Abstract
Orthopaedic implants are applied daily in our orthopaedic clinics for treatment of musculoskeletal injuries, especially for bone fracture fixation. To realise the multiple functions of orthopaedic implants, hybrid system that contains several different materials or parts have also been designed for application, such as prosthesis for total hip arthroplasty. Fixation of osteoporotic fracture is challenging as the current metal implants made of stainless steel or titanium that are rather rigid and bioinert, which are not favourable for enhancing fracture healing and subsequent remodelling. Magnesium (Mg) and its alloys are reported to possess good biocompatibility, biodegradability and osteopromotive effects during its in vivo degradation and now tested as a new generation of degradable metallic biomaterials. Several recent clinical studies reported the Mg-based screws for bone fixation, although the history of testing Mg as fixation implant was documented more than 100 years ago. Truthfully, Mg has its limitations as fixation implant, especially when applied at load-bearing sites because of rather rapid degradation. Currently developed Mg-based implants have only been designed for application at less or non–loading-bearing skeletal site(s). Therefore, after years research and development, the authors propose an innovative hybrid fixation system with parts composed of Mg and titanium or stainless steel to maximise the biological benefits of Mg; titanium or stainless steel in this hybrid system can provide enough mechanical support for fractures at load-bearing site(s) while Mg promotes the fracture healing through novel mechanisms during its degradation, especially in patients with osteoporosis and other metabolic disorders that are unfavourable conditions for fracture healing. This hybrid fixation strategy is designed to effectively enhance the osteoporotic fracture healing and may potentially also reduce the refracture rate.
The translational potential of this article: This article systemically reviewed the combination utility of different metallic implants in orthopaedic applications. It will do great contribution to the further development of internal orthopaedic implants for fracture fixation. Meanwhile, it also introduced a titanium–magnesium hybrid fixation system as an alternative fixation strategy, especially for osteoporotic patients.
Keywords: Fracture repair, Hybrid fixation system, Magnesium, Orthopaedic implants
Introduction
Orthopaedic implant occupies a special and vital position in both clinical applications and biomedical industry. In 2016, the orthopaedic device market in the United States has reached 15.8 billion USD, [1] while this value was 6.1 billion USD in Asia [2]. The global orthopaedic device market value was estimated to reach 45.0 billion USD by 2020 [3]. Besides, the global orthopaedic trauma devices, such as plates, screws and external fixators, have possessed a market of 5.7 billion in 2013, which was forecasted to have a highest compound annual growth rate of 7.2% until 2020 [4].
Orthopaedic implants could be categorised into the following four main applications, including joint replacement, spinal implants, orthobiologics and trauma implants [5]. Those different types of the orthopaedic implants are basically derived from nearly all the clinical needs and developed with an extraordinary speed. Orthopaedic implants are Class III medical devices that shall be well-designed, tested and developed based on specific clinical indications to realise their expected functions within body [5]. This article mainly focuses on the internal implants designed for bone fracture fixation.
Bone fractures and fracture healing
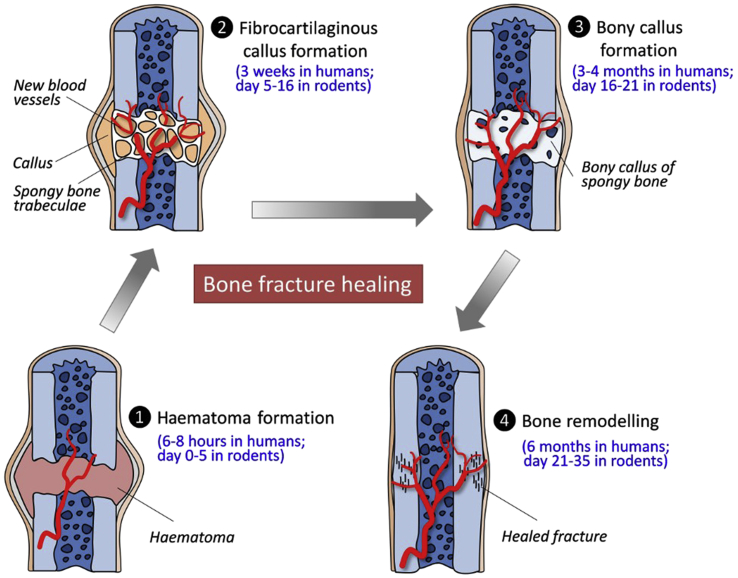
Bone fractures can be categorised as complete or incomplete, simple or comminuted, close or open fractures [6], [7]. There are normally three phases in the fracture healing irrespective of differences in their fracture types, including reactive phase (haematoma and inflammatory stage), reparative phase (callus formation stage) and remodelling phase (remodelling stage) (Figure 1) [8], [9].
Figure 1.
Fracture healing phases and tissue responses in both humans and rodents.
The initial reactive phase of fracture healing can be regarded as an anabolic phase with the activities of inflammatory cells and stem cells. This phase can last several hours in humans, which mainly consists of the recruitments and differentiation of stem cells that form the subsequent skeletal tissues and vessels. After this phase, a cartilaginous callus is formed adjacent to the fracture line immediately to restore an early stability of the fractured bones. Afterwards, endochondral ossification of the formed cartilaginous tissue occurs, and nascent blood vessels are simultaneously formed in the surrounding tissue. Cartilage extracellular matrix will then undergo mineralisation and reduction in the volume of the callus tissues, indicating bone callus remodelling. Bone remodelling from woven bone towards lamellar bone is a relatively long phase which can start about 6 months after the fracture and last up to 1–2 years [9], [10], [11], [12], [13]. At last, the bone returns to its original shape with sufficient mechanical strength, with blood supply towards its pre-injury level [14]. This fracture healing process might be accelerated if favourable micromotion is presented at the fracture sites [10].
Nevertheless, these healing phases and healing mechanisms become quite different in patients with osteoporosis, where nearly all the reaction and healing phases are prolonged, and the healing abilities are limited in osteoporotic fractures [15]. Conventional orthopaedic implants made of permanent rigid metals with high stiffness are not favourable for osteoporotic fracture fixations because of deteriorated bone structure and poor mechanical stability. Too rigid fixation on the fracture site is unfavourable for fracture healing and its remodelling outcome due to limited cellular responses in periosteum and bone marrow [16]. Both clinical and experimental studies indicated that rigid internal fixators implanted over a longer period of time were unfavourable for fracture repair by increasing cortical resorption, which was even more obvious in osteoporotic fractures [17], [18]. Interfragmentary micromotion would be beneficial for callus stimulation and subsequent bone healing [19]. Therefore, bioactive materials with lower rigidity as orthopaedic implants would be preferred to improve the fracture healing for osteoporotic patients.
Fixation classifications and strategies
Bone fractures need to be fixed by stabilising the fracture fragments [6], [7], [20]. Although not all bone fractures need to be treated surgically, fracture fixation with implants is still quite common and essential to maintain a proper bone alignment and fundamental function [7], [20]. Therefore, the purpose of the fracture fixation is to restore the stability and alignment of the fractured bones, enable the healing process and avoid damages to the surrounding tissues. There are mainly two types of fracture fixations clinically, i.e., internal fixation and external fixation [7]. Meanwhile, all internal and external fixation strategies that allow proper interfragmentary movement under functional weight bearing are considered to be flexible fixation and those involving compression mechanisms are regarded as rigid fixation [6], [7], [20], [21].
In some cases, fracture healing has a close connection with the fixation stability or rigidity of the fixation. Primary fracture healing is induced by rigid fixation with direct cortical bone remodelling via “cutting-cone” mechanism on the fracture site, while secondary fracture healing is induced by flexible fixation with endochondral ossification over the fracture site [22]. Since most fractures are treated or fixed with certain degree of local micromovement, primary healing is rare [23].
The external fixations are generally regarded as flexible fixation. External fixator consists of pins, wires (Schanz screws, Steinman pins, Kirschner wires) and belts that are widely used as a dynamic fixation of fractured bones. This strategy mainly applies for open fractures with substantial soft-tissue injuries, open Type II, Type III fractures and even in joint arthrodesis, in which the surgical trauma to the limb during fixation is minimised (Figure 2A) [7], [24], [25].
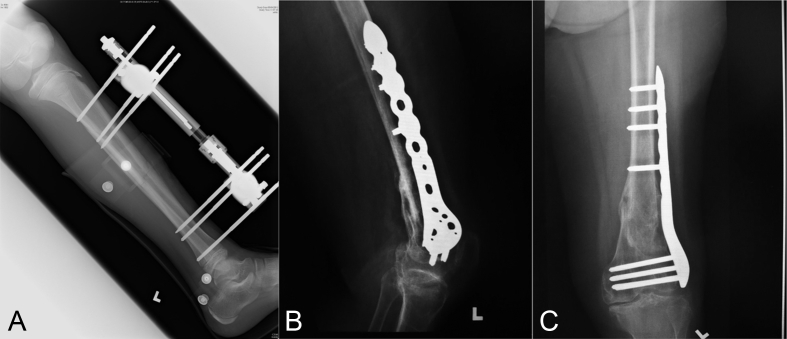
Figure 2.
(A) Typical external fixators applied in tibial shaft fracture; (B) and (C) internal fixators used for fixation of distal femoral fracture.
