Abstract
Viral infection in the brain can be acute or chronic, with the responses often producing foci of increasingly cytotoxic inflammation. This can lead to effects beyond the central nervous system (CNS). To stimulate discussion, this commentary addresses four questions: What drives the development of human immunodeficiency virus (HIV)-associated neurocognitive disorders, does the phenotype of macrophages in the CNS spur development of HIV encephalitis (HIVE), does continual activation of astrocytes drive the development of HIV-associated neurocognitive disorders/subclinical disease, and neuroinflammation: friend or foe? A unifying theory that connects each question is the issue of continued activation of glial cells, even in the apparent absence of simian immunodeficiency virus/HIV in the CNS. As the CNS innate immune system is distinct from the rest of the body, it is likely there could be a number of activation profiles not observed elsewhere.
Keywords: HIV, neuropathogenesis, astrocyte
Introduction
Although inflammation promotes the recruitment of immune cells to combat bacterial and viral infection, the product of these immune responses can damage tissues and organs of the body. “Danger signals” are released by injured and dying cells recruiting more immune cells to the site of inflammation where they can further drive the immune response, resulting in a positive feedback loop and chronic inflammatory spiral (as reviewed in Ref. 76). When this inflammatory response occurs in the brain in response to bacterial or viral infection, the damage can be widespread, with effects beyond the central nervous system (CNS).
Inflammation in the Brain
Inflammation in the brain can be acute or chronic. Acute CNS inflammation is characterized by activation of astrocytes and microglia with increased secretion of proinflammatory cytokines, including interleukin-1β (IL-1β), tumor necrosis factor alpha (TNFα), and IL-6. This local inflammatory response begins the recruitment of leukocytes, including monocyte-derived macrophages (MDMs). Once inside the CNS, macrophages can secrete cytokines and chemokines, activating astrocytes that can then secrete more cytokines and chemokines, leading to the positive feedback loop alluded to earlier (82).
If the infectious agent is not completely controlled and eradicated, it can lead to a localized increasingly cytotoxic inflammation, which can have a cumulative effect over time and result in chronic inflammation (6,82). In chronic inflammation, the prolonged glial activation leads to tissue toxicity and neuron damage (82). To prevent this damage from spreading, reactive astrocytes can proliferate and create glial scars to block contact with healthy neurons; however, such a scar can also hinder axonal regeneration (27,58,95). That said, not all reactive astrocytes are protective, as long-term activation of astrocytes can lead to sustained innate immune activation. This will be addressed further in Question 3. In several viral infections, including simian immunodeficiency virus (SIV) and chikungunya, astrocytes can remain in an activated phenotype after immune clearance of virus, leading to chronic implications of inflammation (47,56,99).
Toll-Like Receptors
As one of the major factors of innate immunity in the CNS, Toll-like receptors (TLRs) are expressed on endothelial cells, astrocytes, microglia, and subsets of neurons. Of the 11 TLRs identified in humans, increased expression of TLR2, TLR3, and TLR9 has been reported in CNS viral infections (11–13,38,47,56–58). TLRs are professional molecular pattern recognition receptors that elicit the innate immune response, and later adaptive immune response, upon encountering highly specific antigens, referred to as pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs) (38). The release of DAMPs, as a result of cell death or tissue remodeling, can trigger activation of TLRs, assuming the correct pairing of DAMP and TLR is present at the same time (38). It is also possible that the strength of the binding between the TLR and DAMP can influence the outcome, either neuroprotective or neurodegenerative (69).
Although TLR2 is activated by interaction with bacterial products, including cell wall components and lipoprotein, TLR4 can be activated by damaged tissue markers such as soluble CD14 (sCD14) and high mobility group box-1, both of which can be shed from activated monocytes (64). Thus, TLRs contribute to brain injury through activation of microglia and astrocytes, downstream release of proinflammatory cytokines, and further recruit peripheral immune cells to the CNS. Increasing evidence is showing that once activated, astrocytes can have a prolonged increase in expression of TLR2. This is likely to have long-term effects, including macrophage recruitment and retention in the CNS, which is addressed in Question 2.
Encephalitic Viruses
Numerous viruses can cause encephalitis, including herpes simplex, cytomegalovirus (CMV), the Togaviridae and Flaviviridae, and human immunodeficiency virus (HIV). CMV has been shown to be a cause of mental retardation, cerebral palsy, and has been linked to the development of brain tumors (19,84). Togaviridae, similar to chikungunya, have neurological sequelae, including immune activation of astrocytes, even in the absence of apparent virus in tissues (47). Flaviviridae, similar to Dengue and Zika, showed hypertrophy of astrocytes in the absence of neuroinflammation and vascular leakage, indicating activation of astrocytes that continued after viral control (57).
Perhaps the most widely studied virus associated with encephalitis is HIV. HIV and the parent virus SIV subvert the innate and adaptive immune responses, in part, through infection and reprogramming of myeloid cells (49,82). Because of this, HIV and its model, SIV, are the focus of this commentary.
Studies of HIV infection of CD4+ T cell subpopulations have become increasingly popular (22). However, too often analyses of other cell types are excluded. Once CD4+ T cells become infected, they experience cytopathic effects and often die, which is why a CD4+ latent reservoir model is losing popularity. Viral outgrowth analyses have shown that there are greater number of infected monocytes and MDMs than CD4+ T cells in blood, bronchoalveolar lavage, lungs, spleen, and brain (3). It has almost become forgotten that HIV is a lentivirus and, therefore, infects monocytes and traffics to the CNS within a Trojan horse (21,92). Beyond this, there is much debate, fueled by several studies that suggest, but without proving, a lesser role of macrophages and other cell types. This commentary seeks to pose some questions that some may see as controversial.
