Abstract
Viral infections of the central nervous system are accompanied by the expression of cytokines and chemokines that can be critical for the control of viral replication in the brain. The outcomes of cytokine/chemokine signaling in neural cells vary widely, with cell-specific effects on cellular activity, proliferation, and survival. Neural stem/progenitor cells (NSPCs) are often altered during viral infections, through direct infection by the virus or by the influence of immune cell activity or cytokine/chemokine signaling. However, it has been challenging to dissect the contribution of the virus and specific inflammatory mediators during an infection. In addition to initiating an antiviral program in infected NSPCs, cytokines/chemokines can induce multiple changes in NSPC behavior that can perturb NSPC numbers, differentiation into other neural cells, and migration to sites of injury, and ultimately brain development and repair. The focus of this review was to dissect the effects of common antiviral cytokines and chemokines on NSPC activity, and to consider the subsequent pathological consequences for the host from changes in NSPC function.
Keywords: neural stem/progenitor cells, virus, cytokines, chemokines, neurodevelopment, inflammation
Introduction
Viral infections of the central nervous system (CNS) can profoundly impact brain development and function, particularly in younger hosts where new neural cells are still actively produced and synaptic connections are undergoing refinement. Viruses generally cause CNS disease in two, nonexclusive ways: (a) replicating in and killing CNS cells (e.g., Zika virus, Semliki Forest virus), and/or (b) inducing an immune response that causes excessive inflammation or encephalitis in the brain (e.g., human immunodeficiency virus [HIV], West Nile virus [WNV]). Neuronal loss is prevalent in many viral CNS infections, in which damage to postmitotic neurons may lead to a range of pathologies depending upon the affected brain region and the age of the host (30,40,86,116). In addition to neurons that may be damaged or killed, neural stem/progenitor cells (NSPCs) are a target for many neurotropic viruses, including murine and human cytomegalovirus (CMV), herpes simplex virus (HSV), lymphocytic choriomeningitis virus (LCMV), Japanese encephalitic virus (JEV), Borna disease virus, and Zika virus (5,17,18,32,85,98,117). During many viral infections, NSPC proliferation decreases and production of new neurons declines that could impair maintenance of the NSPC pool, brain growth, and replacement of dying neurons (18,58,85,101,125,127).
NSPCs are characterized by cellular plasticity and by the ability to self-renew, and they play critical roles in the embryonic, neonatal, and adult brain. In the developing brain, NSPCs are controlled by a finely orchestrated series of signals that coordinate proliferation and differentiation into different neural cell types (neurons, astrocytes, and oligodendrocytes) that ultimately populate the mature brain. Depending upon the developmental stage, NSPCs can divide symmetrically to give rise to two daughter NSPCs or asymmetrically to produce one NSPC and one neuron or glial cell. NSPCs are driven into the appropriate lineage by tightly timed expression of developmental growth factors and cytokines in specific brain regions, ultimately building the finely patterned network of the CNS. Disruption of either NSPC proliferation or differentiation can lead to profound neurodevelopmental disorders, including microcephaly (52). The potency and localization of NSPCs change with age, with more widespread expression in the embryonic brain followed by a gradual restriction into neurogenic niches in adulthood [reviewed in Stevens et al. (110) and Temple (118)]. In the adult brain, NSPCs are restricted to neurogenic niches in the subgranular zone of the hippocampus, and the subventricular zone (SVZ) (61,93,94,124,126), where they are involved in the production of new neurons for long-term memory, learning, and repair (36,63,80). In addition to producing most of the neural cells in the brain, NSPCs also play important roles in direct replacement of damaged or dying neurons, secretion of trophic factors, and even modulation of resident and infiltrating immune cells (65,75,82). Much of the cross talk between NSPCs and immune cells results from cytokines produced by both cell types, with potential protective or toxic effects depending upon the cytokine profile (24,34,55). Thus, disruptions in NSPC function have significant physiological consequences for the host at any age.
When NSPCs are infected by a virus, there are a range of cytopathic effects by which the virus can alter NSPC function, including apoptosis or inhibition of growth [reviewed in Das and Basu (32)]. However, indirect mechanisms by which NSPCs are disrupted during a viral infection, and the role that antiviral mediators play in this process, remain largely undefined. Our goal in this review was to highlight common antiviral cytokines and chemokines that are expressed in neurotropic infections and to address how NSPCs would be predicted to respond to the inflammatory milieu. By better understanding the factors that contribute to alterations in NSPC behavior, we hope to elucidate potential therapeutic targets to prevent or limit neurological sequelae associated with viral infections.
Cytokines
Tumor necrosis factor alpha
Tumor necrosis factor alpha (TNFα) has significant physiological roles in the healthy brain and immunoregulatory roles in infected or diseased brain (97). Exposure of the neonatal brain to TNFα can result in long-term behavioral deficits and high concentrations of TNFα are associated with anxiety and depression-like behavior in adulthood (7). In models of viral CNS infections, TNFα is upregulated in various in vivo and in vitro studies of HSV1, CMV, HIV, and tick-borne encephalitis virus (1,22,74,104,122,141). Importantly, during murine CMV infection, TNFα reduces the numbers of granule NSPCs in the cerebellar external granule layer, suggesting that TNFα may affect survival or proliferation during CNS infections (104). As there are very few studies that have investigated the effects of TNFα on NSPCs in the presence of a viral infection, we consider studies that examine the effect of TNFα treatment on NSPC activity.
The effects of TNFα on NSPC proliferation seem to be dependent on the dose and the duration of exposure. Low concentrations of TNFα induce proliferation of postnatal SVZ NSPCs in vitro, whereas high concentrations of TNFα trigger apoptosis in the same cells (73). Similarly, short exposure to TNFα causes an initial increase in proliferation, followed subsequently by apoptosis in longer exposures in vitro and in vivo. During TNFα treatment of adult SVZ NSPCs, increased proliferation was observed 24 h after treatment, whereas at 72 h there was a moderate increase in apoptosis of NSPCs (135). Distinct signaling pathways appear to be responsible at each stage (Fig. 1). TNFα mediates proliferative changes in NSPCs through activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and inhibitor of NF-κB kinase subunit beta (IKK-β) signaling with an increase in cyclin D1 activity (135), whereas TNFα causes apoptosis of NSPCs through activation of the p38 mitogen-activated protein kinase (MAPK) pathway. Activation of p38-MAPK is coupled with an increase in B cell lymphoma 2 (Bcl2)-associated protein (Bax) and cleaved caspase-3 and a decrease in Bcl2, suggestive of a disruption of mitochondrial integrity (25). Similarly, in vivo administration of TNFα to adult mice resulted in an increase in newly formed NSPCs in the SVZ at 24 h postadministration, followed by a reduction in NSPCs in the SVZ at 48 h. The authors attribute this decrease to either apoptosis of the NSPCs or migration of NSPCs from the SVZ to the site of injection (138). Thus, brief exposure of NSPCs to TNFα seems to increase NSPC proliferation, whereas long-term exposure may be detrimental to the NSPC pool.
