Abstract
A variety of viruses can induce central nervous system (CNS) infections and neurological diseases, although the incidence is rare. Similar to peripheral infections, IFNα/β induction and signaling constitutes a first line of defense to limit virus dissemination. However, CNS-resident cells differ widely in their repertoire and magnitude of both basal and inducible components in the IFNα/β pathway. While microglia as resident myeloid cells have been implicated as prominent sentinels of CNS invading pathogens or insults, astrocytes are emerging as key responders to many neurotropic RNA virus infections. Focusing on RNA viruses, this review discusses the role of astrocytes as IFNα/β inducers and responders and touches on the role of IFNα/β receptor signaling in regulating myeloid cell activation and IFNγ responsiveness. A summary picture emerges implicating IFNα/β not only as key in establishing the classical “antiviral” state, but also orchestrating cell mobility and IFNγ-mediated effector functions.
Keywords: astrocytes, IFNα/β, IFNγ, viral encephalitis
Introduction
Challenges for immune responses in the central nervous system
The central nervous system (CNS) harbors fully differentiated, nonregenerating postmitotic cells, such as neurons and oligodendrocytes, as well as microglia and astrocytes dedicated to providing trophic and metabolic support. While a complex endothelial cell barrier system physically protects the CNS from invading pathogens, astrocytes and microglia are vital participants in early innate responses to virus penetrating the CNS through neuronal transport or a breached barrier system. Their rapid innate response not only controls virus dissemination directly by IFNα/β, but also indirectly by promoting leukocyte infiltration and effector function.
Many neurotropic viruses infect neurons as primary targets, but glia cells are also susceptible (26,38,41). Immune inflammatory responses to clear virus infection must therefore be tightly regulated to minimize direct viral as well as immune-mediated damage to neurons; the inability to achieve an appropriate balance manifests in breakdown of neuronal function and even mortality (26,41). A rapid but delicately orchestrated innate immune response tailored for each infection is thus critical to limit early virus replication and spread, ultimately tipping the balance in favor of subsequent control by adaptive immune effector cells.
An integral component of the acute innate immune response is production of IFNα/β and signaling through the IFNα/β receptor (IFNAR). Autocrine and paracrine binding of IFNα/β to IFNAR and signal transduction through STAT1 and STAT2 induces transcription of hundreds of IFN-stimulated genes (ISGs). In addition to encoding antiviral factors interfering with viral replication and assembly, ISGs encode pathogen recognition receptors (PRRs), factors associated with IFNα/β signaling, as well as IFNαs themselves. Induction of ISGs thus restricts viral replication in infected cells and promotes an antiviral state in neighboring uninfected cells (25). By amplifying the IFNα/β response, the positive feedback loop is essential to establish an antiviral state (25,39).
IFNα/β also shapes the transition from innate to adaptive immune response by activating dendritic cells (DCs) and enhancing major histocompatibility complex (MHC) class I antigen (Ag) processing and presentation to promote T cell activation (25). Swift class I Ag presentation specifically by non-DC resident cells in the target tissue in turn triggers effective antiviral CD8 T cell responses (14,43). However, similar to overexuberant innate responses, unchecked CD8 T cell responses can lead to extensive tissue damage. The IFNα/β response thus also comprises negative regulators, such as suppressors of cytokine signaling (SOCSs), or PD-L1 and PD-L2 that downregulate ongoing activation and control excessive T cell function (15,31,36).
Aside from numerous studies on individual IFNAR-mediated functions, the impact of IFNα/β on the global antiviral program was demonstrated using a model of CNS infection by lymphocytic choriomeningitis virus (LCMV), a noncytopathic arenavirus (27). Although intracranial infection by LCMV causes a fatal meningitis due to effector functions of infiltrating cytotoxic T lymphocytes and myeloid cells, it establishes asymptomatic long-term persistence in mice with a highly restricted CD8 T cell repertoire specific for ovalbumin (OT-I mice). In OT-I mice sufficient in IFNAR expression, infection was associated with a prominent IFNα/β gene expression signature and a vigorous dynamic patrolling behavior by myeloid cells in the meninges.
