Abstract
An outbreak of methicillin-resistant Staphylococcus aureus (MRSA) infections on the skin and soft tissues of experimental macaques in the vivarium of The Rockefeller University, New York, triggered this observational and interventional study. We screened 14 macaques in the colony (samples from head, nares, and rectum) and their housing (40 environmental surfaces) four times in 1 year, for S. aureus colonization or contamination, while implementing enhanced decolonization and decontamination procedures. A total of 114 isolates of S. aureus were recovered and characterized (antibiograms, spa typing, multilocus sequence typing, pulsed-field gel electrophoresis [PFGE], mecA, Panton–Valentine Leukocidin, and arginine catabolic mobile element). Based on these results, six strains of S. aureus were identified: two MRSA strains (t16708/ST3862/PFGE-A, t16709/ST3862/PFGE-C) and one methicillin-sensitive S. aureus (t8397/ST3884/PFGE-D) were characterized for the first time in this study; strains belonging to spa types t189 and t4167 have been identified in primates in previous studies. None of these strains was common to the neighboring New York City human community. Thus, it seems probable that the animals were already colonized upon arrival to the University. We suggest screening primates for S. aureus carriage upon arrival to University vivaria and possible implementation of extensive decolonization procedures before any surgical interventions.
Keywords: Staphylococcus aureus, veterinary infections, MRSA
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) is a major human pathogen and a leading cause of severe infections like bacteremia. MRSA carriage (colonization) increases the risk of infection, and skin-to-skin contact is the main route of transmission.1–4
Macaques (Macaca mulatta) are frequently bred in captivity for research purposes. Animal care, comfort, and safety are strictly controlled following state and federal regulations in accordance with the Guide for the Care and Use of Laboratory Animals.5 Macaques can be asymptomatic carriers of S. aureus,6–9 and like in humans, severe infections often occur.2,3 Veterinary standard operating procedures require that all animals be screened for carriage of certain infectious agents upon arrival to Animal Facilities. These agents include Mycobacterium tuberculosis, enteric pathogens, simian retroviruses, and herpes viruses. Although S. aureus carriage is routinely screened in other research animals such as mice, this is not part of the standard care for macaques, and there is little information on the actual prevalence of S. aureus carriage among research macaques. Available evidence points to a few clones of S. aureus, which are frequently detected among research macaques, but these are rarely identified as causing infections in humans.6,8,9 Unfortunately, a thorough epidemiological comparison is difficult, because different studies utilize different molecular methods to characterize the strains.
In this study we present a case where six different strains of S. aureus were identified as colonizing and/or infecting 13 of the 14 macaques in one of the colonies in the animal facility during a 1 year study: four MRSA and two methicillin-sensitive Staphylococcus aureus (MSSA) strains. Five out of the six strains were multidrug resistant.
During the spring of 2016, four macaques from The Rockefeller University animal vivarium developed skin and soft-tissue infections (SSTIs) by MRSA in the margins of transcranial exogenous implants. Preliminary screening showed that 9 of the 14 animals housed in the affected rooms carried two different clones of S. aureus—one MSSA and one MRSA, which are very uncommon in the New York City human community.10,11 These strains (t189/ST188 and t4167/ST3862) have been described previously among research macaques in Europe9; t189/ST188 was also described in a vivarium in Seattle, Washington, and United States8 and among wild animals in Africa.7
The affected animals are subjects of a neuroscience research program at The Rockefeller University. Transcranial implants are required for this research. After four animals developed MRSA SSTIs in the margins of their implants, the devices were removed from two of the infected animals, and the surgical procedures to install implants to four additional macaques were postponed for several months to prevent infections.
This was the first registry of MRSA in the vivarium, so this study was designed to characterize the strains present in the facilities and to test decolonization and decontamination procedures to eliminate them.
We prospectively studied the carriage of S. aureus among the macaques in the affected rooms of the vivarium during four sampling times between July 2016 and May 2017 and established an enhanced routine of decolonization and decontamination of the facilities to prevent spread and infection. This also included the reorganization of the animal colony with the aim of restricting bacterial spread.
Materials and Methods
Animal routine care
The Comparative Bioscience Center at The Rockefeller University hosts 21 macaques (M. mulatta), ages 5–19 years. A group of 14 of those macaques were included in this study. These animals were 4–8 years old in 2016. Animals are maintained in accordance with the Animal Welfare Act, as amended in 2013 (https://nal.usda.gov/awic/animal-welfare-act) and the Guide for the Care and Use of Laboratory Animals5 in an AAALAC-accredited facility. All procedures in the study were approved by The Rockefeller University Institutional Animal Care and Use Committee. The macaques originated from two breeding colonies as follows: Covance, Inc. (Alice, TX), and Three Springs Scientific, Inc. (Perkasie, PA). Macaques were either pair or single housed in stainless steel mobile caging (Apartment Module with conversion floor, 3 m2, Primate Products, Inc., Immokalee, FL). Cage components were sanitized in a mechanical washer (BetterBuilt; Northwest Systems, Inc., Delta, British Columbia) that provided a 82°C final rinse water and used a combination of organic acid detergent (Acidulate 28; Quip Laboratories, Wilmington, DE) and a chlorinated cleaning compound (ENVIRO-KLEEN 900S; Quip Laboratories) during the wash cycle. The macaques received a commercial diet (Monkey Diet Jumbo 5037; LabDiet, St. Louis, MO) and were maintained on municipal water. They were housed at 22 ± 1°C at 30–70% relative humidity and a 12-h light/12-h dark cycle (07:00–19:00 hours). Daily environmental enrichment included structural enrichment and manipulanda; novel food items (cereals, nuts, dried pasta, yogurt, etc.); occupational devices; and sensory enrichment (alternating between visual and auditory). All macaques were seronegative for simian type D retrovirus, simian immunodeficiency virus, simian T cell leukemia virus type 1, and herpes B virus as determined by enzyme linked immunosorbent assay (VRL Laboratories, San Antonio, TX). They were also negative for M. tuberculosis tested through an intradermal tuberculin skin test (Tuberculin Mammalian, Human Isolates Intradermic; Zoetis, San Diego, CA) and were measles vaccinated.