-
1.
What drives the development of HIV-associated neurocognitive disorders (HANDs)?
-
2.
Does the phenotype of macrophages in the CNS spur development of HIV encephalitis (HIVE)?
-
3.
Does continual activation of astrocytes drive the development of HANDs/subclinical disease?
-
4.
Neuroinflammation: friend or foe?
Q1: What Drives the Development of HANDs?
HANDs are the collective term for HIV-related neurological impairment. HANDs are broken down into asymptomatic, referred to as asymptomatic neurocognitive impairment (ANI), and symptomatic, which is further broken down into the classifications mild neurocognitive disorder/impairment (MND/I) and HIV-associated dementia (HAD). Pathologically, HIVE has been defined by perivascular leukocytic infiltrates, perivascular cuffing, and gliosis, but the unique diagnostic criteria are the presence of multinucleated giant cells (MNGCs) in the brain (80). MNGCs are giant cells created by the cell–cell fusion of macrophages in response to chronic inflammation, as is seen in chronic HIV infections (61,63).
Despite the advent of combination antiretroviral therapies (cARTs) and the resulting suppression of viral replication, the prevalence of HIV-associated neurocognitive impairment has increased (5), most likely due to ongoing neuroinflammation. The 2010 CHARTER study found that >52% of HIV-infected individuals had a neurocognitive disorder, with 7% diagnosed with HAD, 12% diagnosed with MND/I, and 33% with ANI (5,41). Patients with HAND and HIVE experience worse adherence to treatment, lower quality of life, and a higher rate of mortality than patients without it (5,65,66). Another study found that 25–30% of untreated adults with HIV-1 and 15% of patients treated with cART had HAND (77). Many hypotheses have been proposed, such as a legacy effect, which suggests damage was already done before the initiation of therapy (78), cART neurotoxicity, and the inability of cART to cross the blood–brain barrier (BBB) (5). It is possible that this damage is due to chronic activation of astrocytes and other glial cells of the brain, which are chronically activated in SIV/HIV infection and can persist in the absence of virus in CNS (56). We will return to this idea in Question 3.
Monocyte invasion is linked to the development and pathogenesis of HIVE (40,62), and increased monocyte turnover predicts the progression of HIV infection to AIDS, and is also correlated with the severity of HIVE (10). There are three subsets of monocytes, which have since been termed classical, nonclassical, and intermediate monocytes. Classical monocytes are characterized by high CD14 expression, low-to-no CD16 expression, and moderate CD64 expression and comprise about 90% of the circulating monocytes in blood. Intermediate and nonclassical monocytes make up the rest of the monocytes circulating and differ in that they express CD16. Intermediate monocytes are distinguished by high CD14 and CD64 expression, whereas nonclassical monocytes have low CD14 expression and no CD64 expression (2). CD16+ monocytes are selectively infected with HIV, are preferentially attracted to the endothelial receptors of the BBB, and have increased junctional proteins such as JAM-A, ALCAM, and PECAM-1 to mediate the diapedesis (60,87,88,91). CD16+ monocytes have also been shown to promote higher viral replication than CD16− monocytes when they differentiate into macrophages and interact with T cells (2).
There are two overlapping theories for how monocytes enter the CNS, the “Trojan horse” and the “Trojan herd.” The monocyte “Trojan horse” model theorizes that an infected monocyte traffics across the BBB during normal immune surveillance early in infection [usually around 10–14 days postinfection (55)]. Once this infected monocyte enters the CNS, it becomes a productively infected macrophage, inducing more (potentially infected) monocytes to cross the BBB, forming perivascular cuffs of cells and triggering limited breakdown of the BBB, including the choroid plexus (26,70). It is believed these infected monocytes cross the BBB and differentiate into MDM and infect CNS resident macro- and microglia and lead to viral latency (49,54). Recently, the “late invasion” or “Trojan herd” model was proposed, where changes in the circulating monocyte phenotype described earlier can lead to increased cycles of invasion of monocytes in late-stage infection (30,34).
Macrophages and microglia are the primary targets of HIV infection in the CNS (18,97). Since both activated microglia and MDMs secrete proinflammatory cytokines, it has been debated over which drove SIV infection of the brain; however, in vivo and in vitro (74) studies suggest it is more likely that infiltrating macrophages drive the microglial response to SIV infection. These microglia activated by infiltrating MDMs had increased levels of monocyte chemoattractant protein-1 (MCP-1/CCL2), granulocyte–macrophage colony stimulating factor, and TNFα, regardless of whether the macrophages were SIV infected or not, although there were increased levels of IL-6, IL-8, and vascular endothelial growth factor when SIV-infected macrophages were introduced (74).
Q2: Does the Phenotype of Macrophages in the CNS Spur Development of HIVE?
SIV encephalitis is only seen in chronic infection with macrotrophic strains of SIV, providing further evidence of macrophage involvement in AIDS disease progression (83). At least three types of macrophages have been identified within the brain: microglia, perivascular macrophages, and choroid plexus macrophages (36,50). It was once thought that microglia populated the brain early in utero with very little turnover during the life span (94). Recent evidence using selective depletion of microglia (24,45), however, shows the presence of a population of microglia progenitor cells.