By contrast, OT-I mice deficient in IFNAR (IFNAR−/−) did not exhibit differentially regulated genes following infection relative to uninfected control mice; moreover, myeloid cells were unresponsive during both acute and chronic infection. This study showed that almost the entire gene expression program induced by a non cytolytic virus in the CNS was either directly or indirectly linked to IFNα/β signaling (27). Furthermore, microglia in OT-I IFNAR−/− mice did not show a dynamic cellular response to LCMV infection and vascular patrolling by CX3CR1-GFP+/− myeloid cells remained similar to uninfected mice (27). Given that the responses of microglia to perturbation in the brain are too rapid to depend on gene expression changes (12), this particular LCMV infection model indicated that IFNAR signaling is not only essential for global antiviral activity, but also for physical myeloid cell dynamics.
Cell Type-Dependent IFNα/β Responses in the CNS
Low but constitutive expression of IFNα/βs and their homeostatic activity in the CNS implied that CNS-resident cells are prepared to rapidly induce protective IFN responses. Moreover, high susceptibility of IFNAR−/− mice to neurotropic viruses highlights the essential role of IFNAR for protection (26,38). As plasmacytoid DCs, potent peripheral IFNα/β inducers, are absent from the brain parenchyma, resident CNS cells rely on intrinsic induction of IFNα/β (4,38).
Numerous studies have revealed that almost all CNS-resident cells are capable of inducing and responding to IFNα/β (28,46). However, they differ widely in both the repertoire and magnitude of basal and inducible transcripts encoding PRRs and factors associated with the IFNα/β pathway, including the receptor chains IFNAR1 and IFNAR2, as well as STAT1 (16,22,28,35,46,48). Responses can even be different within neuronal cell subtypes (6,26). Nevertheless the rapid induction of genes involved in pathogen sensing and their signaling components by the positive IFNAR signaling feedback loop assures that poor IFNα/β inducer cells nevertheless make vital contributions to overall innate antiviral protection (26,28,53). However, the interdependence of CNS cells in optimizing protective IFNα/β function requires further investigation.
Astrocyte-Mediated Innate Immune Response During RNA Virus Infections
Astrocytes are the predominant glia population within the CNS. They play a crucial role in maintaining homeostatic CNS functions as well as regulating immune responses following insults and display regional functional heterogeneity. For example, astrocytes are involved in regulating neuronal synaptic plasticity and blood–brain barrier (BBB) integrity (44). In response to infection, reactive astrocytes upregulate PRRs, including various toll-like receptors, and produce chemokines and cytokines, such as CXCL10, CXCL1, CCL2, and IL-6 to modulate inflammation (8). Therefore, the dysregulation of astrocyte homeostatic function by microbial infection contributes to dysfunction of the CNS and neurological complication.
Neurotropic viruses predominantly infect neurons, but also target glia cells (11,16,21,26,27,29,38,41). Studies of CNS infections by RNA viruses, including La Crosse, rabies virus (RABV), vesicular stomatitis virus (VSV), and Theiler's encephalomyelitis virus (TMEV) demonstrated that astrocytes and microglia/macrophages are the main source of IFNβ, despite their prominent neuronal tropism (21,29). Infection of IFNβ reporter mice to effectively track IFNβ confirmed that neuronal IFNβ production is highly controlled in vivo and that astrocytes are essential contributors to virus control through PRR activation pathways (29).
Importantly, not only productively, but also abortively VSV-infected astrocytes produced protective IFNα/β. IFNβ production was limited to the local site of infection and was not detected in other brain areas or draining lymph nodes at early stages of infection (13,42). Locally induced IFNα/β in VSV-infected olfactory bulbs triggered ISG activation in distal parts of the brain, sparing them from infection (13). This long distance warning to establish a highly alert state in distal brain regions, was also previously reported by van den Pol et al. (42); this group demonstrated that IFNβ produced by infected olfactory sensory neurons following VSV infection activated ISGs in uninfected regions.
