Abstract
Significance: Nanomedicine is an application of nanotechnology that provides solutions to unmet medical challenges. The unique features of nanoparticles, such as their small size, modifiable components, and diverse functionality, make them attractive and suitable materials for novel diagnostic, therapeutic, or theranostic applications. Cardiovascular diseases (CVDs) are the major cause of noncommunicable illness in both developing and developed countries. Nanomedicine offers novel theranostic options for the treatment of CVDs.
Recent Advances: Many innovative nanoparticles to target reactive oxygen species (ROS) have been developed. In this article, we review the characteristics of nanoparticles that are responsive to ROS, their limitations, and their potential clinical uses. Significant advances made in diagnosis of atherosclerosis and treatment of acute coronary syndrome using nanoparticles are discussed.
Critical Issues: Although there is a tremendous potential for the nanoparticle applications in medicine, their safety should be considered while using in humans. We discuss the challenges that may be encountered with some of the innovative nanoparticles used in CVDs.
Future Directions: The unique properties of nanoparticles offer novel diagnostic tool and potential therapeutic strategies. However, nanomedicine is still in its infancy, and further in-depth studies are needed before wide clinical application is achieved.
Keywords: nanomedicine, cardiovascular diseases, oxidative stress, theranostics
Introduction
The role of nanotechnology in various disease conditions has been well recognized (60). Nanomedicine is an application of nanotechnology that provides solutions to various medical challenges. Generally, nanomedicine involves the use of nanomaterials to diagnose, treat, and prevent diseases (11). The potential uses of nanotechnology in medicine are vast, including diagnostic tests, novel medical devices, biosensors, bioimaging agents, drug delivery systems (DDSs), and therapeutic drugs. The term “theranostic” refers to the dual capacity of a single agent to be both diagnostic and therapeutic (13).
Cardiovascular diseases (CVDs) are the major cause of noncommunicable illness in both developing and developed countries and are responsible for 30% of deaths globally (6). Despite many advances in diagnostic and therapeutic technologies for CVDs, the overall grim statistics of CVDs has not significantly improved over the last decade and is expected to further increase as the population ages. Thus, additional therapeutic strategies for cardiovascular care are needed.
Overproduction of reactive oxygen species (ROS) has been recognized in many CVDs, such as myocardial infarction and acute coronary syndrome (ACS) (57, 68, 77, 105). Thus, ROS have become a critical target for diagnostics, therapeutics, and theranostics. In this review, we provide an overview of nanoparticles that are responsive to ROS by reviewing the profile of their special features, advantages, and limitations in potential clinical applications. We also provide a literature review of recent developments in nanoparticles that may potentially have roles in clinical applications.
Characterization of Nanoparticles
Size advantage
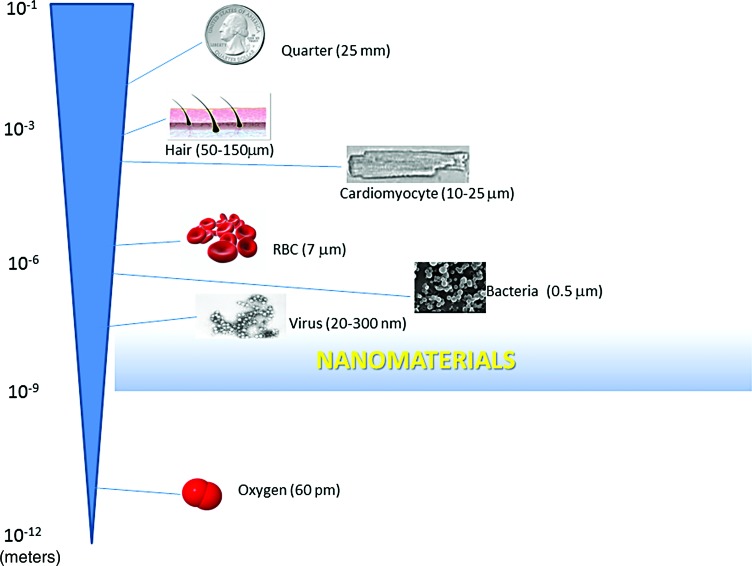
A major advantage of nanoparticles is their small size. Nanoparticles are typically in the 1–100 nm diameter range, although they can be up to ∼400 nm. Figure 1 provides relative size comparisons of various common items. The small size of nanoparticles allows for an exponential increase in surface area per volume of materials (92, 95). For example, a decrease by a factor of 10 in radius results in greater than a 1020-fold increase in the surface area-to-volume ratio of spherically shaped particles. This enables further modifications and a significant increase in the direct contact area of nanomaterials with target cells or biomaterials. Furthermore, the very small size of nanoparticles may also lead to vascular permeability because they can go through anatomic barriers that macromolecules cannot cross. Particles up to 400 nm may experience some degree of enhanced vascular permeability (92). However, a substance greater than several nanometers can be recognized as “foreign” by the body and may be taken up by the reticuloendothelial system (RES), which could significantly lower the half-life.
FIG. 1.
Relative size comparison of common items and nanomaterials. The shaded area represents the size range of nanomaterials. RBC, red blood cell. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The specific half-life of nanoparticles in plasma also depends on how the particles are engineered and what surface modifications are present. These characteristics affect the potency of the drug. Some nanoparticles may allow a similar efficacy that is achieved using a lower drug dose, which could result in less toxicity and lower cost.
Nanoparticle composition
The composition of nanoparticles is also important. For example, depending on the surface properties (hydrophilic or hydrophobic) of the nanomaterials, the compound that a nanoparticle can carry may be different (81). Surface modification of nanoparticles can affect their circulation in the plasma as well as their ability to penetrate target tissues (92). The shape of the nanomaterial also affects its permeability to anatomic barriers, such as alveolar, hepatic, sinusoidal, and glomerular membranes.
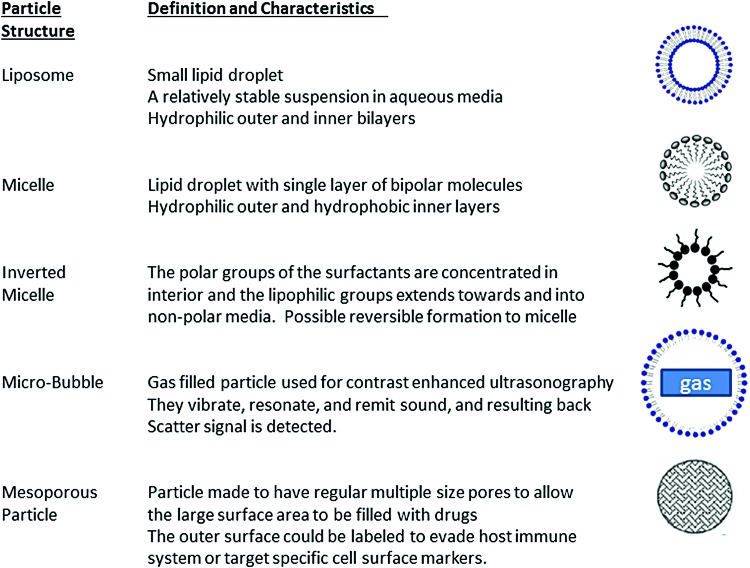
The degree of deformability is another feature that allows vesicles to navigate different compartments in response to various cellular changes. On the contrary, the degree of nondeformability, uniformity, or stability also influences the predictability of particle response. More deformable and thus less stable nanoparticles are generally associated with less predictable responses. The available basic particles are summarized with their special features in Figure 2. Matching these specific and unique characteristics of nanoparticles to respective functions will allow better interactions with cellular molecules in a more predictable manner.
FIG. 2.
Structures of nanoparticles. Simplified liposome, micelle, inverted micelle, microbubble, and mesoporous particles are depicted to show their structural features. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Administration strategy
The route of nanoparticle administration is also an important factor. The most common administration route is intravenous injection, but direct administration into the regional blood supply or the tissue itself is also possible. Common considerations when determining the administration strategy include the percentage of cardiac output distribution, the organ-specific control of circulation, and the degree of influence of a specific condition, such as hypoxia (Table 1) (18). Precise timing to target an opportune period may also be a critical factor in the setting of ischemia/reperfusion injury.
Table 1.
Distribution (%) of Cardiac Output at Rest and Organ-Specific Control of Major Circulatory Beds
| Organs | Percent of CO at rest | Degree of control by local metabolites | Influence by hypoxia |
|---|---|---|---|
| Heart | 5 | +++ | +++ |
| Lungs | 100 | +++ | +++ |
| Brain | 15 | +++ | +++ |
| Muscles | 20 | ++ (During exercise) | + |
From the right ventricle, 100% of the blood volume is delivered to the lungs (unless there is a right to left shunt). From the left ventricle, the percent of blood flow, given as CO, is listed. These blood flows are controlled by the local metabolites and the degree of hypoxia (18).
CO, cardiac output.
If nanoparticles are administered via peripheral vessels, they must remain in the plasma to circulate long enough to exert their functions. A commonly used method to increase the half-life of nanoparticles in the plasma is to incorporate polyethylene glycol (PEG), termed PEGylation, in which PEG is combined with the drug to enhance the availability of the drug in the plasma (84, 106, 118). PEG is generally considered a nontoxic compound. Covalent attachment of PEG polymer chains has been shown to increase hydrophilicity, decrease renal clearance, reduce immunogenicity, and increase stability of PEGylated particles in the body by “disguising” them from being recognized by macrophages.