Screening for S. aureus colonization and contamination
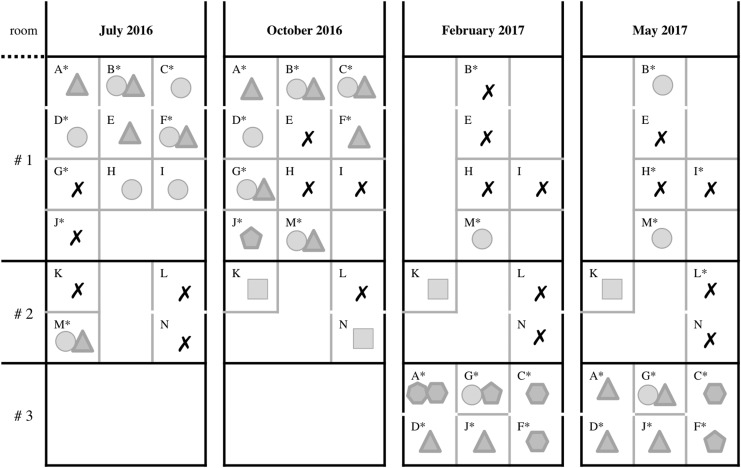
Between April and June 2016, four macaques (identified with letters G, H, I, and J in this article) carrying transcranial implants presented with SSTIs in the margins of their implants; they were caused by MRSA and were treated with clindamycin. The implants of two of these animals—H and I—were removed before this study started, due to the progression of the infection. Starting in July 2016, the 14 macaques in their animal colony (ages 4–8 years), including the four infected animals, were included as part of this study. These animals were initially housed in two rooms. A third room was also used after the second sampling time in October–November 2016, and seven macaques (A, C, D, F, G, J, M) were moved between rooms during the study. Movement of the animals was based on the results of microbiological sampling, with the intention of preventing the spread of MRSA (Fig. 1).
FIG. 1.
Staphylococcus aureus colonization of the 14 macaques located in three different rooms, at the four sampling times. Each letter represents one macaque. Gray lines separate cages. Note that some animals are caged in pairs (A and D, B and E, C and F, L and N). Some animals were moved to different rooms during the study in an attempt to control bacterial spread (M was moved from room #2 to room #1; then A, C, D, F, G, and J from room #1 to room #3). A star by the letter designating each macaque means that the animal was wearing a transcranial implant at the moment of sampling. Each geometric shape corresponds to one strain of S. aureus detected at each time, in each animal. An “X” means no S. aureus was detected on a particular animal at a particular screening time; circle: MA-I (t189/ST188/PFGE-A/mecA−); triangle: MA-II (t4167/ST3862/PFGE-B/mecA+); pentagon: MA-III (t4167/ST3862/PFGE-C/mecA+); hexagon: MA-IV (t16708/ST3862/PFGE-C/mecA+); heptagon: MA-V (t16709/ST3862/PFGE-C/mecA+); and square: MA-VI (t8397/ST3884/PFGE-D/mecA−). Animals marked with two shapes carried two strains of S. aureus at the corresponding screening. PFGE, pulsed-field gel electrophoresis.
To collect samples, macaques were chaired (closed-chair design) with the use of positive reinforcement training. Sampling from animals and their environment took place four times during a 1-year period between July 2016 and May 2017 (July–August, October–November, February, and May). At each sampling time, three specimens (anterior nasal, rectal, and head) were taken from each animal. Eight macaques carried transcranial implants at the beginning of the study (A, B, C, D, F, G, J, and M). In these cases, the head samples were taken by swabbing the margins of the implants. The other six animals without implants were swabbed at equivalent areas around their heads. Following animal swabbing, at each one of the sampling times, an average of 40 environmental specimens were also collected from the cages, chairs, transport containers, and other surfaces in the colony rooms before and after the implementation of decolonization and decontamination procedures. All biological samples were taken using BBL™ CultureSwab™ Plus swabs (Copan, for Becton Dickinson, Sparks, MD) and were transported to the Laboratory of Microbiology and Infectious Diseases for processing.
In the laboratory, each swab was used to inoculate 6 mL of Tryptic Soy Broth (TSB, Difco, BBL; Becton Dickinson, Franklin Lakes) by vortexing for 10 seconds. The TSB cultures were next incubated with agitation at 37°C overnight. Stocks were prepared in 70% glycerol and stored at −80°C, and at the same time, plates of Mannitol Salt Agar (Difco, BBL; Becton Dickinson) were inoculated and incubated at 37°C for 48 hours. Yellow colonies were examined for coagulase agglutination (Staphaurex; Thermo Fisher Scientific, Lenexa, KS) to confirm S. aureus. Except when colonies with different sizes were observed, only one colony was recovered.