Apart from external fixation, another strategy for bone fracture fixation is internal fixation, which has been widely used to restore bone anatomy and also enable early mobilisation of the bone fragments. This strategy is able to restore the function of the injured bones and provide an instant mechanical support for physiological load at the fracture site. The majority of internal fixation implants are currently made of stainless steel and titanium (Ti). These devices can be roughly divided into wires, plates and screws, pins and intramedullary nails or rods (Figure 2B and C) for fracture fixation at various skeletal sites (Table 1) [6], [7], [20], [21], [26], [27], [28], [29].
Table 1.
| Fracture sites | Internal fixators | |
|---|---|---|
| Head | Skull fracture | Wires, pins and plates |
| Craniofacial fracture | Wires, screws and plates | |
| Trunk | Clavicle fracture | Intramedullary nail and plates |
| Scapular fracture | Screws and plates | |
| Pelvic fracture | Screws, plates and external fixators | |
| Spinal fracture | Fixation device consists of rods, pedicle screws and plates | |
| Upper limb fracture | Humeral fracture | Open reduction with plate and screws/close reduction with intramedullary nail |
| Radius, ulnar fracture | Open reduction with plate and screws/close reduction with intramedullary nail | |
| Metacarpal and phalangeal fracture | Close reduction with external fixators, open reduction with intramedullary nail, screws and plates | |
| Lower limb fracture | Femoral fracture | Open reduction with plate and screws/close reduction with intramedullary nail |
| Tibial and fibular fracture | Open reduction with plate and screws and intramedullary nail | |
| Metatarsus fracture | Open reduction with plate and screws and intramedullary nail | |
| Calcaneal fracture | Close reduction and fixation with screws or wires | |
Internal fixation has low incidence of malunion, high stability and most importantly no need for external immobilisation to realise an immediate movement of nearby joints. Patients with internal fixators can move freely and much less bound to the fixators, which greatly contributes to fast recovery in some circumstances [27].
However, internal fixators made of stainless steel or Ti also have some limitations. They are rigid materials with much higher Young's modulus and stiffness compared to human cortical bones. Fixation with these high rigidity materials prevents load transfer to the healing bone that is unfavourable for fracture callus remodelling [31]. Since internal fixators are implanted into the human body, surgical removal procedures can also be a concern for the patients. Recently, biocompetitive, biodegradable and bioactive materials have been reported to be absorbed gradually in vivo during the fracture healing process and therefore extensively tested as internal implants for orthopaedic applications [32], [33], [34], [35]. As the application of biodegradable biomaterials may avoid later implant-removal surgery or also known as “second operation”, this is very attractive for both orthopaedic surgeons and patients.
Research and development history of orthopaedic plates and screws
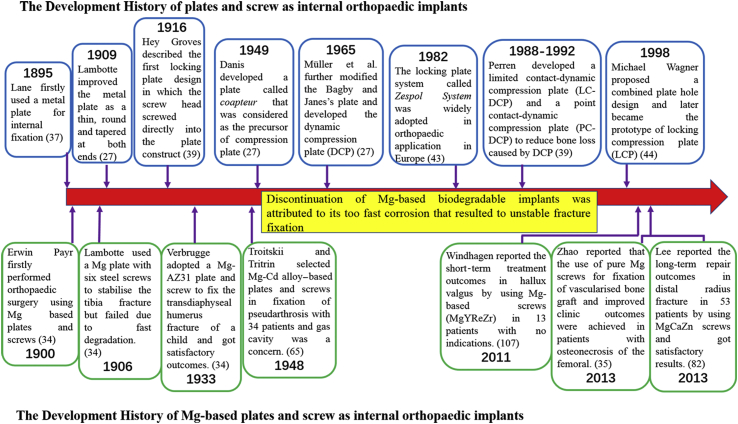
Orthopaedic plates as internal fixators have been tested for more than 100 years (Figure 4) [27]. The first report by Hansman was back in 1886, introduced a case of fracture fixed with a bone plate made of an alloy of nickel, copper and tin [36]. Lane also reported his innovative steel plate and lag screws for bone fracture fixation in 1895, which was considered to be the precursor of current cancellous thread forms [37]. Corrosion of the implants and incompatibility of these nickel–copper alloy have been solved by the invention and application of stainless steel as orthopaedic implants since 1931 [38], [39]. Surgical grade stainless steel alloys, also termed as 316L, was made of iron, chromium and nickel and have excellent corrosion resistance, high mechanical strength and better biocompatibility compared to the silver–aluminium alloys used in the past [39]. Danis introduced the world's first compression plate in 1949 [27], and titanium (Ti) has been developed as orthopaedic implants since 1951 [40]. Compared to 316L stainless steel and other alloys used before, Ti has very high biocompatibility and low corrosion rate, which has showed quite good long-term results with few controversies. In 1958, Swiss surgeon Maurice Müller and his team studied the process of bone healing and the influence of rigid fixation on fracture repair, which gave birth to the subsequent world's famous organisation, the Association for the Study of Internal Fixation [41], [42]. In 1965, Müller et al. successfully developed a plate with a tensioner to provide interfragmentary compression during tightening processing, known as which, was called the dynamic compression plate (DCP) and was officially documented in AO technique in 1973 [27]. In 1988, Perren et al. developed another compression plate which had limited contact with the bone surface, which was to preserve the blood supply and protect the periosteum [39]. Apart from the DCP, Hey Groves introduced the first locking plate construct in which the screw heads screwed directly into the plate in 1916 [39]. In 1982, a locking plate system named as Zespol System was finally widely used in clinical after years of developments [43]. In 1998, Michael Wagner proposed a design of combined plate hole which intended to develop a plate that possessed the advantages of both DCP and locking plate system, which gave birth to the development of locking compression plate [44]. During the past several decades, some new metals such as tantalum, some nonmetallic materials such as poly-lactic acid have been studied and developed as internal orthopaedic implants and also applied to clinical applications [39], [45], [46], [47], [48].
Figure 4.
Development of orthopaedic metallic implants (screw–plate) for internal fixation of bone fractures from the published representative clinic studies.
Hybrid fixation implants designed for orthopaedic applications
With healing over time, the fractured bone callus becomes more rigid. Apart from implant designs, the ideal metallic implants for fracture fixation should be made with metals of different Young's modules to match the mechanical properties of healing tissues [49]. Alloying technology and combined utilisations of different metallic materials became the solution, and great efforts were made to improve the performances of the implants. As mentioned above, the 316L stainless steel and Ti-6Al-4V are the most commonly used alloy materials for fabricating orthopaedic implants, which show good mechanical properties and corrosion resistances in orthopaedic applications. However, single material can hardly fulfil the task due to the multiple requirements of the complicated situations in the human body. For example, Ti-6Al-4V has very good biocompatibility but not high wear resistance, while cobalt–chrome (Co–Cr) alloy has the reversed mechanical properties. There were recently some reports that indicated that the generated wear debris from Co–Cr during wear process could stimulate tissue reactions, e.g., lymphocytic infiltrations and cell necrosis that can affect implant performance [50]. Therefore, the combined use of these different materials has been studied and tested for various orthopaedic applications since 1960s [5]. The total hip arthroplasty is a typical successful application that involves various materials to cope with the complex in vivo environment. A total hip arthroplasty prosthesis is made of miscellaneous materials, such as Ti, Co–Cr alloy, polyethylene and polymethylmethacrylate, which showed both high mechanical strength and wear resistance [51]. Over the past century, different combinations of materials have been developed as hybrid fixation implants. These implants not only provided enough mechanical strength to support the broken bones or anatomical structure around, but showed good wear resistance to realise a long-term fixation stability as well [5], [52], [53]. Besides, interactions between the materials and the living cells and tissues are even more important. The non-living materials are regarded as foreign bodies within a biological system, which induced series reactions of the surrounding cells and tissues. After series studies and experiments, a significant number of materials were identified for testing their biocompatibilities and mechanical properties for different clinical applications [54].
In recent years, biodegradable materials, such as iron (Fe), magnesium (Mg), poly-caprolactone (PCL) and poly-lactic acid, have been developed for musculoskeletal applications [5], [33], [47]. These innovative biodegradable materials degrade gradually in vivo with no implant residues, and the “second operation” for implant removal can be avoided. These biodegradable materials show different mechanical properties, in vivo degradation rates and tissue reactions [47], [55], [56], [57]. Proper combined utilisations could be a promising method to develop an ideal biodegradable orthopaedic implant for clinical applications.
In general, hybrid fixation implants have been widely used over several decades and contributed a lot in orthopaedic applications. Nevertheless, adverse effects arising from interactions at the implant surface might result in implant failures.
Limitations of current fixation implants
Implant removal surgical procedure and concerns
Clinically, patients are mostly concerned about the secondary surgical procedure for removing the permanent implants. Operative therapy for fracture fixation using metal implants has gradually replaced the conservative and nonoperative treatments for achieving early mobility since the World War II [58]. Necrosis, inflammation, pain, allergic reactions and even cancer potentially result from the fixation implants have been reported frequently in 1970s and 1980s [40], [59]. All these adverse effects and indications have made the implant removal as a clinical routine ever since [58]. Taking implant removal cases from the upper extremity as an example, two reports declared that the complication rates among 23 and 37 cases of implant removals (mostly plates removal) were 26% and 19%, respectively, due to indications like nerve injuries, implant breakage or refracture [60], [61]. In lower extremity, the implant removal (mostly nails) due to such indications was however much less but more with complications of necrosis and infections [62], [63], [64]. Besides, cut-out of the implants were also an indication which forced the surgeon to perform implant removal surgery for our patients. By analysing refracture cases, the poor quality of newly formed bone was the main explanation that might even further weaken during the removal procedures.