Targeted delivery
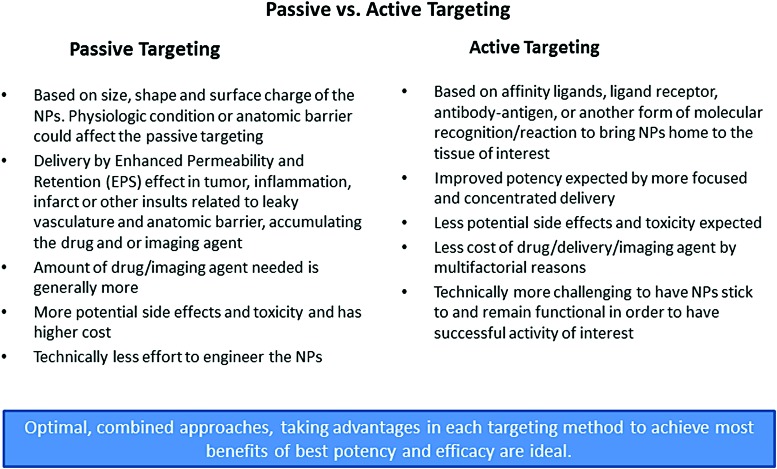
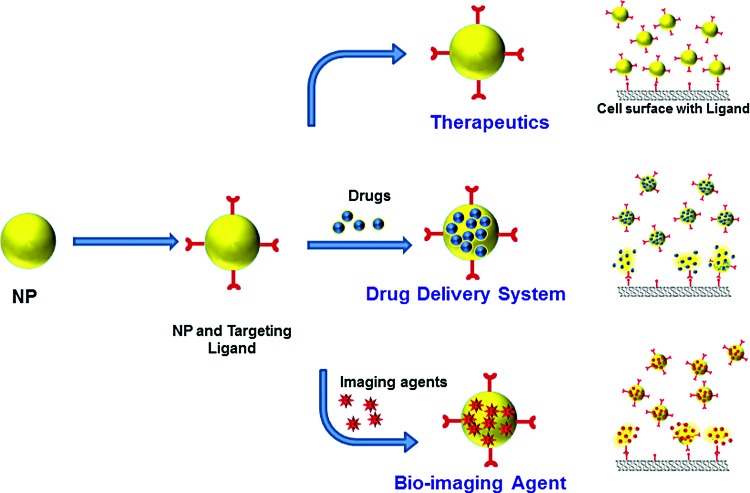
Targeting can be either active or passive (20, 41). The characteristics of active and passive targeting are summarized in Figure 3. Targeting applies to the way in which delivery is made, either passively or actively, to the target of interest, such as a specific microenvironment, biological material, or cell surface modifier. This also enables an agent to be multifunctional in therapeutics, diagnostics, or theranostics, depending on the modifications (Fig. 4).
FIG. 3.
Passive versus active targeting. Characteristics of passive and active targeting. Advantages and disadvantages of passive and active targeting are listed. Based on needs, a diagnostic, therapeutic, or theranostic agent can be designed for active or passive targeting to achieve the best possible potency and efficacy (112). NPs, nanoparticles. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
FIG. 4.
Multifunctional properties of targeted nanoparticles. Nanoparticles can be used as therapeutics, drug delivery systems, or diagnostic bioimaging agents. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Passive targeting is commonly used in oncology. The effects of tumors on microvasculature increase permeability, which results in preferential nanoparticle accumulation through the enhanced permeability and retention (EPR) effect (20). The EPR effect can also be present in ischemic myocardial tissues and may facilitate delivery of nanoparticles to ischemic areas. Studies in mice have shown that micelles, liposomes, and PEGylated polysaccharide compounds accumulate preferentially in areas of infarcted myocardium and muscles (24, 29, 32, 46).
There is also delayed enhancement in the infarct bed of human hearts as demonstrated by magnetic resonance imaging (MRI), although the findings are not specific to the infarcted area. The most important features of nanoparticles for passive targeting are their size, shape, and surface characteristics. Lipophilicity as well as modifications that alter membrane permeability can also determine the capacity of the nanoparticles to cross through physiological membrane barriers.
Active targeting results from surface modifiers (or affinity ligands), such as ligand/receptor, antibody/antigen, or another form of molecular recognition, to guide particles into the tissue of interest (3, 19, 99). By concentrating a drug in the tissue of interest, nanoparticles with active targeting can potentially reduce the total amount of drug that is needed for comparable efficacy. This should in turn reduce side effects and cost. The biocompatibility of surface modifiers is important to decrease the removal of the nanoparticles through uptake by the mononuclear phagocytic system (for larger and foreign substances), renal elimination (for smaller particles), or endosomal uptake and degradation.
Once the nanoparticles have reached the tissue of interest, an additional control method to release the payload could be devised. The nature of the bonding between the drug component and the nanoparticle can also be manipulated to fine tune drug release. For example, a molecule can be attached to a nanoparticle by a pH-labile bond to keep the drug bound until it reaches an acidic microenvironment, such as the location of a tumor or an infection (58, 63, 123). In our studies, we have used nanoparticles that were designed to target an excess amount of hydrogen peroxide (H2O2). These nanoparticles have peroxalate bonds that are cleaved by excessive levels of H2O2. Once these peroxalate bonds are cleaved, there is a release of a potent antioxidant molecule, such as vanillyl alcohol, which has both general antioxidative and anti-inflammatory effects. Thus, these nanoparticles have two-step antioxidative effects at the site of oxidative stress (61, 62, 82). Other targeted systems that are sensitive to specific stimuli, such as temperature, magnetic field, and sheer stress, have also been reported (65, 71, 90).
Nanoparticle formulations
Biocompatibility
Both biocompatibility and biodegradability are critical factors in clinical applications. Biological polymer-based nanomaterials are usually biocompatible, which make them attractive materials for diagnostic and/or therapeutic applications. They are also usually biodegradable without significant accumulation. For these reasons, polymer-based agents have been used safely in food and drug formulations for many years.
One of the commonly used organic nanoparticles is liposomes, which have been successfully applied clinically, primarily in the establishment of nanoparticle-based DDSs (114, 130). Liposomes are both biocompatible and biodegradable. They can be modified to entrap both hydrophilic and hydrophobic drugs, and they often have higher efficacy and lower toxicity than their nonliposomal analogs. However, they have several limitations. They have low encapsulation efficiency and are associated with fast-burst drug release. They also have limited storage capacity and poor circulation stability. These issues can be partially overcome by surface modifications, such as PEGylation, to decrease their removal by the RES and increase their circulation time (106, 118).
Polymeric formulation
Polymeric nanoparticles have several different shapes and can be engineered through anionic or cationic synthesis (16, 34, 63). They can be organic and can be tuned to release their encapsulated drug in response to different types of local stimuli to exert different functions (Fig. 4). Polymer/drug conjugates can be created to increase drug solubility and prolong circulation time. However, this process is not trivial. It may require chemical modification of existing drugs, which technically creates a new chemical entity that may need new FDA approval even though it was created using an approved drug. These particles can be degraded under stress, similar to liposomes. They can cause hypersensitivity reactions, and the polymers usually need to be synthesized within a certain size range. If the size is too small (<400 Da), the particle may be metabolized to toxic alcohols; if the size is too large (>20–60 kDa), the particle may not be excreted by the kidney and accumulate in the liver.
Other organic nanoparticle subtypes include polymeric micelles, hydrogels, dendrimers, and protein-based nanoparticles. Polymeric micelles are composed of a hydrophilic outer shell and a hydrophobic inner core produced by spontaneous self-organization of amphiphilic polymers (10, 127).
The inner hydrophobic core can serve as a reservoir for water soluble and amphiphilic drugs. The outer hydrophilic shell is where hydrophilic drugs can be incorporated via covalent bonding. They are relatively simple to prepare and can be loaded without modifying the encapsulated drug, and thus, the drug release can be controlled. Polymeric micelles tend to be smaller and are able to better incorporate hydrophobic drugs (114, 130). However, inversion is possible when the hydrophilic layer becomes the inner component. They can also break into smaller particles. Hydrogels are three-dimensional polymer networks of hydrophilic monomers that can absorb and retain a large amount of water while maintaining a particular shape (28, 50). Because they can mimic native tissue architecture and functions, they have been used as a matrix for tissue engineering and as drug-delivery vehicles (79, 88, 117). In addition, other nanoparticles can be incorporated into hydrogels to engineer nanocomposites (31, 64). However, it is challenging to maintain a consistent release profile in the setting of constant physiochemical changes.
Inorganic nanoparticles
Inorganic nanoparticles typically include precious metals (gold, silver, palladium, and platinum), superparamagnetic iron oxide (SPIO), zinc, ceramic, and carbon (5, 54, 72). Gold nanoparticles have long been used and extensively studied for applications in cancer therapy.
The advantages of inorganic nanoparticles include their relative inertness, easy synthesis, and well-established properties and principles for surface functionalization. They have been used as imaging agents and DDSs (97, 116). In addition, they can be delivered to a tissue and heated using a laser to provide thermal disruption (12, 30). However, the main disadvantages of inorganic nanoparticles are their high cost and potential toxicity, which have limited their clinical use (43). Platinum-based particles are most commonly used as cancer chemotherapeutic agents, but they have limited use in CVDs (124). Silver nanoparticles may result in vascular dysfunction through potentially maladaptive sensitization of the immune system (2).