Antimicrobial susceptibility
The initial suspicion was that S. aureus infecting the macaques might have a human origin, so antimicrobial susceptibility testing was performed to compare the results with all human-borne strains previously analyzed in the laboratory.10,12
All staphylococcal specimens were tested by disc-diffusion method for susceptibility to the following 12 antibiotics: penicillin, cefoxitin (to detect MRSA), ciprofloxacin, clindamycin, erythromycin, gentamicin, rifampicin, tetracycline, cotrimoxazole (trimethoprim/sulfamethoxazole), linezolid, chloramphenicol, and vancomycin; and by E-test to mupirocin, following the Clinical and Laboratory Standards Institute (CLSI) recommendations.13
Molecular identification: spa typing, multilocus sequence typing, and pulsed-field gel electrophoresis
Molecular characterization of the S. aureus isolates was performed initially by spa typing,14 and isolates were assigned spa types using the RIDOM web server (http://spaserver.ridom.de). Sequence types (STs) were assigned based on data available on the spa server or bibliography if available. For those spa types without a designated ST, multilocus sequence typing (MLST) was performed as previously described.15 STs were then assigned by DNA amplification and sequencing of seven housekeeping genes (arcC, aroE, glpF, gmk, pta, tpi, yqiL) and using the online MLST database (https://pubmlst.org).16
Pulsed-field gel electrophoresis (PFGE) was performed to further confirm the relatedness of all the isolates. Bacterial DNA was restricted with SmaI enzyme, and the resulting fragments were separated by electrophoresis.17 Band patterns were compared manually following guidelines to establish strain or PFGE type classifications.18,19
Molecular characterization: detection of mecA, SCCmec typing Panton–Valentine Leukocidin, and arginine catabolic mobile element
The mecA gene, responsible for resistance to oxacillin and other beta-lactam antibiotics, was detected following the protocol of Okuma.20 Typing of the SCCmec cassette was performed by multiplex PCR as previously described.21
According to our initial hypothesis of a human origin for the S. aureus colonization, we also searched for the main virulence factors observed among human-borne strains: PVL and arginine catabolic mobile element (ACME). The lukS and lukF genes, which encode PVL, were identified by PCR.20,22 The ACME was detected using primers that target its two main loci (arcA and opp3) in USA300 strain FPR375723 and classified according to its structure: type I (arcA and opp3 operons), type II (arcA operon only), and type III (opp3 operon only).24
Decolonization and decontamination procedures
Starting in July 2016, our study began extensive procedures to try to decolonize the animals and decontaminate their housing. The 5-day decolonization procedures described below were implemented on four different occasions during the study, always following the sampling times (July, October–November, February, and May).
The primate chair (closed-chair design) was decontaminated with a hydrogen-peroxide disinfectant (Accel TB; Fisher Scientific, Pittsburgh, PA) before and after decolonization of each animal. Next, the animal was chaired with the use of positive reinforcement training. Alternatively, sedation was used as needed (combination of ketamine HCl [Henry Schein Animal Health, Dublin, OH] and dexmedetomidine HCL [Zoetis, Inc., Florham Park, NJ]). The animal decolonization procedures consisted of four steps as follows: (1) cleaning the transcranial implant margins (when applicable) with chlorhexidine solution 2% (First Priority, Inc., Elgin, IL), followed by rinsing with sterile 0.9% sodium chloride solution, (2) bathing the animals with 2% chlorhexidine scrub, (3) mouth washing with 3 mL of chlorhexidine gluconate 0.12% oral rinse (Best Pet Rx, New York, NY), and (4) application of 2% topical mupirocin ointment (NYCOMED US, Inc., Melville, NY) in the nostrils and around the transcranial implants (as applicable). These procedures were repeated once daily for five consecutive days, with the exception of treatment with mupirocin, which was applied twice daily for 2 weeks.
In addition, an extensive decontamination protocol of 5 days was implemented after the second sampling time in October–November 2016. On days 1 and 5 of this decontamination protocol, all working areas, equipments, and cages were decontaminated with hydrogen-peroxide disinfectants and the cages sanitized with a mechanical washer. Furthermore, the housing room (walls, floors, and ceiling) was decontaminated with a hydrogen-peroxide disinfectant followed by and then with SaniGuard® Antimicrobial Fogger (AliMed, Inc., Dedham, MA).
Results
A total of 168 predecolonization samples were taken from the 14 animals over four time points, from three body sites (head/margin of transcranial implant, nares, and rectum). Additional samples were taken 2 weeks after each decolonization to verify short-term efficacy, bringing the total number of specimens to 210. In parallel, 199 environmental samples were taken from the housing and working rooms of the animals. A total of 114 specimens showed growth of S. aureus: 77 of animal origin (36% of 210) and 37 from the environment (18% of 199). From the animal specimens, 33 isolates were MSSA and 44 were MRSA. During the different sampling times, 47 were “margin” samples (around the transcranial implant) and 27 were “head” samples (fur on the same part of the head, but in absence of implant). Thirty-six of the 47 “margin” samples (76%) were positive for S. aureus (24 MRSA), while only 6 of the 27 “head” samples (22%) were positive (1 MRSA); 19 of 71 (27%) nasal samples rendered S. aureus growth (10 MRSA); and 17 of 71 (24%) rectal samples gave positive S. aureus growth (10 MRSA). Among the environmental specimens 14 (7%) were MSSA and 23 (12%) MRSA, recovered from cage surfaces, work chairs, and transport carts.