Although different types of metallic materials have been developed and tested, one problem shared by most of them is their too high rigidity and Young's modulus that affects the fracture healing and callus remodelling [65], [66].
Meanwhile, a survey in UK. indicated that the routine implant removal was mostly objected (only 3% advocated) by the orthopaedic surgeons when dealing with patients aged over 35 years [67]. It is obvious that the routine implant removal is unfavourable for both surgeons and patients. However, there is still a variety of view points with huge differences in the routine implant removal among the surgeons over the world [58].
Impaired bone fracture healings due to ageing and osteoporosis
Apart from surgical fixation, fracture healing showed quite different situations with respect to differences in age and/or bone quality, such as in healthy or osteoporotic conditions. Current fixation implants have their limitations for effective applications on several challenging conditions, such as osteoporosis of elderly people. Patients with osteoporotic fractures have limited osteogenic potential and therefore show delay in healing, and they are also at high risk of suffering further fragility fractures due to impaired mobility [68].
Rigid fixation via current stiff steel or Ti implants developed for bone fracture repair has its significant drawback for fixation of osteoporotic bone [69]. Furthermore, refracture of osteoporotic bone caused by the removal of the fixation plate is also a critical issue especially for elderly people as compared to those in younger patients due to significant poor bone quality [70]. Although routine implant removal is unnecessary for elderly patients, indications (pain, infection, aseptic loosening) caused by these bioinert metal implants need to be controlled to avoid complication-induced removal [58], [71].
Apart from the challenges in fixation of osteoporotic fractures, its healing process is also prolonged as compared with normal one, in spite of similarity in all three healing stages of osteoporotic fracture compared to normal bone fracture repair [15], [72]. This has also been proved in many animal studies [73], [74], [75]. Reduced callus formation around the fracture site and low bone mineral density has been found in long bone fractures of osteoporotic animals. Meanwhile, a clinical study also showed significant increase in fixation failure rate in osteoporotic patients [76].
There were several possible explanations for the fracture healing disturbances in osteoporosis situations. One mechanism was limited availability of mesenchymal stem cells observed in osteoporotic individuals, implying limited cell sources for facilitating fracture repair [77], [78]. Clinical case study also reported that mesenchymal stem cells in postmenopausal women with osteoporosis had a lower rate of growth and lack of differentiation ability compared to premenopausal women [79]. Even bone cells from osteoporotic patients were discovered to be less active and had an impaired response to mechanical stress [72], [80].
Development of innovative orthopaedic implants as potential internal fixators
The orthopaedic implants have been developed to overcome the above limitations over the years. Generally, there are several research directions to improve the bioactivity, osteo-integrity and application methods of the orthopaedic implants. From the aspect of implant material, bioactive and biodegradable materials have been developed as orthopaedic implants and showed great application potentials [32], [33]. Bioactive and biodegradable metallic materials like Mg, strontium and iron have been introduced to orthopaedic applications [35], [81], [82], [83]. Besides, nanotechnology has been revealed to show important potentials in modifying the interaction between orthopaedic implants and host bone [84]. It was reported that a single inorganic mineral matrix of mature bone was within nanoscale, which made the application of a nanotextured material reduce the risk of implant failure [85], [86]. Surface coating is another method to improve the performances of orthopaedic implants. Inorganic coatings like calcium phosphate–based coatings can effectively enhance the osteointegration of orthopaedic implants [87]. Biomolecular coatings on the implants enhance not only bioactivity but promote the bone formation as well [88], [89].
Research and development of Mg-based biodegradable metallic and hybrid orthopaedic implants
Research and development of Mg-based biodegradable orthopaedic implants
Mg, as biodegradable implants, has attracted much attention from material scientists, biomedical scientists and clinicians in recent decades. Mg is the fourth most abundant element in human body and distributed mostly in skeletons and also in soft tissues especially skeletal muscles where Mg ions are involved in about 300 known enzymatic reactions [90], [91]. Mg also possesses good mechanical properties compared to currently used stainless steel and titanium with regards to bone material and mechanical properties (Table 2).
Table 2.
Summary of the mechanical properties of various implant materials compared to natural bone (adopted from studies by Husain et al [63], Staiger et al [65]).
| Properties | Natural bone | Magnesium | Ti alloy | Stainless steel | Polymers |
|---|---|---|---|---|---|
| Density (g/cm3) | 1.8–2.1 | 1.74–2.0 | 4.4–4.5 | 7.9–8.1 | 1.2–1.3 |
| Elastic modulus (Gpa) | 3–20 | 41–45 | 110–117 | 189–205 | 3–4 |
| Compressive yield strength (Mpa) | 130–180 | 65–100 | 758–1117 | 170–310 | 40–60 |
Mg is active chemically and can degrade in aqueous solutions with the formation of magnesium hydroxide (Mg(OH)2) and hydrogen (H2), which gives Mg the biodegradability in vivo. It also has excellent biocompatibility and osteopromotion effect through periosteum (Figure 3, adopted from study by Zhang et al [16]), which makes it the most potential biodegradable metallic implants.
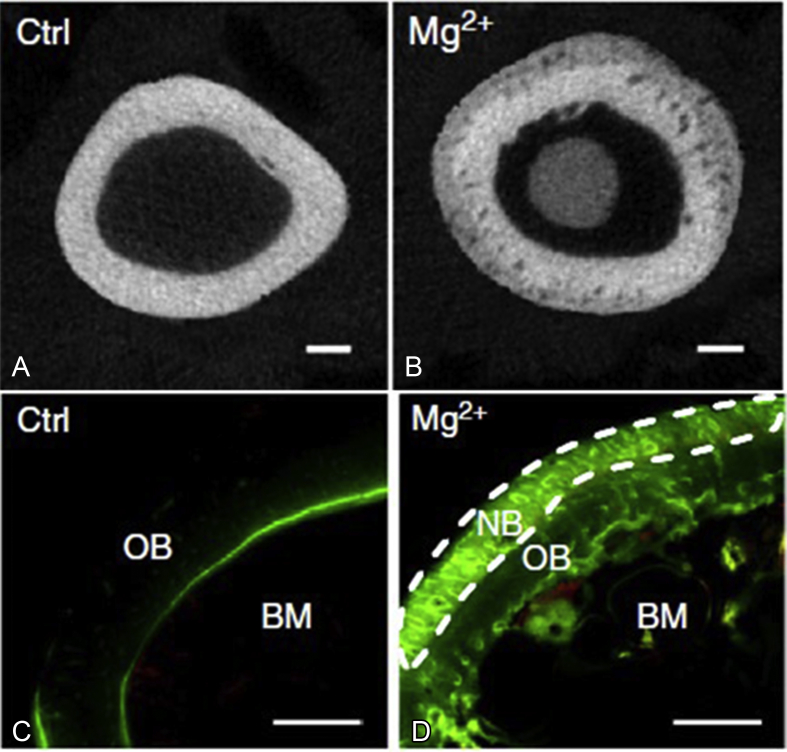
Figure 3.
(A) X-ray cross-sectional image of the rats' femora without implantations of Mg pins in the bone marrow cavities; (B) X-ray cross-sectional image of the rats' femora with implantations of Mg pins in the bone marrow cavities; (C) fluorescent labelling image of rats' femora without implantations of Mg pins in the bone marrow cavities; (D) fluorescent labelling image of rats' femora with implantations of Mg pins in the bone marrow cavities. Old bone area (OB), new bone area (NB) and bone marrow area (BM) were marked. Both the X-ray and fluorescent labelling images showed more new bone formations in the rats implanted with Mg pins (B, D), as compared to those without Mg pins (A, C) [16].
From the first Mg clinical trial in 1892 [34], Mg-based implants have been tested both in human and in animals for over 100 years. In 1900, Payr first proposed the Mg implants for orthopaedic applications, such as pins, nails, plates, sheets, etc. [34]. Tremendous efforts have been made to the development and application of Mg implants in orthopaedics over the past century although the progress was rather slow (Figure 4).
The main limitation of Mg tested in the past was its lower purity that led to rapid degradation after implantation and therefore lost its mechanical support too early to maintain its function, e.g., for fracture fixation, together with local hydrogen accumulation [34], [65]. To enhance the degradation resistance of Mg, there are several approaches, including purifying, alloying with another or more metals and surface modification [34], [92]. With advancement in metallurgy, high purity Mg is now available with significantly reduced degradation properties. It is reported that fewer alloying elements in alloy materials provide higher corrosion-resistance due to much reduced galvanic corrosion [93]. Different kinds and combinations of Mg alloys were also developed, and their properties were studied in vitro, which also showed comparable high corrosion resistance and better mechanical properties [94], [95]. An appropriate alloying composition can generate a fine grain structure of the developed alloy that could truly enhance the corrosion resistance [65].