Iron-based nanoparticles, such as SPIO nanoparticles, have been extensively studied in cardiovascular research. One of the advantages of SPIOs is that they can be magnetically manipulated. They have been used as imaging agents for various imaging modalities. Iron-based nanoparticles can be functionalized to carry a drug and tuned for drug release. However, their use in humans to diagnose and treat oxidative stress-related conditions has been limited due to potential generation of harmful radicals (Fenton reaction). SPIOs were previously approved by the FDA as a stand-alone MRI contrast agent (ferumoxides, marketed as Feridex/Endorem) for a brief period of time, but they are currently off the clinical market in the United States.
Ceramic nanoparticles are inorganic compounds with porous surfaces (111). Silica nanoparticles have received significant attention due to their biocompatibility as well as their relative ease of synthesis and modification (67, 76, 120). Silica nanoparticles have been used to carry antibiotics and to increase the biocompatibility of other types of nanoparticles. Gold and silica have also been combined to produce gold nanoparticles within a silica shell (39, 40, 83, 101). By varying the thickness of the gold shell relative to the silica core, the nanoparticle properties can be adjusted. These nanoparticles can be designed to either absorb or scatter resonant light, creating useful diagnostic capabilities in optical scattering, optical coherence tomography, and therapeutic photothermal cancer therapy (101, 115). However, despite being biocompatible, they are not biodegradable. Therefore, there is concern that they may accumulate in the body and exert toxicity.
Other nonbiodegradable agents include carbon-based nanoparticles, such as carbon nanotubes, fullerenes, and diamond-based particles. In particular, carbon nanotubes have shown promise as nanocarriers that can transport chemotherapeutics and nucleic acids (66, 129). However, they are not biodegradable and require surface modification for aqueous solubility.
Nanotechnology in CVDs
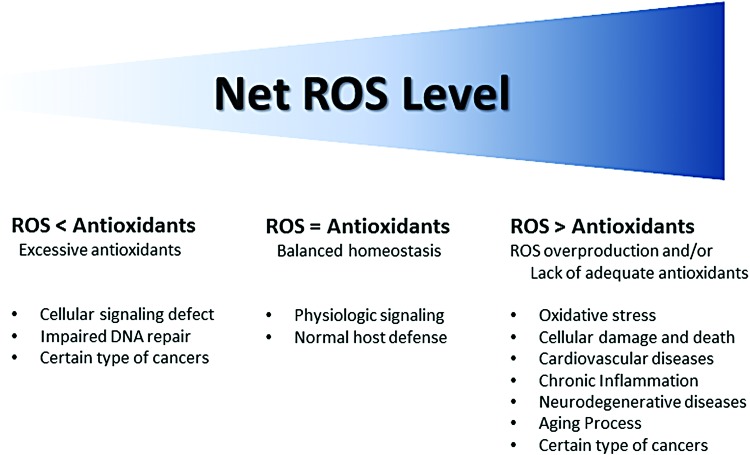
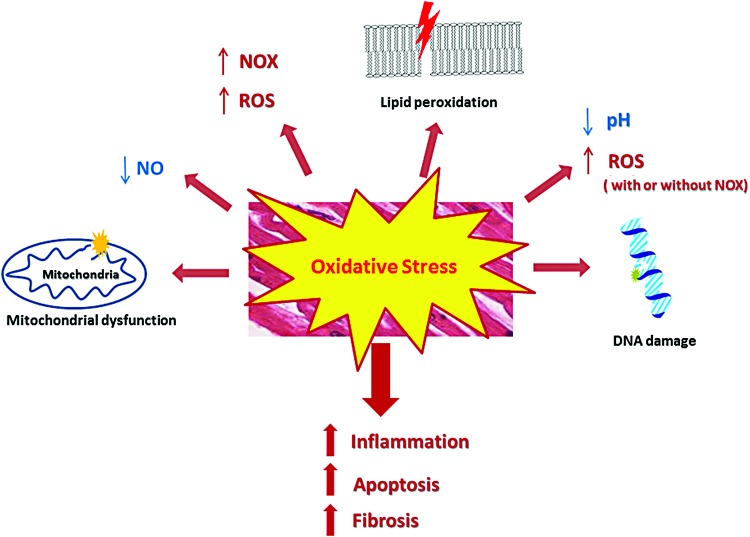
The intracellular net ROS (e.g., H2O2, superoxide anion radical [O2−], .OH) level is tightly regulated by cellular defense mechanisms to maintain homeostasis (Fig. 5). The physiological ROS level facilitates cellular signaling and maintains normal host defense. However, overproduction of ROS or lack of antioxidants can cause oxidative stress, which could lead to various pathological conditions (Fig. 6). Superoxide can be generated with or without an enzyme, such as nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) oxidase (NOX). Superoxide conversion to H2O2 can also occur independent of enzymatic actions. For example, in a very acidic microenvironment, superoxide is nonenzymatically converted to H2O2. On the contrary, if there is an excessive level of antioxidants, cellular signaling and DNA repair mechanisms can be affected, which could potentially lead to cancer. Figure 7 illustrates common oxidative insults in CVDs and their effects.
FIG. 5.
Schematic representation of net ROS balance between ROS generation and the status of the host antioxidant defense system. When there is a balanced homeostasis between ROS and antioxidants, ROS play important roles in health maintenance through appropriate signaling, biosynthesis, and host defense. A net ROS balance that is either too low or too high may result in pathological processes (55). ROS, reactive oxygen species. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
FIG. 6.
Oxidative stress and biological effects. Oxidative stress causes a number of deleterious effects, such as acidosis, mitochondrial dysfunction, inhibition of NO, DNA damage, and lipid peroxidation. Acidosis results from cellular death and inflammation. These pathological effects of ROS lead to inflammation, apoptosis, and fibrosis (78). NO, nitric oxide; NOX, nicotinamide adenine dinucleotide phosphate hydrogen oxidase. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
FIG. 7.
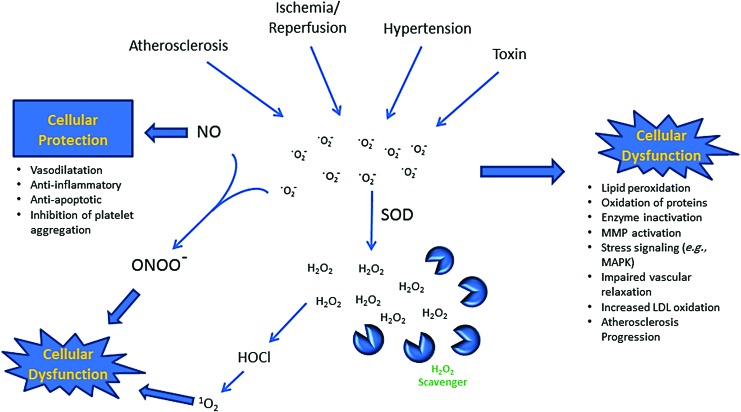
Oxidative insults in cardiovascular diseases. Atherosclerosis, ischemia/reperfusion, hypertension, and toxins can cause excessive ROS production, such as O2−, rapidly converting to neutral H2O2. H2O2 can then permeate the cell membrane freely and cause cellular dysfunction (49). H2O2, hydrogen peroxide; HOCl, hypochlorous acid; LDL, low-density lipoprotein; MAPK, mitogen-activated protein kinase; MMP, matrix metalloproteinase; 1O2, singlet oxygen; O2−, superoxide anion radical; ONOO−, peroxynitrite anion; SOD, superoxide dismutase. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Atherosclerosis
Atherosclerosis is a systemic process that involves accumulation of cholesterol beneath the intima of the arteries, followed by local activation of the immune system, proliferation of vascular smooth muscle cells, and deposition of connective tissues (52, 85, 93). When the atheromatous plaque ruptures, it could lead to thromboembolic ischemia.
There is mounting evidence showing that ROS have a dual role: as signaling messengers in maintaining physiologic vascular homeostasis and as harmful culprits in oxidative stress. H2O2 has been shown to be an important signaling messenger in maintaining endothelial function by regulating the activity of signaling proteins, enzymes, and ion channels (47). However, dysfunction occurs when ROS levels exceed the maximum amount that intrinsic defense mechanisms can handle, which may lead to a disease state. In explanted human hearts, the coronary arteries from patients with atherosclerosis had increased expression of NOX proteins, such as gp91phox and NOX4, compared with nonatherosclerotic coronary arteries (102).
There have been investigations to characterize the biological process in CVDs at the cellular and molecular level. The increased number of macrophages expressing CD163 has been demonstrated in atherosclerosis plaques using gold-coated nanoparticles (2, 80, 109, 122). PEGylated nanoparticles also have been developed to image macrophages in atherosclerosis (25, 126). The enzymatic reaction of myeloperoxidase, an enzyme secreted by activated macrophages in the plaque, produces ROS, causing the progression and rupture of the atherosclerotic plaques (53, 96). H2O2 also reacts with chloride and myeloperoxidase to form hypochlorous acid (HOCl) that can further react with another molecule of H2O2 to produce harmful singlet oxygen (1O2) in addition to the macrophage-mediated ROS production. Gadolinium-linked gold nanoparticles have been used to quantitate macrophage-rich atherosclerosis plaques using MRI (86). These data demonstrate the feasibility of using ROS imaging techniques to monitor the progression of atherosclerosis during early and late plaque formation in animals. However, translation of these techniques to humans requires further investigation.
