Abstract
Significance: Compounds derived from plants are gaining importance for the treatment of several diseases. Many plants from the Convolvulaceae family contain compounds that have demonstrated wound healing and antidiabetic activity. Such compounds can be effectively used as a part of treatments to promote wound healing in diabetics and used in combination with antimicrobial therapy to reduce the likelihood of drug resistance and allergic reactions. Novel strategies for developing herbal formulations such as nanoparticles and adhesive patches can improve the delivery of plant-based therapeutic agents.
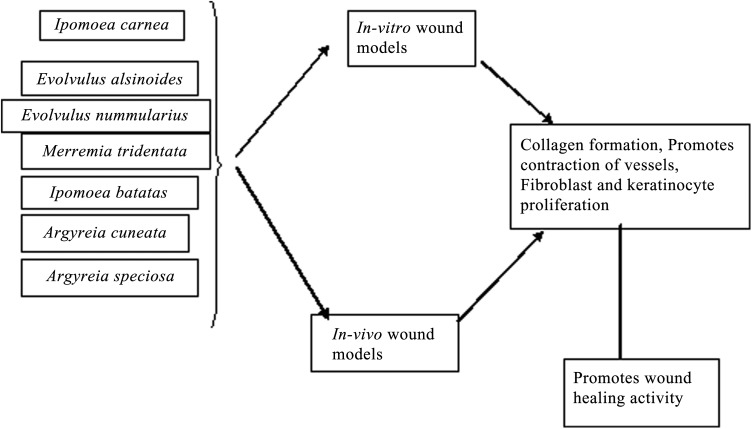
Recent Advances: Studies have confirmed the antidiabetic and wound healing activities of Merremia tridentata, Argyreia speciosa, and Ipomoea batatas, whereas Evolvulus alsinoides, Evolvulus nummularius, Argyreia cuneata, and Ipomoea carnea have wound healing activity.
Critical Issues: Drug resistance is a major problem associated with antimicrobial therapy and can affect wound healing processes. Phytoconstituents can facilitate healing processes and reduce reliance on antibiotics.
Future Directions: Plants from the Convolvulaceae family have had frequent traditional uses, and all plants selected for this study have antimicrobial, antidiabetic, and wound healing properties. Detailed phytochemical studies of these plants can help develop novel wound healing therapies.
Keywords: wound healing activity, antidiabetic, drug resistance, nanoparticle, adhesive patches
Anitha P. Ambika, MPharm.

Scope and Significance
Phytochemicals are gaining importance and can be incorporated into treatments to lower the incidence of side effects from antibiotics and other drugs. In diabetic patients, chronic infections caused by drug-resistant microorganisms can affect wound healing. Plants from the Convolvulaceae family have antidiabetic, wound healing, and antimicrobial activities. Thus, the use of phytoconstituents, in polyherbal formulations or delivered as nanoparticles, neosomes, or adhesive patches, can be used to mitigate problems associated with drug resistance.
Transational Relevance
Drug resistance, particularly to antibiotics, is a major problem that affects more than 10 million patients annually. Plants produce a variety of secondary metabolites, including alkaloids, glycosides, tannins, terpenoids, steroids, flavonoids, quinones, and saponins. Such phytoconstituents can be combined with antimicrobials to enhance overall effectiveness.
Clinical Relevance
There are fewer standardized herbal drugs due to a lack of regulatory standards and implementation protocols, which remain a major obstacle to the development of plant-based compounds as alternative therapies. An in-depth analysis of each step/stage of natural drug development is necessary to ascertain quality, safety, and reproducibility. Limitations related to solubility and bioavailability of phytochemicals can be avoided using novel delivery strategies, such as liposomes, nanoparticles, micelles, and adhesive patches.