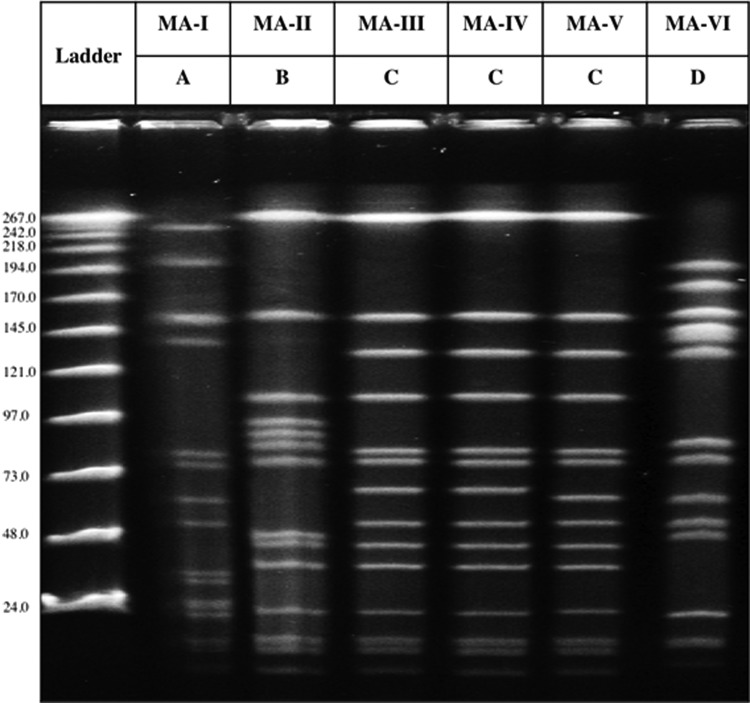
Five different spa types were detected among S. aureus recovered in this study: t189, t4167, t8397, t16708, and t16709; the latter two types are described in this study for the first time. In addition, the 114 S. aureus specimens identified in this study were also grouped into three different ST types: ST188, ST3862, and ST3884. Four different PFGE profiles were also identified and named by capital letters A–D (Fig. 2). MSSA isolates were grouped in two different clones: MA-I (t189/ST188/PFGE-A) and MA-VI (t8397/ST3884/PFGE-D), and MRSA isolates were grouped in four clones: MA-II (t4167/ST3862/PFGE-B), MA-III (t4167/ST3862/PFGE-C), MA-IV (t16708/ST3862/PFGE-C), and MA-V (t16709/ST3862/PFGE-C). The ST3862 was observed for the first time in this study (submission number 32235 in PubMLST.org). Table 1 shows the complete molecular characterization of the six S. aureus strains or clones.
FIG. 2.
The six different PFGE profiles detected among the S. aureus isolates in this study. MA-I to MA-VI are the names of the six S. aureus strains observed in this study. A–D represent the four different PFGE profiles observed for them. MA-I and MA-VI are methicillin-sensitive S. aureus, while MA-II through MA-V are methicillin-resistant S. aureus. On the side of the figure, the kilobases correspond to each band in the ladder.
Table 1.
Characterization of the Six Clones of Staphylococcus aureus Colonizing the Macaques
| Strain | N | Room | PFGE | spa type | MLST | mecA | Antibiogram |
|---|---|---|---|---|---|---|---|
| MA-I | 40 | 1, 2, 3 | A | t189 (07-23-12-21-17-34) | ST188 (3, 1, 1, 8, 1, 1, 1) | − | PEN, CLI, ERY, GEN, CIP, SXT, MUPa |
| MA-II | 47 | 1, 2, 3 | B | t4167 (07-23-17-22-249-12-117-24-25-17) | ST3862 (1, 1, 1, 214, 451, 303, 117) | + | PEN, FOX, GEN, CIP, TET, SXT, MUPa |
| MA-III | 9 | 3 | C | t4167 (07-23-17-22-249-12-117-24-25-17) | ST3862 (1, 1, 1, 214, 451, 303, 117) | + | PEN, FOX, GEN, CIP, TET, SXT |
| MA-IV | 10 | 3 | C | t16708 (07-23-17-22-249-12-117-25-17) | ST3862 (1, 1, 1, 214, 451, 303, 117) | + | PEN, FOX, GEN, CIP, TET, SXT |
| MA-V | 1 | 3 | C | t16709 (07-22-249-12-117-24-25-17) | ST3862 (1, 1, 1, 214, 451, 303, 117) | + | PEN, FOX, GEN, CIP, SXT |
| MA-VI | 7 | 2 | D | t8397 (04-20-24-17-17-25) | ST3884 (22, 23, 1, 8, 1, 1, 1) | − | All S |
In parenthesis, spa repeats and MLST profiles.
Five of the 40 isolates of MA-I clone and one of the 47 isolates of MA-II clone showed reduced susceptibility to mupirocin (MICs of 12–32 μg/mL) at the two last sampling times (February and May 2017).