Acute coronary syndrome
ACS encompasses a spectrum of ischemic heart disease from unstable angina to acute myocardial infarction. ACS is usually caused by the disruption of a plaque. An unstable or ruptured plaque can activate an occlusive thromboembolic event. Myocardial infarction is the result of a complete or subtotal thrombotic occlusion in the artery. Reperfusion of the previously ischemic tissues is accompanied by the generation of a large amount of ROS. This overwhelms the cellular defense system and subsequently damages normal cellular functions, leading to apoptosis or even necrosis of the cells.
In particular, H2O2, the most abundant form of ROS, plays an important role in the process by inducing proinflammatory cytokine release as well as cell apoptosis, causing further tissue damage (70). Overproduction of H2O2 exceeding the local antioxidant capacity may result in oxidative damage. Superoxide converts rapidly to H2O2 and could have harmful effects and lead to apoptosis and tissue damage. This can be minimized if there is a temporal- or spatial-specific decrease in H2O2. Therefore, focusing on local overproduction of H2O2 has tremendous potential as a therapeutic target to halt oxidative stress injury.
Ischemic and reperfusion injuries
Reperfusion injuries are associated with ischemic heart disease (45, 89). Current treatments include medical, endovascular, or surgical revascularization, with the aim of restoring blood supply in the ischemic area. Ischemia initiates a number of damaging cellular events, including acidosis, elevation of intracellular calcium, release of intracellular contents, and production of damaging substances, such as oxygen free radicals (38, 47, 56, 107). Particularly, ROS generation has been implicated as the main cause of reperfusion injuries and cell death. The expression of NOX has been shown to be induced by ischemia and leads to activation of apoptosis in the human heart (9, 128). Thus, by developing targeted nanomaterials that could deliver therapeutic drugs to treat specific cellular events during oxidative stress, we could minimize the tissue damage during reperfusion injury.
Innovative nanoparticle-based approaches have been developed to mitigate reperfusion injury following revascularization procedures to minimize endothelial injury, prevent microvascular injury, and inhibit apoptosis and necrosis of cardiac myocytes (26). The innovative modifications include using targeted nanoparticles to deliver drugs and small interfering RNA (siRNA) to the ischemic area (69).
Our group has designed several H2O2-responsive nanoparticles based on polyoxalate to limit the effects of H2O2 in ischemia/reperfusion injury (61, 62, 82). These nanoparticles have peroxalate bonds that are cleaved by an excessive level of H2O2, and thus, they can be used to target excess levels of H2O2. Once these peroxalate bonds are cleaved, these nanoparticles break apart and release a potent antioxidant molecule at the site of oxidative stress, which has both antioxidative and anti-inflammatory effects. In addition, overexpression of intracellular superoxide dismutase 1 (SOD1, Cu/Zn SOD) has been found to protect against ischemia in a mouse myocardial infarction model (108). Investigators have subsequently designed nanoparticles carrying SOD1, which can scavenge both intra- and extracellular antioxidants, to decrease myocyte apoptosis and improve postmyocardial infarction cardiac function (94).
Apoptosis is associated with the development of various CVDs. Blocking apoptosis has been demonstrated to prevent cellular damage after oxidative insults (38, 89, 107). Externalization of the cellular protein annexin V is an early step in the apoptotic process. Annexin V is found in the membranes of platelet- and lipid-laden cells of plaques. The annexin-based nanoparticles consisting of iron oxide linked to annexin V and near-infrared fluorophore have been used to image cardiomyocyte apoptosis in animal models of reperfusion injury (14, 103). In addition, annexin V conjugated with very small iron oxide nanoparticles has been shown to effectively detect apoptotic cells (27).
Myocardial infarction and thromboembolic conditions
Nanoparticles can be modified to target specific cell surface markers or biological substances, such as thrombus. This modification technique has been used to target a specific cell surface antigen in cancer therapeutics (3, 17, 22). Similarly, targeting a biological substance, such as a thrombus, will allow accumulation of nanoparticles at the site of thrombus formation. Thrombus formation is the key pathological feature in ACS, deep vein thrombosis, pulmonary embolism, and stroke. Thus, use of nanomaterials to deliver specific antithrombotic agents that can be directed specifically to the area of the acute event could be a new avenue of therapy.
Although therapies with various antithrombotic regimens have been developed to treat these conditions clinically, the nonspecific nature of these therapies has been associated with a number of complications, most commonly bleeding. Studies have shown promising results for targeting nanoparticles that deliver a thrombolytic drug after thrombotic events (1, 48). In addition, determining the specific sites of emboli formation would be helpful to guide therapeutic decisions and to directly improve therapeutic outcomes through a targeted approach. Nanoparticles can also be targeted to a small-caliber vascular event that may not be amenable to catheter-directed intervention.
The conversion of fibrinogen to fibrin by thrombin is a critical step in thrombus formation (113, 121). Various investigators have used nanoparticles targeting fibrin to image clots in different animal and human models of thrombus formation (51, 75, 110). EP-2104R, an MRI contrast agent designed to detect thrombus by binding to the fibrin, has been showed to effectively detect thrombi with high sensitivity (4, 104). Nanomaterial-based imaging agents that target other components of the blood clotting factors, such as thrombin and factor XIIIA, have been also studied (59, 110). The pentapeptide Cys-Arg-Glu-Lys-Ala has been shown to be specific for the fibrinogen/fibrin complex in a newly formed clot and has been used for bioimaging of active thrombus formation (51).
Platelet activation is another critical step in thrombus formation (21). P-selectin is a cell adhesion protein that is expressed on the surface of activated platelets. The gadolinium-based P-selectin-targeted paramagnetic nanoparticles have been shown to detect and to localize microthrombi with high sensitivity in dog model of thrombus formation (119). Also, novel ligand-conjugated microparticles of iron oxide to P-selectin have been developed as an MR contrast agent to image thrombi atherosclerotic plaques (74).
In patients treated with thrombolysis for acute stroke, the incidence of intracranial hemorrhage is ∼6% (35). Thus, nanomaterial-based DDS of thrombolytic agents only to the affected area could significantly reduce bleeding complications in acute stroke patients. Targeted thrombolysis using magnetic nanoparticles (MNPs) conjugated to urokinase (UK) showed a decrease in thrombus size compared to saline (7). Also, the thrombolytic activity of the conjugated UK/MNPs directed by a magnetic field was found to be 5.0-fold more effective than treatment with UK alone and 2.6-fold more effective than UK/MNPs without a magnetic field. Silica-coated MNPs as a nanocarrier of tissue plasminogen activator (tPA) also demonstrated 40% reductions in blood clot lysis time compared with the same drug dosage of free tPA (15).
Heart failure
Our laboratory has demonstrated that H2O2-targeted nanoparticles can effectively decrease myocardial damage caused by doxorubicin in a mouse model of heart failure (82). In addition to preventing myocardial damage caused by doxorubicin administration, nanoparticles have also been designed to target tissues of interest. In such studies, doxorubicin is bound to the nanoparticle core in peripheral circulation and is only released at the tumor site (37, 44, 98). These applications greatly decrease the systemic concentration of doxorubicin and limit myocardial damage. N-acetylcysteine conjugated to nanoparticles has also been investigated in its capacity to attenuate the development of cardiac fibrosis in heart failure in mice (33).
Other therapeutic applications in CVDs
Oxidative stress injuries are seen in cardiovascular and other vascular surgeries. Since reperfusion injury during these surgeries is predictable, with well-defined time and duration, suppressing ROS-induced injury using nanoparticles will be a good strategy to limit reperfusion injury during these surgeries. The use of nanoparticles to decrease chronic heart transplant rejection has been investigated (36). Using a chitosan-DNA nanoparticle encoding the major histocompatibility class I antigen molecule of the donors abdominal aorta, the investigators showed improvement in the degree of transplant-associated intimal proliferation in their nanoparticle treatment group compared with the control group.
Another area of study is related to aging and the regeneration potential of nanoparticles. The regenerating potential of the heart is very limited in adults. Animal studies have demonstrated that myocardial delivery of stimulating material can lead to the formation of myocytes and coronary vessels and improve cardiac function after a period of ischemia (100). However, one of the main problems of many early strategies, which delivered either stem cells or proteins to stimulate cardiac stem cells, is the use of local injections, which limits their therapeutic effects.
Using nanoparticles could overcome this issue as they may contribute either as targeted drug delivery vehicles or as intravenously delivered sustained release preparations (e.g., hydrogels) (23, 42). Because hydrogels can mimic a functional extracellular matrix, they represent an implanted construct that facilitates the delivery of nutrients and the formation of tissue and capillaries (73). Hydrogels can also be made into nanogels that are responsive to the redox effect (125), although covalently bound systems consist of stronger bonds than hydrogen bonds and offer more stability.
Limitations in Clinical Translation of Nanotechnology
The applications of nanotechnology in medicine are still in their infancy. Despite much advancement, translation to clinical use has been slow due to several reasons (87, 91). First, the data obtained through animal research cannot be directly applied to humans. For example, the typical optical and fluorescence techniques used in preclinical studies to image molecular changes cannot be directly applied in clinical imaging.
Second, there is uncertainty about the actual behavior of nanoparticles in response to dynamic physiological and pathological changes in vivo. Plasma survivability and the permeability of the nanoparticles in nontargeted organs and tissues need to be investigated. The specific behaviors and mechanisms of developed nanoparticles also need to be elucidated in not only the disease condition but also in the normal physiological state.
