS could be a novel risk factor of IBD and IBD-associated colon tumorigenesis. It would be important to further evaluate the impact of TCS exposure on gut health in humans.
FIG. 1.
Proposal model of TCS on gut microbiota, gut permeability, colonic inflammation, and colon cancer. TCS, triclosan. Color images available online at www.liebertpub.com/dna
Although the precise causes of IBD remain unknown, IBD is widely regarded as abnormal mucosal immune response toward the gut microbiota. Substantial studies have shown that the gut microbiota is deregulated in IBD patients and contributes to the pathogenesis of IBD (Kostic et al., 2014). In this study, we find that the gut microbiota plays an important role in the proinflammatory effects of TCS. Exposure to TCS disrupts gut microbiota, with reduced diversity and changed composition of the gut microbiota. In addition, TCS exposure increases basal colonic inflammation in mice with the gut microbiota (conventionally raised mice), but has no such effect on mice lacking the gut microbiota (germ-free mice), supporting that gut microbiota is required for the proinflammatory effects of TCS (Yang et al., 2018). In agreement with our finding, previous studies have shown that TCS exposure alters the gut microbiota in various animal models, including fathead minnows (Narrowe et al., 2015), zebrafish (Gaulke et al., 2016), mice (Gao et al., 2017), and rats (Hu et al., 2016). More importantly, recent human studies have shown that the usage of TCS-containing toothpaste alters the fecal microbiota (Ribado et al., 2017), and consumption of TCS-containing breast milk could change the fecal microbiota in infants (Bever et al., 2018). Together, these results support the critical importance of gut microbiota in the toxicology of TCS, and emphasize the need to better assess the potential impact of TCS exposure on the gut microbiota and gut health.
Furthermore, we find that the innate immunity receptor, Toll-like receptor 4 (TLR4), contributes to the pro-IBD effects of TCS. TLR4 recognizes lipopolysaccharide, which is a common component of many Gram-negative bacteria and certain Gram-positive bacteria and plays an important role in gut bacteria-host interactions (Abreu, 2010). Upon ligand binding, TLR4 activates NF-κB signaling pathway and induces the production of an array of proinflammatory cytokines, leading to enhanced inflammatory responses (Abreu, 2010). The activation of TLR4 signaling in the gut tissues is tightly regulated: TLR4 is generally expressed at the basolateral surface of intestinal epithelial cells, and therefore is physically separated from the gut bacteria, and this ensures that an immune response would only be activated when the gut bacteria penetrate the intestinal epithelium layer (Abreu, 2010; Kubinak and Round, 2012). We find that, in a chemically induced IBD model, exposure to TCS reduces colonic expression of tight junction proteins, impairs intestinal barrier function, and enhances bacterial translocation through the intestinal epithelium layer, and this leads to enhanced activation of TLR4 signaling in the systemic circulation. Finally, we demonstrate that TCS enhances IBD in wild-type mice, but not in TLR4-knockout mice, supporting that TLR4 signaling is important for the pro-IBD effects of TCS (Yang et al., 2018). Considering the critical roles of TLR4 in recognizing gut bacteria, these data further support that the gut microbiota contributes to the adverse effects of TCS.
Beyond TCS, many other antimicrobials are also used as high-volume chemicals and are incorporated into many consumer products as antimicrobial ingredients. Compared with TCS, we know less about the biological actions of these compounds. We recently also studied the actions of benzalkonium chloride and benzethonium chloride, which are antimicrobial compounds used in many personal care products and are potential candidates to replace TCS, and found that these two compounds also exaggerated IBD and IBD-induced colon tumorigenesis in mice (Sanidad et al., 2018). These results support that it is important to evaluate other antimicrobials, as well as other compounds that could disrupt the gut microbiota, on gut health.
The ultimate fates of these compounds, including TCS, need to be determined from human studies. A better understanding of the mechanisms by which TCS exposure increases colonic inflammation and colon tumorigenesis could help design human studies to validate its potential adverse effects. Our study has shown that the gut microbiota is essentially required for the proinflammatory effects of TCS in the colon (Yang et al., 2018); however, the functional roles of the gut microbiota and the specific gut bacteria involved are unknown. An intriguing question is the roles of gut microbiota in the colonic metabolism of TCS. Previous studies have demonstrated that once TCS enters the body, TCS is known to be rapidly metabolized by Phase II detoxification enzymes to generate water-soluble conjugates (e.g., TCS glucuronide and TCS sulfate), which are biologically inactive and are rapidly removed from the body through the kidney or gastrointestinal tract (Yueh and Tukey, 2016). However, we argue that in the colon tissues, the β-glucuronidase- and sulfatase-expressing gut bacteria could catalyze hydrolysis of TCS glucuronide and TCS sulfate, and regenerate the parent, biologically active, TCS in the colon tissues (Pellock and Redinbo, 2017). Therefore, there could be a completely different pattern of TCS metabolism in the colon tissues, which could contribute to its gut toxicology. Elucidation of the specific bacteria involved in the biological actions and colonic metabolism of TCS could help us design better human trials, or understand interindividual variations to TCS exposure. This will greatly help to clarify the potential health effects of TCS and facilitate the development of the further regulatory policies of TCS.