Introduction
Plants produce a variety of secondary metabolites, including alkaloids, glycosides, tannins, terpenoids, steroids, flavonoids, quinones, and saponins. These plant products may exert their action by resembling endogenous metabolites, ligands, hormones, signal transduction molecules, or neurotransmitters, and lead to beneficial effects on would healing and diabetes. Plants also produce antimicrobial peptides having structures that are stabilized by eight disulphide-linked cysteines, which could be a factor in alternative approaches for microbial therapy. Previous studies demonstrated that secondary metabolites such as coumarins specifically act on Staphylococcus aureus and that plant-derived agents such as phenolic compounds exhibit antimicrobial activity through the reduction of pH, increases in membrane permeability, or alterations in efflux pumping.1 Gram-negative bacteria develop drug resistance through changes in the permeability of outer membranes that are composed of hydrophilic polysaccharides and control the entry of hydrophobic quinolones, alkaloids, and amphipathic antibiotic compounds. Efflux of antimicrobials mediated by membrane-bound pumps also contributes to drug resistance. These resistance mechanisms can be addressed through the use of plant-derived antimicrobials with traditional antibiotics.2 In India, combinations of several medicinal plants have long been used to treat a range of conditions.3 Many of the plant materials used in traditional medicines are readily available in rural areas and at lower cost than modern medicines.4,5 Considering this wide use of herbal medicines, standardization methods are essential to ensure that active ingredients isolated from different parts of the plant and from plants obtained from different sources are consistent. Such standardization procedures include: (1) good agricultural practices; (2) good manufacturing practices for extraction; (3) quality assurance by determining the presence of adulterants, such as pesticide residues, aflatoxin content, bacterial/fungal growth, and heavy metal contamination; (4) evaluation of pharmacodynamics, pharmacokinetics, dosage, stability, self-life, and toxicity to prevent adverse reactions; (5) repetitive testing using different batches to control batch-to-batch variation to increase reproducibility; and (6) cheminformatic approaches to ensure that pharmacological profiles match activity profiles of active constituents of the drugs themselves.6
Wounds disrupt the functional continuity of cells and tissues at the site of injury and can be caused by insults to tissue that occur via changes to physical, chemical, microbiological, or immunological processes. Wound healing can involve inflammation, granulation, epithelialization, and maturation. In diabetic patients, delays in wound healing can lead to chronic wounds. Drug-resistant bacterial strains such as methicillin-resistant S. aureus are often found in chronic wounds.7–10 Drugs derived from plant compounds can address these issues. Curcumin is a promising phytochemical that could promote wound healing due to its inhibitory activity toward nuclear factor-κB and ability to enhance collagen deposition as well as increase fibroblast numbers and vascular density. Although the solubility of curcumin is limited, new curcumin formulations such as nanocrystals or amorphous solid dispersion or nanoemulsions increase its solubility, while also improving the bioavailability and photostability.11 Phytochemicals derived from aloe vera, propolis, banana leaf, cocoa, and honey also possess wound healing activity12 The number of phytochemicals used for therapeutic applications is likely to increase based on the growing interest in various uses of plant extracts and studies on active plant constituents that promote or modulate healing processes.
Use of Convolvulaceae Family Plants to Promote Wound Healing
Ipomoea carnea
Ipomoea carnea, commonly known as morning glory, is used traditionally to treat a range of diseases from fungal infections of the skin to arthritis. Leaves of I. carnea are used as purgative, and milky juice from these leaves is traditionally used to treat leukoderma. Several tribes in India use I. carnea leaf material suspended in coconut oil or sesame oil for treatment of wounds. I. carnea also contains multiple secondary metabolites, such as glycosides, alkaloids, phytosterols, proteins, saponins, and the polysaccharide ipomose.13 Gas chromatography-mass spectrometry (GC-MS) studies on chloroform and benzene extracts of these leaves indicated the presence of neophytadiene, 1-decanol, tetradecanoic acid, pentadecane, 1-iodo-2-methylundecane, trans-caryophyllene, eicosane, 2-butenoic acid, and cholestan-3-one, which have insecticidal properties. Furthermore, quantitative studies of I. carnea phytochemistry revealed that these plants contain high amount of total phenols, tannins, and flavonoids other plants.14
A comparison of the relative effectiveness of a common antibiotic nitrofurazone (0.2% w/w) with I. carnea methanol extracts (Fig. 1) showed that the plant extract contained tannins that could be responsible for the observed wound healing activity of these plants. These tannins could affect several mechanisms involved in would healing, including chelation of free radicals and reactive oxygen species, promotion of blood vessel contraction, and proliferation of fibroblasts and keratinocytes. However, the extracts did not affect differentiation of cornified cells. Results from this study showed that methanol extracts were more effective in promotion of wound healing than ethyl acetate and chloroform fractions (Fig. 2).4
Figure 1.

Ipomoea carnea.
Figure 2.