Contrasting the multitude of neurotropic RNA virus infections, sublethal neurotropic mouse hepatitis virus (MHV) strains have varying glia tropism and provide valuable encephalomyelitis models to study crosstalk between IFNα/β induction and responsiveness among distinct cell types. MHV belongs to the family of enveloped single-positive stranded RNA coronaviruses.
Prevalent strains used to study acute and persistent CNS infection are the dual hepato- and neurotropic MHV-A59 strain and the JHM 2.2v-1 monoclonal antibody-derived neutralization escape variant of the highly virulent MHV-JHM strain; both are sublethal in adult mice and cause immune-mediated demyelination (1–3). While MHV-A59 infects microglia, astrocytes, neurons, and to a lesser extent oligodendroglia, MHV-JHM 2.2v-1, infects predominantly oligodendroglia and microglia with sparse neuronal infection (23,51). Although both MHVs are poor inducers of IFNα/β in vivo and in vitro (1,33), they nevertheless induce IFNβ in myeloid cells in culture and in vivo (22,34,52). Importantly, even the low levels of IFNα/β are essential to prevent viral dissemination and mortality following both intracranial MHV-A59 and MHV-JHM 2.2v-1 infection (3,18).
To better understand how individual glia populations respond to CNS infection, our group analyzed gene expression patterns of microglia, astrocytes, and oligodendrocytes isolated from the MHV-infected CNS. Following MHV-JHM 2.2v-1 infection, microglia were the main initial inducers of IFNα/β mRNA, whereas oligodendroglia exhibited limited and delayed innate antiviral responses. Moreover, microglia from naive mice exhibited higher constitutive expression levels of mRNAs encoding for PRRs and factors associated with IFNα/β pathway than oligodendrocytes (22). Naive adult brain astrocytes also expressed lower basal levels of IFNα/β-associated genes relative to microglia (16,47). Nevertheless, following MHV-A59-mediated IFNα/β production in the CNS, astrocytes showed delayed but substantially upregulated IFNα/β-associated gene expression (16). This supported that astrocytes are poor initial sensors of MHV-A59 to induce IFNα/β, but effective IFNα/β responders. Primary mouse astrocytes have also been shown to trigger IFNα synthesis to TMEV infection (37).
Active participation of astrocytes in paving the way for lymphocyte-mediated immunity is also demonstrated by their strong capacity to produce CXCL10, a chemokine involved in the recruitment of CXCR3-expressing lymphocytes, including NK cells, T cells, and plasma cells. During MHV-JHM 2.2v-1 infection, astrocyte-mediated CXCL10 production is independent of their direct infection (30) and acts on CD4 T cell and plasma blast recruitment. In the LCMV model, CXCL10 production by infected astrocytes is associated with IFNγ producing CD8 T cells and disease severity (7). These data highlight the early responder role of astrocytes in orchestrating both IFNα/β as well as subsequent lymphocyte-mediated protection.
Contributions of IFNAR Signaling in Astrocytes
Astrocytes from different anatomical CNS regions display functional heterogeneity (47). In their role as integral components of the BBB ensheathing the CNS microvasculature with endfeet, region-specific astrocyte function also manifests in distinct regulation of BBB integrity. Contrasting disruption of endothelial cell tight-junction integrity in the BBB by proinflammatory cytokines, IFNα/β strengthens and stabilizes BBB function during West Nile virus (WNV) infection (10). Genetically induced loss of IFNAR signaling in astrocytes specifically enhanced BBB permeability in the hindbrain region, especially the cerebellum, which was associated with earlier detection of WNV in this area (11). This study also demonstrated that astrocyte IFNAR signaling regulates infiltration of inflammatory cells specifically in the cerebellum. The underlying mechanisms involved IFNα/β-mediated suppression of vascular cell adhesion molecule 1 expression, an integrin that facilitates immune cell trafficking during CNS infection (11).