Third, there is a need to establish biocompatibility and biodegradability in a systematic way, which has not yet been performed. Establishing criteria or guidelines in nanomedicine research is a complex process because different types of nanostructures have different properties that can be altered by surface moieties.
Fourth, ischemia/reperfusion injury is a vascular event that leads to sequential molecular changes and signaling, including redox reactions associated with oxidative stress that are time sensitive. In addition to targeting relatively stable ROS, timely diagnosis and intervention are a critical factor and can potentially be achieved using theranostic nanoparticles.
Fifth, manufacturing nanoparticles is much more complex than current biopharmaceutical manufacturing. It is challenging to determine a scalable manufacturing system and to standardize drug quality and functionality tests for clinical use.
Sixth, safety is the primary concern for nanomaterial use, particularly for human use (8). Finally, properly acknowledging the intellectual property of nanomedicine will be a challenge because each nanoparticle component, for example, may be the subject of multiple unique patents.
Future Perspectives
Nanoparticles have many attractive features, including their small size (and thus large surface area per volume), relative ease of modification, and surface components. These features enable the attachment of ligands to create targeted molecules, allowing diverse functionalities, such as theranostic properties. These unique properties make nanoparticles a truly novel entity with vast potential for future therapeutics. Many of the challenges described above require close collaboration among different disciplines, which may not be an easy task. Nanoparticles can be a successful addition to the diagnosis and treatment approaches for CVDs only after rigorous testing and screening to verify their safety and lack of short- and long-term toxicity. Despite these challenges, we remain optimistic about the future of nanomedicine.
Conclusion
Nanoparticles represent one of the most important and exciting new developments in modern medicine. Further studies are needed in chemistry, materials science, biology, and medicine, as well as in fusion biotechnology, to further develop the truly unique role of nanomedicine. We are optimistic that nanomedicine will become a valuable contributor in solving the many important unmet needs in medicine.
Acknowledgments
This study was supported, in part, by grants from the National Institutes of Health R44DK103389-01 (P.M.K.), American Heart Association Grant in Aid 17GRNT33680110 (P.M.K.), and the Technology Innovation Program No. 10052749 from the Ministry of Trade, Industry and Energy of Korea (P.M.K. and C.G.S.).
Abbreviations Used
- ACS
acute coronary syndrome
- CVDs
cardiovascular diseases
- DDS
drug delivery system
- EPR
enhanced permeability and retention
- H2O2
hydrogen peroxide
- HOCl
hypochlorous acid
- MNPs
magnetic nanoparticles
- MRI
magnetic resonance imaging
- NOX
NADPH oxidase
- PEG
polyethylene glycol
- RES
reticuloendothelial system
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- SOD1, Cu/Zn SOD
superoxide dismutase 1
- SPIO
superparamagnetic iron oxide
- tPA
tissue plasminogen activator
- UK
urokinase
References
- 1. Agyare E. and Kandimalla K. Delivery of polymeric nanoparticles to target vascular diseases. J Biomol Res Ther 3: S1–001, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahamed M, Alsalhi MS, and Siddiqui MK. Silver nanoparticle applications and human health. Clin Chim Acta 411: 1841–1848, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Alibakhshi A, Abarghooi Kahaki F, Ahangarzadeh S, Yaghoobi H, Yarian F, Arezumand R, Ranjbari J, Mokhtarzadeh A, and de la Guardia M. Targeted cancer therapy through antibody fragments-decorated nanomedicines. J Control Release 268: 323–334, 2017 [DOI] [PubMed] [Google Scholar]
- 4. Andia ME, Saha P, Jenkins J, Modarai B, Wiethoff AJ, Phinikaridou A, Grover SP, Patel AS, Schaeffter T, Smith A, and Botnar RM. Fibrin-targeted magnetic resonance imaging allows in vivo quantification of thrombus fibrin content and identifies thrombi amenable for thrombolysis. Arterioscler Thromb Vasc Biol 34: 1193–1198, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anselmo AC. and Mitragotri S. A review of clinical translation of inorganic nanoparticles. AAPS J 17: 1041–1054, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, and Muntner P. Heart disease and stroke statistics-2017 update: a Report From the American Heart Association. Circulation 135: e146–e603, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bi F, Zhang J, Su Y, Tang YC, and Liu JN. Chemical conjugation of urokinase to magnetic nanoparticles for targeted thrombolysis. Biomaterials 30: 5125–5130, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Bostan HB, Rezaee R, Valokala MG, Tsarouhas K, Golokhvast K, Tsatsakis AM, and Karimi G. Cardiotoxicity of nano-particles. Life Sci 165: 91–99, 2016 [DOI] [PubMed] [Google Scholar]
- 9. Braunersreuther V, Montecucco F, Asrih M, Pelli G, Galan K, Frias M, Burger F, Quindere AL, Montessuit C, Krause KH, Mach F, and Jaquet V. Role of NADPH oxidase isoforms NOX1, NOX2 and NOX4 in myocardial ischemia/reperfusion injury. J Mol Cell Cardiol 64: 99–107, 2013 [DOI] [PubMed] [Google Scholar]
- 10. Cabral H. and Kataoka K. Progress of drug-loaded polymeric micelles into clinical studies. J Control Release 190: 465–476, 2014 [DOI] [PubMed] [Google Scholar]
- 11. Chang EH, Harford JB, Eaton MA, Boisseau PM, Dube A, Hayeshi R, Swai H, and Lee DS. Nanomedicine: past, present and future—a global perspective. Biochem Biophys Res Commun 468: 511–517, 2015 [DOI] [PubMed] [Google Scholar]
- 12. Chauhan G, Chopra V, Tyagi A, Rath G, Sharma RK, and Goyal AK. “Gold nanoparticles composite-folic acid conjugated graphene oxide nanohybrids” for targeted chemo-thermal cancer ablation: in vitro screening and in vivo studies. Eur J Pharm Sci 96: 351–361, 2017 [DOI] [PubMed] [Google Scholar]
- 13. Chen F, Ehlerding EB, and Cai W. Theranostic nanoparticles. J Nucl Med 55: 1919–1922, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen HH, Feng Y, Zhang M, Chao W, Josephson L, Shaw SY, and Sosnovik DE. Protective effect of the apoptosis-sensing nanoparticle AnxCLIO-Cy5.5. Nanomedicine 8: 291–298, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen JP, Yang PC, Ma YH, Tu SJ, and Lu YJ. Targeted delivery of tissue plasminogen activator by binding to silica-coated magnetic nanoparticle. Int J Nanomedicine 7: 5137–5149, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cheng CJ, Tietjen GT, Saucier-Sawyer JK, and Saltzman WM. A holistic approach to targeting disease with polymeric nanoparticles. Nat Rev Drug Discov 14: 239–247, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cisterna BA, Kamaly N, Choi WI, Tavakkoli A, Farokhzad OC, and Vilos C. Targeted nanoparticles for colorectal cancer. Nanomedicine (Lond) 11: 2443–2456, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Costanzo L. Cardiovascular physiology. In: Physiology, 5th ed., edited by Costanzo L. Philadelphia, PA: Lippincott Williams & Wilkins, 2011, pp. 64–99 [Google Scholar]
- 19. Dai Q, Bertleff-Zieschang N, Braunger JA, Bjornmalm M, Cortez-Jugo C, and Caruso F. Particle targeting in complex biological media. Adv Healthc Mater 7: 1700575, 2018 [DOI] [PubMed] [Google Scholar]
- 20. Danhier F, Feron O, and Preat V. To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release 148: 135–146, 2010 [DOI] [PubMed] [Google Scholar]
- 21. Davi G. and Patrono C. Platelet activation and atherothrombosis. N Engl J Med 357: 2482–2494, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Dim N, Perepelyuk M, Gomes O, Thangavel C, Liu Y, Den R, Lakshmikuttyamma A, and Shoyele SA. Novel targeted siRNA-loaded hybrid nanoparticles: preparation, characterization and in vitro evaluation. J Nanobiotechnology 13: 61, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Don CW. and Murry CE. Improving survival and efficacy of pluripotent stem cell-derived cardiac grafts. J Cell Mol Med 17: 1355–1362, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. England CG, Im HJ, Feng L, Chen F, Graves SA, Hernandez R, Orbay H, Xu C, Cho SY, Nickles RJ, Liu Z, Lee DS, and Cai W. Re-assessing the enhanced permeability and retention effect in peripheral arterial disease using radiolabeled long circulating nanoparticles. Biomaterials 100: 101–109, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Erogbogbo F, Yong KT, Hu R, Law WC, Ding H, Chang CW, Prasad PN, and Swihart MT. Biocompatible magnetofluorescent probes: luminescent silicon quantum dots coupled with superparamagnetic iron(III) oxide. ACS Nano 4: 5131–5138, 2010 [DOI] [PubMed] [Google Scholar]