During the past two decades, many research groups worldwide devoted themselves into Mg and related studies for orthopaedic applications [94], [95], [96], [97], [98], [99], [100], [101], [102], [103], [104], [105], [106]. All published work indicated their potentials to develop Mg-based orthopaedic implants. In 2013, the world's first biodegradable Mg-based screw (MgYReZr, Mg–yttrium–neodymium–gadolinium–zirconium) called MAGNEZIX by Syntellix was approved in Germany with CE mark for application in less load-bearing skeleton sites without requirement for implant removal [107]. In 2015, another Mg alloy screw product (Mg–Ca–Zn) was approved in Korea for successful fixation of knuckle in patients with no complications [82]. During the past several years, Zhao et al have conducted the bone grafting fixation using biodegradable pure Mg screw for successful fixation of bony flap used for treatment of osteonecrosis of the femoral head and with detailed published in 2016 [35] although multicentre clinical trials are required before official registration of Mg-related products [108].
However, all the three successful clinical applications of Mg-based screws described above were tested at low load-bearing skeletal sites. The current available Mg-based implants have limited range of application. For long bone fractures and other critical load-bearing sites, there are still no published reports on their successful applications. Meanwhile, there are few published articles about fracture fixation using Mg plates and screws in animal studies [109], [110], [111]. Fast degradation and limited mechanical strength are the main concerns for Mg-based implants applied at critical load-bearing skeletal sites. Recently, an innovative Mg-based “super-nanometre-sized dual-phase glass-crystal” (SNDP-GC) alloy has been developed by magnetron sputtering [112]. This developed Mg-based SNDP-GC alloy showed an ultimate stress of 3.3 GPa, which was much higher than those of conventional Mg-based metallic glass (around 1 GPa) and Mg-based crystalline alloy (0.46 GPa) [113]. Besides, both Young's modulus and strain limit of the Mg-based SNDP-GC alloy were improved, as compared to the conventional Mg-based metallic glass and crystalline alloys [112]. This innovative Mg-based SNDP-GC alloy showed great potential in development of internal fixators for fracture fixation at critical load-bearing skeletal site.
R&D of Mg–containing hybrid fixation system
From the above studies about Mg-based implants, it is obvious that Mg implants provide comparable fixation efficiencies and osteopromotion effect in fracture fixation. However, concerns and complications from patients of using Mg-based implants at crucial load-bearing skeletal site(s) is predictable. To expand the application of Mg-based implants, a combination use of Mg and other metallic material to develop an Mg-related hybrid fixation system is a potential method.
Mg-related hybrid fixation implants were firstly tested clinically in 1906 by Lambotte, who used one Mg plate with six steel screws together to fix a fractured tibia. Unfortunately, this trial failed due to extensive subcutaneous gas formation and accumulation, local swelling and pain of the patient only 1 day after its implantation [34]. Therefore, Lambotte realised that Mg corroded extremely fast when combined with other metals for application, which had a great impact on the subsequent Mg-related researches. Since then, nearly all the orthopaedic surgeons agreed that Mg could only be implanted without combined use with other metallic implants, which was later proved to be caused by electrolytic corrosion [34], [39]. Meanwhile, it was also accepted that Mg alone was not strong enough to support the fractured bones at load-bearing skeletal sites and the fast degradation made the clinical application more challenging. Since then, surgeons and metallurgists have started to work on other metallic materials as fixation implants for clinical applications [39].
As reviewed above, osteoporotic fracture fixation and repair is a great challenge for orthopaedic surgeons because of poor bone quality and the impaired fracture healing abilities. Too rigid fixation is not favourable for osteoporotic fracture fixation. Patients with osteoporosis have lower bone mass and architecture deterioration leading to poor bone quality. The compromised bone strength affects anchorage of the orthopaedic implants that weakens the fracture fixation stability. Several studies reported that osteoporotic patients showed delay in callus formation, its ossification and remodelling. The failure rate of fixation in patients with osteoporosis range from 10% to 25% [114], and orthopaedic surgeons reported unfavourable prognosis of osteoporotic fracture fixation using current implants that were suitable for fracture fixation of younger patients [115], [116]. To solve these challenging problems in osteoporotic fracture fixation and repair, authors’ group has proposed an innovative construct of Ti/Mg hybrid fixation system. The mature Ti internal fixators and bioactive Mg implants are combined to realise a strong and osteopromotive hybrid fixation system, which has demonstrated great potential for its application in osteoporotic fracture healing.
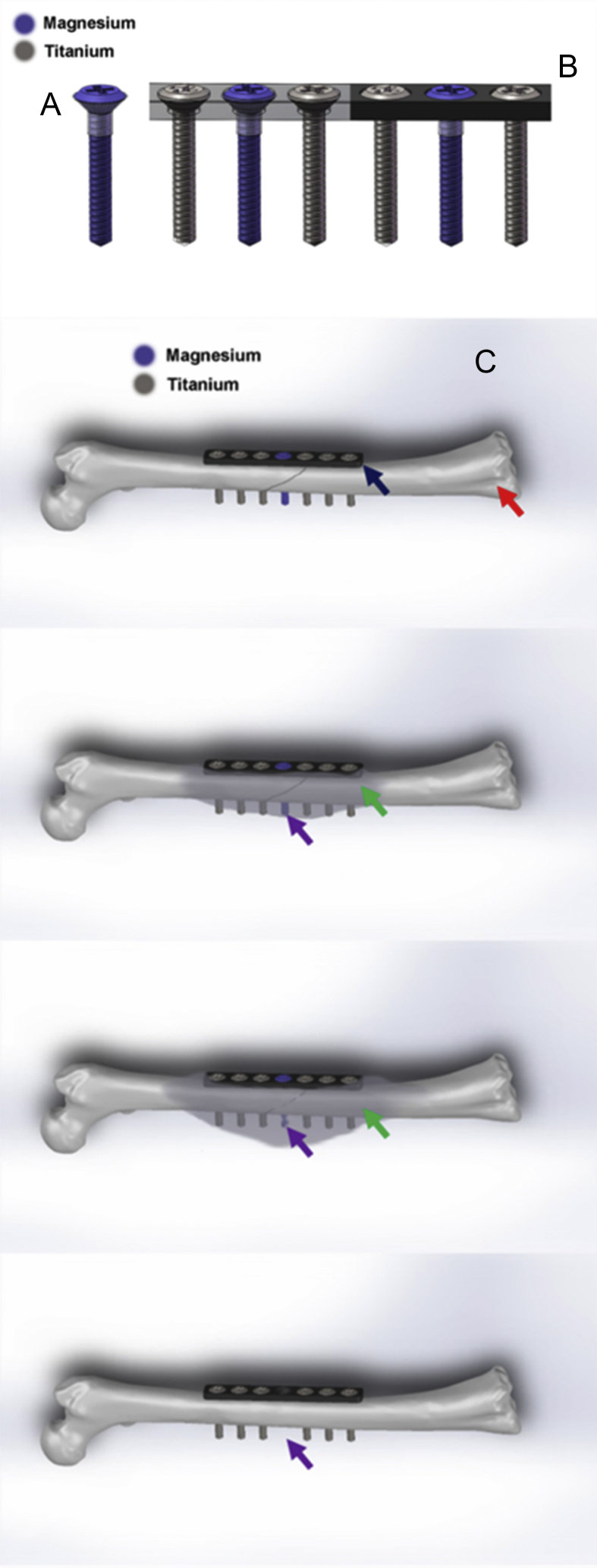
With recent huge advancement in metallurgy, it enabled us to develop a high-purity Mg (99.99%) for developing fixation implants. Yet high-purity Mg has rather weak mechanical properties, a combined use of Mg and other routine fixation implants made of Ti could be a solution for achieving both essential mechanical support and osteogenic function as the degradation of Mg after implantation. To realise the combination use, the electrolytic corrosion (galvanic corrosion) needs to be controlled due to the different equilibrium potentials of these two metallic materials [117], [118]. Therefore, a thin compact layer of polymer coating film has been developed on the surface of Mg screw, which works as a barrier to prevent direct contact between Mg and Ti implants (Figure 5A and B).
Figure 5.
(A) The Mg screw coated with a thin layer of polymer coating film on the screw head (transparent part); (B) the plate with coated Mg screw (purple one) and Ti screws; (C) hypothesised working mechanisms of the proposed innovative Mg-containing hybrid fixation system for fracture fixation at long bone fracture. The fracture was fixed by Ti plate (pointed by dark blue arrow) and six Ti screws (demonstrated with dark grey). The purple screw next to the fracture gap is Mg screw. The positions and number of the Mg screws can be justified according to the fracture types. After fixation, the Mg screw degrades gradually (pointed by purple arrows) and larger fracture callus (pointed by green arrows) is induced to promote fracture healing, based on previous published articles [16], [111]. When the fracture heals, the Mg screw is fully absorbed and no screw hole is remained (pointed by purple arrow in the last figure). With less fixation screws remained, the fixation system can be easily removed and the healing efficiency and quality can be both enhanced by the degradation of Mg screws.