Acknowledgments
This research is supported by a new faculty start-up from the University of Massachusetts Amherst, USDA NIFA 2016-67017-24423, and NIH/NCI R03CA218520 (to G.Z.), and National Natural Science Foundation of China 21676212 (to H.Y.).
Disclosure Statement
No competing financial interests exist.
References
- Abreu M.T. (2010). Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol 10, 131–144 [DOI] [PubMed] [Google Scholar]
- Bever C.S., Rand A.A., Nording M., Taft D., Kalanetra K.M., Mills D.A., et al. (2018). Effects of triclosan in breast milk on the infant fecal microbiome. Chemosphere 203, 467–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat A.M., Ye X., Wong L.Y., Reidy J.A., and Needham L.L. (2008). Urinary concentrations of triclosan in the U.S. population: 2003–2004. Environ Health Perspect 116, 303–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassaing B., Koren O., Goodrich J.K., Poole A.C., Srinivasan S., Ley R.E., et al. (2015). Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 519, 92–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlhamer J.M., Zammitti E.P., Ward B.W., Wheaton A.G., and Croft J.B. (2016). Prevalence of inflammatory bowel disease among adults aged >18 years—United States, 2015. MMWR Morb Mortal Wkly Rep 65, 1166–1169 [DOI] [PubMed] [Google Scholar]
- Devkota S., Wang Y., Musch M.W., Leone V., Fehlner-Peach H., Nadimpalli A., et al. (2012). Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature 487, 104–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food Drug Administration HHS. (2016). Safety and effectiveness of consumer antiseptics; topical antimicrobial drug products for over-the-counter human use. Final rule. Fed Regist 81, 61106–61130 [PubMed] [Google Scholar]
- Gao B., Tu P., Bian X., Chi L., Ru H., and Lu K. (2017). Profound perturbation induced by triclosan exposure in mouse gut microbiome: a less resilient microbial community with elevated antibiotic and metal resistomes. BMC Pharmacol Toxicol 18, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaulke C.A., Barton C.L., Proffitt S., Tanguay R.L., and Sharpton T.J. (2016). Triclosan exposure is associated with rapid restructuring of the microbiome in adult zebrafish. PLoS One 11, e0154632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halden R.U. (2014). On the need and speed of regulating triclosan and triclocarban in the United States. Environ Sci Technol 48, 3603–3611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Raikhel V., Gopalakrishnan K., Fernandez-Hernandez H., Lambertini L., Manservisi F., et al. (2016). Effect of postnatal low-dose exposure to environmental chemicals on the gut microbiome in a rodent model. Microbiome 4, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostic A.D., Xavier R.J., and Gevers D. (2014). The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology 146, 1489–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubinak J.L., and Round J.L. (2012). Toll-like receptors promote mutually beneficial commensal-host interactions. PLoS Pathog 8, e1002785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molodecky N.A., Soon I.S., Rabi D.M., Ghali W.A., Ferris M., Chernoff G., et al. (2012). Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 142, 46–54 [DOI] [PubMed] [Google Scholar]
- Najdi R., Holcombe R.F., and Waterman M.L. (2011). Wnt signaling and colon carcinogenesis: beyond APC. J Carcinog 10, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narrowe A.B., Albuthi-Lantz M., Smith E.P., Bower K.J., Roane T.M., Vajda A.M., et al. (2015). Perturbation and restoration of the fathead minnow gut microbiome after low-level triclosan exposure. Microbiome 3, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen G.C., Chong C.A., and Chong R.Y. (2014). National estimates of the burden of inflammatory bowel disease among racial and ethnic groups in the United States. J Crohns Colitis 8, 288–295 [DOI] [PubMed] [Google Scholar]
- Pellock S.J., and Redinbo M.R. (2017). Glucuronides in the gut: sugar-driven symbioses between microbe and host. J Biol Chem 292, 8569–8576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribado J.V., Ley C., Haggerty T.D., Tkachenko E., Bhatt A.S., and Parsonnet J. (2017). Household triclosan and triclocarban effects on the infant and maternal microbiome. EMBO Mol Med 9, 1732–1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanidad K.Z., Yang H., Wang W., Ozay E.I., Yang J., Gu M., et al. (2018). Effects of consumer antimicrobials benzalkonium chloride, benzethonium chloride, and chloroxylenol on colonic inflammation and colitis-associated colon tumorigenesis in mice. Toxicol Sci 163, 490–499 [DOI] [PubMed] [Google Scholar]
- Yang H., Wang W., Romano K.A., Gu M., Sanidad K.Z., Kim D., et al. (2018). A common antimicrobial additive increases colonic inflammation and colitis-associated colon tumorigenesis in mice. Sci Transl Med 10, pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yueh M.F., Taniguchi K., Chen S., Evans R.M., Hammock B.D., Karin M., et al. (2014). The commonly used antimicrobial additive triclosan is a liver tumor promoter. Proc Natl Acad Sci U S A 111, 17200–17205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yueh M.F., and Tukey R.H. (2016). Triclosan: a widespread environmental toxicant with many biological effects. Annu Rev Pharmacol Toxicol 56, 251–272 [DOI] [PMC free article] [PubMed] [Google Scholar]