Perivascular macrophages have a comparatively short turnover: within 14 weeks, based on labeling studies (4). Perivascular macrophages were identified as a major cell productively infected during acute and terminal SIV infection and form the characteristic macrophage cuffs and MNGCs that are the defining pathological characteristic of HIVE (93).
Within the choroid plexus, there are two distinct populations of macrophages: choroid plexus macrophages are believed to be long-term tissue established macrophages, whereas perivascular macrophages, which lie along the fenestrated capillaries inside the choroid plexus, are the short-lived MDMs (36).
Changes to monocyte and macrophage phenotype and differentiation can lead to the development of HANDs (20,29). The dogma used to be that there were two phenotypes of CNS macrophages, proinflammatory M1, and anti-inflammatory M2, although recent studies have expanded the macrophage polarization to also include MØ and M4. M1 and M2 are the most widely characterized, with increasing number of subsets for M2 phenotypes proposed, including M2a, M2b, and M2c. M1 is believed to be classically activated and proinflammatory (triggering Th1 responses and secretion of IL-1, IL-12, TNFα, and ROS), whereas M2 is alternatively activated and anti-inflammatory (triggering Th2 responses and secretion of IL-10) (17,48). Ourselves and others have shown the presence of TNFα-producing macrophages in macaques with SIVE (67), yet M1 polarization may inhibit HIV from even integrating into macrophages (81). The time of monocyte recruitment, early or late in infection, can polarize the monocytes to either M1 or M2, respectively (37).
The type, degree, and length of macrophage activation can drive neuroprotection or neurodegeneration (1), leading to apparently contradictory phenotypes. For example, a receptor for haptoglobin–hemoglobin, CD163, has been described as a marker of M2 macrophages (25,31). Conversely, upregulation of CD163 on monocytes may follow activation of TLR2 (89). M2-polarized perivascular macrophages, double positive for CD163 and CD16 (which we described earlier as being preferentially infected with HIV), can be found in the CNS early in infection (7,25), and correlated with plasma viral load and negatively correlated with CD4+ T cells (10,31). However, CD163 is not an exclusive marker for perivascular macrophages; it can also identify activated microglia and choroid plexus macrophages (7,53). To complicate the issue further, CD163 is shed from monocytes/macrophages after exposure to TNFα, soluble CD163 is elevated in chronic HIV infection (9,25) and correlates with the percentage of intermediate and nonclassical monocytes in HIV infection (9). Thus, it is possible that M1 polarization, traditionally considered proinflammatory, actually prevents HIV replication, whereas the anti-inflammatory M2 phenotype may be more permissive to HIV replication and disease progression (68).
CD206 was originally thought of as a selective marker for perivascular macrophages, linked to an M2 phenotype. A phenotypic shift from CD206+ to CD206− perivascular macrophages has also been observed in SIV-infected rhesus macaques, indicating that the virus might cause changes in macrophage phenotype (46). Increased migration and proliferation of macrophages [a hallmark of M1 polarization (48)] lead to their accumulation and ultimately the formation of MNGCs, which are usually positive for viral RNA (39). As productive viral infection in macrophages potentially indicates an M2 phenotype, then the proliferative M1 neuroprotective response might be futile (28,46). It is also possible that the accumulation of macrophages could contribute to the damage of HIVE by polarizing more M2 macrophages from infiltrating myeloid cells (96).
Q3: Does Continual Activation of Astrocytes Drive the Development of HANDs/Subclinical Disease?
Another potential source of increased monocyte invasion in both acute and chronic HIV infection is activated astrocytes. For too long, astrocytes were considered support cells maintaining neuron health and contributing to BBB integrity (85). A protective role was acknowledged by protecting neurons from inflammation by helping repair the BBB and preserving surrounding tissue through the formation of glial scars (32,85), as discussed earlier. Indeed, there may be no direct counterpart for astrocytes in the peripheral immune system (72). However, astrocytes also play a role in the inflammatory damage linked to HIVE. Although astrocytes have not been reported to be productively infected in adults, they are postulated to be a latent reservoir even under ART. Furthermore, once astrocytes are infected during acute infection, they do not return to normal function as evidenced by long-term dysregulation of TLRs (56). Astrocytes are capable of being infected as they express both the CXCR4 and CCR5 coreceptors needed in HIV infection with restricted infection of astrocytes reported in neonatal rhesus macaques (59,90), although this may require specific tropism of viral strains (59).
Astrocyte activation could drive the increased monocyte turnover seen in HIV infection and HIVE. Astrocytes can be activated through binding transactivator of transcription (Tat) or cytokines secreted by neighboring microglia/MDMs. This leads to increased expression of the adhesion molecules intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 (86). These adhesion molecules then facilitate increased monocyte migration into the brain through chemotaxis and haptotaxis (35,52). Increased proinflammatory cytokines have been observed in brain tissue after SIV/HIV infection, including CCL2/MCP-1 and MCP-3/CCL7 (73,79,98). Although MCP-1/CCL2 recruits both monocytes and T cells, CD4+ T cells are not found in large quantities in the CNS in HIV infection (16,51). It is interesting to note that astrocytic supernatant had increased CCL7 and induced preferential migration of MDMs versus T cells (73). This could explain why monocytes, rather than CD4+ T cells, are preferentially selected to the CNS during HIV infection.