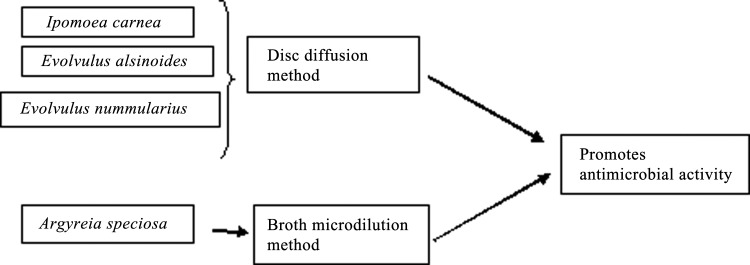
An assessment of the antimicrobial activity of I. carnea using the disk diffusion method with streptomycin as a standard revealed that a crude acetone extract of I. carnea had antimicrobial activity against Salmonella typhimurium and Proteus vulgaris as evidenced by a 7 and 9 mm, respectively, zone of inhibition (ZOI). Meanwhile, an I. carnea ethanol extract was active against Pseudomonas aeruginosa, producing an 8 mm ZOI.15 Furthermore, silver nanoparticles generated using aqueous extracts of I. carnea leaves were shown to have antibacterial activity. These extracts contain polyphenols and alkaloids that act as reducing agents, demonstrated by the ability of 500 mL of extract to reduce 100 mL of 1 mM silver nitrate solution to silver nanoparticles within 5 min. In the disk diffusion assay, these particles produced a 14 mm ZOI against S. aureus and produced a 13 mm ZOI against nanoparticles loaded per disk. This inhibitory activity is likely due to the release of free radicals from the surface of silver nanoparticles that could attack membrane lipids of the bacteria to affect membrane function.16
Evolvulus alsinoides
Evolvulus alsinoides (Fig. 3) has traditional applications for treatment of coughs and colds and for illnesses associated with the nervous system, including epilepsy, memory loss, and mental disorders. The Kani ethnic group in South Western Ghats in India uses these plants to treat venereal diseases.17,18 As with other plants in the Convolvulaceae family, E. alsinoides contains several phytoconstituents and alkaloids, flavonoids, saponins, glycosides, carbohydrates, phenolic compounds, and steroids as determined by preliminary phytochemical screening. GC-MS studies on whole plant extracts indicated the presence of lupeol, betulin, viridiflorol, glycerol, anthocyanidin, 1,2,4-butanetriol, n-hexadecanoic acid, quinic acid, 1-[2-(2-methoxy-1-methylethoxy)-1-methylethoxy]-2-propanol, squalene, phytol, octadecanoic acid, 9-octadecenoate, copaene, terpinolene, conhydrin, bis(2-ethylhexyl) phthalate, diethyl phthalate, 3,5-dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one, ethyl icosanoate, pyrogallol, triethyl (5-benzoyl-1H-pyrrol-2-yl) methanetricarboxylate, cycloheptatriene, 1-(2-piperidinyl)-1-propanol, 3-methylcyclopentane-1,2-diol, 4-(3,6-dimethyl-3-heptanyl)phenol, cinnamaldehyde, and ethyl 3,7,12-trihydroxycholan-24-oate.19,20
Figure 3.

Evolvulus alsinoides.
To evaluate the wound healing activity of E. alsinoides, excision wounds (2 × 2 cm) were made in albino rats weighing about 180–200 g. The animals were divided into two groups with one group serving as the control and the other treated with topical application of E. alsinoides extract. Histological analysis revealed increased collagen formation and fewer macrophages in tissues from extract-treated animals relative to the control. The study demonstrated that the treated tissues contained hexosamine and hydroxyproline, a component of collagen, and that the amounts of these compounds increased during wound healing. These results indicated that E. alsinoides could have potent wound healing activity, although the phytoconstituent responsible for this action awaits more detailed phytochemical studies.21,22
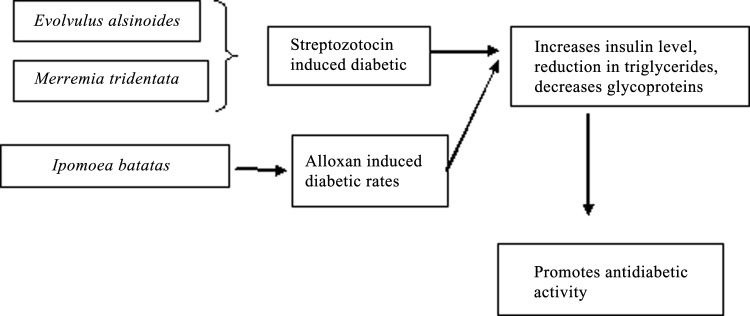
In diabetic studies rat a model, in which diabetes is induced by streptozotocin, after dietary supplementation for 45 days with E. alsinoides, the animals showed decreased glycoprotein content that was comparable to that seen for the antidiabetic agent glibenclamide (1.25 mg/kg). The hypoglycemic state with increased levels of plasma insulin observed in E. alsinoides-treated diabetic rats may be responsible for the decrease in glycoprotein levels in liver, kidney, and pancreas.23
E. alsinoides extracts from roots, stems, and flowers also have antimicrobial activity against S. aureus, Bacillus cereus, Escherichia coli, and P. aeruginosa with ZOIs ranging from 10 to 17 mm. Root extracts had the highest activity against S. aureus and B. cereus, with ZOIs of 17 and 14 mm, respectively.24 Gold nanoparticles produced using E. alsinoides also showed antimicrobial activity against S. typhimurium, Micrococcus luteus, and the fungus Trichophyton rubrum in the disk diffusion assay.25
Evolvulus nummularius
Evolvulus nummularius (Fig. 4) has traditionally been used to treat burns, cuts, wounds, and scorpion stings. Studies showed that E. nummularius has anthelmintic and wound healing properties, but poor sedative and anticonvulsant properties. Phytochemical screening of methanolic extracts of air-dried areal portions of E. nummularius plants indicated the presence of β-sitosterol, stigmasterol, ursolic acid, oleanolic acid, nummularic acid, and palmitic acid.26
Figure 4.