N, number of isolates belonging to each clone colonizing the macaques; Room, room in which the clones were found colonizing macaques; PEN, penicillin; CLI, clindamycin; ERY, erythromycin; GEN, gentamicin; CIP, ciprofloxacin; SXT, cotrimoxazole; MUP, mupirocin; FOX, cefoxitin; TET, tetracycline; All S, susceptible to all antibiotics tested; MLST, multilocus sequence typing; ST, sequence type; MICs, minimal inhibitory concentrations.
Only one strain, MA-VI (t8397/ST3884/PFGE-D), was susceptible to all antibiotics tested, including penicillin. The other MSSA strain, MA-I (t189/ST188/PFGE-A), was resistant to penicillin, clindamycin, erythromycin, gentamicin, ciprofloxacin, and cotrimoxazole. Three MRSA strains, MA-II, MA-III, and MA-IV, were resistant to beta-lactams (MRSA), gentamicin, ciprofloxacin, tetracycline, and cotrimoxazole. Strain MA-V was resistant to beta-lactams (MRSA), gentamicin, ciprofloxacin, and cotrimoxazole, but not to tetracycline. A reduction in susceptibility to mupirocin was detected in six specimens during the sampling times in February and May 2017 (minimal inhibitory concentrations [MICs] of 16–32 mg/L). Five of them were obtained from macaque M and its cage surface and all belonged to clone MA-I (MSSA). The other specimen, which belonged to MA-II (MRSA), was obtained from the cage of macaque C that was housed in a different room.
Of the 14 macaques in this study, only macaque L was consistently negative for S. aureus. The other 13 animals were colonized at least once during the study, either by MRSA or MSSA strains, or by more than one strain at the same time (Fig. 1). Only for macaque G in May two strains (MA-I and MA-II) were colonizing the margins of the transcranial implant. That was the only time that two different strains were detected in one sample. For two animals, the rectum was the only carriage site in November–December, but in further sampling the strain appeared also in the head. Of the 45 times that a screened animal was colonized at least in one body site, 22 times it was in only one site (mainly the head); 14 times being colonized in two or three body sites, the strains detected were concordant; only in 9 cases two different strains were obtained from the different body sites colonized. In the first screening performed on July 2016, macaques E, H, and I were carriers of S. aureus, but after decolonization they remained negative in all subsequent screens until the end of the study. By the completion of the study in May 2017, the number of animals equipped with the transcranial implants had increased from 8 to 11 (H, I, and L received implants in May 2017). The newly implanted devices were sterile for S. aureus at the last screening (head samples were on the margin of implants, when present), but devices that were contaminated at the beginning of the study (A, B, C, D, F, and M) remained positive with S. aureus throughout the study ending on May 2017. Macaques G and J, which had implants, gave negative cultures in July 2016, when undergoing antibiotic treatment for their previously diagnosed MRSA infections. Interestingly, after treatment ended, they were identified as culture positive during the following screening, and they remained colonized or infected until the end of the study. Three macaques (B, K, and M) were positive for MSSA (MA-I and MA-VI); five (A, C, D, F, and J) carried MRSA (MA-II, MA-III, and MA-IV); and one animal (G) was colonized with both MRSA and MSSA (MA-I and MA-II) by the end of the study. Of the three animals without implants only macaque K has remained a positive carrier for MSSA and was the only animal colonized by strain MA-VI during the last three sampling times (October 2016 and February and May 2017).
The animals were initially housed in two rooms, with 10 macaques in room #1 (A, B, C, D, E, F, G, H, I, and J) and four macaques in room #2 (K, L, M, and N) (Fig. 1). At the time of the first screening, in July 2016, two different strains of S. aureus MA-I (MSSA) and MA-II (MRSA) were colonizing nine animals: C, D, H, and I were colonized by MA-I; A and E by MA-II; and B, F, and M by both MA-I and MA-II. Since macaque M was the only colonized animal in room #2, he was subsequently moved to room #1, where 8 of 10 animals were positive (A, B, C, D, E, F, H, and I). As mentioned before, macaques G and J were negative for S. aureus at this first sampling time, possibly because they were undergoing systemic antibiotic treatment for MRSA infection, diagnosed immediately before the beginning of this study. These two animals eventually became S. aureus positive (after the treatment course) at the time of the second sampling and then remained positive throughout the entire study. Given that the treatments and enhanced decontamination procedures failed to eliminate S. aureus between the first and the second screening, macaques A, C, D, F, G, and J were relocated to a third room (room #3) before the third sampling took place. Only macaque M (colonized by MA-I and MA-II) remained in room #2 after the second sampling time (February). One pan-susceptible strain (MA-VI) was detected during the study in two animals: macaque K was positive at three sampling times (October, February, and May) and macaque N for one sampling time (October). This strain remained restricted to room #2 and was not detected in the other rooms at any of the time points. Of note, macaque L was also housed in room #2 with the MA-VI colonized macaques and always remained negative to S. aureus. At the end of the study nine macaques (A, B, C, D, F, G, J, K, and M) were still carriers of a total of four different strains of S. aureus, two MRSA (MA-II and MA-IV) and two MSSA (MA-I and MA-VI). Macaques E, H, and I were colonized by S. aureus at the beginning of the study, but they were S. aureus free at the end, while G, J, K, and N were negative at first then became colonized during the study. Increased efforts to control contamination and prevent further S. aureus spread were successful in restricting the MRSA to room #3, while rooms #1 and #2 showed the presence of MSSA strains only. Also the number of contaminated cages was reduced with these procedures from eight in August and October 2016 to four in May 2017.