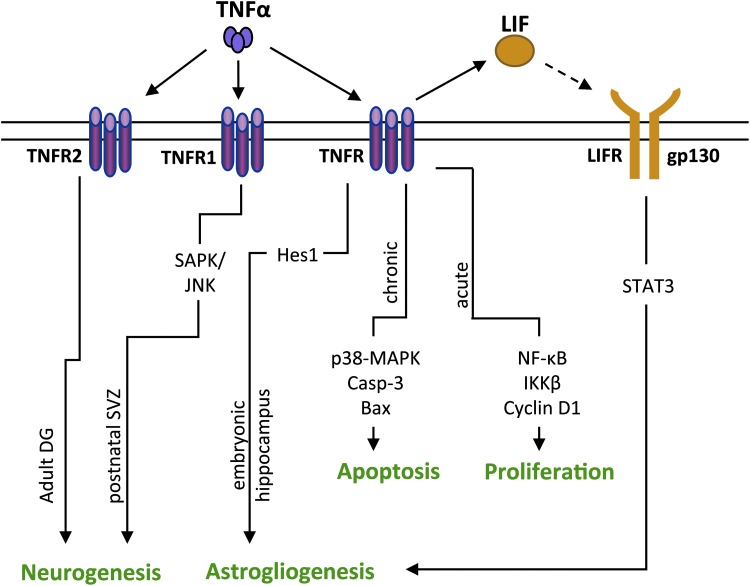
FIG. 1.
Effects of TNFα on NSPC activity. TNFα treatment results in disparities in differentiation because of the subtype of TNFR (TNFR1 or TNFR2) and the age/brain region of the NSPCs. In the adult dentate gyrus (DG), TNFα induces increased neurogenesis in NSPCs through TNFR2 binding. No changes in differentiation are observed when TNFα binds to TNFR1 in the same model. In postnatal SVZ NSPCs, TNFα activates the SAPK/JNK pathway through TNFR1, resulting in increased neurogenesis. Many studies do not address the relative roles of TNFR1/TNFR2 specifically, but rather focus on intracellular signaling pathways that are activated by TNFα. In embryonic hippocampal NSPCs, TNFα results in astrogliogenesis through the activation of Hes1, an antineurogenic transcription factor. Chronic TNFα treatment results in increased expression of the proapoptotic protein, Bax, which is accompanied by activation/cleavage of caspase-3 in NSPCs. In contrast, acute TNFα treatment results in increased proliferation through NF-κB signaling. TNFα also leads to the release of LIF that activates STAT3 signaling through LIFR and promotes astrogliogenesis in NSPCs. Solid lines represent pathways that are defined in the literature, and the dotted line represents a proposed pathway. Bax, B cell lymphoma 2 associated protein; Hes1, Hairy and enhancer of split-1; LIF, leukemia inhibitory factor; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NSPCs, neural stem/progenitor cells; SAPK/JNK, stress activated protein kinase/c-Jun N-terminal kinase; SVZ, subventricular zone; TNFα, tumor necrosis factor alpha.
TNFα also promotes differentiation of NSPCs, but with different cell fates depending upon cell type and the receptor profile. TNFα increases neurogenesis of postnatal SVZ NSPCs activation of the stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) pathway in vitro (73). In contrast, TNFα treatment of human and rat fetal NSPCs enhances astrogliogenesis and reduces neurogenesis (62,69). The increase in astrogliogenesis in rat fetal NSPCs can be attributed to elevated expression of the antineurogenic transcription factor, Hairy and enhancer of split-1 (Hes1) (62). In human fetal NSPCs, TNFα leads to an increase in production of leukemia inhibitory factor (LIF) that leads to astrogliogenesis in an autocrine manner (69). Thus, whether TNFα induces neurogenesis or gliogenesis of NSPCs may depend partially on the age of the host. It is also possible that these distinct cell fate commitments are the result of the subtypes of TNFα receptors (TNFR) expressed on the NSPCs. Iosif et al. observed that TNFα binding to TNFR2, a subtype of TNFR, leads to an increase in neurogenesis in adult rat dentate gyrus. No changes in differentiation were observed with binding to TNFR1 in the same cell type (56). However, TNFα also induces neurogenesis in the postnatal SVZ NSPCs, although it binds to TNFR1 (73). Interestingly, TNFα may also increase NSPC numbers through dedifferentiation of astrocytes, where astrocytes are reprogrammed into pluripotent, NSPC-like cells (41). Hence, TNFα may alter the NSPC pool through direct modulation of proliferation or through indirect mechanisms such as dedifferentiation of other neural cells.
Interleukin-6
Interleukin-6 (IL-6) is expressed basally in the brain where it plays a role in learning and memory (35). However, inflammatory changes in IL-6 can have protective and deleterious effects. IL-6 overexpression impairs avoidance learning and mediates autism-like behavior, whereas mice lacking IL-6 demonstrate increased sensitivity to infection and deficits in fear conditioning (50,132). IL-6 expression has been observed in numerous CNS infections such as HIV, CMV, WNV, JEV, and Zika virus (4,13,71,87,136). NSPCs infected by JEV also produce IL-6 that may then act in an autocrine matter to trigger apoptosis (83).
Multiple studies suggest that IL-6 exposure can trigger neurogenesis in primary NSPCs (12,57,60,103). IL-6 treatment of human NSPC lines also results in an increase in neurogenesis and neurite growth (103). However, the context of the inflammatory milieu, and the other cytokines that are expressed, may influence the effects of IL-6. When activated microglia release IL-6 and LIF, astrocytic differentiation results in NSPCs without any changes in proliferation (79). Similarly, Zika virus infection of microglia results in the production and release of IL-6 and TNFα, which causes an increase in NSPC proliferation and astrogliogenesis and a decrease in neurogenesis (129). Hence, although studying the effects of individual cytokines is important, evaluating the effects of IL-6 in combination with other cytokines is imperative for understanding NSPC activity.
In vivo treatment with IL-6 can result in long-term changes in NSPC function and activity that might be detrimental to the cellular ratio or composition of the brain. Storer et al. showed that IL-6 administration in postnatal and adult mice results in an initial increase in NSPC proliferation and neurogenesis. Ultimately, prolonged IL-6 exposure led to an increase in neurogenesis that was associated with a depletion of NSPC pools in both age groups (111). In addition, in utero exposure to IL-6 results in long-term changes in the NSPC pools of the offspring. Maternal IL-6 administration causes an increase in cortical and forebrain precursors in the embryo. These perturbations in NSPC activity last into adulthood, where an increase in proliferation and neurogenesis is observed in the SVZ pools of the adult offspring (42). Thus, IL-6 has the potential to cause life-long effects on NSPC activity that may or may not be reversible. It is also important to note that IL-6 may play a protective role for NSPCs, particularly during certain viral infections. HSV1 infection leads to a decrease in the NSPC pool and the number of immature neurons in vitro. However, these effects are blocked by microglial-derived IL-6 that preserves the NSPC pool during an active infection (27).
Interleukin 1 beta
The IL-1 family contains 11 cytokines including the proinflammatory interleukin 1 beta (IL-1β) (89). IL-1β is often seen in models of CNS viral infection including WNV, CMV, HSV, and HIV (4,28,74,95,99). In these viral models, IL-1β has been shown to have a variety of effects in the CNS. In WNV infection, IL-1β signaling has been implicated in control of both viral replication and immune cell recruitment and activation within the CNS (37,99). Similarly, in HSV infection, the absence of IL-1β is associated with increased viral replication in the brain (106). The impact of IL-1β on NSPCs could be relevant in the context of viral infection; however, this has not been extensively studied in viral models. Despite this, the effects of IL-1β on NSPCs in nonviral models can be evaluated and used to speculate on the nature of the NSPC response to IL-1β during a viral infection.