Evolvulus nummularius.
The wound healing activity of an aqueous-alcoholic extract of E. nummularius leaf juice (10% w/w in simple ointment Indian Pharmacopeia) and juice from fresh leaves was compared with that for control and 0.2% nitrofurazone as a standard in rats. The standard and the extracts were applied topically to the wound area. Measurement of the wound area on days 1, 4, 8, 12, and 15 after treatment showed that animals treated with leaf juice had 94.4% wound healing, whereas animals that received the hydroalcoholic extract had 87.12% wound healing.27
The disk diffusion assay to test the antimicrobial activity showed that E. nummularius had a minimum bactericidal concentration against E. coli and Bacillus subtilis of 25 and 50 mg/mL, respectively. The tannins and flavonoids present in this plant are likely responsible for the observed anti-inflammatory, antioxidant, and wound healing properties (Fig. 5).28
Figure 5.
Merremia tridentata
Oil derived from whole Merremia tridentata (Fig. 6) plants or the leaves alone mixed with coconut oil is used to treat various skin disorders, including dandruff. The areal parts of the plant contain several flavonoids, including diosmetin, diosmetin-7-o-diosmetin-7-o-β-d-glucoside, and luteolin-7-o-β-d-glucoside.29 In Ayurvedic medicine, M. tridentata is used to treat hemorrhoids, general body pain, toothache, urinary infections, and joint stiffness. Preliminary phytochemical screening carried out on petroleum ether, chloroform, acetone, and methanolic M. tridentata extracts showed that the chloroform extracts contain steroids and carbohydrates, whereas both the acetone and methanolic extracts contain carbohydrates, alkaloids, phenols, tannins, and flavonoids. The methanolic extract, such as the chloroform extract, also contains steroids.29,30
Figure 6.

Merremia tridentate.
The promotion of incision, excision, and dead space wound healing by M. tridentata in albino mice was tested. For the sutured incision model, a 6 cm long wound of 1.5 cm thickness was made, and for the excision wound model, a wound area of 500 mm2 was made. The dead space model involved implantation of sterilized cylindrical glass piths between the axilla and groin, and the formation of granulation tissues was used to evaluate the tensile strength. Mice were treated with petroleum ether, solvent ether, butanol, butanone, or ethyl acetate M. tridentata fractions in suspension using Tween-80 and were compared with control animals. The ethyl acetate fraction produced high tensile strength that is essential for effective wound healing activity. Again, flavonoids present in the plant may promote this wound healing activity.31,32
The antidiabetic action of aqueous M. tridentata root extracts was tested in streptozotocin-induced diabetes in rats. Using 10 mg/kg glibenclamide as a standard, 50 mg/kg extract had the highest antidiabetic activity among the three concentrations tested (50, 100, and 150 mg/kg) when freshly prepared extracts were given orally. Body weight and blood glucose levels were determined after an overnight fast, and the animals were sacrificed after 21 days, at which point, biochemical analyses were conducted on the liver, pancreas, and skeletal muscle tissues. Extract-treated animals showed increased body weight and insulin level, with a concurrent decrease and increase in triglyceride and glycogen level, respectively (Fig. 7).33
Figure 7.
Ipomoea batatas (sweet potato)
Ipomoea batatas (Fig. 8) is considered to be a nutraceutical due to its nutritional and medicinal activities. Leaves of these plants have laxative, fungicidal, bactericidal, and astringent properties. The use of I. batatas to treat dengue patients increases platelet counts. I. batatas reportedly protects cigarette smokers from emphysema and can relieve muscle spasms arising from high potassium levels. I. batatas tubers also have antidiabetic activity. The tubers are rich in minerals (zinc, potassium, sodium, manganese, calcium, magnesium, and iron), carbohydrates, dietary fiber, iron, vitamins (beta carotenes [provitamin-A], vitamin C, and vitamin E), anthocyanins (peonidins and cyanidins), caffeoylquinic acid derivatives, coumarins (aesculetin, scopoletin, and umbelliferone), triterpenoids, and furanoterpenoids, which have all been shown to have beneficial health effects.34,35
Figure 8.

Ipomoea batatas.