Discussion
The newly characterized strain MA-VI (t8397/ST3884/PFGE-D) was MSSA, like the MA-I (t189/ST188/PFGE-A) strain, which was also detected in this study. Interestingly, ST3884 (MA-VI) is a double locus variant of ST188 seen in MA-I, with new arcC and aroE alleles. This suggests that the two strains may be phylogenetically related. However, the PFGE profiles are quite different, and while MA-VI was susceptible to all antibiotics tested in this study, MA-I was resistant to several antimicrobials (clindamycin, erythromycin, gentamicin, ciprofloxacin, and cotrimoxazole), and in three isolates a decrease in susceptibility to mupirocin was also detected (Table 1). The molecular profile t189/ST188, as well as the spa type t189 alone,9 was previously described in S. aureus strains infecting or colonizing macaques.7,8 However, none of those studies performed PFGE, so we cannot conclude whether we identified the same strains.
The close molecular relatedness among the strains suggest that there might have been initially two original strains, one MSSA and one MRSA, which then may have evolved into six different derivatives after being subjected to a variety of antibiotic pressures, and the captive lifestyle of the different hosts they came in contact with, during their stay at the animal breeding facilities.
Only one of the strains of S. aureus identified in this study, MA-I, has been recently isolated from human patients or their households in a currently ongoing study in New York City (M.P., unpublished data). MA-I has been described, as MSSA or MRSA, with higher prevalence in Asia,25,26 but it was not identified in a nearby hospital nor in community settings—in two recent surveillance studies–performed in New York City.10,12 The other five strains detected in the present study (MA-II, MA-III, MA-IV, MA-V, and MA-VI) have not been described in humans before. However, the strains are similar or closely related to S. aureus previously described in association to primates in different locations worldwide.7–9 None of the six strains carried PVL or ACME virulence genes, frequent among human community-associated strains. The SCCmec cassette was nontypable; however, we could conclude that it did not belong to types IV and II, which are the SCCmec types identified in human strains in New York City.
This study raises two major concerns. First, it seems quite likely that the animals arrived to the animal care facility at the University already colonized by S. aureus. Screening for S. aureus was not part of the routine testing of macaques arriving to the vivarium, although as a result of the study presented here, this may become an added procedure for new incoming macaques to an animal care facility. Second, S. aureus is a well-known human pathogen, so animal-care workers could be at risk of colonization or infection during animal handling. Moreover, five of the six strains of S. aureus detected in this study were resistant to a number of antimicrobial agents (multidrug resistant), which present increased difficulty for treatment of both animals and/or humans if further infection occurs.
These macaques come from breeding farms, which provide animals to research institutions. The fact that the bacterial strains detected in this study are unrelated to strain colonizing humans in America, but common to other research animals in the country, suggests that those bacteria are selected in the indoor breeding premises. However, the authors ignore whether antibiotics are used in such facilities before animal delivery, which could explain the multidrug resistance profile of several of the strains.
To prevent the spread of S. aureus, additional environmental disinfection with hydrogen peroxide based disinfectants and decolonizing procedures (daily bathing with chlorhexidine and topical mupirocin treatment) was implemented in the vivarium during this study.
It was observed that these protocols, together with the relocation of animals based on their colonization status, worked well and managed to eliminate S. aureus colonization, as long as the animals did not carry an exogenous implant during the procedure, and reduced the number of cages providing S. aureus positive cultures from eight (in August and October) to four (in February and May). However, cages are open and these animals require socialization, so isolation was not possible, and therefore, cross-contamination between sampling times could not be prevented, but only minimized.
Out of the original five animals found with MRSA colonization in July 2016 (A, B, E, F, and M), four were carrying implants at the time (A, B, F, and M), while only one of them was implant free (E). Two of those carrying implants (B and M) were cleared of MRSA after the environmental disinfection and decolonizing procedures, but they switched to MSSA colonization in February 2017. Only the implant-free animal (E) cleared from MRSA to S. aureus negative carriage by the end of the study.
An additional concern in treating the animals to reduce S. aureus colonization or infections was that six bacterial isolates obtained during the second half of the study (February and May 2017) showed decreased susceptibility to mupirocin (MICs increased to 12–32 μg/mL). Since, this topical antibiotic was used throughout the study we hypothesize that this practice could have selected for low-level resistance. Therefore, mupirocin use was restricted in prospective decolonization protocols of nares in this facility.
The nares are the main carriage site for S. aureus in humans. However, exogenous devices are well known to be an important risk factor for colonization and infection. In our study 76% of “margin” samples (around implants) were positive for S. aureus compared to 22% of “head” samples (when there was no implant) and to the 19 (27%) nasal or the 17 (24%) rectal samples that were positive for S. aureus at screening.
None of the procedures to decolonize the animals and decontaminate the housing surfaces achieved a complete remission for all animals. Infections and colonization of the animals continued throughout the study period. Control of this small S. aureus outbreak had many consequences on the research being performed, animal technician time workload, and a significant cost in animal care while attempting to cure the infections and prevent bacterial spread in the colony.
We suggest that the presence of exogenous implants may be an important colonization risk factor for S. aureus in animals as much as in humans, and therefore, screening for S. aureus should be compulsory before any future surgical procedures in this type of research animal.