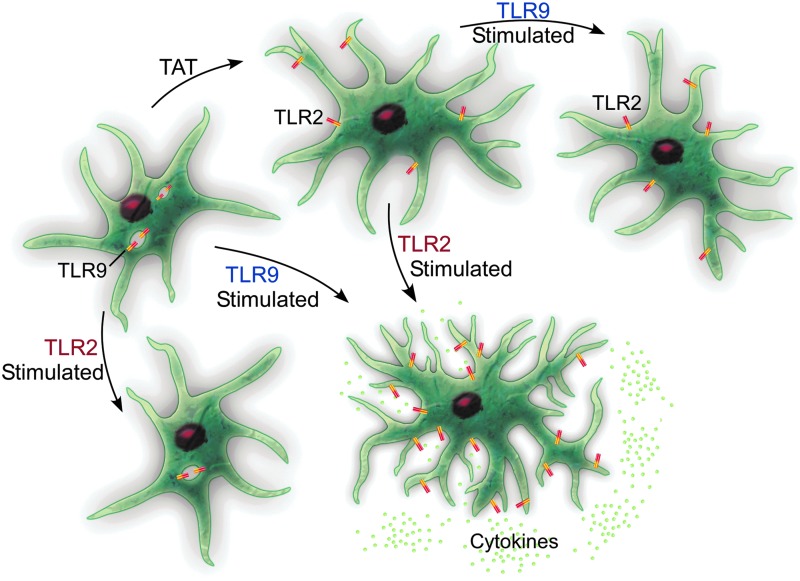
Under resting conditions, astrocytes express several TLRs that can bind DAMPs and PAMPs, activating an immune response (71). In HIV infection of the CNS, TLR2 is upregulated in astrocytes, which has been linked to sustained inflammation due to the continuous secretion of proinflammatory cytokines, and sustained TLR2 expression, (8,23,42,56) even when no virus is evident in the CNS (56). This is combined with decreased TLR9 expression (23). It is easy to imagine how HIV hijacking of the normal innate immune function of astrocytes would have long-standing effects (Fig. 1).
FIG. 1.
Under normal healthy conditions, astrocytes express high levels of TLR9 (generally thought of as antiviral) and low levels of TLR2 (antibacterial). When stimulated through TLR2, cytokine secretion is low, whereas exposure to TLR9 agonists produces a higher secretion of cytokines. When astrocytes are exposed to HIV proteins, including Tat, there is a phenotypic switch, both in vitro and in vivo. This means that if the astrocytes are then stimulated with TLR9 agonists, they fail to produce cytokines, yet when stimulated through TLR2, they produce a cytokine response. This implies that Tat protein hijacks the glia and creates increased activation through TLR2, while limiting the response to HIV viral antigens through TLR9. HIV, human immunodeficiency virus; TLR, Toll-like receptor.
Under normal conditions, astrocytes have high TLR9 (considered antiviral) and low TLR2 (considered antibacterial) (23,42,43). Thus, stimulation with a TLR2 agonist results in fairly low levels of cytokines, but TLR9 binding would be anticipated to induce higher secretion of cytokines. Incubation with Tat protein induces decreased expression of TLR9 (23) and increased TLR2; stimulation with TLR2 agonists induces increased cytokines (23,44). Thus, Tat effectively hides astrocytes from the very infectious agent they are stimulated by, making the astrocytes refractory to stimulation through viral antigens! This could provide an avenue through which astrocytes remain continually primed for activation, just not to the very virus that has primed them.
Q4: Neuroinflammation: Friend or Foe?
Arguably, the damage associated with HANDs is not caused by the virus, per se, but rather by the double-edged sword that comprises the immunological response(s) within the CNS. Obviously, inflammation is needed to control infection and the damage caused by it; however, HIV may have subverted this system, allowing for long-term and progressive damage to the CNS. The HIV proteins glycoprotein 120 (gp120), Tat, and negative regulatory factor (Nef) may do more damage than the actual HIV infection of the CNS cells.
HIV infection and damage of the brain is a multifaceted positive and negative feedback system composed of monocyte/macrophage, microglial, and astrocytic components. As we have shown earlier, classically activated proinflammatory M1-polarized macrophages (including microglia) are refractory to HIV replication; however, M2 macrophages, which should be resolving neuroinflammation, allow HIV to replicate (Q2). The result is an endless cycle of resolving and relapsing activation of microglia and astrocytes (Q3), waves of infiltrating monocytes (Q1), leading to chronic damage.
Once neuroinflammation is resolved, it is possible that the damage might still exist. A difficulty in assessing the presence of active inflammation in animal models is the histology at necropsy. If MNGCs are not identified on the hematoxylin and eosin slide, does that mean the animal never experienced neuroinflammation? It is not even known whether the brain returns to normal once MNGCs are present or whether this is a “point of no return?” Are other nonimmune cells of the brain damaged permanently? Pathologists are limited by the timing of necropsies, and H&Es do not always identify activation of nonimmune cells, including astrocytes and endothelium.