Based on the prevalent role of astrocytes in IFNα/β-mediated antiviral activities discussed above, we evaluated the role of astrocyte-specific IFNAR signaling in modulating MHV-A59-mediated pathogenesis. Infection of adult mice lacking IFNAR on astrocytes resulted in acute, severe encephalitis and early mortality within 1 week (16). The delayed mortality by 5 days relative to global IFNAR−/− mice (3) indicated that other CNS cells participate in limiting early virus dissemination. Irrespectively, mortality was associated with uncontrolled virus replication and spread throughout the CNS, unlike distinct foci of infection in IFNAR-sufficient mice. Importantly, uncontrolled virus spread was not restricted to astrocytes but also affected neurons and microglia, despite overall elevated IFNα/β responses (16).
As virus was administered directly into the CNS, elevated viral entry into the CNS due to loss of astrocyte IFNAR-mediated BBB integrity was excluded in this model. These results confirmed that IFNAR responsiveness by astrocytes, independent of their initially poor sensing of MHV infection, was vital in blocking viral spread. Of note, increased viral spread to other cell types was irrespective of an environment enriched in IFNα/β and otherwise competent in IFNAR signaling.
Crosstalk Between IFNα/β and IFNγ
During neurotropic MHV-A59 and JHM 2.2v-1 infections, IFNα/β stalls virus spread, but CD4 and CD8 T cell effector functions, including IFNγ activity, are vital to reduce infectious virus below detectable levels (2,9). Lack of virus control in the absence of astrocyte IFNAR was thus attributed to impaired T cell recruitment and/or local function. However, although IFNAR abrogation in astrocytes modestly reduced T cell recruitment, it enhanced, rather than impaired, IFNγ production (16). Uncontrolled virus replication despite high IFNγ levels, suggested IFNγ could not exert effector function, such as promoting MHC molecule expression (5). Microglia indeed exhibited significantly reduced MHC class II surface expression coincident with a failure to induce IFNγ-dependent class II transactivator (CIITA), the master regulator of MHC class II expression.
These surprising results supported defective IFNγ signaling in microglia (16), which may not only be limited to myeloid cells, but also affect other CNS-resident cells. For example, oligodendrocytes require IFNγ to upregulate MHC class I antigen processing and presentation molecules during MHV-JHM 2.2v-1 infection (24). Failure to upregulate MHC class I and class II may thus contribute to failed T cell-mediated virus control.
The notion that elevated and sustained IFNα/β signaling acts as a negative regulator of IFNγ signaling is supported by earlier reports showing antagonistic effects of IFNα/β on IFNγ-induced activation of macrophages (17,32,45). In this context it is critical to note that IFN desensitization is necessary to limit sustained detrimental cytokine signaling. One IFN desensitization mechanism involves activation of ISGs that act as negative regulators of the signaling cascade to limit sustained toxic IFN responses (19). Negative regulators, such as SOCSs and ubiquitin-specific peptidase 18 (USP18) compete with STATs or JAK1 binding to the IFN receptor, respectively (36). Other mechanisms rely on receptor endocytosis and turnover as well as phosphatase activity to reduce the levels of phosphorylated JAK-STATs, essential for IFNAR signal transduction. Downregulation of the IFNγ receptor (IFNGR) to reduce IFNGR signaling has indeed been shown during Listeria infection (32). IFNα/β induced by Listeria monocytogenes suppresses IFNγ-mediated macrophage activation by inhibiting induction of CIITA mRNA and MHCII and also downregulating the expression of genes involved in IFNγ responsiveness, including IFNGRs (32).
IFNα/β-dependent sensitization of microglia/macrophages to subsequent IFNγ responses may provide a mechanism to limit excessive immune responses leading to tissue damage. Although it is well established that IFNα/β and IFNγ responses alone are tightly controlled by multiple mechanisms, crosstalk between IFNα/β and IFNγ responses in vivo may provide additional cues to protect the host from exacerbated proinflammatory responses.