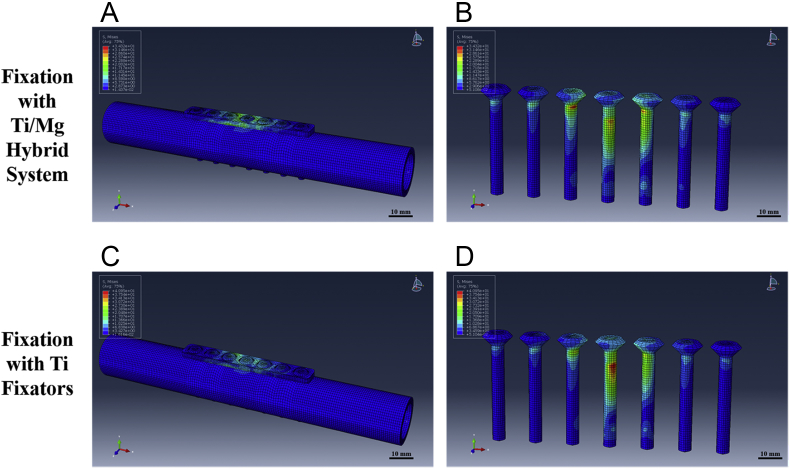
In this design, Ti implants could provide enough mechanical support, and the Mg implants are able to support and contribute to the fracture healing. Mg has similar Young's modulus and yield strength compared to human cortical bones. Therefore, the fixation with Mg implant is not rigid for osteoporotic patients, which can induce more callus formation with degradation of Mg implants over time during fracture healing. The coated Mg implants can stabilise the fragments at early stage and then promote the fracture healing with Mg degradation. A finite element analysis was performed through Abaqus 6.13.1 (Dassault Systèmes Simulia Corp., Willits, France) to test the feasibility of this hybrid fixation system. Two cylinders were established as the mimic models of cortical bone segments with the size of rabbit tibiae based on published work [110], [119]; the Ti/Mg hybrid fixation system were established based on AO 2.0 mm dynamic compression plate (DCP) and the matched cortical screws [6], [30]. Another model fixed with Ti plates and screws was also established as the control. A certain torque of 30 Nm (around 1.5 times the body weight of rabbit) was added to both groups, and the generated maximum stress was recorded. Our modelling results indicated that the maximum stress applied on the hybrid system was even lower than that on the Ti fixators, and no failure was observed after the simulation, suggesting that the hybrid fixation system was suitable to fix the fracture of cortical bones (Figure 6).
Figure 6.
Finite element analysis results of the Ti/Mg hybrid fixation system. No fixation failure was observed in the simulation results and the maximum stress of the Ti/Mg hybrid fixation system was comparable to that in Ti group; (A) and (B), the stress distribution of the models fixed with Ti/Mg hybrid fixation system; (C) and (D), the stress distribution of the models fixed with Ti fixators.
As previously reported, Mg can stimulate periosteum to generate more callus tissues via upregulation of local Calcitonin Gene-Related Peptide (CGRP) secretion of a known neuronal protein for acceleration of fracture healing process [16]. An innovative Mg-containing Ti intramedullary nail has been developed to fix the fractured femur in rats, and the fracture healing process was found accelerated and enhanced [16]. Therefore, this innovative Ti/Mg hybrid fixation construct might have potential to be extended to plate–screw system, where the degrading Mg implant will provide less fixation stability at the fracture site with healing over time. In addition, at the time of implant removal, less screws need to be removed that would prevent a second damage to the newly healed tissues due to screw removal from the fracture site. Nevertheless, implant removal is not essential in some cases, thus the Mg implant can degrade thoroughly and merge into the surrounding tissues. In this scenario, there will be no screw hole remained at the fracture site, which can further improve the healing quality (Figure 5C). Therefore, this system will reduce the refracture rate and enhance the healing quality of fracture site.
Summary
It has been nearly 130 years after the invention of first metal plate for bone fracture fixation attributed to collective efforts of orthopaedic surgeons, metallurgists and engineers. Apart from traditional and even widely used metallic materials, such as gold, silver, cooper, stainless steel and titanium, biodegradable polymers or magnesium become attractive as they can be developed to biodegradable fixation implants. However, implant made of only one type of material can hardly fulfil multiple demands of fracture fixations, especially at load-bearing skeletal sites. However, electrochemical corrosion of the implants, adverse tissue reactions and inferior healing has been always the concerns of our orthopaedic surgeons. Bioactive and biodegradable materials such as Mg and its alloys have also been tested and developed over the past centuries until recently Mg-related orthopaedic products were approved for clinical applications in Europe and Asia. To expand the clinical applications of Mg implants, Mg-related hybrid fixation systems will also attract attention from both surgeons and material scientists after being abandoned for over a hundred years. After years of research and development of Mg-based implants, innovative hybrid system made of Mg and Ti fixation implants are proposed and that may become a cost-effective fracture fixation concept for future applications, especially for biological fixation of challenging osteoporotic fractures and potential reduction of subsequent refracture rate.
Conflicts of interest statement
The authors have no conflicts of interest to declare.
Acknowledgement
This work was supported by Hong Kong RGC Collaborative Research Fund (C4026-17W), Theme-based Research Scheme from General Research Fund (No. T13-402/17-N), Innovation and Technology Fund (No. ITS/350/13).
References
- 1.Americas accounted for $15.8 billion in orthopedic devices market in 2016. 2017. https://trovenlagovau/work/225026940?q&versionId=246827875 [Google Scholar]
- 2.Asia accounted for $6.1 billion in the orthopedic devices market in 2016. 2017. https://trovenlagovau/work/225229211?q&versionId=247038530 [Google Scholar]
- 3.Global orthopedic devices market to grow to $45.0 billion by 2020. 2017. https://trovenlagovau/work/224918748?q&versionId=246712670 [Google Scholar]
- 4.Global market study on orthopedic trauma devices: plate and screw external fixator system to witness highest CAGR of 7.1%. 2014. https://wwwpersistencemarketresearchcom/market-research/orthopedic-trauma-devices-marketasp [Google Scholar]
- 5.Wang W., Ouyang Y., Poh C.K. Orthopaedic implant technology: biomaterials from past to future. Ann Acad Med Singapore. 2011 May;40(5):237–244. [PubMed] [Google Scholar]
- 6.Helfet D.L., Haas N.P., Schatzker J., Matter P., Moser R., Hanson B. AO philosophy and principles of fracture management-its evolution and evaluation. J Bone Jt Surg Am Vol. 2003 Jun;85-A(6):1156–1160. [PubMed] [Google Scholar]
- 7.Taljanovic M.S., Jones M.D., Ruth J.T., Benjamin J.B., Sheppard J.E., Hunter T.B. Fracture fixation. Radiographics. 2003 Nov-Dec;23(6):1569–1590. doi: 10.1148/rg.236035159. [DOI] [PubMed] [Google Scholar]
- 8.Szczesny G. Fracture healing and its disturbances. A literature review. Ortop Traumatol Rehabil. 2015 Oct;17(5):437–454. doi: 10.5604/15093492.1186809. [DOI] [PubMed] [Google Scholar]
- 9.Einhorn T.A., Gerstenfeld L.C. Fracture healing: mechanisms and interventions. Nat Rev Rheumatol. 2015 Jan;11(1):45–54. doi: 10.1038/nrrheum.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phillips A.M. Overview of the fracture healing cascade. Injury. 2005 Nov;36(Suppl. 3):S5–S7. doi: 10.1016/j.injury.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 11.Hausman M.R., Schaffler M.B., Majeska R.J. Prevention of fracture healing in rats by an inhibitor of angiogenesis. Bone. 2001 Dec;29(6):560–564. doi: 10.1016/s8756-3282(01)00608-1. [DOI] [PubMed] [Google Scholar]
- 12.Kurdy N.M., Weiss J.B., Bate A. Endothelial stimulating angiogenic factor in early fracture healing. Injury. 1996 Mar;27(2):143–145. doi: 10.1016/0020-1383(95)00169-7. [DOI] [PubMed] [Google Scholar]
- 13.Lee F.Y., Choi Y.W., Behrens F.F., DeFouw D.O., Einhorn T.A. Programmed removal of chondrocytes during endochondral fracture healing. J Orthop Res. 1998 Jan;16(1):144–150. doi: 10.1002/jor.1100160124. [DOI] [PubMed] [Google Scholar]
- 14.Melnyk M., Henke T., Claes L., Augat P. Revascularisation during fracture healing with soft tissue injury. Arch Orthop Trauma Surg. 2008 Oct;128(10):1159–1165. doi: 10.1007/s00402-007-0543-0. [DOI] [PubMed] [Google Scholar]
- 15.Giannoudis P., Tzioupis C., Almalki T., Buckley R. Fracture healing in osteoporotic fractures: is it really different? A basic science perspective. Injury. 2007 Mar;38(Suppl. 1):S90–S99. doi: 10.1016/j.injury.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y., Xu J., Ruan Y.C., Yu M.K., O'Laughlin M., Wise H. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat Med. 2016 Aug 29 doi: 10.1038/nm.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davidson C.W., Merrilees M.J., Wilkinson T.J., McKie J.S., Gilchrist N.L. Hip fracture mortality and morbidity–can we do better? N Z Med J. 2001 Jul 27;114(1136):329–332. [PubMed] [Google Scholar]
- 18.Sha M., Guo Z., Fu J., Li J., Yuan C.F., Shi L. The effects of nail rigidity on fracture healing in rats with osteoporosis. Acta Orthop. 2009 Feb;80(1):135–138. doi: 10.1080/17453670902807490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bagheri Z.S., Tavakkoli Avval P., Bougherara H., Aziz M.S., Schemitsch E.H., Zdero R. Biomechanical analysis of a new carbon fiber/flax/epoxy bone fracture plate shows less stress shielding compared to a standard clinical metal plate. J Biomech Eng. 2014 Sep;136(9):091002. doi: 10.1115/1.4027669. [DOI] [PubMed] [Google Scholar]
- 20.Hunter T.B., Taljanovic M. Overview of medical devices. Curr Probl Diagn Radiol. 2001 Jul–Aug;30(4):94–139. [PubMed] [Google Scholar]
- 21.Moreau P.G. Book review: master techniques in orthopedic surgery: the spine. Ann Saudi Med. 1997 Nov;17(6):669–670. [Google Scholar]
- 22.McKibbin B. The biology of fracture healing in long bones. J Bone Jt Surg Br Vol. 1978 May;60-B(2):150–162. doi: 10.1302/0301-620X.60B2.350882. [DOI] [PubMed] [Google Scholar]
- 23.Einhorn T.A. The cell and molecular biology of fracture healing. Clin Orthop Relat Res. 1998 Oct;(355 Suppl.):S7–S21. doi: 10.1097/00003086-199810001-00003. [DOI] [PubMed] [Google Scholar]
- 24.McGarvey W.C. The use of external fixation in arthrodesis and salvage of the foot and ankle. Foot Ankle Clin. 2002 Mar;7(1):147–173. doi: 10.1016/s1083-7515(01)00005-5. [DOI] [PubMed] [Google Scholar]
- 25.Toh S., Harata S., Tsubo K., Inoue S., Narita S. Combining free vascularized fibula graft and the Ilizarov external fixator: recent approaches to congenital pseudarthrosis of the tibia. J Reconstr Microsurg. 2001 Oct;17(7):497–508. doi: 10.1055/s-2001-17752. discussion 9. [DOI] [PubMed] [Google Scholar]
- 26.Parker M.J., Stockton G. Internal fixation implants for intracapsular proximal femoral fractures in adults. Cochrane Database Syst Rev. 2001;(4):CD001467. doi: 10.1002/14651858.CD001467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uhthoff H.K., Poitras P., Backman D.S. Internal plate fixation of fractures: short history and recent developments. J Orthop Sci. 2006 Mar;11(2):118–126. doi: 10.1007/s00776-005-0984-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stover M. Distal femoral fractures: current treatment, results and problems. Injury. 2001 Dec;32(Suppl. 3):SC3–SC13. doi: 10.1016/s0020-1383(01)00179-6. [DOI] [PubMed] [Google Scholar]
- 29.Zheng N., Tang N., Qin L. Atypical femoral fractures and current management. J Orthop Transl. 2016;10//(7):7–22. doi: 10.1016/j.jot.2016.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chris Colton SK, et al. AO surgery reference. AO foundation, https://www2aofoundationorg/wps/portal/surgery.