Some potential solutions moving forward would be to target numerous aspects of CNS infection, thus preventing initiation of the positive feedback loop. The best solution remains to prevent HIV prophylactically, and the second should be early and quick detection that allows for treatment with cART with high neuropenetrance. Alternative solutions under active investigation include blocking trafficking of infected monocytes into the CNS (14,15). Another alternative therapy that is gaining popularity is the use of tetrahydrocannabinol (THC). Levels of circulating CD16+hi monocytes are lower in HIV+THC+ patients than in untreated HIV+ patients, possibly by preventing the CD16− to CD16+ transition (75). THC also blocks the migration of microglial-like cells to HIV tat (33). Further studies are needed to elucidate the positive feedback loop in HIV infection, as well as treatment options to target different parts of the positive feedback loop to prevent rampant chronic damaging cellular activation and subsequent neuroinflammation.
Acknowledgments
This commentary was supported by grants OD011104 (formerly RR00164), NS104016, and MH113517 from the National Institutes of Health to the Tulane National Primate Research Center.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Amor S, Peferoen LAN, Vogel DYS, et al. . Inflammation in neurodegenerative diseases—an update. Immunology 2014;142:151–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ancuta P, Kunstman KJ, Autissier P, et al. . CD16+ monocytes exposed to HIV promote highly efficient viral replication upon differentiation into macrophages and interaction with T cells. Virology 2006;344:267–276 [DOI] [PubMed] [Google Scholar]
- 3. Avalos CR, Price SL, Forsyth ER, et al. . Quantitation of productively infected monocytes and macrophages of Simian immunodeficiency virus-infected Macaques. J Virol 2016;90:5643–5656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bechmann I, Priller J, Kovac A, et al. . Immune surveillance of mouse brain perivascular spaces by blood-borne macrophages. Eur J Neurosci 2001;14:1651–1658 [DOI] [PubMed] [Google Scholar]
- 5. Beck SE, Queen SE, Witwer KW, et al. . Paving the path to HIV neurotherapy: predicting SIV CNS disease. Eur J Pharmacol 2015;759:303–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Block ML, Zecca L, and Hong J-S. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 2007;8:57–69 [DOI] [PubMed] [Google Scholar]
- 7. Borda JT, Alvarez X, Mohan M, et al. . CD163, a marker of perivascular macrophages, is up-regulated by microglia in Simian immunodeficiency virus encephalitis after haptoglobin-hemoglobin complex stimulation and is suggestive of breakdown of the blood-brain barrier. Am J Pathol 2008;172:725–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burda JE, Bernstein AM, and Sofroniew MV. Astrocyte roles in traumatic brain injury. Exp Neurol 2016;275:305–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burdo TH, Lentz MR, Autissier P, et al. . Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J Infect Dis 2011;204:154–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burdo TH, Soulas C, Orzechowski K, et al. . Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma. PLoS Pathog 2010;6:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Butchi NB, Du M, and Peterson KE. Interactions between TLR7 and TLR9 agonists and receptors regulate innate immune responses by astrocytes and microglia. Glia 2010;58:650–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Butchi NB, Pourciau S, Du M, et al. . Analysis of the neuroinflammatory response to TLR7 stimulation in the brain: comparison of multiple TLR7 and/or TLR8 agonists. J Immunol 2008;180:7604–7612 [DOI] [PubMed] [Google Scholar]
- 13. Butchi NB, Woods T, Du M, et al. . TLR7 and TLR9 trigger distinct neuroinflammatory responses in the CNS. Am J Pathol 2011;179:783–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Byrareddy SN, Arthos J, Cicala C, et al. . Sustained virologic control in SIV+ macaques after antiretroviral and α4β7 antibody therapy. Science 2016;354:197–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Campbell JH, Ratai E-M, Autissier P, et al. . Anti-α4 antibody treatment blocks virus traffic to the brain and gut early, and stabilizes CNS injury late in infection. PLoS Pathog 2014;10:e1004533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carr MW, Roth SJ, Luther E, et al. . Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci U S A 1994;91:3652–3656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cassol E, Cassetta L, Rizzi C, et al. . M1 and M2a polarization of human monocyte-derived macrophages inhibits HIV-1 replication by distinct mechanisms. J Immunol 2009;182:6237–6246 [DOI] [PubMed] [Google Scholar]
- 18. Chakrabarti L, Hurtrel M, Maire MA, et al. . Early viral replication in the brain of SIV-infected rhesus monkeys. Am J Pathol 1991;139:1273–1280 [PMC free article] [PubMed] [Google Scholar]
- 19. Cheeran MC-J, Lokensgard JR, and Schleiss MR. Neuropathogenesis of congenital cytomegalovirus infection: disease mechanisms and prospects for intervention. Clin Microbiol Rev 2009;22:99–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Collini P, Noursadeghi M, Sabroe I, et al. . Monocyte and macrophage dysfunction as a cause of HIV-1 induced dysfunction of innate immunity. Curr Mol Med 2010;10:727–740 [DOI] [PubMed] [Google Scholar]