Neutrophils together with monocytes are generally the first CNS infiltrating populations following neurotropic infections, including neurotropic MHVs. While innate responses through PRR stimulation induce myeloid cell recruiting chemokines, CXCL1 and CCL2, IFNα/β suppresses recruitment of neutrophil by inhibiting its chemoattractants (20,40).
Astrocytes are efficient inducers of the neutrophil chemoattractant CXCL1 following infection and other insults. Excessive and sustained CXCL1 mRNA levels in MHV-A59-infected mice devoid of IFNAR in astrocytes are thus associated with increased neutrophil accumulation. Interestingly, the fact that neither IFNα/β nor IFNγ could repress neutrophils in this model suggested indirect regulation through sustained CXCL1 expression. Dysregulated CXCL1 and neutrophil infiltration may thus contribute to neuronal toxicity in the absence of astrocyte IFNAR signaling. More apoptotic neurons in the absence of IFNAR in astrocyte indeed suggested direct neuronal damage by uncontrolled virus replication or detrimental neutrophil activity (16). Lastly, astrocyte-derived CXCL1 induced during sterile neuroinflammation also plays a key role in neuropathic pain by ligating its receptor CXCR2 on neurons (49,50).
Conclusions and Perspectives
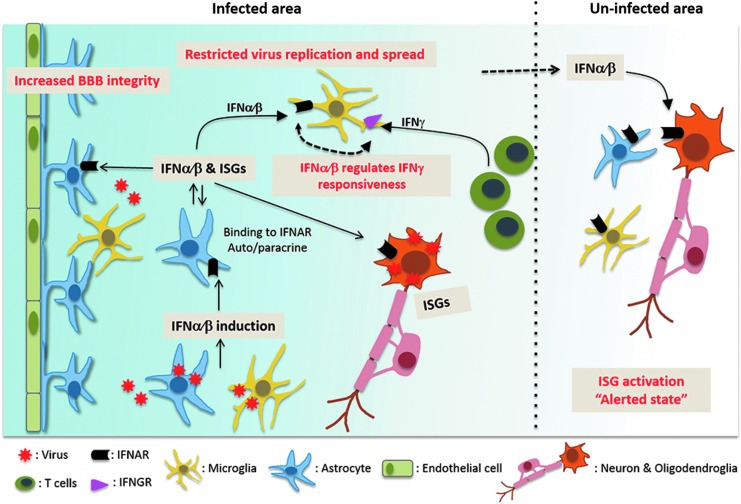
Overall, this review highlights the role of astrocytes in innate immune protection from neurotropic RNA virus infections, including those thought to be primarily neuron-tropic. Detailed analysis of components in the IFNα/β induction and signaling pathway over the past decade revealed significantly diverse expression patterns not only among distinct CNS-resident cell types, but also within neuronal and astrocyte subtypes depending on their regional location. In the case of noncytolytic meningeal infection in the absence of T cell activation, IFNα/β signaling is the sole mediator of altered gene expression as well as myeloid cell dynamics in response to infection. Importantly, the availability of diverse fluorescently marked reporter mice to increase detection of IFNβ, ISG activation, and dynamics of myeloid cell surveillance revealed several novel aspects of intercellular communication (Fig. 1).
FIG. 1.
The interdependence of CNS cells in optimizing protective IFNα/β function. In many neurotropic virus infections, microglia and astrocytes induce initial IFNα/β and are the main sources of IFNβ, despite their prominent neuronal tropism. Locally induced IFNα/β binds to IFNAR on both infected and uninfected cell types, thereby activating an IFNAR signaling cascade, which induces expression of ISGs and amplifies IFNα/β responses. IFNAR signaling specifically on astrocytes promotes BBB integrity, and serves to limit virus replication, thereby restricting infection of other susceptible cells types and stemming spread throughout the CNS. Even focal IFNα/β restricted to the site of infection suffices to activate ISGs at distal sites to alert other connected CNS regions of a potential viral threat. Finally sustained IFNα/β can alter T cell-induced IFNγ function. BBB, blood–brain barrier; CNS, central nervous system; IFNAR, IFNα/β receptor; IFNGR, IFNγ receptor; ISG, IFN-stimulated gene.