IL-1β reduces NSPC proliferation in most in vitro studies (29,45,46,66,102,131), but the proposed signaling mechanisms vary based upon dose and duration (45,102) of IL-1β treatment. Studies by Guadagno et al. and Wang et al. suggested that reduced NSPC proliferation is at least partially because of apoptosis that is mediated by p53 and/or SAPK/JNK signaling (46,131), whereas studies by Koo and Duman (66) and Crampton et al. (29) suggested nonapoptotic mechanisms of inhibition such as reduced cellular respiration and/or induced differentiation. Ultimately, IL-1β appears to inhibit proliferation in short-term treatments while inducing apoptosis in more long-term exposures (>7 days) with higher doses of IL-1β (e.g., 100 ng/mL) (45,102,131). In contrast, human NSPCs respond to IL-1β with increased proliferation, suggesting species-specific differences in the IL-1β response (142). Neurotropic viruses can cause acute or chronic infection in the CNS that may lead to differential expression of IL-1β over time (115). In viral meningitis patients, the cerebrospinal fluid (CSF) concentration of IL-1β ranged from 20 to 700 pg/mL (3). This concentration is lower than the concentrations of IL-1β used for NSPC treatment in in vitro studies (29,45,66,131); however, CSF and local tissue concentrations may not be equivalent. Thus, further studies are needed to elucidate whether viral infections of the CNS generate enough IL-1β to significantly affect NSPC proliferation.
In addition to reducing NSPC proliferation, IL-1β induces differentiation of NSPCs into the glial lineage and/or restricts differentiation into the neuronal lineage in vitro (23,29,45,68,142). This is also supported by in vivo studies where inhibition or knockout of interleukin 1 receptor 1 inhibits the decrease in neurogenesis in models of WNV neuroinvasive disease (44) and acute stress (66). Mechanistically, IL-1β-mediated astrogliogenesis may occur through STAT3 signaling and the suppression of multiple proneural basic helix–loop–helix transcription factors (23,68). In addition, it appears that IL-1β activates the neurotoxic arm of the kynurenine pathway in differentiating NSPCs that reduces neurogenesis during IL-1β exposure (142). Ultimately, it is probable that IL-1β affects NSPC differentiation through multiple mechanisms, and further studies are needed to elucidate which pathways are activated during specific viral infections.
Interferon beta
Interferon beta (IFNβ) is a type I interferon that is secreted in response to viral infection, participates in the innate antiviral response, and induces interferon-stimulated genes that are key for viral control (105). IFNβ is induced in the CNS during many neurotropic infections, including WNV, La Crosse virus, HIV, and HSV-1 (9,33,70,130,136). IFNβ signaling is crucial for viral control in the brain for WNV and HSV-1 infections, and may be neuroprotective and limit viral replication during HIV infection of the CNS (9,10,70,119,130). HSV-infected NSPCs can produce IFNβ, suggesting that the NSPCs may have an autocrine response to IFNβ (114). Indeed, Wellen et al. observed the upregulation of the antiviral genes Myxovirus 1 (Mx1) and viperin in mouse NSPCs following IFNβ treatment (133). Although it is clear that type 1 interferons are produced during many viral infections and can even confer neuroprotection, few NSPC studies have examined protective or toxic roles for IFNβ during infections (119). Thus, here we consider studies that utilized IFNβ treatment of NSPCs in vitro.
The effect of IFNβ on NSPC proliferation and differentiation appears to be species dependent (Fig. 2A). IFNβ has been shown to inhibit or have no effect on mouse NSPC proliferation (51,76,133), but sustain human NSPC proliferation (6). These differences in proliferation are partially explained by distinct gene expression profiles during IFNβ treatment, where human NSPCs specifically upregulate genes related to cell growth and murine NSPCs do not (6,51). Similarly, studies with murine NSPCs suggest that IFNβ has no effect on overall NSPC differentiation (51,76,133), while Arscott et al. observed that IFNβ promoted differentiation in human NSPCs. An additional caveat to these comparisons is that the sole study with human NSPCs generally used higher concentrations of IFNβ than those studies with mouse NSPCs (6,51,76,133). Ultimately, further studies are needed to evaluate whether there are mechanistic differences in IFNβ signaling in NSPCs, and how such differences would impact NSPC function during a viral infection.
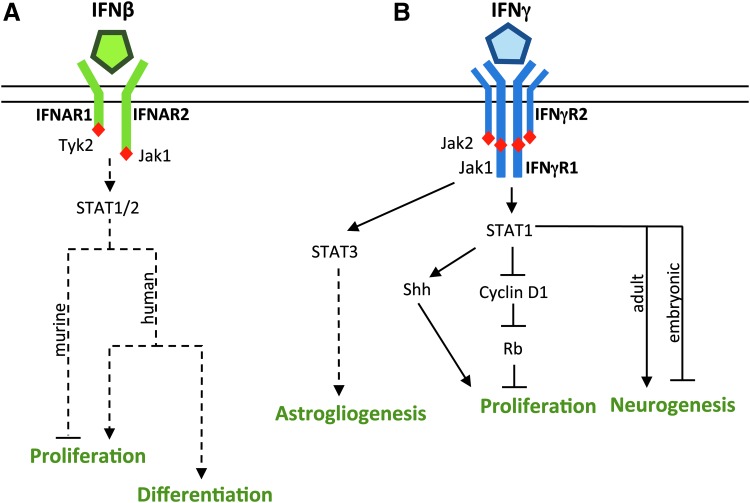
FIG. 2.
Effects of interferon on NSPC activity. (A) The effects of IFNβ on NSPC activity are species dependent. IFNβ generally inhibits proliferation in murine NSPCs, but sustains proliferation and promotes differentiation in human NSPCs. NSPCs express the interferon-α/β receptor (IFNAR), and IFNβ canonically signals through STAT1/2; however, the mechanism of the effects of IFNβ on NSPC activity has not been defined. (B) IFNγ affects NSPC activity predominantly through JAK-STAT signaling. IFNγ inhibits NSPC proliferation and restricts cell cycle progression through STAT1-dependent dephosphorylation of cyclin D1 and of the Rb, but can enhance proliferation through the Shh protein. The effect of IFNγ on neurogenesis is age dependent, with increased neurogenesis seen in adult NSPCs and reduced neurogenesis seen in embryonic NSPCs. IFNγ may induce gliogenesis through STAT3 signaling. Solid lines represent pathways that are defined in the literature, and dotted lines represent proposed pathways. JAK-STAT, Janus kinase-signal transducer and activators of transcription; Rb, retinoblastoma protein; Shh, sonic hedgehog.
Interferon gamma
Interferon gamma (IFNγ) is a pluripotent cytokine that is required for control of many neurotropic viruses in the brain including measles virus (MV), Theiler's virus, HSV, and Sindbis virus (20,91,92,100,109). IFNγ has well-defined roles in the modulation of immune cells and activation of antiviral genes in many neural cell types (19,26,88,90,108). However, it is less clear how IFNγ affects NSPC activity during a viral infection. IFNγ primarily signals through activation of the Janus kinase-signal transducer and activators of transcription (JAK/STAT) signaling pathway that also regulates proliferation and cell fate choice in NSPCs (38,47,128). Recent studies suggest that IFNγ may alter NSPC survival and behavior during viral infections, depending upon the model system and the cell types infected by the virus. During HSV-1 infection, activated CD8+ T cells release IFNγ that restricts growth of embryonic NSPCs and increases expression of astrocytic markers (55). In a model of neuron-restricted MV infection, IFNγ protected neonatal NSPCs and immature neurons from the cytotoxic effects of inflammation without altering neurogenesis (39). Thus, IFNγ may disrupt or protect NSPCs depending upon the type of viral infection and the age of the NSPCs.