- 31.Hayes J.S., Richards R.G. The use of titanium and stainless steel in fracture fixation. Expert Rev Med Dev. 2010 Nov;7(6):843–853. doi: 10.1586/erd.10.53. [DOI] [PubMed] [Google Scholar]
- 32.Zhao D., Witte F., Lu F., Wang J., Li J., Qin L. Current status on clinical applications of magnesium-based orthopaedic implants: a review from clinical translational perspective. Biomaterials. 2016 Oct 11;112:287–302. doi: 10.1016/j.biomaterials.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 33.Zheng Y.F., Gu X.N., Witte F. Biodegradable metals. Mat Sci Eng R. 2014 Mar;77:1–34. [Google Scholar]
- 34.Witte F. The history of biodegradable magnesium implants: a review. Acta Biomater. 2010 May;6(5):1680–1692. doi: 10.1016/j.actbio.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 35.Zhao D., Huang S., Lu F., Wang B., Yang L., Qin L. Vascularized bone grafting fixed by biodegradable magnesium screw for treating osteonecrosis of the femoral head. Biomaterials. 2016 Mar;81:84–92. doi: 10.1016/j.biomaterials.2015.11.038. [DOI] [PubMed] [Google Scholar]
- 36.Uhthoff H.K. Current concepts of internal fixation of fractures. Can J Surg. 1980 May;23(3):213–214. [PubMed] [Google Scholar]
- 37.Lane W.A. Some remarks on the treatment of fractures. Br Med J. 1895 Apr 20;1(1790):861–863. doi: 10.1136/bmj.1.1790.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Venable C.S., Stuck W.G., Beach A. The effects on bone of the presence of metals based upon electrolysis: an experimental study. Ann Surg. 1937;105(6):917–938. doi: 10.1097/00000658-193706000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greenhagen R.M., Johnson A.R., Joseph A. Internal fixation: a historical review. Clin Podiatr Med Surg. 2011 Aug;28(4):607–618. doi: 10.1016/j.cpm.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 40.Leventhal G.S. Titanium, a metal for surgery. J Bone Jt Surg Am Vol. 1951 Apr;33-A(2):473–474. [PubMed] [Google Scholar]
- 41.Matter P. History of the AO and its global effect on operative fracture treatment. Clin Orthop Relat Res. 1998 Feb;(347):11–18. [PubMed] [Google Scholar]
- 42.Roberts T.T., Prummer C.M., Papaliodis D.N., Uhl R.L., Wagner T.A. History of the orthopedic screw. Orthopedics. 2013 Jan;36(1):12–14. doi: 10.3928/01477447-20121217-02. [DOI] [PubMed] [Google Scholar]
- 43.Ramotowski W., Granowski R. Zespol. An original method of stable osteosynthesis. Clin Orthop Relat Res. 1991 Nov;(272):67–75. [PubMed] [Google Scholar]
- 44.Frigg R. Locking compression plate (LCP). An osteosynthesis plate based on the dynamic compression plate and the point contact fixator (PC-Fix) Injury. 2001 Sep;32(Suppl. 2):63–66. doi: 10.1016/s0020-1383(01)00127-9. [DOI] [PubMed] [Google Scholar]
- 45.Harrison A.K., Gioe T.J., Simonelli C., Tatman P.J., Schoeller M.C. Do porous tantalum implants help preserve bone?: evaluation of tibial bone density surrounding tantalum tibial implants in TKA. Clin Orthop Relat Res. 2010;468(10):2739–2745. doi: 10.1007/s11999-009-1222-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levine B., Sporer S., Della Valle C.J., Jacobs J.J., Paprosky W. Porous tantalum in reconstructive surgery of the knee: a review. J Knee Surg. 2007 Jul;20(3):185–194. doi: 10.1055/s-0030-1248041. [DOI] [PubMed] [Google Scholar]
- 47.Kontakis G.M., Pagkalos J.E., Tosounidis T.I., Melissas J., Katonis P. Bioabsorbable materials in orthopaedics. Acta Orthop Belg. 2007 Apr;73(2):159–169. [PubMed] [Google Scholar]
- 48.Ashammakhi N., Peltoniemi H., Waris E., Suuronen R., Serlo W., Kellomaki M. Developments in craniomaxillofacial surgery: use of self-reinforced bioabsorbable osteofixation devices. Plast Reconstr Surg. 2001 Jul;108(1):167–180. doi: 10.1097/00006534-200107000-00027. [DOI] [PubMed] [Google Scholar]
- 49.Hoeppner D.W., Chandrasekaran V. Fretting in orthopedic implants – a review. Wear. 1994 Apr;173(1–2):189–197. [Google Scholar]
- 50.Mahendra G., Pandit H., Kliskey K., Murray D., Gill H.S., Athanasou N. Necrotic and inflammatory changes in metal-on-metal resurfacing hip arthroplasties. Acta Orthop. 2009 Dec;80(6):653–659. doi: 10.3109/17453670903473016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Knight S.R., Aujla R., Biswas S.P. Total hip arthroplasty – over 100 years of operative history. Orthop Rev. 2011 Sep 6;3(2):e16. doi: 10.4081/or.2011.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eglin D., Alini M., de Bruijn J., Gautrot J., Grijpma D.W., Kamer L. The RAPIDOS project—European and Chinese collaborative research on biomaterials. J Orthop Transl. 2015;3(2):78–84. doi: 10.1016/j.jot.2015.02.001. 4// [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Y., Chen S.-K., Li L., Qin L., Wang X.-L., Lai Y.-X. Bone defect animal models for testing efficacy of bone substitute biomaterials. J Orthop Transl. 2015;3(3):95–104. doi: 10.1016/j.jot.2015.05.002. 7// [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shah A.K., Sinha R.K., Hickok N.J., Tuan R.S. High-resolution morphometric analysis of human osteoblastic cell adhesion on clinically relevant orthopedic alloys. Bone. 1999 May;24(5):499–506. doi: 10.1016/s8756-3282(99)00077-0. [DOI] [PubMed] [Google Scholar]
- 55.Agarwal S., Curtin J., Duffy B., Jaiswal S. Biodegradable magnesium alloys for orthopaedic applications: a review on corrosion, biocompatibility and surface modifications. Mater Sci Eng C Mater Biol Appl. 2016 Nov 01;68:948–963. doi: 10.1016/j.msec.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 56.Geetha M., Singh A.K., Asokamani R., Gogia A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants – a review. Prog Mater Sci. 2009 May;54(3):397–425. [Google Scholar]
- 57.Bostman O., Pihlajamaki H. Clinical biocompatibility of biodegradable orthopaedic implants for internal fixation: a review. Biomaterials. 2000 Dec;21(24):2615–2621. doi: 10.1016/s0142-9612(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 58.Vos D.I., Verhofstad M.H. Indications for implant removal after fracture healing: a review of the literature. Eur J Trauma Emerg Surg. 2013 Aug;39(4):327–337. doi: 10.1007/s00068-013-0283-5. [DOI] [PubMed] [Google Scholar]
- 59.Meningaud J.P., Poupon J., Bertrand J.C., Chenevier C., Galliot-Guilley M., Guilbert F. Dynamic study about metal release from titanium miniplates in maxillofacial surgery. Int J Oral Maxillofac Surg. 2001 Jun;30(3):185–188. doi: 10.1054/ijom.2000.0039. [DOI] [PubMed] [Google Scholar]
- 60.Hidaka S., Gustilo R.B. Refracture of bones of the forearm after plate removal. J Bone Jt Surg Am Vol. 1984 Oct;66(8):1241–1243. [PubMed] [Google Scholar]
- 61.Deluca P.A., Lindsey R.W., Ruwe P.A. Refracture of bones of the forearm after the removal of compression plates. J Bone Jt Surg Am Vol. 1988 Oct;70(9):1372–1376. [PubMed] [Google Scholar]
- 62.Boerger T.O., Patel G., Murphy J.P. Is routine removal of intramedullary nails justified. Injury. 1999 Mar;30(2):79–81. doi: 10.1016/s0020-1383(98)00200-9. [DOI] [PubMed] [Google Scholar]
- 63.Husain A., Pollak A.N., Moehring H.D., Olson S.A., Chapman M.W. Removal of intramedullary nails from the femur: a review of 45 cases. J Orthop Trauma. 1996;10(8):560–562. doi: 10.1097/00005131-199611000-00009. [DOI] [PubMed] [Google Scholar]