Microglia and astrocytes are primary sentinels of infection initiating an IFNα/β-positive feedback loop through both autocrine and paracrine IFNα/β signaling. Especially nonproductively infected astrocytes can be more potent sources of IFNβ than productively infected neurons. Importantly, local IFNα/β induction at the initial site of replication can have long-range protective effects by triggering an “alerted” state in distal parts of the brain. IFNAR signaling specifically in astrocytes can lead to tightening of the BBB, leading to enhanced physical protection from virus invasion in certain brain regions. However, this protection is clearly dependent on the balance of upregulated cytokines disrupting the BBB. Once virus replicates in the CNS, IFNAR signaling in astrocytes is crucial to limit virus dissemination not only within astrocytes, but also IFNAR-competent cells.
In addition to astrocyte-dependent direct antiviral responses, IFNα/β also downregulates detrimental sustained chemokine expression, which signal to both neurons as well as infiltrating neutrophils. The multitude of interconnected functions highlights how effective crosstalk between IFNα/β-inducing and -responder cells, as well as the IFNγ pathway, determine pathogenic outcome.
Acknowledgments
This work was supported by the U.S. National Institutes of Health grant P01NS064932. The funding source had no involvement in the study design, writing of the article, decision to submit, or collection, analysis, and interpretation of data.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Bender SJ, and Weiss SR. Pathogenesis of murine coronavirus in the central nervous system. J Neuroimmune Pharmacol 2010;5:336–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bergmann CC, Lane TE, and Stohlman SA. Coronavirus infection of the central nervous system: Host-virus stand-off. Nat Rev Microbiol 2006;4:121–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Butchi NB, Hinton DR, Stohlman SA, et al. Ifit2 deficiency results in uncontrolled neurotropic coronavirus replication and enhanced encephalitis via impaired alpha/beta interferon induction in macrophages. J Virol 2014;88:1051–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carty M, Reinert L, Paludan SR, et al. Innate antiviral signalling in the central nervous system. Trends Immunol 2014;35:79–87 [DOI] [PubMed] [Google Scholar]
- 5. Chesler DA, and Reiss CS. The role of IFN-gamma in immune responses to viral infections of the central nervous system. Cytokine Growth Factor Rev 2002;13:441–454 [DOI] [PubMed] [Google Scholar]
- 6. Cho H, Proll SC, Szretter KJ, et al. Differential innate immune response programs in neuronal subtypes determine susceptibility to infection in the brain by positive-stranded RNA viruses. Nat Med 2013;19:458–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Christensen JE, Simonsen S, Fenger C, et al. Fulminant lymphocytic choriomeningitis virus-induced inflammation of the CNS involves a cytokine-chemokine-cytokine-chemokine cascade. J Immunol 2009;182:1079–1087 [DOI] [PubMed] [Google Scholar]
- 8. Claycomb KI, Johnson KM, Winokur PN, et al. Astrocyte regulation of CNS inflammation and remyelination. Brain Sci 2013;3:1109–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cupovic J, Onder L, Gil-Cruz C, et al. Central nervous system stromal cells control local CD8(+) T cell responses during virus-induced neuroinflammation. Immunity 2016;44:622–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Daniels BP, Holman DW, Cruz-Orengo L, et al. Viral pathogen-associated molecular patterns regulate blood-brain barrier integrity via competing innate cytokine signals. MBio 2014;5:e01476–e01414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Daniels BP, Jujjavarapu H, Durrant DM, et al. Regional astrocyte IFN signaling restricts pathogenesis during neurotropic viral infection. J Clin Invest 2017;127:843–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Davalos D, Grutzendler J, Yang G, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 2005;8:752–758 [DOI] [PubMed] [Google Scholar]