Studies of wound healing activity of I. batatas extracts in Wistar albino rats with excision and incision wounds were also conducted. For treatment of excision wounds, methanolic extracts of I. batatas tuber peel were applied on bandages and as gels prepared from Carbopol 974P NF and glycerol containing 3%, 6%, and 10% (w/w) peel extract and were compared with betadine as a reference standard. Wounds having 500 mm2 area and 2 mm depth were made on shaved skin surfaces, and wound closure was followed by tracing the wound area on every fourth day for 16 days. On day 10, the scab was removed and used for hydroxyproline estimation. Malondialdehyde and ascorbic acid levels were determined in plasma. Animals treated with I. batatas extract had higher and lower hydroxyproline and ascorbic acid levels, respectively, relative to untreated controls, indicative of an anti-lipid peroxidative effect that is predicted to promote wound healing. In an incision wound model, animals carrying 6 × 2 cm wounds and treated with betadine standard, 3% and 6% peel extract gel or bandages carrying peel extract were compared with wound healing in untreated control animals. To measure the tensile strength, the parted skin of animals bearing incision wounds was sutured using surgical thread, which was removed on day 8. At that time, the animals were sacrificed and the wound tensile strength was determined using a tensiometer. This study confirmed that the tensile strength is indeed an indicator of wound healing promotion. I. batatas was also shown to have potent antioxidant activity as would be expected based on the high content of beta-carotene, anthocyanins, caffeoyldaucic acid, and caffeoylquinic acid derivatives that all have documented wound healing activity.36 For cutaneous wounds, treatment with ointment containing 2.5% I. batatas extract for 4 and 10 days showed reepithelialization of 43% and 75% relative to untreated controls. A study of in vivo antiulcer properties of I. batatas revealed that an aqueous suspension containing 75 and 100 mg/kg tuber flour prevented edema formation by 75% and 100%, respectively, compared with untreated controls.37
The effect of I. batatas leaf flavonoids on antidiabetic activity showed an effect for male mice with alloxan-induced diabetes and treated with I. batatas leaf extract. The animals were treated with 50, 100, or 150 mg/kg leaf extract for 28 days and compared with animals given glibenclamide (0.25 mg/kg) and untreated animals. Blood glucose level and body weight were also monitored. Mice treated with the extract for 28 days showed lower fasting blood glucose, total cholesterol, and triglyceride levels, and increased body weight and levels of serum high-density lipoprotein cholesterol, indicating the antidiabetic activity of these extracts.38
Argyreia cuneata
Argyreia cuneata (Fig. 9) is traditionally used to treat diabetes, burns, and fever and contains alkaloids, phytosterols, and sitosterol. In healing of excision wounds in Wistar rats treated with either 10% (w/w) hydroalcoholic A. cuneata extract or 0.2% (w/w) nitrofurantoin and leaf juice extract of A. cuneata found that on day 14, the leaf juice extract induced 96.07% wound healing, while nitrofurantoin (0.2% w/w) and hydroalcoholic extract of A. cuneata (10% w/w) showed 95.19% and 91.29%, respectively.
Figure 9.

Argyreia cuneata.
Argyreia speciosa
Argyreia speciosa, which is also known as Argyreia nervosa (Fig. 10), is traditionally used for the treatment of gonorrhea and chronic ulcers. A. speciosa seeds have hypotensive, spasmolytic, and anti-inflammatory actions. The roots are used as an appetite stimulant, digestive, carminative, cardiotonic, expectorant, brain tonic, aphrodisiac, and emollient. Anemia, obesity, diabetes, tuberculosis, ulcer, and wounds have also been treated using A. speciosa roots.40 A. speciosa leaves contain beta-sitosterol, epifriedelinol and its acetate, quercetin, and kaemperol and its glycoside kaemperol-3-o-lrhamnopyranoside. Hexane extracts of A. speciosa roots contain tetradecanylpalminate, sigmasteryl p-hydroxy cinnamate, hexadecanyl p-hydroxy cinnamate, and scopoletin. The coumarin glycoside l ester coumarin, 6-methoxy-7-o-alpha-D-glu, was also isolated from root. Fatty oils present in A. speciosa seeds contain glycosides of palmitic, oleic, stearic, behenic, stearic, and linolenic acids. Gas layer chromatography of seed oil confirmed the presence of myristoleic, myristic, palmitic, linoleic, linolenic, oleic, stearic, nonadecanoic, eicosenoic, heneicosanoic, and behenic acids. Ethanolic extracts of A. speciosa seeds contain three alkaloids characterized as ergometrine, caffeic acid, and ethyl caffeate. Ergoline alkaloids such as ergometrine, ergometrinine, lysergic acid-α-hydroxy ethyl amide agroclavine, chanoclavine-I, chanoclavine-II, festuclavine, lysergene, lysergol, isolysergol, setoclavine, iso-setoclavine, ergine, and isoergine are also present in A. speciosa seed extracts. The amino acid fraction of seed extracts includes glutamic acid, glycine, isoleucine, leucine, lysine, phenylalanine, tyrosine, praline, and α-aminobutyric acid. A. speciosa seeds also contain crude proteins, such as albumin, globulin, and glutelin. As such, A. speciosa seeds have good nutritive value, although the presence of lysergic acid may be responsible for the known hallucinogenic effects of these seeds.40–42
Figure 10.

Argyreia speciosa.