When NSPCs are treated in vitro, IFNγ exerts predominately antiproliferative effects through activation of JAK/STAT1 signaling (Fig. 2B). IFNγ arrests the growth of embryonic and adult NSPCs derived from the SVZ (14,67,76,96,123,137). Consistent with these observations, NSPCs derived from mice lacking IFNγ or STAT1 show greater proliferation than wild-type NSPCs (67,72). In embryonic NSPCs, IFNγ restricts cell cycle progression through STAT1-dependent dephosphorylation of cyclin D1 and of the retinoblastoma protein (Rb), thus blocking cell progression at a late-stage G1/S-phase transition (67). Although most studies suggest that IFNγ restricts NSPC proliferation, studies in cerebellar NSPCs show that IFNγ can enhance proliferation through the sonic hedgehog (Shh) protein (112), suggesting that the impact of IFNγ on proliferation may depend on the brain region in which the NSPCs reside (112).
The effects of IFNγ on NSPC differentiation are less clear. In vitro and in vivo studies of adult murine NSPCs show that IFNγ induces neuronal differentiation in a STAT1-dependent manner (76,96,123,137). However, NSPCs derived from neonatal rat striatum do not exhibit any changes in differentiation in response to IFNγ (14). In contrast to many studies on adult NSPCs, embryonic NSPCs exhibit decreased neuronal differentiation in response to IFNγ (2). A potential explanation for these different outcomes in differentiation may be age-dependent regulation of STAT signaling in NSPCs. Other developmentally regulated cytokines, such as LIF and ciliary neurotropic factor, induce astrogliogenesis in NSPCs through STAT1 and STAT3 signaling. However, their ability to induce glial differentiation is limited in early embryonic stages, when neurogenesis dominates and glial gene expression is inhibited at the epigenetic level (16,47). Another possibility is that IFNγ acts synergistically with other developmental cytokines to drive glial differentiation in younger NSPCs, whereas IFNγ induces neurogenesis in adult NSPCs that have more limited potency. In the context of a viral infection, the inappropriate activation of STAT signaling by IFNγ could interrupt neurogenesis in the embryonic or neonatal CNS that could have long-term neurodevelopmental consequences on cortical growth or hippocampal structure. Regardless, these studies suggest that effects of IFNγ on NSPC differentiation are at least partially age dependent.
Chemokines
C-X-C motif chemokine ligand 10
Chemokines such as C-X-C motif chemokine ligand 10 (CXCL10), which is also known as IFNγ-induced protein 10, are secreted by resident CNS cells and are instrumental for leukocyte trafficking into the brain (54). CXCL10 secretion by neurons, glia, and NSPCs is induced by cytokines such as TNFα and IFNγ (84,107). CXCL10 acts primarily through the C-X-C motif chemokine receptor 3 (CXCR3) receptor, which is found on activated T cells and natural killer cells as well as neurons, astrocytes, and microglia (15,84,139). CXCL10 expression is seen in many models of CNS infection, including MV, rabies virus, WNV, and HSV-1 (21,43,64,78). In a murine model of MV infection, CXCL10 is the most highly induced cytokine in neonatal and adult brains (43). In a model of WNV encephalitis and rabies virus infection, CXCL10 is important for T cell recruitment and control of viral replication (21,64). In addition to a well-established role in immune cell recruitment, CXCL10 expression is also associated with reduced neuroblast numbers in an in vivo model of LCMV infection, suggesting that CXCL10 may have direct or indirect effects on NSPC function (113). NSPCs express the CXCR3 receptor and follow a CXCL10 gradient in vitro, suggesting that NSPCs may use CXCL10 to home regions of neuronal damage during CNS infection or injury (120,121).
In addition to effects on NSPC migration, CXCL10 may affect NSPC survival. CXCL10 treatment of a rat NSPC line induced extracellular regulate kinase (ERK)-1/2 phosphorylation, and chronic CXCL10 exposure in neurons induces ERK-1/2 activation and the expression of the antiapoptotic proteins Bcl2 and SOD2 (8,53). ERK-1/2 signaling is generally neuroprotective and thus CXCL10 stimulation may protect NSPCs from apoptosis (49). CXCL10 also reduces differentiation of pluripotent stem cells and oligodendrocyte precursor cells, suggesting that CXCL10 may maintain pluripotency (59,81). In the context of viral infections where CXCL10 is highly expressed in the brain, one possibility is that NSPCs migrate toward areas of infection or damage but are protected by prosurvival signals from the CXCL10 expression. Such a scenario would provide a pool of NSPCs near brain regions where repair of damaged neurons or modulation of infiltrating immune cells may be beneficial for the host. Although further studies would be needed to establish whether CXCL10 induces migration in vivo, the responsiveness of stem cells to CXCL10 suggests that NSPCs are likely to be sensitive to its upregulation during infection.
C-C motif chemokine ligand 2
C-C motif chemokine ligand 2 (CCL2), also known as monocyte chemoattractant protein-1, is a chemokine expressed in the brain in inflammatory conditions (31,140), including viral infections such as JEV, MV, WNV, and neurotropic mouse hepatitis virus (11,43,71,82), where it primarily acts as a chemoattractant for monocytes (11,48). Although there are not yet studies that investigate the effects of CCL2 on NSPCs in the context of viral infection, there are studies assessing the role of CCL2 on NSPC activity. CCL2 increases neurogenesis in adult murine NSPCs from the SVZ (123). During glial differentiation of NSPCs, it was observed that there was an upregulation of CCL2 that occurred through the NF-κB pathway (77). CCL2 also induces migration of rat hippocampal NSPCs but does not induce significant migration of NSPCs from the SVZ at the same concentration, suggesting that there are region-specific differences in NSPC responses to chemokines (134). If considered in the context of high CXCL10 expression during viral infections, it is conceivable that gradients of different chemokines will differentially attract NSPCs from distinct niches in the brain. Whether such a scenario could have an impact on repair or upon the type of neurons that are produced by infiltrating NSPCs is a question for future study.
Conclusions
Viral infections can profoundly impact brain function, whether it is through perturbations in developmental processes or irreparable damage to existing neural cells. NSPCs produce many of the cells in the developing brain and also provide new neural cells in response to physiological and pathological stimuli. A growing body of research demonstrates that NSPCs are responsive to cytokines and chemokines produced during viral infections in addition to being cellular targets for some neurotropic viruses. Although many of these cytokines and chemokines have been studied independently, it is likely that the outcomes for the NSPCs will depend on the cumulative effects of the inflammatory milieu that bears further study in in vivo models of infection.
Although cytokines can have different effects on NSPC proliferation depending on the model, our overall observations are that most antiviral cytokines inhibit NSPC proliferation. Given that NSPCs support replication of many CNS viruses, it is conceivable that limiting proliferation of these cells could also limit viral replication, as viruses generally prefer cells that are actively dividing. Furthermore, many of these antiviral cytokines limit neurogenesis or enhance gliogenosis. Immature neurons are especially susceptible to the neurotoxic effects of inflammation, and many viruses spread more readily in immature neurons than in fully differentiated neurons. A transient reduction in the pool of newly born neurons may be beneficial to the host in terms of avoiding neuronal death and limiting viral spread. As with any inflammatory condition, the immune system must strike a balance between preserving the host cell tissue and creating an unfavorable environment for the virus. If inflammatory mediators were to act at a sensitive window in brain development or for a prolonged period during a chronic infection, alterations in NSPC function may lead to long-term pathological consequences for the host. Evaluating the impact of the initial infection and successive immunological milestones on NSPC function, particularly in the developing brain, may provide a foundation for future therapeutics.