- 26. Evans CW, Iyer KS, and Hool LC. The potential for nanotechnology to improve delivery of therapy to the acute ischemic heart. Nanomedicine (Lond) 11: 817–832, 2016 [DOI] [PubMed] [Google Scholar]
- 27. Figge L, Appler F, Chen HH, Sosnovik DE, Schnorr J, Seitz O, Taupitz M, Hamm B, and Schellenberger E. Direct coupling of annexin A5 to VSOP yields small, protein-covered nanoprobes for MR imaging of apoptosis. Contrast Media Mol Imaging 9: 291–299, 2014 [DOI] [PubMed] [Google Scholar]
- 28. Gaharwar AK, Peppas NA, and Khademhosseini A. Nanocomposite hydrogels for biomedical applications. Biotechnol Bioeng 111: 441–453, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Galagudza M, Korolev D, Postnov V, Naumisheva E, Grigorova Y, Uskov I, and Shlyakhto E. Passive targeting of ischemic-reperfused myocardium with adenosine-loaded silica nanoparticles. Int J Nanomedicine 7: 1671–1678, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gannon CJ, Patra CR, Bhattacharya R, Mukherjee P, and Curley SA. Intracellular gold nanoparticles enhance non-invasive radiofrequency thermal destruction of human gastrointestinal cancer cells. J Nanobiotechnology 6: 2, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gao W, Vecchio D, Li J, Zhu J, Zhang Q, Fu V, Li J, Thamphiwatana S, Lu D, and Zhang L. Hydrogel containing nanoparticle-stabilized liposomes for topical antimicrobial delivery. ACS Nano 8: 2900–2907, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Geelen T, Paulis LE, Coolen BF, Nicolay K, and Strijkers GJ. Passive targeting of lipid-based nanoparticles to mouse cardiac ischemia-reperfusion injury. Contrast Media Mol Imaging 8: 117–126, 2013 [DOI] [PubMed] [Google Scholar]
- 33. Gray WD, Che P, Brown M, Ning X, Murthy N, and Davis ME. N-acetylglucosamine conjugated to nanoparticles enhances myocyte uptake and improves delivery of a small molecule p38 inhibitor for post-infarct healing. J Cardiovasc Transl Res 4: 631–643, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grottkau BE, Cai X, Wang J, Yang X, and Lin Y. Polymeric nanoparticles for a drug delivery system. Curr Drug Metab 14: 840–846, 2013 [DOI] [PubMed] [Google Scholar]
- 35. Group TNt-PSS. Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. The NINDS t-PA Stroke Study Group. Stroke 28: 2109–2118, 1997 [DOI] [PubMed] [Google Scholar]
- 36. Guo Y, Chen W, Wang W, Shen J, Guo R, Gong F, Lin S, Cheng D, Chen G, and Shuai X. Simultaneous diagnosis and gene therapy of immuno-rejection in rat allogeneic heart transplantation model using a T-cell-targeted theranostic nanosystem. ACS Nano 6: 10646–10657, 2012 [DOI] [PubMed] [Google Scholar]
- 37. Han HS, Lee J, Kim HR, Chae SY, Kim M, Saravanakumar G, Yoon HY, You DG, Ko H, Kim K, Kwon IC, Park JC, and Park JH. Robust PEGylated hyaluronic acid nanoparticles as the carrier of doxorubicin: mineralization and its effect on tumor targetability in vivo. J Control Release 168: 105–114, 2013 [DOI] [PubMed] [Google Scholar]
- 38. Hausenloy DJ. and Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest 123: 92–100, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hayashi K, Nakamura M, Miki H, Ozaki S, Abe M, Matsumoto T, and Ishimura K. Gold nanoparticle cluster-plasmon-enhanced fluorescent silica core-shell nanoparticles for X-ray computed tomography-fluorescence dual-mode imaging of tumors. Chem Commun (Camb) 49: 5334–5336, 2013 [DOI] [PubMed] [Google Scholar]
- 40. Hembury M, Chiappini C, Bertazzo S, Kalber TL, Drisko GL, Ogunlade O, Walker-Samuel S, Krishna KS, Jumeaux C, Beard P, Kumar CS, Porter AE, Lythgoe MF, Boissiere C, Sanchez C, and Stevens MM. Gold-silica quantum rattles for multimodal imaging and therapy. Proc Natl Acad Sci U S A 112: 1959–1964, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hirsjarvi S, Passirani C, and Benoit JP. Passive and active tumour targeting with nanocarriers. Curr Drug Discov Technol 8: 188–196, 2011 [DOI] [PubMed] [Google Scholar]
- 42. Ho YT, Poinard B, and Kah JC. Nanoparticle drug delivery systems and their use in cardiac tissue therapy. Nanomedicine (Lond) 11: 693–714, 2016 [DOI] [PubMed] [Google Scholar]
- 43. Hofmann-Amtenbrink M, Grainger DW, and Hofmann H. Nanoparticles in medicine: current challenges facing inorganic nanoparticle toxicity assessments and standardizations. Nanomedicine 11: 1689–1694, 2015 [DOI] [PubMed] [Google Scholar]
- 44. Hu J, Xie L, Zhao W, Sun M, Liu X, and Gao W. Design of tumor-homing and pH-responsive polypeptide-doxorubicin nanoparticles with enhanced anticancer efficacy and reduced side effects. Chem Commun (Camb) 51: 11405–11408, 2015 [DOI] [PubMed] [Google Scholar]
- 45. Ibanez B, Heusch G, Ovize M, and Van de Werf F. Evolving therapies for myocardial ischemia/reperfusion injury. J Am Coll Cardiol 65: 1454–1471, 2015 [DOI] [PubMed] [Google Scholar]
- 46. Im HJ, England CG, Feng L, Graves SA, Hernandez R, Nickles RJ, Liu Z, Lee DS, Cho SY, and Cai W. Accelerated blood clearance phenomenon reduces the passive targeting of PEGylated nanoparticles in peripheral arterial disease. ACS Appl Mater Interfaces 8: 17955–17963, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jain AK, Mehra NK, and Swarnakar NK. Role of antioxidants for the treatment of cardiovascular diseases: challenges and opportunities. Curr Pharm Des 21: 4441–4455, 2015 [DOI] [PubMed] [Google Scholar]
- 48. Jin S, Wang Y, Zhu H, Wang Y, Zhao S, Zhao M, Liu J, Wu J, Gao W, and Peng S. Nanosized aspirin-Arg-Gly-Asp-Val: delivery of aspirin to thrombus by the target carrier Arg-Gly-Asp-Val tetrapeptide. ACS Nano 7: 7664–7673, 2013 [DOI] [PubMed] [Google Scholar]
- 49. Joshi-Barr S, de Gracia Lux C, Mahmoud E, and Almutairi A. Exploiting oxidative microenvironments in the body as triggers for drug delivery systems. Antioxid Redox Signal 21: 730–754, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kamata H, Li X, Chung UI, and Sakai T. Design of hydrogels for biomedical applications. Adv Healthc Mater 4: 2360–2374, 2015 [DOI] [PubMed] [Google Scholar]
- 51. Kang C, Gwon S, Song C, Kang PM, Park SC, Jeon J, Hwang DW, and Lee D. Fibrin-targeted and H2O2-responsive nanoparticles as a theranostics for thrombosed vessels. ACS Nano 11: 6194–6203, 2017 [DOI] [PubMed] [Google Scholar]
- 52. Karagkiozaki V, Logothetidis S, and Pappa AM. Nanomedicine for atherosclerosis: molecular imaging and treatment. J Biomed Nanotechnol 11: 191–210, 2015 [DOI] [PubMed] [Google Scholar]
- 53. Karakas M. and Koenig W. Myeloperoxidase production by macrophage and risk of atherosclerosis. Curr Atheroscler Rep 14: 277–283, 2012 [DOI] [PubMed] [Google Scholar]
- 54. Kim T. and Hyeon T. Applications of inorganic nanoparticles as therapeutic agents. Nanotechnology 25: 012001, 2014 [DOI] [PubMed] [Google Scholar]
- 55. Kimura W, Muralidhar S, Canseco DC, Puente B, Zhang CC, Xiao F, Abderrahman YH, and Sadek HA. Redox signaling in cardiac renewal. Antioxid Redox Signal 21: 1660–1673, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kloner RA, Brown DA, Csete M, Dai W, Downey JM, Gottlieb RA, Hale SL, and Shi J. New and revisited approaches to preserving the reperfused myocardium. Nat Rev Cardiol 14: 679–693, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kornfeld OS, Hwang S, Disatnik MH, Chen CH, Qvit N, and Mochly-Rosen D. Mitochondrial reactive oxygen species at the heart of the matter: new therapeutic approaches for cardiovascular diseases. Circ Res 116: 1783–1799, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kumar S, Aswal VK, and Callow P. pH-dependent interaction and resultant structures of silica nanoparticles and lysozyme protein. Langmuir 30: 1588–1598, 2014 [DOI] [PubMed] [Google Scholar]
- 59. Kwon SP, Jeon S, Lee SH, Yoon HY, Ryu JH, Choi D, Kim JY, Kim J, Park JH, Kim DE, Kwon IC, Kim K, and Ahn CH. Thrombin-activatable fluorescent peptide incorporated gold nanoparticles for dual optical/computed tomography thrombus imaging. Biomaterials 150: 125–136, 2018 [DOI] [PubMed] [Google Scholar]