Ethanolic extracts of A. speciosa leaves were tested for the wound healing activity in healthy and alloxan-induced diabetes in Wistar rats. Excision wounds (300 mm2 and 2 mm deep) were made, and the animals are divided into four groups that were left untreated (control) or treated with mupirocin ointment (2% w/w) as a standard, ointment containing 15% (w/w) A. speciosa extract, or 200 mg/kg extract given orally. Healthy animals treated with topical ointment had good wound healing compared with rats given the extract orally. By day 16 of treatment, the wound closure was 92.96% and 94.4% for extract ointment and mupirocin, respectively. For diabetic rats, ethanol extracts promoted 76.20% and 74.36% for ointment and oral treatment, respectively, which was higher than that for the control group. Together, these results show that A. speciosa extracts promote wound healing and that topical treatments had slightly better outcomes than did oral adminstration.43
Antimicrobial activity of A. speciosa was tested using the broth microdilution method with ciprofloxacin and norfloxacin used as standards. The ethyl acetate fraction and flavonoid sulfates (quercetin 3'7 di-O methyl 3-sulfate and kaempferol 7-O methyl 3-sulfate) extracted from A. speciosa roots inhibited Mycobacterium tuberculosis growth with a minimum inhibitory concentration of 50 and 25 μg/mL (Table 1).44
Table 1.
The main constituents responsible for the activities
| Sl. No | Plant name | Biological activities | Active constituents | Refs. |
|---|---|---|---|---|
| 1 | Ipomoea carnea | Wound healing activity | Tannins | 4 |
| Antimicrobial activity | Polyphenols, alkaloids | 15 | ||
| 2 | Evolvulus alsinoides | Wound healing activity | — | 21,22 |
| Antimicrobial activity | — | 24 | ||
| Antidiabetic activity | — | 23 | ||
| 3 | Evolvulus nummularius | Wound healing activity | Flavonoids, tannins | 27 |
| Antimicrobial activity | 28 | |||
| 4 | Merremia tridentata | Wound healing activity | Flavonoid | 31,32 |
| Antidiabetic activity | — | 33 | ||
| 5 | Ipomoea batatas | Wound healing activity | Beta-carotene, anthocyanins, caffeoyldaucic acid, and caffeoylquinic acid derivatives | 36 |
| Antidiabetic activity | — | 38 | ||
| 6 | Argyreia cuneata | Wound healing activity | — | 39 |
| 7 | Argyreia speciosa | Wound healing activity | — | 43 |
| Antimicrobial activity | Flavonoid sulfates | 44 |
Conclusion
The above-mentioned plants have various therapeutic uses and wound healing activities as demonstrated by multiple studies (Table 1). Given the wound healing properties of these phytocompounds, as well as their antimicrobial activity and antidiabetic activity, formulations containing these agents could be developed and used to treat wounds in both diabetic and nondiabetic patients (Figs. 2, 5, and 7). Bacteria infiltrating some diabetic wounds often develop drug resistance that can negatively impact wound healing. Phytocompounds can be used in combination with antibiotic therapy to reduce the incidence of resistance. However, detailed phytochemical studies are needed to determine the mechanism by which these agents exert their effects. M. tridentata, A. speciosa, and I. batatas have each demonstrated antidiabetic and wound healing properties. Future studies are needed to gain insight into the action of these plant-derived agents to promote healing of diabetic wounds and strategies for the development of novel formulations containing plant extracts and phytochemicals that will reduce the likelihood of drug resistance and drug allergies that affect wound healing.
Take-Home Messages.
Diabetic and drug resistance are major problems for wound healing.
Phytotherapy is an alternative approach to promote wound healing in diabetics.
Phytochemicals are gaining importance and can be taken as dietary supplements or in polyherbal formulations.
Novel phytochemical formulations such as liposomes, nanoparticles, and adhesive patches can be used for effective delivery of phytotherapy.
Basic Science.
Phytochemicals are present in plants and exhibit a range of pharmacological actions.
Phytochemicals can be developed for use as alternate medical treatments that have fewer side effects and lower likelihood of drug resistance.
Clinical Science.
Combinations of phytochemicals with other antimicrobials can increase the effectiveness of wound healing therapy.
Phytochemicals can be part of cost‐effective therapies to treat a range of conditions.
Abbreviation and Acronym
- ZOI
zone of inhibition
Author Disclosure and Ghostwriting
No conflict of interest. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
About the Authors
Anitha P. Ambika, MPharm, and Sreesha N. Nair, MPharm, are lecturers in the Department of Pharmacognosy at the Amrita School of Pharmacy, Kochi, India. Their research concerns the actions of phytochemicals in medicinal plants.