- 64.Brumback R.J., Ellison T.S., Poka A., Bathon G.H., Burgess A.R. Intramedullary nailing of femoral shaft fractures. Part III: long-term effects of static interlocking fixation. J Bone Jt Surg Am Vol. 1992 Jan;74(1):106–112. [PubMed] [Google Scholar]
- 65.Staiger M.P., Pietak A.M., Huadmai J., Dias G. Magnesium and its alloys as orthopedic biomaterials: a review. Biomaterials. 2006 Mar;27(9):1728–1734. doi: 10.1016/j.biomaterials.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 66.Sumitomo N., Noritake K., Hattori T., Morikawa K., Niwa S., Sato K. Experiment study on fracture fixation with low rigidity titanium alloy: plate fixation of tibia fracture model in rabbit. J Mater Sci Mater Med. 2008 Apr;19(4):1581–1586. doi: 10.1007/s10856-008-3372-y. [DOI] [PubMed] [Google Scholar]
- 67.Jamil W., Allami M., Choudhury M.Z., Mann C., Bagga T., Roberts A. Do orthopaedic surgeons need a policy on the removal of metalwork? A descriptive national survey of practicing surgeons in the United Kingdom. Injury. 2008 Mar;39(3):362–367. doi: 10.1016/j.injury.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 68.Gruber R., Koch H., Doll B.A., Tegtmeier F., Einhorn T.A., Hollinger J.O. Fracture healing in the elderly patient. Exp Gerontol. 2006 Nov;41(11):1080–1093. doi: 10.1016/j.exger.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 69.Goldhahn J., Feron J.M., Kanis J., Papapoulos S., Reginster J.Y., Rizzoli R. Implications for fracture healing of current and new osteoporosis treatments: an ESCEO consensus paper. Calcif Tissue Int. 2012 May;90(5):343–353. doi: 10.1007/s00223-012-9587-4. [DOI] [PubMed] [Google Scholar]
- 70.Hanafusa S., Matsusue Y., Yasunaga T., Yamamuro T., Oka M., Shikinami Y. Biodegradable plate fixation of rabbit femoral shaft osteotomies. A comparative study. Clin Orthop Relat Res. 1995 Jun;(315):262–271. [PubMed] [Google Scholar]
- 71.Goriainov V., Cook R., ML J., GD D., Oreffo R.O. Bone and metal: an orthopaedic perspective on osseointegration of metals. Acta Biomater. 2014 Oct;10(10):4043–4057. doi: 10.1016/j.actbio.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 72.Giannoudis P.V., Schneider E. Principles of fixation of osteoporotic fractures. J Bone Jt Surg Br Vol. 2006 Oct;88(10):1272–1278. doi: 10.1302/0301-620X.88B10.17683. [DOI] [PubMed] [Google Scholar]
- 73.Namkung-Matthai H., Appleyard R., Jansen J., Hao Lin J., Maastricht S., Swain M. Osteoporosis influences the early period of fracture healing in a rat osteoporotic model. Bone. 2001 Jan;28(1):80–86. doi: 10.1016/s8756-3282(00)00414-2. [DOI] [PubMed] [Google Scholar]
- 74.Meyer R.A., Jr., Tsahakis P.J., Martin D.F., Banks D.M., Harrow M.E., Kiebzak G.M. Age and ovariectomy impair both the normalization of mechanical properties and the accretion of mineral by the fracture callus in rats. J Orthop Res. 2001 May;19(3):428–435. doi: 10.1016/S0736-0266(00)90034-2. [DOI] [PubMed] [Google Scholar]
- 75.Chow D.H., Leung K.S., Qin L., Leung A.H., Cheung W.H. Low-magnitude high-frequency vibration (LMHFV) enhances bone remodeling in osteoporotic rat femoral fracture healing. J Orthop Res. 2011 May;29(5):746–752. doi: 10.1002/jor.21303. [DOI] [PubMed] [Google Scholar]
- 76.Barrios C., Brostrom L.A., Stark A., Walheim G. Healing complications after internal fixation of trochanteric hip fractures: the prognostic value of osteoporosis. J Orthop Trauma. 1993;7(5):438–442. doi: 10.1097/00005131-199310000-00006. [DOI] [PubMed] [Google Scholar]
- 77.Bergman R.J., Gazit D., Kahn A.J., Gruber H., McDougall S., Hahn T.J. Age-related changes in osteogenic stem cells in mice. J Bone Miner Res. 1996 May;11(5):568–577. doi: 10.1002/jbmr.5650110504. [DOI] [PubMed] [Google Scholar]
- 78.D'Ippolito G., Schiller P.C., Ricordi C., Roos B.A., Howard G.A. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res. 1999 Jul;14(7):1115–1122. doi: 10.1359/jbmr.1999.14.7.1115. [DOI] [PubMed] [Google Scholar]
- 79.Rodriguez J.P., Garat S., Gajardo H., Pino A.M., Seitz G. Abnormal osteogenesis in osteoporotic patients is reflected by altered mesenchymal stem cells dynamics. J Cell Biochem. 1999 Dec 1;75(3):414–423. doi: 10.1002/(sici)1097-4644(19991201)75:3<414::aid-jcb7>3.3.co;2-3. [DOI] [PubMed] [Google Scholar]
- 80.Sterck J.G., Klein-Nulend J., Lips P., Burger E.H. Response of normal and osteoporotic human bone cells to mechanical stress in vitro. Am J Physiol. 1998 Jun;274(6 Pt 1):E1113–E1120. doi: 10.1152/ajpendo.1998.274.6.E1113. [DOI] [PubMed] [Google Scholar]
- 81.Lai Y., Cao H., Wang X., Chen S., Zhang M., Wang N. Porous composite scaffold incorporating osteogenic phytomolecule icariin for promoting skeletal regeneration in challenging osteonecrotic bone in rabbits. Biomaterials. 2018 Jan;153:1–13. doi: 10.1016/j.biomaterials.2017.10.025. [DOI] [PubMed] [Google Scholar]
- 82.Lee J.W., Han H.S., Han K.J., Park J., Jeon H., Ok M.R. Long-term clinical study and multiscale analysis of in vivo biodegradation mechanism of Mg alloy. Proc Natl Acad Sci U S A. 2016 Jan 19;113(3):716–721. doi: 10.1073/pnas.1518238113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cheng P., Han P., Zhao C., Zhang S., Wu H., Ni J. High-purity magnesium interference screws promote fibrocartilaginous entheses regeneration in the anterior cruciate ligament reconstruction rabbit model via accumulation of BMP-2 and VEGF. Biomaterials. 2016 Mar;81:14–26. doi: 10.1016/j.biomaterials.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 84.Sullivan M.P., McHale K.J., Parvizi J., Mehta S. Nanotechnology: current concepts in orthopaedic surgery and future directions. Bone Jt J. 2014 May;96-B(5):569–573. doi: 10.1302/0301-620X.96B5.33606. [DOI] [PubMed] [Google Scholar]
- 85.Harvey E.J., Henderson J.E., Vengallatore S.T. Nanotechnology and bone healing. J Orthop Trauma. 2010 Mar;24(Suppl. 1):S25–S30. doi: 10.1097/BOT.0b013e3181ca3b58. [DOI] [PubMed] [Google Scholar]
- 86.Streicher R.M., Schmidt M., Fiorito S. Nanosurfaces and nanostructures for artificial orthopedic implants. Nanomedicine (Lond) 2007 Dec;2(6):861–874. doi: 10.2217/17435889.2.6.861. [DOI] [PubMed] [Google Scholar]
- 87.Goodman S.B., Yao Z., Keeney M., Yang F. The future of biologic coatings for orthopaedic implants. Biomaterials. 2013 Apr;34(13):3174–3183. doi: 10.1016/j.biomaterials.2013.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mathews S., Bhonde R., Gupta P.K., Totey S. A novel tripolymer coating demonstrating the synergistic effect of chitosan, collagen type 1 and hyaluronic acid on osteogenic differentiation of human bone marrow derived mesenchymal stem cells. Biochem Biophys Res Commun. 2011 Oct 14;414(1):270–276. doi: 10.1016/j.bbrc.2011.09.071. [DOI] [PubMed] [Google Scholar]
- 89.Liu Y., de Groot K., Hunziker E.B. BMP-2 liberated from biomimetic implant coatings induces and sustains direct ossification in an ectopic rat model. Bone. 2005 May;36(5):745–757. doi: 10.1016/j.bone.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 90.Vormann J. Magnesium: nutrition and metabolism. Mol Aspects Med. 2003 Feb-Jun;24(1–3):27–37. doi: 10.1016/s0098-2997(02)00089-4. [DOI] [PubMed] [Google Scholar]