Acknowledgments
The authors are funded by the Duquesne University School of Pharmacy (M.N.C., P.S.C., L.A.O.) and R15-NS087606-01A1 (L.A.O.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Abassi M, Morawski BM, Nakigozi G, et al. . Cerebrospinal fluid biomarkers and HIV-associated neurocognitive disorders in HIV-infected individuals in Rakai, Uganda. J Neurovirol 2017;23:369–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahn J, Lee J, and Kim S. Interferon-gamma inhibits the neuronal differentiation of neural progenitor cells by inhibiting the expression of Neurogenin2 via the JAK/STAT1 pathway. Biochem Biophys Res Commun 2015;466:52–59 [DOI] [PubMed] [Google Scholar]
- 3. Akalin H, Akdiş AC, Mistik R, et al. . Cerebrospinal fluid interleukin-1β/interleukin-1 receptor antagonist balance and tumor necrosis factor-α concentrations in tuberculous, viral and acute bacterial meningitis. Scand J Infect Dis 1994;26:667–674 [DOI] [PubMed] [Google Scholar]
- 4. Alcendor DJ, Charest AM, Zhu WQ, et al. . Infection and upregulation of proinflammatory cytokines in human brain vascular pericytes by human cytomegalovirus. J Neuroinflammation 2012;9:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ariff IM, Thounaojam MC, Das S, et al. . Japanese encephalitis virus infection alters both neuronal and astrocytic differentiation of neural stem/progenitor cells. J Neuroimmune Pharmacol 2013;8:664–676 [DOI] [PubMed] [Google Scholar]
- 6. Arscott WT, Soltys J, Knight J, et al. . Interferon β-1b directly modulates human neural stem/progenitor cell fate. Brain Res 2011;1413:1–8 [DOI] [PubMed] [Google Scholar]
- 7. Babri S, Doosti M-H, and Salari A-A. Tumor necrosis factor-alpha during neonatal brain development affects anxiety- and depression-related behaviors in adult male and female mice. Behav Brain Res 2014;261:305–314 [DOI] [PubMed] [Google Scholar]
- 8. Bajova H, Nelson TE, and Gruol DL. Chronic CXCL10 alters the level of activated ERK1/2 and transcriptional factors CREB and NF-kB in hippocampal neuronal cell culture. J Neuroimmunol 2008;195:36–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barber SA, Gama L, Dudaronek JM, et al. . Mechanism for the establishment of transcriptional HIV latency in the brain in a simian immunodeficiency virus–macaque model. J Infect Dis 2006;193:963–970 [DOI] [PubMed] [Google Scholar]
- 10. Barber SA, Herbst DS, Bullock BT, et al. . Innate immune responses and control of acute simian immunodeficiency virus replication in the central nervous system. J Neurovirol 2004;10:15–20 [DOI] [PubMed] [Google Scholar]
- 11. Bardina SV, Michlmayr D, Hoffman KW, et al. . Differential roles of chemokines CCL2 and CCL7 in monocytosis and leukocyte migration during West Nile virus infection. J Immunol 2015;195:4306–4318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barkho BZ, Song H, Aimone JB, et al. . Identification of astrocyte-expressed factors that modulate neural stem/progenitor cell differentiation. Stem Cells Dev 2006;15:407–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bayless NL, Greenberg RS, Swigut T, et al. . Zika virus infection induces cranial neural crest cells to produce cytokines at levels detrimental for neurogenesis. Cell Host Microbe 2016;20:423–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ben-Hur T, Ben-Menachem O, Furer V, et al. . Effects of proinflammatory cytokines on the growth, fate, and motility of multipotential neural precursor cells. Mol Cell Neurosci 2003;24:623–631 [DOI] [PubMed] [Google Scholar]
- 15. Biber K, Dijkstra I, Trebst C, et al. . Functional expression of CXCR3 in cultured mouse and human astrocytes and microglia. Neuroscience 2002;112:487–497 [DOI] [PubMed] [Google Scholar]
- 16. Bonni A, Sun Y, Nadal-Vicens M, et al. . Regulation of gliogenesis in the central nervous system by the JAK-STAT signaling pathway. Science 1997;278:477–483 [DOI] [PubMed] [Google Scholar]
- 17. Bonthius DJ, and Perlman S. Congenital viral infections of the brain: lessons learned from lymphocytic choriomeningitis virus in the neonatal rat. PLoS Pathog 2007;3:e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brnic D, Stevanovic V, Cochet M, et al. . Borna disease virus infects human neural progenitor cells and impairs neurogenesis. J Virol 2012;86:2512–2522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Burdeinick-Kerr R, Govindarajan D, and Griffin DE. Noncytolytic clearance of sindbis virus infection from neurons by gamma interferon is dependent on Jak/STAT signaling. J Virol 2009;83:3429–3435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Burdeinick-Kerr R, and Griffin DE. Gamma interferon-dependent, noncytolytic clearance of sindbis virus infection from neurons in vitro. J Virol 2005;79:5374–5385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chai Q, She R, Huang Y, et al. . Expression of neuronal CXCL10 induced by rabies virus infection initiates infiltration of inflammatory cells, production of chemokines and cytokines, and enhancement of blood-brain barrier permeability. J Virol 2015;89:870–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cheeran MC-J, Hu S, Yager SL, et al. . Cytomegalovirus induces cytokine and chemokine production differentially in microglia and astrocytes: antiviral implications. J Neurovirol 2001;7:135–147 [DOI] [PubMed] [Google Scholar]
- 23. Chen E, Xu D, Lan X, et al. . A novel role of the STAT3 pathway in brain inflammation-induced human neural progenitor cell differentiation. Curr Mol Med 2013;13:1474–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen L, Coleman R, Leang R, et al. . Human neural precursor cells promote neurologic recovery in a viral model of multiple sclerosis. Stem Cell Reports 2014;2:825–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen N-N, Wei F, Wang L, et al. . Tumor necrosis factor alpha induces neural stem cell apoptosis through activating p38 MAPK pathway. Neurochem Res 2016;41:3052–3062 [DOI] [PubMed] [Google Scholar]
- 26. Chesler DA, and Reiss CS. The role of IFN-gamma in immune responses to viral infections of the central nervous system. Cytokine Growth Factor Rev 2002;13:441–454 [DOI] [PubMed] [Google Scholar]
- 27. Chucair-Elliott AJ, Conrady C, Zheng M, et al. . Microglia-induced IL-6 protects against neuronal loss following HSV-1 infection of neural progenitor cells. Glia 2014;62:1418–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Corasaniti MT, Bilotta A, Strongoli MC, et al. . HIV-1 coat protein gp120 stimulates interleukin-1β secretion from human neuroblastoma cells: evidence for a role in the mechanism of cell death. Br J Pharmacol 2001;134:1344–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Crampton SJ, Collins LM, Toulouse A, et al. . Exposure of foetal neural progenitor cells to IL-1β impairs their proliferation and alters their differentiation—a role for maternal inflammation? J Neurochem 2011;120:964–973 [DOI] [PubMed] [Google Scholar]
- 30. Daley JK, Gechman LA, Skipworth J, et al. . Poliovirus replication and spread in primary neuron cultures. Virology 2005;340:10–20 [DOI] [PubMed] [Google Scholar]
- 31. Daly C, and Rollins BJ. Monocyte chemoattractant protein-1 (CCL2) in inflammatory disease and adaptive immunity: therapeutic opportunities and controversies. Microcirculation 2003;10:247–257 [DOI] [PubMed] [Google Scholar]
- 32. Das S, and Basu A. Viral infection and neural stem/progenitor cell's fate: implications in brain development and neurological disorders. Neurochem Int 2011;59:357–366 [DOI] [PubMed] [Google Scholar]
- 33. Delhaye S, Paul S, Blakqori G, et al. . Neurons produce type I interferon during viral encephalitis. Proc Natl Acad Sci U S A 2006;103:7835–7840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Deverman BE, and Patterson PH. Cytokines and CNS development. Neuron 2009;64:61–78 [DOI] [PubMed] [Google Scholar]