References
- 1. Ramar PS, Ponnampalam G. Therapeutic potential of plants as anti-microbials for drug discovery. Evid Based Complement Alternat Med 2010;7:283–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jatin S, Harish C, Anant RN, Swinder JS. Antimicrobial resistance (AMR) and plant-derived antimicrobials (PDAms) as an alternative drug line to control infections. Biotech 2014;4:451–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Surendra KS, Ajay PS. Antimicrobial investigations on rhizomes of Cyperusrotundus Linn. Der Pharmacia Lettre 2011;3:427–431 [Google Scholar]
- 4. Susanta KS, Durga MK. Antimicrobial, antioxidant and wound healing activity of the crude extract and different fractions of methanolic extract of Ipomoea carnea. Der Pharmacia Lettre 2015;7:1–9 [Google Scholar]
- 5. Ashly U, Sreesha NN. A review on ten sacred flowers in Kerala: Dashapushpam. Res J Pharm And Tech 2017;10:1555–1562 [Google Scholar]
- 6. Raman C, Pallavi T, Ayush C, Sarita J, Anamika S, Rajeev G, et al. Evidence based herbal drug standardization approach in coping with challenges of holistic management of diabetes: a dreadful lifestyle disorder of 21st century. J Diabetes Metab Disord 2013;12:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Payal DP, Anis K. Evaluation of wound healing potential of Cynodondactylon. Asian J Pharm Clin Res 2012;5:161–164 [Google Scholar]
- 8. Andrew SP, Anthony PC. The treatment of diabetic foot infections. J Antimicrob Chemother 2010;65:3–9 [DOI] [PubMed] [Google Scholar]
- 9. Sreesha NN, Alexeyena V, Meenu B, Greeshma R. Comparative evaluation of Coriandrum sativum Linn. snd Apiumgraveolens for antimicrobial activity. Res J Pharm Tech 2017;10:541–544 [Google Scholar]
- 10. Ashok D. Why diabetic ulcers do not heal? JIMSA 2011;24:205–206 [Google Scholar]
- 11. Rajesh LT, Shashwat S, Radha K. Phytochemicals in wound healing. Adv Wound Care (New Rochelle) 2016;5:230–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Raja KS, Brian RM, Lisa NW, Emanual M. Phytochemicals and naturally derived substances for wound healing. Adv Wound Care (New Rochelle) 2012;1:213–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vellingiri V, Pemaiah B. Wound healing potential of Ipomoea carnea Jacq. an un-explored herb used in Indian Traditional System of Medicine. Glob J Pharmaceu Sci 2017;3:1–5 [Google Scholar]
- 14. Sahayaraj K, Ravi C. Preliminary phytochemistry of Ipomea carnea Jacq. and Vitex egundo Linn. leaves. Int J Chem Sci 2008;6:1–6 [Google Scholar]
- 15. Adsull VB, Khatiwora E, Rasika T, Deshpande NR. Antimicrobial activities of Ipomoea carnea leaves. J Nat Prod Plant Resour 2012;2:597–600 [Google Scholar]
- 16. Ganeshb SK, Sivakumar M. Ipomea carnea-based silver nanoparticle synthesis for antibacterial activity against selected human pathogens. J Exp Nanosci 2014;9:197–209 [Google Scholar]
- 17. Indhumol VG, Pradeep HR, Sushrutha CK, Jyothi T, Shavas MM. Ethnomedicinal, phytochemical, and therapeutic applications of Evolvulus alsinoides Linn.—a review. Int Res J Pharm Plant Sci 2013;1:1–9 [Google Scholar]
- 18. Daniel FA. Evolvulus alsinoides (Convolvulaceae): an American herb in the Old World. J Ethnopharmacol 2008;117:185–198 [DOI] [PubMed] [Google Scholar]
- 19. Elangovan K, Supriya K, Murugesan K, Aravind R. Screening of phytochemicals and in vitro antioxidant activity of Evolvulus alsinoides L. J Acad Industr Res 2013;2:230–234 [Google Scholar]
- 20. Zahir HA, Kumaresan S. GC–MS Analysis and phytochemical studies of Evolvulus alsinoides L. Int J Nano Corros Sci Eng 2015;2:58–63 [Google Scholar]
- 21. Ramya S, Alaguchamy N, Maruthappan VM, Sivaperumal R, Sivalingam M, Krishnan A, et al. Wound healing ethnomedicinal plants popular among the malayali tribes in Vattal Hills, Dharmapuri, TN, India. Ethnobotanical Leaflets 2009;13:1257–1271 [Google Scholar]
- 22. Dhanalekshmi UM, Kishore GP, Raja MD, Reddy PN. Evaluation of wound healing potential and antimicrobial activityof ethanolic extract of Evolvulus alsinoides. Ann Biol Res 2010;1:49–61 [Google Scholar]