- 21. Collman R, Nassef T, Hassan F, et al. . Infection of monocyte-derived macrophages with human immunodeficiency virus type 1 (HIV-1): monocyte-tropic and lymphocyte-tropic strains of HIV-1 show distinctive patterns of replication in a panel of cell types. J Exp Med 1989;170:1149–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Douek DC, Brenchley JM, Betts MR, et al. . HIV preferentially infects HIV-specific CD4+ T cells. Nature 2002;417:95–98 [DOI] [PubMed] [Google Scholar]
- 23. El-Hage N, Podhaizer EM, Sturgill J, et al. . Toll-like receptor expression and activation in astroglia: differential regulation by HIV-1 Tat, gp120, and morphine. Immunol Invest 2011;40:498–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Elmore MRP, Lee RJ, West BL, et al. . Characterizing newly repopulated microglia in the adult mouse: impacts on animal behavior, cell morphology, and neuroinflammation. PLoS One 2015;10:e0122912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Etzerodt A, and Moestrup SK. CD163 and inflammation: biological, diagnostic, and therapeutic aspects. Antioxid Redox Signal 2013;18:2352–2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Falangola MF, Hanly A, Galvao-Castro B, et al. . HIV infection of human choroid plexus: a possible mechanism of viral entry into the CNS. J Neuropathol Exp Neurol 1995;54:497–503 [DOI] [PubMed] [Google Scholar]
- 27. Fawcett JW, and Asher RA. The glial scar and central nervous system repair. Brain Res Bull 1999;49:377–391 [DOI] [PubMed] [Google Scholar]
- 28. Filipowicz AR, Mcgary CM, Holder GE, et al. . Proliferation of perivascular macrophages contributes to the development of encephalitic lesions in HIV-infected humans and in SIV-infected Macaques. Sci Rep 2016;6:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fischer-Smith T, Bell C, Croul S, et al. . Monocyte/macrophage trafficking in acquired immunodeficiency syndrome encephalitis: lessons from human and nonhuman primate studies. J Neurovirol 2008;14:318–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fischer-Smith T, and Rappaport J. Evolving paradigms in the pathogenesis of HIV-1-associated dementia. Expert Rev Mol Med 2005;7:1–26 [DOI] [PubMed] [Google Scholar]
- 31. Fischer-Smith T, Tedaldi EM, and Rappaport J. CD163/CD16 coexpression by circulating monocytes/macrophages in HIV: potential biomarkers for HIV infection and AIDS progression. AIDS Res Hum Retroviruses 2008;24:417–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fitch MT, and Silver J. CNS injury, glial scars, and inflammation: inhibitory extracellular matrices and regeneration failure. Exp Neurol 2008;209:294–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fraga D, Raborn ES, Ferreira GA, et al. . Cannabinoids inhibit migration of microglial-like cells to the HIV protein Tat. J Neuroimmune Pharmacol 2011;6:566–577 [DOI] [PubMed] [Google Scholar]
- 34. Gartner S. HIV infection and dementia. Science 2000;287:602–604 [DOI] [PubMed] [Google Scholar]
- 35. Gerszten RE, Luscinskas FW, Ding HT, et al. . Adhesion of memory lymphocytes to vascular cell adhesion molecule-1-transduced human vascular endothelial cells under simulated physiological flow conditions in vitro. Circ Res 1996;79:1205–1215 [DOI] [PubMed] [Google Scholar]
- 36. Goldmann T, Jordão MJC, Wieghofer P, et al. . Origin, fate and dynamics of macrophages at CNS interfaces. Nat Immunol 2016;17:797–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Graziano F, Vicenzi E, and Poli G. Plastic restriction of HIV-1 replication in human macrophages derived from M1/M2 polarized monocytes. J Leukoc Biol 2016;100:1147–1153 [DOI] [PubMed] [Google Scholar]
- 38. Hanke ML, and Kielian T. Toll-like receptors in health and disease in the brain: mechanisms and therapeutic potential. Clin Sci (Lond) 2011;121:367–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Harbison C, Zhuang K, Gettie A, et al. . Giant cell encephalitis and microglial infection with mucosally transmitted simian-human immunodeficiency virus SHIV SF162P3 N in rhesus macaques. J Neurovirol 2014;20:62–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hasegawa A, Liu H, Ling B, et al. . The level of monocyte turnover predicts disease progression in the macaque model of AIDS. Immunobiology 2009;114:2917–2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Heaton RK, Clifford DB, Franklin DR, et al. . HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010;75:2087–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Henn A, Kirner S, and Leist M. TLR2 hypersensitivity of astrocytes as functional consequence of previous inflammatory episodes. J Immunol 2011;186:3237–3247 [DOI] [PubMed] [Google Scholar]
- 43. Henn A, Lund S, Hedtjärn M, et al. . The suitability of BV2 cells as alternative model system for primary microglia cultures or for animal experiments examining brain inflammation. ALTEX 2009;26:83–94 [DOI] [PubMed] [Google Scholar]
- 44. Hernández JC, Stevenson M, Latz E, et al. . HIV type 1 infection up-regulates TLR2 and TLR4 expression and function in vivo and in vitro. AIDS Res Hum Retroviruses 2012;28:1313–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hillmer AT, Holden D, Fowles K, et al. . Microglial depletion and activation: a [11 C]PBR28 PET study in nonhuman primates. EJNMMI Res 2017;7:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Holder GE, McGary CM, Johnson EM, et al. . Expression of the mannose receptor CD206 in HIV and SIV encephalitis: a phenotypic switch of brain perivascular macrophages with virus infection. J Neuroimmune Pharmacol 2014;9:716–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Inglis FM, Lee KM, Chiu KB, et al. . Neuropathogenesis of Chikungunya infection: astrogliosis and innate immune activation. J Neurovirol 2016;22:140–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Italiani P, Boraschi D, Ley K, et al. . From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front Immunol 2014;5:1–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ivey NS, Maclean AG, and Lackner AA. Acquired immunodeficiency syndrome and the blood-brain barrier. J Neurovirol 2009;15:111–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jordan FL, and Thomas WE. Brain macrophages: questions of origin and interrelationship. Brain Res 1988;472:165–178 [DOI] [PubMed] [Google Scholar]