- 13. Detje CN, Lienenklaus S, Chhatbar C, et al. Upon intranasal vesicular stomatitis virus infection, astrocytes in the olfactory bulb are important interferon Beta producers that protect from lethal encephalitis. J Virol 2015;89:2731–2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fitzgerald-Bocarsly P, and Feng D. The role of type I interferon production by dendritic cells in host defense. Biochimie 2007;89:843–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garcia-Diaz A, Shin DS, Moreno BH, et al. Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. Cell Rep 2017;19:1189–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hwang M, and Bergmann CC. Alpha/beta interferon (IFN-alpha/beta) signaling in astrocytes mediates protection against viral encephalomyelitis and regulates IFN-gamma-dependent responses. J Virol 2018;92:e1901–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Inaba K, Kitaura M, Kato T, et al. Contrasting effect of alpha/beta- and gamma-interferons on expression of macrophage Ia antigens. J Exp Med 1986;163:1030–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ireland DD, Stohlman SA, Hinton DR, et al. Type I interferons are essential in controlling neurotropic coronavirus infection irrespective of functional CD8 T cells. J Virol 2008;82:300–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ivashkiv LB, and Donlin LT. Regulation of type I interferon responses. Nat Rev Immunol 2014;14:36–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jablonska J, Wu CF, Andzinski L, et al. CXCR2-mediated tumor-associated neutrophil recruitment is regulated by IFN-beta. Int J Cancer 2014;134:1346–1358 [DOI] [PubMed] [Google Scholar]
- 21. Kallfass C, Ackerman A, Lienenklaus S, et al. Visualizing production of beta interferon by astrocytes and microglia in brain of La Crosse virus-infected mice. J Virol 2012;86:11223–11230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kapil P, Butchi NB, Stohlman SA, et al. Oligodendroglia are limited in type I interferon induction and responsiveness in vivo. Glia 2012;60:1555–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lavi E, Gilden DH, Highkin MK, et al. The organ tropism of mouse hepatitis virus A59 in mice is dependent on dose and route of inoculation. Lab Anim Sci 1986;36:130–135 [PubMed] [Google Scholar]
- 24. Malone KE, Stohlman SA, Ramakrishna C, et al. Induction of class I antigen processing components in oligodendroglia and microglia during viral encephalomyelitis. Glia 2008;56:426–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McNab F, Mayer-Barber K, Sher A, et al. Type I interferons in infectious disease. Nat Rev Immunol 2015;15:87–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miller KD, Schnell MJ, and Rall GF. Keeping it in check: Chronic viral infection and antiviral immunity in the brain. Nat Rev Neurosci 2016;17:766–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nayak D, Johnson KR, Heydari S, et al. Type I interferon programs innate myeloid dynamics and gene expression in the virally infected nervous system. PLoS Pathog 2013;9:e1003395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Paul S, Ricour C, Sommereyns C, et al. Type I interferon response in the central nervous system. Biochimie 2007;89:770–778 [DOI] [PubMed] [Google Scholar]
- 29. Pfefferkorn C, Kallfass C, Lienenklaus S, et al. Abortively infected astrocytes appear to represent the main source of interferon beta in the virus-infected brain. J Virol 2016;90:2031–2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Phares TW, Stohlman SA, Hinton DR, et al. Astrocyte-derived CXCL10 drives accumulation of antibody-secreting cells in the central nervous system during viral encephalomyelitis. J Virol 2013;87:3382–3392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Randall RE, and Goodbourn S. Interferons and viruses: An interplay between induction, signalling, antiviral responses and virus countermeasures. J Gen Virol 2008;89:1–47 [DOI] [PubMed] [Google Scholar]