- 35. Donegan JJ, Girotti M, Weinberg MS, et al. . A novel role for brain interleukin-6: facilitation of cognitive flexibility in rat orbitofrontal cortex. J Neurosci 2014;34:953–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dupret D, Revest JM, Koehl M, et al. . Spatial relational memory requires hippocampal adult neurogenesis. PLoS One 2008;3:e1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Durrant DM, Robinette ML, and Klein RS. IL-1R1 is required for dendritic cell-mediated T cell reactivation within the CNS during West Nile virus encephalitis. J Exp Med 2013;210:503–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fan G, Martinowich K, Chin MH, et al. . DNA methylation controls the timing of astrogliogenesis through regulation of JAK-STAT signaling. Development 2005;132:3345–3356 [DOI] [PubMed] [Google Scholar]
- 39. Fantetti KN, Gray EL, Ganesan P, et al. . Interferon gamma protects neonatal neural stem/progenitor cells during measles virus infection of the brain. J Neuroinflammation 2016;13:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fazakerley JK, and Allsopp TE. Programmed cell death in virus infections of the nervous system. Curr Top Microbiol Immunol 2001;253:95–119 [DOI] [PubMed] [Google Scholar]
- 41. Gabel S, Koncina E, Dorban G, et al. . Inflammation promotes a conversion of astrocytes into neural progenitor cells via NF-κB activation. Mol Neurobiol 2016;53:5041–5055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gallagher D, Norman AA, Woodard CL, et al. . Transient maternal IL-6 mediates long-lasting changes in neural stem cell pools by deregulating an endogenous self-renewal pathway. Cell Stem Cell 2013;13:564–576 [DOI] [PubMed] [Google Scholar]
- 43. Ganesan P, Chandwani MN, Creisher PS, et al. . The neonatal anti-viral response fails to control measles virus spread in neurons despite interferon-gamma expression and a Th1-like cytokine profile. J Neuroimmunol 2018;316:80–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Garber C, Vasek MJ, Vollmer LL, et al. . Astrocytes decrease adult neurogenesis during virus-induced memory dysfunction via IL-1. Nat Immunol 2018;19:151–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Green HF, Treacy E, Keohane AK, et al. . A role for interleukin-1β in determining the lineage fate of embryonic rat hippocampal neural precursor cells. Mol Cell Neurosci 2012;49:311–321 [DOI] [PubMed] [Google Scholar]
- 46. Guadagno J, Swan P, Shaikh R, et al. . Microglia-derived IL-1β triggers p53-mediated cell cycle arrest and apoptosis in neural precursor cells. Cell Death Dis 2015;6:e1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. He F, Ge W, Martinowich K, et al. . A positive autoregulatory loop of Jak-STAT signaling controls the onset of astrogliogenesis. Nat Neurosci 2005;8:616–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Held KS, Chen BP, Kuziel WA, et al. . Differential roles of CCL2 and CCR2 in host defense to coronavirus infection. Virology 2004;329:251–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hetman M, and Gozdz A. Role of extracellular signal regulated kinases 1 and 2 in neuronal survival. Eur J Biochem 2004;271:2050–2055 [DOI] [PubMed] [Google Scholar]
- 50. Heyser CJ, Masliah E, Samimi A, et al. . Progressive decline in avoidance learning paralleled by inflammatory neurodegeneration in transgenic mice expressing interleukin 6 in the brain. Proc Natl Acad Sci U S A 1997;94:1500–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hirsch M, Knight J, Tobita M, et al. . The effect of interferon-β on mouse neural progenitor cell survival and differentiation. Biochem Biophys Res Commun 2009;388:181–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Homem CC, Repic M, and Knoblich JA. Proliferation control in neural stem and progenitor cells. Nat Rev Neurosci 2015;16:647–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Honeth G, Staflin K, Kalliomäki S, et al. . Chemokine-directed migration of tumor-inhibitory neural progenitor cells towards an intracranially growing glioma. Exp Cell Res 2006;312:1265–1276 [DOI] [PubMed] [Google Scholar]
- 54. Hosking MP, and Lane TE. The role of chemokines during viral infection of the CNS. PLoS Pathog 2010;6:e1000937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hu S, Rotschafer JH, Lokensgard JR, et al. . Activated CD8+ T lymphocytes inhibit neural stem/progenitor cell proliferation: role of interferon-gamma. PLoS One 2014;9:e105219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Iosif RE, Ekdahl CT, Ahlenius H, et al. . Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J Neurosci 2006;26:9703–9712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Islam O, Gong X, Rose-John S, et al. . Interleukin-6 and neural stem cells: more than gliogenesis. Mol Biol Cell 2009;20:188–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jafari M, Haist V, Baumgartner W, et al. . Impact of Theiler's virus infection on hippocampal neuronal progenitor cells: differential effects in two mouse strains. Neuropathol Appl Neurobiol 2012;38:647–664 [DOI] [PubMed] [Google Scholar]
- 59. Jiang Z, Li Y, Ji X, et al. . Protein profiling identified key chemokines that regulate the maintenance of human pluripotent stem cells. Sci Rep 2017;7:14510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jisun O, McCloskey MA, Blong CC, et al. . Astrocyte-derived interleukin-6 promotes specific neuronal differentiation of neural progenitor cells from adult hippocampus. J Neurosci Res 2010;88:2798–2809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kempermann G, and Gage FH. Neurogenesis in the adult hippocampus. Novartis Found Symp 2000;231:220–235 ; discussion 235–241, 302–306 [PubMed] [Google Scholar]
- 62. Keohane A, Ryan S, Maloney E, et al. . Tumour necrosis factor-α impairs neuronal differentiation but not proliferation of hippocampal neural precursor cells: role of Hes1. Mol Cell Neurosci 2010;43:127–135 [DOI] [PubMed] [Google Scholar]
- 63. Kitamura T, Saitoh Y, Takashima N, et al. . Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell 2009;139:814–827 [DOI] [PubMed] [Google Scholar]
- 64. Klein RS, Lin E, Zhang B, et al. . Neuronal CXCL10 directs CD8(+) T-cell recruitment and control of West Nile virus encephalitis. J Virol 2005;79:11457–11466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Knight J, Hackett C, Breton J, et al. . Cross-talk between CD4+ T-cells and neural stem/progenitor cells. J Neurol Sci 2011;306:121–128 [DOI] [PubMed] [Google Scholar]
- 66. Koo JW, and Duman RS. IL-1β is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A 2008;105:751–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kulkarni A, Scully TJ, and O'Donnell LA. The antiviral cytokine interferon-gamma restricts neural stem/progenitor cell proliferation through activation of STAT1 and modulation of retinoblastoma protein phosphorylation. J Neurosci Res 2017;95:1582–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kuzumaki N, Ikegami D, Imai S, et al. . Enhanced IL-1β production in response to the activation of hippocampal glial cells impairs neurogenesis in aged mice. Synapse 2010;64:721–728 [DOI] [PubMed] [Google Scholar]