- 51. Joseph SB, Arrildt KT, Sturdevant CB, et al. . HIV-1 target cells in the CNS. J Neurovirol 2015;21:276–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kevil CG, Patel RP, and Bullard DC. Essential role of ICAM-1 in mediating monocyte adhesion to aortic endothelial cells. Am J Physiol Cell Physiol 2001;281:C1442–C1447 [DOI] [PubMed] [Google Scholar]
- 53. Kim W-K, Alvarez X, Fisher J, et al. . CD163 identifies perivascular macrophages in normal and viral encephalitic brains and potential precursors to perivascular macrophages in blood. Immunopathol Infect Dis 2006;168:822–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kim W-K, Corey S, Alvarez X, et al. . Monocyte/macrophage traffic in HIV and SIV encephalitis. J Leukoc Biol 2003;74:650–656 [DOI] [PubMed] [Google Scholar]
- 55. Lane JH, Sasseville VG, Smith MO, et al. . Neuroinvasion by simian immunodeficiency virus coincides with increased numbers of perivascular macrophages/microglia and intrathecal immune activation. J Neurovirol 1996;2:423–432 [DOI] [PubMed] [Google Scholar]
- 56. Lee KM, Chiu KB, Renner NA, et al. . Form follows function: astrocyte morphology and immune dysfunction in SIV neuroAIDS. J Neurovirol 2014;20:474–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lee KM, Chiu KB, Sansing HA, et al. . The flavivirus dengue induces hypertrophy of white matter astrocytes. J Neurovirol 2016;22:831–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lee KM, and MacLean AG. New advances on glial activation in health and disease. World J Virol 2015;4:42–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Liu Y, Liu H, Kim BO, et al. . CD4-independent infection of astrocytes by human immunodeficiency virus type 1: requirement for the human mannose receptor. J Virol 2004;78:4120–4133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Maslin CL, Kedzierska K, Webster NL, et al. . Transendothelial migration of monocytes: the underlying molecular mechanisms and consequences of HIV-1 infection. Curr HIV Res 2005;3:303–317 [DOI] [PubMed] [Google Scholar]
- 61. McNally AK, and Anderson JM. Macrophage fusion and multinucleated giant cells of inflammation. In: Dittmar T, Zanker K, eds. Cell Fusion in Health and Disease: Advances in Experimental Medicine and Biology. Dordrecht: Springer, 2011:97–111 [DOI] [PubMed] [Google Scholar]
- 62. Meeker RB, Williams K, Killebrew DA, et al. . Cell trafficking through the choroid plexus. Cell Adh Migr 2012;6:390–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Milde R, Ritter J, Tennent GA, et al. . Multinucleated giant cells are specialized for complement-mediated phagocytosis and large target destruction. Cell Rep 2015;13:1937–1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Min H, Hong J, Cho I-H, et al. . TLR2-induced astrocyte MMP9 activation compromises the blood brain barrier and exacerbates intracerebral hemorrhage in animal models. Mol Brain 2015;8:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mind Exchange Working Group TMEW, Antinori A, Arendt G, et al. . Assessment, diagnosis, and treatment of HIV-associated neurocognitive disorder: a consensus report of the mind exchange program. Clin Infect Dis 2013;56:1004–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mothobi NZ, and Brew BJ. Neurocognitive dysfunction in the highly active antiretroviral therapy era. Curr Opin Infect Dis 2012;25:4–9 [DOI] [PubMed] [Google Scholar]
- 67. Orandle MS, MacLean AG, Sasseville VG, et al. . Enhanced expression of proinflammatory cytokines in the central nervous system is associated with neuroinvasion by simian immunodeficiency virus and the development of encephalitis. J Virol 2002;76:5797–5802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Osman A, Bhuyan F, Hashimoto M, et al. . M-CSF inhibits anti-HIV-1 activity of IL-32, but they enhance M2-like phenotypes of macrophages. J Immunol 2014;192:5083–5089 [DOI] [PubMed] [Google Scholar]
- 69. Owens T. Toll-like receptors in neurodegeneration. In: Kielian T, ed. Current Topics in Microbiology and Immunology. Berlin, Heidelberg: Springer, 2009:105–120 [DOI] [PubMed] [Google Scholar]
- 70. Peluso R, Haase A, Stowring L, et al. . A Trojan Horse mechanism for the spread of visna virus in monocytes. Virology 1985;147:231–236 [DOI] [PubMed] [Google Scholar]
- 71. Phulwani NK, Esen N, Syed MM, et al. . TLR2 expression in astrocytes is induced by TNF-alpha- and NF-kappa B-dependent pathways. J Immunol 2008;181:3841–3849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ransohoff RM, and Brown MA. Innate immunity in the central nervous system. J Clin Invest 2012;122:1164–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Renner NA, Ivey NS, Redmann RK, et al. . MCP-3/CCL7 production by astrocytes: implications for SIV neuroinvasion and AIDS encephalitis. J Neurovirol 2011;17:146–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Renner NA, Sansing HA, Morici LA, et al. . Microglia activation by SIV-infected macrophages: alterations in morphology and cytokine secretion. J Neurovirol 2012;18:213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rizzo MD, Crawford RB, Henriquez JE, et al. . HIV-infected cannabis users have lower circulating CD16+ monocytes and IFN-g-inducible protein 10 levels compared with nonusing HIV patients. AIDS 2018;32:419–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rock KL, and Kono H. The inflammatory response to cell death. Annu Rev Pathol 2008;3:99–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sacktor N, Lyles RH, Skolasky R, et al. . HIV-associated neurologic disease incidence changes: multicenter AIDS Cohort Study, 1990–1998. Neurology 2001;56:257–260 [DOI] [PubMed] [Google Scholar]