- 60. Langer R. and Weissleder R. Nanotechnology. JAMA 313: 135–136, 2015 [DOI] [PubMed] [Google Scholar]
- 61. Lee D, Bae S, Hong D, Lim H, Yoon JH, Hwang O, Park S, Ke Q, Khang G, and Kang PM. H2O2-responsive molecularly engineered polymer nanoparticles as ischemia/reperfusion-targeted nanotherapeutic agents. Sci Rep 3: 2233, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lee D, Bae S, Ke Q, Lee J, Song B, Karumanchi SA, Khang G, Choi HS, and Kang PM. Hydrogen peroxide-responsive copolyoxalate nanoparticles for detection and therapy of ischemia-reperfusion injury. J Control Release 172: 1102–1110, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Li Y, Gao GH, and Lee DS. Stimulus-sensitive polymeric nanoparticles and their applications as drug and gene carriers. Adv Healthc Mater 2: 388–417, 2013 [DOI] [PubMed] [Google Scholar]
- 64. Lin CW, Tseng SJ, Kempson IM, Yang SC, Hong TM, and Yang PC. Extracellular delivery of modified oligonucleotide and superparamagnetic iron oxide nanoparticles from a degradable hydrogel triggered by tumor acidosis. Biomaterials 34: 4387–4393, 2013 [DOI] [PubMed] [Google Scholar]
- 65. Liu Y, Qiao L, Zhang S, Wan G, Chen B, Zhou P, Zhang N, and Wang Y. Dual pH-responsive multifunctional nanoparticles for targeted treatment of breast cancer by combining immunotherapy and chemotherapy. Acta Biomater S1742-7061(17)30696–30697, 2017 [DOI] [PubMed] [Google Scholar]
- 66. Luo E, Song G, Li Y, Shi P, Hu J, and Lin Y. The toxicity and pharmacokinetics of carbon nanotubes as an effective drug carrier. Curr Drug Metab 14: 879–890, 2013 [DOI] [PubMed] [Google Scholar]
- 67. Luo GF, Chen WH, Liu Y, Lei Q, Zhuo RX, and Zhang XZ. Multifunctional enveloped mesoporous silica nanoparticles for subcellular co-delivery of drug and therapeutic peptide. Sci Rep 4: 6064, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Madamanchi NR. and Runge MS. Redox signaling in cardiovascular health and disease. Free Radic Biol Med 61: 473–501, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Maheshwari R, Tekade M, Sharma PA, and Tekade RK. Nanocarriers assisted siRNA gene therapy for the management of cardiovascular disorders. Curr Pharm Des 21: 4427–4440, 2015 [DOI] [PubMed] [Google Scholar]
- 70. Maneechote C, Palee S, Chattipakorn SC, and Chattipakorn N. Roles of mitochondrial dynamics modulators in cardiac ischaemia/reperfusion injury. J Cell Mol Med 21: 2643–2653, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Marosfoi MG, Korin N, Gounis MJ, Uzun O, Vedantham S, Langan ET, Papa AL, Brooks OW, Johnson C, Puri AS, Bhatta D, Kanapathipillai M, Bronstein BR, Chueh JY, Ingber DE, and Wakhloo AK. Shear-activated nanoparticle aggregates combined with temporary endovascular bypass to treat large vessel occlusion. Stroke 46: 3507–3513, 2015 [DOI] [PubMed] [Google Scholar]
- 72. Mattoussi H. and Rotello VM. Inorganic nanoparticles in drug delivery. Adv Drug Deliv Rev 65: 605–606, 2013 [DOI] [PubMed] [Google Scholar]
- 73. Mayfield AE, Tilokee EL, Latham N, McNeill B, Lam BK, Ruel M, Suuronen EJ, Courtman DW, Stewart DJ, and Davis DR. The effect of encapsulation of cardiac stem cells within matrix-enriched hydrogel capsules on cell survival, post-ischemic cell retention and cardiac function. Biomaterials 35: 133–142, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. McAteer MA, Akhtar AM, von Zur Muhlen C, and Choudhury RP. An approach to molecular imaging of atherosclerosis, thrombosis, and vascular inflammation using microparticles of iron oxide. Atherosclerosis 209: 18–27, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. McCarthy JR, Sazonova IY, Erdem SS, Hara T, Thompson BD, Patel P, Botnaru I, Lin CP, Reed GL, Weissleder R, and Jaffer FA. Multifunctional nanoagent for thrombus-targeted fibrinolytic therapy. Nanomedicine (Lond) 7: 1017–1028, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mekaru H, Lu J, and Tamanoi F. Development of mesoporous silica-based nanoparticles with controlled release capability for cancer therapy. Adv Drug Deliv Rev 95: 40–49, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Moris D, Spartalis M, Tzatzaki E, Spartalis E, Karachaliou GS, Triantafyllis AS, Karaolanis GI, Tsilimigras DI, and Theocharis S. The role of reactive oxygen species in myocardial redox signaling and regulation. Ann Transl Med 5: 324, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nishida K. and Otsu K. Inflammation and metabolic cardiomyopathy. Cardiovasc Res 113: 389–398, 2017 [DOI] [PubMed] [Google Scholar]
- 79. Nishikawa M, Ogawa K, Umeki Y, Mohri K, Kawasaki Y, Watanabe H, Takahashi N, Kusuki E, Takahashi R, Takahashi Y, and Takakura Y. Injectable, self-gelling, biodegradable, and immunomodulatory DNA hydrogel for antigen delivery. J Control Release 180: 25–32, 2014 [DOI] [PubMed] [Google Scholar]
- 80. Ouimet T, Lancelot E, Hyafil F, Rienzo M, Deux F, Lemaitre M, Duquesnoy S, Garot J, Roques BP, Michel JB, Corot C, and Ballet S. Molecular and cellular targets of the MRI contrast agent P947 for atherosclerosis imaging. Mol Pharm 9: 850–861, 2012 [DOI] [PubMed] [Google Scholar]
- 81. Pagels RF. and Prud'homme RK. Polymeric nanoparticles and microparticles for the delivery of peptides, biologics, and soluble therapeutics. J Control Release 219: 519–535, 2015 [DOI] [PubMed] [Google Scholar]
- 82. Park S, Yoon J, Bae S, Park M, Kang C, Ke Q, Lee D, and Kang PM. Therapeutic use of H2O2-responsive anti-oxidant polymer nanoparticles for doxorubicin-induced cardiomyopathy. Biomaterials 35: 5944–5953, 2014 [DOI] [PubMed] [Google Scholar]
- 83. Park YS. Preparation of concentrated colloids of gold core-silica shell nanoparticles for biomedical applications. Methods Mol Biol 906: 21–31, 2012 [DOI] [PubMed] [Google Scholar]
- 84. Pasut G. Pegylation of biological molecules and potential benefits: pharmacological properties of certolizumab pegol. BioDrugs 28 Suppl 1: S15–S23, 2014 [DOI] [PubMed] [Google Scholar]
- 85. Psarros C, Lee R, Margaritis M, and Antoniades C. Nanomedicine for the prevention, treatment and imaging of atherosclerosis. Nanomedicine 8 Suppl 1: S59–S68, 2012 [DOI] [PubMed] [Google Scholar]
- 86. Qin H, Zhou T, Yang S, Chen Q, and Xing D. Gadolinium(III)-gold nanorods for MRI and photoacoustic imaging dual-modality detection of macrophages in atherosclerotic inflammation. Nanomedicine (Lond) 8: 1611–1624, 2013 [DOI] [PubMed] [Google Scholar]
- 87. Ragelle H, Danhier F, Preat V, Langer R, and Anderson DG. Nanoparticle-based drug delivery systems: a commercial and regulatory outlook as the field matures. Expert Opin Drug Deliv 14: 851–864, 2017 [DOI] [PubMed] [Google Scholar]
- 88. Ren K, He C, Xiao C, Li G, and Chen X. Injectable glycopolypeptide hydrogels as biomimetic scaffolds for cartilage tissue engineering. Biomaterials 51: 238–249, 2015 [DOI] [PubMed] [Google Scholar]
- 89. Rentrop KP. and Feit F. Reperfusion therapy for acute myocardial infarction: concepts and controversies from inception to acceptance. Am Heart J 170: 971–980, 2015 [DOI] [PubMed] [Google Scholar]
- 90. Sahoo B, Devi KS, Banerjee R, Maiti TK, Pramanik P, and Dhara D. Thermal and pH responsive polymer-tethered multifunctional magnetic nanoparticles for targeted delivery of anticancer drug. ACS Appl Mater Interfaces 5: 3884–3893, 2013 [DOI] [PubMed] [Google Scholar]
- 91. Sainz V, Conniot J, Matos AI, Peres C, Zupancic E, Moura L, Silva LC, Florindo HF, and Gaspar RS. Regulatory aspects on nanomedicines. Biochem Biophys Res Commun 468: 504–510, 2015 [DOI] [PubMed] [Google Scholar]
- 92. Salatin S, Maleki Dizaj S, and Yari Khosroushahi A. Effect of the surface modification, size, and shape on cellular uptake of nanoparticles. Cell Biol Int 39: 881–890, 2015 [DOI] [PubMed] [Google Scholar]
- 93. Schiener M, Hossann M, Viola JR, Ortega-Gomez A, Weber C, Lauber K, Lindner LH, and Soehnlein O. Nanomedicine-based strategies for treatment of atherosclerosis. Trends Mol Med 20: 271–281, 2014 [DOI] [PubMed] [Google Scholar]