- 69. Lan X, Chen Q, Wang Y, et al. . TNF-α affects human cortical neural progenitor cell differentiation through the autocrine secretion of leukemia inhibitory factor. PLoS One 2012;7:e50783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lazear HM, Pinto AK, Vogt MR, et al. . Beta interferon controls West Nile virus infection and pathogenesis in mice. J Virol 2011;85:7186–7194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Li F, Wang Y, Yu L, et al. . Viral infection of the central nervous system and neuroinflammation precede blood-brain barrier disruption during Japanese encephalitis virus infection. J Virol 2015;89:5602–5614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Li L, Walker TL, Zhang Y, et al. . Endogenous interferon gamma directly regulates neural precursors in the non-inflammatory brain. J Neurosci 2010;30:9038–9050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Liliana B, Fabienne A, Bruno S, et al. . Tumor necrosis factor-α modulates survival, proliferation, and neuronal differentiation in neonatal subventricular zone cell cultures. Stem Cells 2008;26:2361–2371 [DOI] [PubMed] [Google Scholar]
- 74. Lokensgard JR, Hu S, Sheng W, et al. . Robust expression of TNF-α, IL-1β, RANTES, and IP-10 by human microglial cells during nonproductive infection with herpes simplex virus. J Neurovirol 2001;7:208–219 [DOI] [PubMed] [Google Scholar]
- 75. Lu P, Jones LL, Snyder EY, et al. . Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol 2003;181:115–129 [DOI] [PubMed] [Google Scholar]
- 76. Lum M, Croze E, Wagner C, et al. . Inhibition of neurosphere proliferation by IFNgamma but not IFNbeta is coupled to neuronal differentiation. J Neuroimmunol 2009;206:32–38 [DOI] [PubMed] [Google Scholar]
- 77. Lawrence DM, Seth P, Durham L, et al. . Astrocyte differentiation selectively upregulates CCL2/monocyte chemoattractant protein-1 in cultured human brain-derived progenitor cells. Glia 2006;53:81–91 [DOI] [PubMed] [Google Scholar]
- 78. Marques CP, Hu S, Sheng W, et al. . Microglial cells initiate vigorous yet non-protective immune responses during HSV-1 brain infection. Virus Res 2006;121:1–10 [DOI] [PubMed] [Google Scholar]
- 79. Masaya N, Tetsuhiro N, Satoru M, et al. . Microglia-derived interleukin-6 and leukaemia inhibitory factor promote astrocytic differentiation of neural stem/progenitor cells. Eur J Neurosci 2007;25:649–658 [DOI] [PubMed] [Google Scholar]
- 80. Ming GL, and Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 2011;70:687–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Moore CS, Cui Q-L, Warsi NM, et al. . Direct and indirect effects of immune and central nervous system—resident cells on human oligodendrocyte progenitor cell differentiation. J Immunol 2015;194:761. [DOI] [PubMed] [Google Scholar]
- 82. Mosher KI, Andres RH, Fukuhara T, et al. . Neural progenitor cells regulate microglia functions and activity. Nat Neurosci 2012;15:1485–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mukherjee S, Singh N, Sengupta N, et al. . Japanese encephalitis virus induces human neural stem/progenitor cell death by elevating GRP78, PHB and hnRNPC through ER stress. Cell Death Dis 2017;8:e2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Müller M, Carter S, Hofer MJ, et al. . Review: the chemokine receptor CXCR3 and its ligands CXCL9, CXCL10 and CXCL11 in neuroimmunity—a tale of conflict and conundrum. Neuropathol Appl Neurobiol 2010;36:368–387 [DOI] [PubMed] [Google Scholar]
- 85. Mutnal MB, Cheeran MC, Hu S, et al. . Murine cytomegalovirus infection of neural stem cells alters neurogenesis in the developing brain. PLoS One 2011;6:e16211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Nargi-Aizenman JL, and Griffin DE. Sindbis virus-induced neuronal death is both necrotic and apoptotic and is ameliorated by N-methyl-d-aspartate receptor antagonists. J Virol 2001;75:7114–7121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Nitkiewicz J, Borjabad A, Morgello S, et al. . HIV induces expression of complement component C3 in astrocytes by NF-κB-dependent activation of interleukin-6 synthesis. J Neuroinflammation 2017;14:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. O'Donnell LA, Conway S, Rose RW, et al. . STAT1-independent control of a neurotropic measles virus challenge in primary neurons and infected mice. J Immunol 2012;188:1915–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Palomo J, Dietrich D, Martin P, et al. . The interleukin (IL)-1 cytokine family—balance between agonists and antagonists in inflammatory diseases. Cytokine 2015;76:25–37 [DOI] [PubMed] [Google Scholar]
- 90. Parra B, Hinton DR, Marten NW, et al. . IFN-gamma is required for viral clearance from central nervous system oligodendroglia. J Immunol 1999;162:1641–1647 [PubMed] [Google Scholar]
- 91. Patterson CE, Daley JK, Echols LA, et al. . Measles virus infection induces chemokine synthesis by neurons. J Immunol 2003;171:3102–3109 [DOI] [PubMed] [Google Scholar]
- 92. Patterson CE, Lawrence DM, Echols LA, et al. . Immune-mediated protection from measles virus-induced central nervous system disease is noncytolytic and gamma interferon dependent. J Virol 2002;76:4497–4506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Pencea V, Bingaman KD, Freedman LJ, et al. . Neurogenesis in the subventricular zone and rostral migratory stream of the neonatal and adult primate forebrain. Exp Neurol 2001;172:1–16 [DOI] [PubMed] [Google Scholar]
- 94. Pencea V, Bingaman KD, Wiegand SJ, et al. . Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci 2001;21:6706–6717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Peng HUI, Whitney N, Wu Y, et al. . HIV-1-infected and/or immune-activated macrophage-secreted TNF-α affects human fetal cortical neural progenitor cell proliferation and differentiation. Glia 2008;56:903–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Pereira L, Medina R, Baena M, et al. . IFN gamma regulates proliferation and neuronal differentiation by STAT1 in adult SVZ niche. Front Cell Neurosci 2015;9:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Perry SW, Dewhurst S, Bellizzi MJ, et al. . Tumor necrosis factor-alpha in normal and diseased brain: conflicting effects via intraneuronal receptor crosstalk? J Neurovirol 2002;8:611–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Puccini JM, Ruller CM, Robinson SM, et al. . Distinct neural stem cell tropism, early immune activation, and choroid plexus pathology following coxsackievirus infection in the neonatal central nervous system. Lab Invest 2014;94:161–181 [DOI] [PubMed] [Google Scholar]
- 99. Ramos HJ, Lanteri MC, Blahnik G, et al. . IL-1β signaling promotes CNS-intrinsic immune control of West Nile virus infection. PLoS Pathog 2012;8:e1003039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Rodriguez M, Zoecklein LJ, Howe CL, et al. . Gamma interferon is critical for neuronal viral clearance and protection in a susceptible mouse strain following early intracranial Theiler's murine encephalomyelitis virus infection. J Virol 2003;77:12252–12265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ruller CM, Tabor-Godwin JM, Van Deren DA Jr., et al. . Neural stem cell depletion and CNS developmental defects after enteroviral infection. Am J Pathol 2012;180:1107–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ryan SM, O'Keeffe GW, O'Connor C, et al. . Negative regulation of TLX by IL-1β correlates with an inhibition of adult hippocampal neural precursor cell proliferation. Brain Behav Immun 2013;33:7–13 [DOI] [PubMed] [Google Scholar]
- 103. Saga J, Jack P, and Michel M. Effect of inflammatory cytokines on major histocompatibility complex expression and differentiation of human neural stem/progenitor cells. Stem Cells 2008;26:2444–2454 [DOI] [PubMed] [Google Scholar]
- 104. Seleme MC, Kosmac K, Jonjic S, et al. . Tumor necrosis factor alpha-induced recruitment of inflammatory mononuclear cells leads to inflammation and altered brain development in murine cytomegalovirus-infected newborn mice. J Virol 2017;91:e01983-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Sen GC. Viruses and interferons. Annu Rev Microbiol 2001;55:255–281 [DOI] [PubMed] [Google Scholar]