- 78. Sanford R, Ances BM, Meyerhoff DJ, et al. . Longitudinal trajectories of brain volume and cortical thickness in treated and untreated primary human immunodeficiency virus infection. Clin Infect Dis 2018. [Epub ahead of print]; DOI: 10.1093/cid/ciy362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sasseville VG, Smith MM, Mackay CR, et al. . Chemokine expression in simian immunodeficiency virus-induced AIDS encephalitis. Am J Pathol 1996;149:1459–1467 [PMC free article] [PubMed] [Google Scholar]
- 80. Saylor D, Dickens AM, Sacktor N, et al. . HIV-associated neurocognitive disorder—pathogenesis and prospects for treatment. Nat Rev Neurol 2016;12:234–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Schlaepfer E, Rochat M-A, Duo L, et al. . Triggering TLR2, -3, -4, -5, and -8 reinforces the restrictive nature of M1- and M2-polarized macrophages to HIV. J Virol 2014;88:9769–9781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Schwartz M, and Baruch K. The resolution of neuroinflammation in neurodegeneration: leukocyte recruitment via the choroid plexus. EMBO J 2014;33:7–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sharma DP, Zink MC, Anderson M, et al. . Derivation of neurotropic simian immunodeficiency virus from exclusively lymphocytetropic parental virus: pathogenesis of infection in macaques. J Virol 1992;66:3550–3556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Söderberg-Nauclér C, and Johnsen JI. Cytomegalovirus in human brain tumors: role in pathogenesis and potential treatment options. World J Exp Med 2015;5:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sofroniew MV, and Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol 2010;119:7–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Song HY, Ryu J, Ju SM, et al. . Extracellular HIV-1 Tat enhances monocyte adhesion by up-regulation of ICAM-1 and VCAM-1 gene expression via ROS-dependent NF-κB activation in astrocytes. Exp Mol Med 2007;39:27–37 [DOI] [PubMed] [Google Scholar]
- 87. Veenstra M, Byrd DA, Inglese M, et al. . CCR2 on peripheral blood CD14+CD16+ monocytes correlates with neuronal damage, HIV-associated neurocognitive disorders, and peripheral HIV DNA: reseeding of CNS reservoirs? J Neuroimmune Pharmacol 2018. [Epub ahead of print]; DOI: 10.1007/s11481-018-9792-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Veenstra M, León-Rivera R, Li M, et al. . Mechanisms of CNS viral seeding by HIV+ CD14+ CD16+ monocytes: establishment and reseeding of viral reservoirs contributing to HIV-associated neurocognitive disorders. MBio 2017;8:e01280–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Weaver LK, Pioli PA, Wardwell K, et al. . Up-regulation of human monocyte CD163 upon activation of cell-surface Toll-like receptors. J Leukoc Biol 2007;81:663–671 [DOI] [PubMed] [Google Scholar]
- 90. Westmoreland SV, Williams KC, Simon MA, et al. . Neuropathogenesis of simian immunodeficiency virus in neonatal rhesus macaques. Am J Pathol 1999;155:1217–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Williams DW, Anastos K, Morgello S, et al. . JAM-A and ALCAM are therapeutic targets to inhibit diapedesis across the BBB of CD14+ CD16+ monocytes in HIV-infected individuals. J Leukoc Biol 2015;97:401–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Williams DW, Eugenin EA, Calderon TM, et al. . Monocyte maturation, HIV susceptibility, and transmigration across the blood brain barrier are critical in HIV neuropathogenesis. J Leukoc Biol 2012;91:401–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Williams KC, Corey S, Westmoreland SV, et al. . Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J Exp Med 2001;193:905–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Williams KC, and Hickey WF. Traffic of hematogenous cells through the central nervous system. Berlin, Heidelberg: Springer, 1995:221–245 [DOI] [PubMed] [Google Scholar]
- 95. Yiu G, and He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci 2006;7:617–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhang Y, Choksi S, Chen K, et al. . ROS play a critical role in the differentiation of alternatively activated macrophages and the occurrence of tumor-associated macrophages. Cell Res 2013;23:898–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zink MC, Amedee AM, Mankowski JL, et al. . Pathogenesis of SIV encephalitis selection and replication of neurovirulent SIV. Am J Pathol 1997;151:793–803 [PMC free article] [PubMed] [Google Scholar]
- 98. Zink MC, Coleman GD, Mankowski JL, et al. . Increased macrophage chemoattractant protein–1 in cerebrospinal fluid precedes and predicts simian immunodeficiency virus encephalitis. J Infect Dis 2001;184:1015–1021 [DOI] [PubMed] [Google Scholar]
- 99. Zlotnik I. The reaction of astrocytes to acute virus infections of the central nervous system. Br J Exp Pathol 1968;49:555–564 [PMC free article] [PubMed] [Google Scholar]