- 23. Duraisamy G, Ganesan R, Manokaran K, Kanakasabapathi D, Chandrasekar U. Protective effect of the whole plant extract of Evolvulus alsinoides on glycoprotein alterations in streptozotocin induced diabetic rats. J Acute Dis 2013;2:148–150 [Google Scholar]
- 24. Saranya B, Sarathadevi D, Somasundaram SSN. Investigation of antibacterial activities of Evolvulus alsinoides (L.) against clinical pathogens. Int J Curr Microbiol App Sci 2015;4:491–497 [Google Scholar]
- 25. Anbarasu R, Selvan G, Baskar S, Raja V. Synthesis of Evolvulus alsinoides derived gold nanoparticles for medical applications. Int J Adv Sci Res 2016;2:038–044 [Google Scholar]
- 26. Biswanath D, Biplab G, Arima S, Nariko S, Hsrigaya Y. Chemical constituents of Evolvulus nummularis. Indian J Chem 2007;46:492–498 [Google Scholar]
- 27. Saini V, Kinge HK, Sharma DK, Ahuja N, Middha A, Gupta VB. Wound healing activity of Evolvulus numularius Linn. Asian J Chem 2007;19:5772–5774d [Google Scholar]
- 28. Pavithra PS, Sreevidya N, Rama SV. Antibacterial and antioxidant activity of methanol extract of Evolvulus nummularius. Indian J Pharmacol 2009;41:233–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Neyanila SK, Prakash Y, Gopal V. Preliminary phytochemical and pharmacognostical standardization of aerial parts of Merremia tridentata (Linn) Hallier.f. Convolvulaceae. Int J Pharm Res Anal 2013;3:99–105 [Google Scholar]
- 30. Aron S, Maria N, Francis JB, Mehalingam P. Pharmacognostical evaluation of stem, leaves and roots Merremia tridentate (L) Hallier f. Indian J Tradit Know 2013;12:693–698 [Google Scholar]
- 31. Neyanila SK, Prakash YG, Gopal V. Medicinal value of Merremia tridentata (L.) Hallier. f. (Convolvulaceae)—a pharmacognostic approach. Int J Pharm 2013;3:36–40 [Google Scholar]
- 32. Bidkar AA, Sherje AP, Gujar KN, Bagul US, Miniyar PB, Aphale SA. Phytochemical and pharmacological investigation of extracts of Merremia tridentata Linn. (Convolvulaceae). J Nat Remed 2009;9:79–84 [Google Scholar]
- 33. Karuppusamy A, Thangaraj P. Antidiabetic activity of aqueous root extract of Merremia tridentata (L.) Hall. f. in streptozotocin-induced-diabetic rats. Asian Pac J Trop Med 2012;5:175–179 [DOI] [PubMed] [Google Scholar]
- 34. Remya M, Subha S. Sweet Potato (Ipomoea batatas [L.] Lam)—a valuable medicinal food: a review. J Med Food 2014;17:733–741 [DOI] [PubMed] [Google Scholar]
- 35. Milind P, Monika Sweet potato as a super food. Int J Res Ayurveda Pharma 2015;6:557–562 [Google Scholar]
- 36. Vandana P, Madhav S, Swati P. Wound healing activity of Ipomoea batatas tubers (sweet potato). Funct Foods Health Dis 2011;10:403–415 [Google Scholar]
- 37. Daniele H, Débora ND, Mariana DM, Lívia PH, Eliete NL, Angelo de F, et al. In vivo wound healing and antiulcer properties of white sweet potato (Ipomoea batatas). J Adv Res 2013;4:411–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fenglin L, Qingwang L, Dawei G, Yong P. The optimal extraction parameters and anti-diabetic activity of flavonoids from Ipomoea batatas Leaf. Afr J Tradit Complement Altern Med 2009;6:195–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Manda RM, Seru G, Bakshi V. Evaluation of anti-pyretic and wound healing activities of Argyreia cuneata in Wistar albino rats. IJPB 2016;6:113–115 [Google Scholar]
- 40. Milimita P, Sujata M, Janyanaranjan P, Nikunja MK. Traditional uses and phytopharmacological aspects of Argyreia nervosa. J Adv Pharm Res 2013;4:23–32 [Google Scholar]
- 41. Ashish JM, Khadabadi SS, Farooqui IA, Deore SL. Argyreia speciosa Linn.f: phytochemistry, pharmacognosy and pharmacological studies. Int J Pharm Sci Rev Res 2010;2:14–21 [Google Scholar]
- 42. Ancy J, Samuel M, Skaria BP, Sheeja EC. Medicinal uses and biological uses of Argyreia speciose sweet Hawaiian Baby Woodrose—an overview. IJNPR 2011;2:286–291 [Google Scholar]
- 43. Singhal AK, Gupta1 H, Bhati VS. Wound healing activity of Argyreia nervosa leaves extract. Int J App Basic Res 2011;1:36–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Habbu PV, Mahadevan KM, Shastry RA, Manjunatha H. Antimicrobial activity of flavanoid sulphates and other fractions of Argyreia speciosa (Burm.f) Boj. Indian J Exp Biol 2009;47:121–128 [PubMed] [Google Scholar]