- 106. Sergerie Y, Rivest S, and Boivin G. Tumor necrosis factor-α and interleukin-1β play a critical role in the resistance against lethal herpes simplex virus encephalitis. J Infect Dis 2007;196:853–860 [DOI] [PubMed] [Google Scholar]
- 107. Sheng Wen S, Hu S, Ni Hsiao T, et al. . TNF-α-induced chemokine production and apoptosis in human neural precursor cells. J Leukoc Biol 2005;78:1233–1241 [DOI] [PubMed] [Google Scholar]
- 108. Shrestha B, Wang T, Samuel MA, et al. . Gamma interferon plays a crucial early antiviral role in protection against West Nile virus infection. J Virol 2006;80:5338–5348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Smith PM, Wolcott RM, Chervenak R, et al. . Control of acute cutaneous herpes simplex virus infection: T cell-mediated viral clearance is dependent upon interferon-gamma (IFN-gamma). Virology 1994;202:76–88 [DOI] [PubMed] [Google Scholar]
- 110. Stevens HE, Smith KM, Rash BG, et al. . Neural stem cell regulation, fibroblast growth factors, and the developmental origins of neuropsychiatric disorders. Front Neurosci 2010;4: pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Storer MA, Gallagher D, Fatt MP, et al. . Interleukin-6 regulates adult neural stem cell numbers during normal and abnormal post-natal development. Stem Cell Reports 2018;10:1464–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Sun L, Tian Z, and Wang J. A direct cross-talk between interferon-gamma and sonic hedgehog signaling that leads to the proliferation of neuronal precursor cells. Brain Behav Immun 2010;24:220–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Sun T, Vasek MJ, and Klein RS. Congenitally acquired persistent lymphocytic choriomeningitis viral infection reduces neuronal progenitor pools in the adult hippocampus and subventricular zone. PLoS One 2014;9:e96442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Sun X, Shi L, Zhang H, et al. . Effects of toll-like receptor 3 on herpes simplex virus type-1-infected mouse neural stem cells. Can J Microbiol 2014;61:201–208 [DOI] [PubMed] [Google Scholar]
- 115. Swanson PA, and McGavern DB. Viral diseases of the central nervous system. Curr Opin Virol 2015;11:44–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Swarup V, Das S, Ghosh S, et al. . Tumor necrosis factor receptor-1-induced neuronal death by TRADD contributes to the pathogenesis of Japanese encephalitis. J Neurochem 2007;103:771–783 [DOI] [PubMed] [Google Scholar]
- 117. Tang H, Hammack C, Ogden SC, et al. . Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell 2016;18:587–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Temple S. The development of neural stem cells. Nature 2001;414:112–117 [DOI] [PubMed] [Google Scholar]
- 119. Thaney VE, O'Neill AM, Hoefer MM, et al. . IFNbeta protects neurons from damage in a murine model of HIV-1 associated brain injury. Sci Rep 2017;7:46514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Tran PB, Banisadr G, Ren D, et al. . Chemokine receptor expression by neural progenitor cells in neurogenic regions of mouse brain. J Comp Neurol 2007;500:1007–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Tran PB, Ren D, Veldhouse TJ, et al. . Chemokine receptors are expressed widely by embryonic and adult neural progenitor cells. J Neurosci Res 2004;76:20–34 [DOI] [PubMed] [Google Scholar]
- 122. Tun MMN, Aoki K, Senba M, et al. . Protective role of TNF-α, IL-10 and IL-2 in mice infected with the Oshima strain of Tick-borne encephalitis virus. Sci Rep 2014;4:5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Turbic A, Leong SY, and Turnley AM. Chemokines and inflammatory mediators interact to regulate adult murine neural precursor cell proliferation, survival and differentiation. PLoS One 2011;6:e25406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. van Praag H, Shubert T, Zhao C, et al. . Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci 2005;25:8680–8685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Venkatesan A, Nath A, Ming GL, et al. . Adult hippocampal neurogenesis: regulation by HIV and drugs of abuse. Cell Mol Life Sci 2007;64:2120–2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Vivar C, Potter MC, Choi J, et al. . Monosynaptic inputs to new neurons in the dentate gyrus. Nat Commun 2012;3:1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. von Ruden EL, Avemary J, Zellinger C, et al. . Distemper virus encephalitis exerts detrimental effects on hippocampal neurogenesis. Neuropathol Appl Neurobiol 2012;38:426–442 [DOI] [PubMed] [Google Scholar]
- 128. Walter J, and Dihne M. Species-dependent differences of embryonic stem cell-derived neural stem cells after Interferon gamma treatment. Front Cell Neurosci 2012;6:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Wang J, Liu J, Zhou R, et al. . Zika virus infected primary microglia impairs NPCs proliferation and differentiation. Biochem Biophys Res Commun 2018;497:619–625 [DOI] [PubMed] [Google Scholar]
- 130. Wang JP, Bowen GN, Zhou S, et al. . Role of specific innate immune responses in herpes simplex virus infection of the central nervous system. J Virol 2012;86:2273–2281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Wang X, Fu S, Wang Y, et al. . Interleukin-1β mediates proliferation and differentiation of multipotent neural precursor cells through the activation of SAPK/JNK pathway. Mol Cell Neurosci 2007;36:343–354 [DOI] [PubMed] [Google Scholar]
- 132. Wei H, Chadman KK, McCloskey DP, et al. . Brain IL-6 elevation causes neuronal circuitry imbalances and mediates autism-like behaviors. Biochim Biophys Acta 2012;1822:831–842 [DOI] [PubMed] [Google Scholar]
- 133. Wellen J, Walter J, Jangouk P, et al. . Neural precursor cells as a novel target for interferon-beta. Neuropharmacology 2009;56:386–398 [DOI] [PubMed] [Google Scholar]
- 134. Widera D, Holtkamp W, Entschladen F, et al. . MCP-1 induces migration of adult neural stem cells. Eur J Cell Biol 2004;83:381–387 [DOI] [PubMed] [Google Scholar]
- 135. Widera D, Mikenberg I, Elvers M, et al. . Tumor necrosis factor α triggers proliferation of adult neural stem cells via IKK/NF-κB signaling. BMC Neurosci 2006;7:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Winkelmann ER, Luo H, and Wang T. West Nile virus infection in the central nervous system. F1000Res 2016;5:F1000 Faculty Rev-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Wong G, Goldshmit Y, and Turnley AM. Interferon-gamma but not TNF alpha promotes neuronal differentiation and neurite outgrowth of murine adult neural stem cells. Exp Neurol 2004;187:171–177 [DOI] [PubMed] [Google Scholar]
- 138. Wu J-P, Kuo J-S, Liu Y-L, et al. . Tumor necrosis factor-alpha modulates the proliferation of neural progenitors in the subventricular/ventricular zone of adult rat brain. Neurosci Lett 2000;292:203–206 [DOI] [PubMed] [Google Scholar]
- 139. Xia MQ, Bacskai BJ, Knowles RB, et al. . Expression of the chemokine receptor CXCR3 on neurons and the elevated expression of its ligand IP-10 in reactive astrocytes: in vitro ERK1/2 activation and role in Alzheimer's disease. J Neuroimmunol 2000;108:227–235 [DOI] [PubMed] [Google Scholar]
- 140. Yao Y, and Tsirka SE. Monocyte chemoattractant protein-1 and blood-brain barrier. Cell Mol Life Sci 2014;71:683–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Zahr NM. The aging brain with HIV infection: effects of alcoholism or hepatitis C comorbidity. Front Aging Neurosci 2018;10:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Zunszain PA, Anacker C, Cattaneo A, et al. . Interleukin-1β: a new regulator of the kynurenine pathway affecting human hippocampal neurogenesis. Neuropsychopharmacology 2012;37:939–949 [DOI] [PMC free article] [PubMed] [Google Scholar]