- 91.Touyz R.M. Magnesium in clinical medicine. Front Biosci. 2004 May 1;9:1278–1293. doi: 10.2741/1316. [DOI] [PubMed] [Google Scholar]
- 92.Wang J., Tang J., Zhang P., Li Y., Lai Y., Qin L. Surface modification of magnesium alloys developed for bioabsorbable orthopedic implants: a general review. J Biomed Mater Res Part B Appl Biomater. 2012 Aug;100(6):1691–1701. doi: 10.1002/jbm.b.32707. [DOI] [PubMed] [Google Scholar]
- 93.Abidin N.I.Z., Rolfe B., Owen H., Malisano J., Martin D., Hofstetter J. The in vivo and in vitro corrosion of high-purity magnesium and magnesium alloys WZ21 and AZ91. Corros Sci. 2013 Oct;75:354–366. [Google Scholar]
- 94.Gu X.N., Xie X.H., Li N., Zheng Y.F., Qin L. In vitro and in vivo studies on a Mg-Sr binary alloy system developed as a new kind of biodegradable metal. Acta Biomater. 2012 Jul;8(6):2360–2374. doi: 10.1016/j.actbio.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 95.Xu L., Yu G., Zhang E., Pan F., Yang K. In vivo corrosion behavior of Mg-Mn-Zn alloy for bone implant application. J Biomed Mater Res Part A. 2007 Dec 1;83(3):703–711. doi: 10.1002/jbm.a.31273. [DOI] [PubMed] [Google Scholar]
- 96.Li H.F., Xie X.H., Zhao K., Wang Y.B., Zheng Y.F., Wang W.H. In vitro and in vivo studies on biodegradable CaMgZnSrYb high-entropy bulk metallic glass. Acta Biomater. 2013 Nov;9(10):8561–8573. doi: 10.1016/j.actbio.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 97.Li H.F., Xie X.H., Zheng Y.F., Cong Y., Zhou F.Y., Qiu K.J. Development of biodegradable Zn-1X binary alloys with nutrient alloying elements Mg, Ca and Sr. Sci Rep. 2014:5. doi: 10.1038/srep10719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang J., Qin L., Wang K., Wang J., Yue Y., Li Y. Cytotoxicity studies of AZ31D alloy and the effects of carbon dioxide on its biodegradation behavior in vitro. Mater Sci Eng C Mater Biol Appl. 2013 Oct;33(7):4416–4426. doi: 10.1016/j.msec.2013.06.041. [DOI] [PubMed] [Google Scholar]
- 99.Wang J., Tang J., Zhang P., Li Y., Wang J., Lai Y. Surface modification of magnesium alloys developed for bioabsorbable orthopedic implants: a general review. J Biomed Mater Res Part B Appl Biomater. 2012 Aug;100(6):1691–1701. doi: 10.1002/jbm.b.32707. [DOI] [PubMed] [Google Scholar]
- 100.Wang J., Witte F., Xi T., Zheng Y., Yang K., Yang Y. Recommendation for modifying current cytotoxicity testing standards for biodegradable magnesium-based materials. Acta Biomater. 2015 Jul;21:237–249. doi: 10.1016/j.actbio.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 101.Wang Y.B., Xie X.H., Li H.F., Wang X.L., Zhao M.Z., Zhang E.W. Biodegradable CaMgZn bulk metallic glass for potential skeletal application. Acta Biomater. 2011 Aug;7(8):3196–3208. doi: 10.1016/j.actbio.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 102.Witte F., Fischer J., Nellesen J., Crostack H.A., Kaese V., Pisch A. In vitro and in vivo corrosion measurements of magnesium alloys. Biomaterials. 2006 Mar;27(7):1013–1018. doi: 10.1016/j.biomaterials.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 103.Witte F., Fischer J., Nellesen J., Vogt C., Vogt J., Donath T. In vivo corrosion and corrosion protection of magnesium alloy LAE442. Acta Biomater. 2010 May;6(5):1792–1799. doi: 10.1016/j.actbio.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 104.Ma J., Thompson M., Zhao N., Zhu D. Similarities and differences in coatings for magnesium-based stents and orthopaedic implants. J Orthop Transl. 2014;2(3):118–130. doi: 10.1016/j.jot.2014.03.004. 7// [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu C., Wan P., Tan L.L., Wang K., Yang K. Preclinical investigation of an innovative magnesium-based bone graft substitute for potential orthopaedic applications. J Orthop Transl. 2014;2(3):139–148. 7// [Google Scholar]
- 106.Tang J., Wang J., Xie X., Zhang P., Lai Y., Li Y. Surface coating reduces degradation rate of magnesium alloy developed for orthopaedic applications. J Orthop Transl. 2013;1(1):41–48. 10// [Google Scholar]
- 107.Windhagen H., Radtke K., Weizbauer A., Diekmann J., Noll Y., Kreimeyer U. Biodegradable magnesium-based screw clinically equivalent to titanium screw in hallux valgus surgery: short term results of the first prospective, randomized, controlled clinical pilot study. Biomed Eng Online. 2013;12:62. doi: 10.1186/1475-925X-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yuan Y., Lin D., Chen F., Liu C. Clinical translation of biomedical materials and the key factors towards product registration. J Orthop Transl. 2014;2(2):49–55. 4// [Google Scholar]
- 109.Schaller B., Saulacic N., Imwinkelried T., Beck S., Liu E.W., Gralla J. In vivo degradation of magnesium plate/screw osteosynthesis implant systems: soft and hard tissue response in a calvarial model in miniature pigs. J Craniomaxillofac Surg. 2016 Mar;44(3):309–317. doi: 10.1016/j.jcms.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 110.Wolters L., Angrisani N., Seitz J., Helmecke P., Weizbauer A., Reifenrath J. Applicability of degradable magnesium LAE442 alloy plate-screw-systems in a rabbit model. Biomed Tech Biomed Eng. 2013 Sep 7 doi: 10.1515/bmt-2013-4059. [DOI] [PubMed] [Google Scholar]
- 111.Chaya A., Yoshizawa S., Verdelis K., Myers N., Costello B.J., Chou D.T. In vivo study of magnesium plate and screw degradation and bone fracture healing. Acta Biomater. 2015 May;18:262–269. doi: 10.1016/j.actbio.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 112.Wu G., Chan K.C., Zhu L.L., Sun L.G., Lu J. Dual-phase nanostructuring as a route to high-strength magnesium alloys. Nature. 2017 May 4;545(7652) doi: 10.1038/nature21691. 80-+ [DOI] [PubMed] [Google Scholar]
- 113.Zheng Q., Cheng S., Strader J.H., Ma E., Xu J. Critical size and strength of the best bulk metallic glass former in the Mg-Cu-Gd ternary system. Scripta Mater. 2007 Jan;56(2):161–164. [Google Scholar]
- 114.Cornell C.N. Internal fracture fixation in patients with osteoporosis. J Am Acad Orthop Surg. 2003 Mar-Apr;11(2):109–119. doi: 10.5435/00124635-200303000-00005. [DOI] [PubMed] [Google Scholar]
- 115.Bogunovic L., Cherney S.M., Rothermich M.A., Gardner M.J. Biomechanical considerations for surgical stabilization of osteoporotic fractures. Orthop Clin North Am. 2013 Apr;44(2):183–200. doi: 10.1016/j.ocl.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 116.Feron J.M., Mauprivez R. Fracture repair: general aspects and influence of osteoporosis and anti-osteoporosis treatment. Injury. 2016 Jan;47(Suppl. 1):S10–S14. doi: 10.1016/S0020-1383(16)30003-1. [DOI] [PubMed] [Google Scholar]
- 117.Patterson S.P., Daffner R.H., Gallo R.A. Electrochemical corrosion of metal implants. Am J Roentgenol. 2005 Apr;184(4):1219–1222. doi: 10.2214/ajr.184.4.01841219. [DOI] [PubMed] [Google Scholar]
- 118.Cohen J. Current concepts review. Corrosion of metal orthopaedic implants. J Bone Jt Surg Am Vol. 1998 Oct;80(10):1554. [PubMed] [Google Scholar]
- 119.Lee J.Y., Lee J.W., Pang K.M., Kim H.E., Kim S.M., Lee J.H. Biomechanical evaluation of magnesium-based resorbable metallic screw system in a bilateral sagittal split ramus osteotomy model using three-dimensional finite element analysis. J Oral Maxillofac Surg. 2014 Feb;72(2) doi: 10.1016/j.joms.2013.10.003. 402 e1–13. [DOI] [PubMed] [Google Scholar]