- 32. Rayamajhi M, Humann J, Penheiter K, et al. Induction of IFN-alphabeta enables Listeria monocytogenes to suppress macrophage activation by IFN-gamma. J Exp Med 2010;207:327–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rose KM, and Weiss SR. Murine coronavirus cell type dependent interaction with the type I interferon response. Viruses 2009;1:689–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roth-Cross JK, Bender SJ, and Weiss SR. Murine coronavirus mouse hepatitis virus is recognized by MDA5 and induces type I interferon in brain macrophages/microglia. J Virol 2008;82:9829–9838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Russo MV, and McGavern DB. Immune surveillance of the CNS following infection and injury. Trends Immunol 2015;36:637–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schneider WM, Chevillotte MD, and Rice CM. Interferon-stimulated genes: A complex web of host defenses. Annu Rev Immunol 2014;32:513–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. So EY, and Kim BS. Theiler's virus infection induces TLR3-dependent upregulation of TLR2 critical for proinflammatory cytokine production. Glia 2009;57:1216–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sorgeloos F, Kreit M, Hermant P, et al. Antiviral type I and type III interferon responses in the central nervous system. Viruses 2013;5:834–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stark GR, and Darnell JE., Jr The JAK-STAT pathway at twenty. Immunity 2012;36:503–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stock AT, Smith JM, and Carbone FR. Type I IFN suppresses Cxcr2 driven neutrophil recruitment into the sensory ganglia during viral infection. J Exp Med 2014;211:751–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Swanson PA, 2nd, and McGavern DB. Viral diseases of the central nervous system. Curr Opin Virol 2015;11:44–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van den Pol AN, Ding S, and Robek MD. Long-distance interferon signaling within the brain blocks virus spread. J Virol 2014;88:3695–3704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang Y, Swiecki M, Cella M, et al. Timing and magnitude of type I interferon responses by distinct sensors impact CD8 T cell exhaustion and chronic viral infection. Cell Host Microbe 2012;11:631–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yeh TH, Lee DY, Gianino SM, et al. Microarray analyses reveal regional astrocyte heterogeneity with implications for neurofibromatosis type 1 (NF1)-regulated glial proliferation. Glia 2009;57:1239–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yoshida R, Murray HW, and Nathan CF. Agonist and antagonist effects of interferon alpha and beta on activation of human macrophages. Two classes of interferon gamma receptors and blockade of the high-affinity sites by interferon alpha or beta. J Exp Med 1988;167:1171–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zegenhagen L, Kurhade C, Koniszewski N, et al. Brain heterogeneity leads to differential innate immune responses and modulates pathogenesis of viral infections. Cytokine Growth Factor Rev 2016;30:95–101 [DOI] [PubMed] [Google Scholar]
- 47. Zhang Y, and Barres BA. Astrocyte heterogeneity: An underappreciated topic in neurobiology. Curr Opin Neurobiol 2010;20:588–594 [DOI] [PubMed] [Google Scholar]
- 48. Zhang Y, Chen K, Sloan SA, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 2014;34:11929–11947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang ZJ, Cao DL, Zhang X, et al. Chemokine contribution to neuropathic pain: Respective induction of CXCL1 and CXCR2 in spinal cord astrocytes and neurons. Pain 2013;154:2185–2197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang ZJ, Jiang BC, and Gao YJ. Chemokines in neuron-glial cell interaction and pathogenesis of neuropathic pain. Cell Mol Life Sci 2017;74:3275–3291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhao L, Rose KM, Elliott R, et al. Cell-type-specific type I interferon antagonism influences organ tropism of murine coronavirus. J Virol 2011;85:10058–10068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhou H, Zhao J, and Perlman S. Autocrine interferon priming in macrophages but not dendritic cells results in enhanced cytokine and chemokine production after coronavirus infection. MBio 2010;1:pii [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zurney J, Howard KE, and Sherry B. Basal expression levels of IFNAR and Jak-STAT components are determinants of cell-type-specific differences in cardiac antiviral responses. J Virol 2007;81:13668–13680 [DOI] [PMC free article] [PubMed] [Google Scholar]