Conclusion
The impact of undetected S. aureus (MSSA and MRSA) carriage among research macaques can be considerable if infection occurs, not only for the overall health of the animal but also for the treatment or containment costs and the potential to delay the research for which they were bred. Furthermore, while the human caretakers are required to wear personal protective equipment, the underdiagnosed animal carriage of S. aureus, especially when it is MRSA, presents a risk to personnel in frequent contact with the animals.
The observations presented in this study strongly suggest that experimental animals should be tested for S. aureus carriage before any invasive procedure.
This study had several limitations. First, due to bacterial testing and animal treatment before this study began, samples of the bacterial strains infecting the four macaques (G, H, I, and J) before this study began (between April and June 2016) could not be recovered, so it can only be an epidemiological assumption that the strains colonizing the animals in the following months are the same that infected them in the first place. Second, testing of bacterial carriage could not be performed on the human caretakers to determine or measure transmission risks. Caretakers must wear protective clothing because of the other infectious agents both macaques and humans can often carry. No caretakers' illnesses or other zoonotic infections were recorded during the time of the study. Nonetheless, isolation of S. aureus during the environmental surface testing shows that the risk is real for bacterial exposure, should the protective barriers fail. Third, vancomycin susceptibility was tested by disk diffusion, which might fail in detecting non-vanA mechanisms of resistance.
Acknowledgments
The authors thank the group of Dr. Marilyn C. Roberts, from the University of Washington, and the Washington National Primate Research Center, Seattle, WA, for their guidance on decontamination and decolonization of primates and their housing. This study was supported by grant (# UL1 TR0018663) from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program to M.P.d.l.G., (PI). The salary of M.P.d.l.G. was supported by PCORI grant (CER-1402-10800) to J.N. Tobin (PI) and by funds from the RB Roberts Bacterial Antibiotic Resistance Group (BARG) to A.T. V.A.F. was supported by The Rockefeller University laboratory funds.
Disclosure Statement
No competing financial interests exist.
References
- 1. Chambers H.F., and Deleo F.R. 2009. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 7:629–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chertow D.S., Kindrachuk J., Sheng Z.M., Pujanauski L.M., Cooper K., Nogee D., Claire M.S., Solomon J., Perry D., Sayre P., Janosko K.B., Lackemeyer M.G., Bohannon J.K., Kash J.C., Jahrling P.B., and Taubenberger J.K. 2016. Influenza A and methicillin-resistant Staphylococcus aureus co-infection in rhesus macaques—a model of severe pneumonia. Antiviral Res. 129:120–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee J.I., Kim K.S., Oh B.C., Kim N.A., Kim I.H., Park C.G., and Kim S.J. 2011. Acute necrotic stomatitis (noma) associated with methicillin-resistant Staphylococcus aureus infection in a newly acquired rhesus macaque (Macaca mulatta). J. Med. Primatol. 40:188–193 [DOI] [PubMed] [Google Scholar]
- 4. Poovelikunnel T., Gethin G., and Humphreys H. 2015. Mupirocin resistance: clinical implications and potential alternatives for the eradication of MRSA. J. Antimicrob. Chemother. 70:2681–2692 [DOI] [PubMed] [Google Scholar]
- 5. Committee for the Update of the Guide for the Care and Use of Laboratory Animals. 2011. Institute for Laboratory Animal Research Division on Earth and Life Studies. National Research Council of the National Academies. The National Academies Press, Washington, DC: Available at www.nap.edu [Google Scholar]
- 6. Hsu L.Y., Holden M.T.G., Koh T.H., Pettigrew K.A., Cao D., Hon P.Y., Sergio D.M., Pena E., and Ogden B.E. 2017. ST3268: a geographically widespread primate MRSA clone. J. Antimicrob. Chemother. 72:2401–2403 [DOI] [PubMed] [Google Scholar]
- 7. Schaumburg F., Mugisha L., Kappeller P., Fichtel C., Kock R., Kondgen S., Becker K., Boesch C., Peters G., and Leendertz F. 2013. Evaluation of non-invasive biological samples to monitor Staphylococcus aureus colonization in great apes and lemurs. PLoS One 8:e78046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Soge O.O., No D., Michael K.E., Dankoff J., Lane J., Vogel K., Smedley J., and Roberts M.C. 2016. Transmission of MDR MRSA between primates, their environment and personnel at a United States primate centre. J. Antimicrob. Chemother. 71:2798–2803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van den Berg S., van Wamel W.J., Snijders S.V., Ouwerling B., de Vogel C.P., Boelens H.A., Willems R.J., Huijsdens X.W., Verreck F.A., Kondova I., Heidt P.J., Verbrugh H.A., and van Belkum A. 2011. Rhesus macaques (Macaca mulatta) are natural hosts of specific Staphylococcus aureus lineages. PLoS One 6:e26170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pardos de la Gandara M., Curry M., Berger J., Burstein D., Della-Latta P., Kopetz V., Quale J., Spitzer E., Tan R., Urban C., Wang G., Whittier S., de Lencastre H., and Tomasz A. 2016. MRSA causing infections in hospitals in greater metropolitan New York: major shift in the dominant clonal type between 1996 and 2014. PLoS One 11:e0156924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pardos de la Gandara M., Raygoza Garay J.A., Mwangi M., Tobin J.N., Tsang A., Khalida C., D'Orazio B., Kost R.G., Leinberger-Jabari A., Coffran C., Evering T.H., Coller B.S., Balachandra S., Urban T., Parola C., Salvato S., Jenks N., Wu D., Burgess R., Chung M., de Lencastre H., and Tomasz A. 2015. Molecular types of methicillin-resistant Staphylococcus aureus and methicillin-sensitive S. aureus strains causing skin and soft tissue infections and nasal colonization, identified in community health centers in New York city. J. Clin. Microbiol. 