- 94. Seshadri G, Sy JC, Brown M, Dikalov S, Yang SC, Murthy N, and Davis ME. The delivery of superoxide dismutase encapsulated in polyketal microparticles to rat myocardium and protection from myocardial ischemia-reperfusion injury. Biomaterials 31: 1372–1379, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Shang L, Nienhaus K, and Nienhaus GU. Engineered nanoparticles interacting with cells: size matters. J Nanobiotechnology 12: 5, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Shao B, Tang C, Sinha A, Mayer PS, Davenport GD, Brot N, Oda MN, Zhao XQ, and Heinecke JW. Humans with atherosclerosis have impaired ABCA1 cholesterol efflux and enhanced high-density lipoprotein oxidation by myeloperoxidase. Circ Res 114: 1733–1742, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sheng Y, Liao LD, Thakor NV, and Tan MC. Nanoparticles for molecular imaging. J Biomed Nanotechnol 10: 2641–2676, 2014 [DOI] [PubMed] [Google Scholar]
- 98. Shi Z, Guo R, Li W, Zhang Y, Xue W, Tang Y, and Zhang Y. Nanoparticles of deoxycholic acid, polyethylene glycol and folic acid-modified chitosan for targeted delivery of doxorubicin. J Mater Sci Mater Med 25: 723–731, 2014 [DOI] [PubMed] [Google Scholar]
- 99. Siafaka PI, Ustundag Okur N, Karavas E, and Bikiaris DN. Surface modified multifunctional and stimuli responsive nanoparticles for drug targeting: current status and uses. Int J Mol Sci 17: 1440, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Siminiak T. and Kurpisz M. Myocardial replacement therapy. Circulation 108: 1167–1171, 2003 [DOI] [PubMed] [Google Scholar]
- 101. Song JT, Yang XQ, Zhang XS, Yan DM, Yao MH, Qin MY, and Zhao YD. Composite silica coated gold nanosphere and quantum dots nanoparticles for X-ray CT and fluorescence bimodal imaging. Dalton Trans 44: 11314–11320, 2015 [DOI] [PubMed] [Google Scholar]
- 102. Sorescu D, Weiss D, Lassegue B, Clempus RE, Szocs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, Vega JD, Taylor WR, and Griendling KK. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation 105: 1429–1435, 2002 [DOI] [PubMed] [Google Scholar]
- 103. Sosnovik DE, Garanger E, Aikawa E, Nahrendorf M, Figuiredo JL, Dai G, Reynolds F, Rosenzweig A, Weissleder R, and Josephson L. Molecular MRI of cardiomyocyte apoptosis with simultaneous delayed-enhancement MRI distinguishes apoptotic and necrotic myocytes in vivo: potential for midmyocardial salvage in acute ischemia. Circ Cardiovasc Imaging 2: 460–467, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Spuentrup E, Fausten B, Kinzel S, Wiethoff AJ, Botnar RM, Graham PB, Haller S, Katoh M, Parsons EC, Jr., Manning WJ, Busch T, Gunther RW, and Buecker A. Molecular magnetic resonance imaging of atrial clots in a swine model. Circulation 112: 396–399, 2005 [DOI] [PubMed] [Google Scholar]
- 105. Sugamura K. and Keaney JF., Jr. Reactive oxygen species in cardiovascular disease. Free Radic Biol Med 51: 978–992, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Suk JS, Xu Q, Kim N, Hanes J, and Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev 99: 28–51, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Takagi H, Matsui Y, and Sadoshima J. The role of autophagy in mediating cell survival and death during ischemia and reperfusion in the heart. Antioxid Redox Signal 9: 1373–1381, 2007 [DOI] [PubMed] [Google Scholar]
- 108. Tanaka M, Mokhtari GK, Terry RD, Balsam LB, Lee KH, Kofidis T, Tsao PS, and Robbins RC. Overexpression of human copper/zinc superoxide dismutase (SOD1) suppresses ischemia-reperfusion injury and subsequent development of graft coronary artery disease in murine cardiac grafts. Circulation 110: II200–II206, 2004 [DOI] [PubMed] [Google Scholar]
- 109. Tarin C, Carril M, Martin-Ventura JL, Markuerkiaga I, Padro D, Llamas-Granda P, Moreno JA, Garcia I, Genicio N, Plaza-Garcia S, Blanco-Colio LM, Penades S, and Egido J. Targeted gold-coated iron oxide nanoparticles for CD163 detection in atherosclerosis by MRI. Sci Rep 5: 17135, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Temme S, Grapentin C, Quast C, Jacoby C, Grandoch M, Ding Z, Owenier C, Mayenfels F, Fischer JW, Schubert R, Schrader J, and Flogel U. Noninvasive imaging of early venous thrombosis by 19F magnetic resonance imaging with targeted perfluorocarbon nanoemulsions. Circulation 131: 1405–1414, 2015 [DOI] [PubMed] [Google Scholar]
- 111. Thomas SC, Harshita, Mishra PK, and Talegaonkar S. Ceramic nanoparticles: fabrication methods and applications in drug delivery. Curr Pharm Des 21: 6165–6188, 2015 [DOI] [PubMed] [Google Scholar]
- 112. Tyler PD. and Kang PM. Diagnostic and therapeutic nanoparticles in cardiovascular diseases. Curr Pharm Des 21: 6070–6080, 2015 [DOI] [PubMed] [Google Scholar]
- 113. Undas A. and Ariens RA. Fibrin clot structure and function: a role in the pathophysiology of arterial and venous thromboembolic diseases. Arterioscler Thromb Vasc Biol 31: e88–e99, 2011 [DOI] [PubMed] [Google Scholar]
- 114. van der Meel R, Fens MH, Vader P, van Solinge WW, Eniola-Adefeso O, and Schiffelers RM. Extracellular vesicles as drug delivery systems: lessons from the liposome field. J Control Release 195: 72–85, 2014 [DOI] [PubMed] [Google Scholar]
- 115. Vanderkooy A, Chen Y, Gonzaga F, and Brook MA. Silica shell/gold core nanoparticles: correlating shell thickness with the plasmonic red shift upon aggregation. ACS Appl Mater Interfaces 3: 3942–3947, 2011 [DOI] [PubMed] [Google Scholar]
- 116. Varshosaz J. and Taymouri S. Hollow inorganic nanoparticles as efficient carriers for siRNA delivery: a comprehensive review. Curr Pharm Des 21: 4310–4328, 2015 [DOI] [PubMed] [Google Scholar]
- 117. Vashist A. and Ahmad S. Hydrogels in tissue engineering: scope and applications. Curr Pharm Biotechnol 16: 606–620, 2015 [DOI] [PubMed] [Google Scholar]
- 118. Vllasaliu D, Fowler R, and Stolnik S. PEGylated nanomedicines: recent progress and remaining concerns. Expert Opin Drug Deliv 11: 139–154, 2014 [DOI] [PubMed] [Google Scholar]
- 119. Wang XF, Jin PP, Tong Z, Zhao YP, Ding QL, Wang DB, Zhao GM, Jing D, Wang HL, and Ge HL. MR molecular imaging of thrombus: development and application of a Gd-based novel contrast agent targeting to P-selectin. Clin Appl Thromb Hemost 16: 177–183, 2010 [DOI] [PubMed] [Google Scholar]
- 120. Wang Y, Zhao Q, Han N, Bai L, Li J, Liu J, Che E, Hu L, Zhang Q, Jiang T, and Wang S. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomedicine 11: 313–327, 2015 [DOI] [PubMed] [Google Scholar]
- 121. Weisel JW. and Litvinov RI. Mechanisms of fibrin polymerization and clinical implications. Blood 121: 1712–1719, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Weissleder R, Nahrendorf M, and Pittet MJ. Imaging macrophages with nanoparticles. Nat Mater 13: 125–138, 2014 [DOI] [PubMed] [Google Scholar]
- 123. Xu Y, Wang L, Li YK, and Wang CQ. Oxidation and pH responsive nanoparticles based on ferrocene-modified chitosan oligosaccharide for 5-fluorouracil delivery. Carbohydr Polym 114: 27–35, 2014 [DOI] [PubMed] [Google Scholar]
- 124. Yamada M, Foote M, and Prow TW. Therapeutic gold, silver, and platinum nanoparticles. Wiley Interdiscip Rev Nanomed Nanobiotechnol 7: 428–445, 2015 [DOI] [PubMed] [Google Scholar]
- 125. Yao X, Liu Y, Gao J, Yang L, Mao D, Stefanitsch C, Li Y, Zhang J, Ou L, Kong D, Zhao Q, and Li Z. Nitric oxide releasing hydrogel enhances the therapeutic efficacy of mesenchymal stem cells for myocardial infarction. Biomaterials 60: 130–140, 2015 [DOI] [PubMed] [Google Scholar]
- 126. Yi S, Allen SD, Liu YG, Ouyang BZ, Li X, Augsornworawat P, Thorp EB, and Scott EA. Tailoring nanostructure morphology for enhanced targeting of dendritic cells in atherosclerosis. ACS Nano 10: 11290–11303, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Yokoyama M. Polymeric micelles as drug carriers: their lights and shadows. J Drug Target 22: 576–583, 2014 [DOI] [PubMed] [Google Scholar]
- 128. Yu Q, Lee CF, Wang W, Karamanlidis G, Kuroda J, Matsushima S, Sadoshima J, and Tian R. Elimination of NADPH oxidase activity promotes reductive stress and sensitizes the heart to ischemic injury. J Am Heart Assoc 3: e000555, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Zhang Y, Petibone D, Xu Y, Mahmood M, Karmakar A, Casciano D, Ali S, and Biris AS. Toxicity and efficacy of carbon nanotubes and graphene: the utility of carbon-based nanoparticles in nanomedicine. Drug Metab Rev 46: 232–246, 2014 [DOI] [PubMed] [Google Scholar]
- 130. Zylberberg C, Gaskill K, Pasley S, and Matosevic S. Engineering liposomal nanoparticles for targeted gene therapy. Gene Ther 24: 441–452, 2017 [DOI] [PubMed] [Google Scholar]