53:2648–2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Balachandra S., Pardos de la Gandara M., Salvato S., Urban T., Parola C., Khalida C., Kost R.G., Evering T.H., Pastagia M., D'Orazio B.M., Tomasz A., H. and de Lencastre J.N. 2015. Tobin, recurrent furunculosis caused by a community-acquired Staphylococcus aureus strain belonging to the USA300 clone. Microb. Drug Resist. 21:237–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clinical and Laboratory Standards Institute (CLSI). 2013. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Third Informational Supplement. CLSI Document M100-S23. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 14. Aires-de-Sousa M., Boye K., de Lencastre H., Deplano A., Enright M.C., Etienne J., Friedrich A., Harmsen D., Holmes A., Huijsdens X.W., Kearns A.M., Mellmann A., Meugnier H., Rasheed J.K., Spalburg E., Strommenger B., Struelens M.J., Tenover F.C., Thomas J., Vogel U., Westh H., Xu J., and Witte W. 2006. High interlaboratory reproducibility of DNA sequence-based typing of bacteria in a multicenter study. J. Clin. Microbiol. 44:619–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Enright M.C., Day N.P., Davies C.E., Peacock S.J., and Spratt B.G. 2000. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 38:1008–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Feil E.J., Cooper J.E., Grundmann H., Robinson D.A., Enright M.C., Berendt T., Peacock S.J., Smith J.M., Murphy M., Spratt B.G., Moore C.E., and Day N.P. 2003. How clonal is Staphylococcus aureus? J. Bacteriol. 185:3307–3316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chung M., de Lencastre H., Matthews P., Tomasz A., Adamsson I., Aires de Sousa M., Camou T., Cocuzza C., Corso A., Couto I., Dominguez A., Gniadkowski M., Goering R., Gomes A., Kikuchi K., Marchese A., Mato R., Melter O., Oliveira D., Palacio R., Sa-Leao R., Santos Sanches I., Song J.H., Tassios P.T., Villari P., and Multilaboratory Project Collaborators. 2000. Molecular typing of methicillin-resistant Staphylococcus aureus by pulsed-field gel electrophoresis: comparison of results obtained in a multilaboratory effort using identical protocols and MRSA strains. Microb. Drug Resist. 6:189–198 [DOI] [PubMed] [Google Scholar]
- 18. McDougal L.K., Steward C.D., Killgore G.E., Chaitram J.M., Mc Allister S.K., and Tenover F.C. 2003. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J. Clin. Microbiol. 41:5113–5120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tenover F.C., Arbeit R.D., Goering R.V., Mickelsen P.A., Murray B.E., Persing D.H., and Swaminathan B. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Okuma K., Iwakawa K., Turnidge J.D., Grubb W.B., Bell J.M., O'Brien F.G., Coombs G.W., Pearman J.W., Tenover F.C., Kapi M., Tiensasitorn C., Ito T., and Hiramatsu K. 2002. Dissemination of new methicillin-resistant Staphylococcus aureus clones in the community. J. Clin. Microbiol. 40:4289–4294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Milheirico C., Oliveira D.C., and de Lencastre H. 2007. Update to the multiplex PCR strategy for assignment of mec element types in Staphylococcus aureus. Antimicrob. Agents Chemother. 51:3374–3377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lina G., Piemont Y., Godail-Gamot F., Bes M., Peter M.O., Gauduchon V., Vandenesch F., and Etienne J. 1999. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin. Infect. Dis. 29:1128–1132 [DOI] [PubMed] [Google Scholar]
- 23. Diep B.A., Gill S.R., Chang R.F., Phan T.H., Chen J.H., Davidson M.G., Lin F., Lin J., Carleton H.A., Mongodin E.F., Sensabaugh G.F., and Perdreau-Remington F. 2006. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 367:731–739 [DOI] [PubMed] [Google Scholar]
- 24. Diep B.A., Stone G.G., Basuino L., Graber C.J., Miller A., des Etages S.A., Jones A., Palazzolo-Ballance A.M., Perdreau-Remington F. Sensabaugh G.F., DeLeo F.R., and Chambers H.F. 2008. The arginine catabolic mobile element and staphylococcal chromosomal cassette mec linkage: convergence of virulence and resistance in the USA300 clone of methicillin-resistant Staphylococcus aureus. J. Infect. Dis. 197:1523–1530 [DOI] [PubMed] [Google Scholar]
- 25. Ip M., Wang Z., Lam W.Y., Zhou H., and Tsui S. 2014. Draft genome sequence of methicillin-resistant Staphylococcus aureus CUHK_188 (ST188), a health care-associated bacteremic isolate from Hong Kong. Genome Announc. 2:pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Song Z., Gu F.F., Guo X.K., Ni Y.X., He P., and Han L.Z. 2017. Antimicrobial resistance and molecular characterization of Staphylococcus aureus causing childhood pneumonia in Shanghai. Front. Microbiol. 8:455. [DOI] [PMC free article] [PubMed] [Google Scholar]