Abstract
Persistent Human Papillomavirus (HPV) infection is a prerequisite for cervical cancer development. Few studies investigated clearance of high-risk HPV in low-and-middle-income countries. Our study investigated HPV clearance and persistence over four years in women from North Tongu District, Ghana.
In 2010/2011, cervical swabs of 500 patients were collected and HPV genotyped (nested multiplex PCR) in Accra, Ghana. In 2014, 104 women who previously tested positive for high-risk HPV and remained untreated were re-tested for HPV. Cytobrush samples were genotyped (GP5+/6+ PCR & Luminex-MPG readout) in Berlin, Germany. Positively tested patients underwent colposcopy and treatment if indicated.
Of 104 women, who tested high-risk HPV+ in 2010/2011, seven (6,7%; 95%CI: 2.7–13.4%) had ≥1 persistent high-risk‐infection after ~4 years (mean age 39 years). Ninety-seven (93,3%; 95%CI: 86.6–97.3%) had cleared the original infection, while 22 (21.2%; 95%CI: 13.8–30.3%) had acquired new high-risk infections with other genotypes. Persistent types found were HPV 16, 18, 35, 39, 51, 52, 58, and 68. Among those patients, one case of CIN2 (HPV 68) and one micro-invasive cervical cancer (HPV 16) were detected.
This longitudinal observational data suggest that single HPV screening rounds may lead to over-referral. Including type-specific HPV re-testing or additional triage methods could help reduce follow-up rates.
Keywords: Persistence, Cervical screening, Natural history, LMIC, HPV screening
Highlights
-
•
High-risk HPV genotype-specific clearance rates of 93.3% after 4 years.
-
•
New infections with other high-risk HPV were 21.2%.
-
•
New infections may overestimate women at risk if non-genotyping repeat HPV-testing is used for triage.
-
•
Type-specific HPV genotyping reduces follow-up rates, for example by colposcopy.
1. Introduction
Cervical cancer is the fourth most common cancer in women, causing approximately 266.000 registered deaths worldwide every year. In many low-and-middle-income countries (LMICs) it is the second most common cancer in women, representing a major burden with the number of deaths accounting for 85% of the total cervical cancer mortality rate globally [1]. Approximately 99.7% of cervical cancers are caused by persistent infection with HPV genotypes, which are classified as high risk [2]. However, HPV infections and resulting lesions can be transient with, for example, only about 5% of cervical intraepithelial lesion (CIN) grade II lesions progressing to invasion or 22% to carcinoma in situ [3]. It is postulated that probably less than 50% of women with CIN3 develop invasive cervical cancer within 30 years [4]. Nevertheless, it is known that persistent infection with high-risk HPV increase the relative risk of developing high-grade cervical intraepithelial lesions and invasive cancer [5], [6]. Interestingly, the prevalence of different high-risk HPV types differs between infection and disease. In Western Africa HPV 16, 58, 18, 35 and 52 are the most prevalent types in normal cytology, while in cervical cancer HPV 16, 18, 45, 59 and 35 are found most often [7]. One study from Ghana shows HPV 18, 59, 45 and 16 as the most prevalent types in descending order in confirmed cervical cancer cases with HPV 18 being present in 47.4% of the cancer tissues [8]. Compared to the worldwide genotype distribution within cervical cancer samples, for which the most common HPV types were 16, 18, 31, 33, 35, 45, 52, and 58 [9], this order indicates regional differences. It has also been shown that the carcinogenic potential for high-grade lesions differs between the oncogenic HPV types. While the cumulative risk for the development of CIN2+ after 14 years is 42.8 for HPV 16, it is only 8.1 for HPV 59 [10].
Persistence of different HPV types is an important phenomenon for the development of cervical cancer and its natural history. Elfgren et al. showed in a small group that all women who continuously had genotype-specific high-risk HPV persistence developed CIN2+ lesions within six years [11]. Despite this knowledge still relatively few studies have reported persistence or clearance rates of HPV and the associated risk of developing cervical cancer, especially in LMICs. In a population-based cohort in Costa Rica, for example, a clearance rate of 55% of carcinogenic HPV infections was observed after six months of follow-up [12]. In Scotland, a clearance rate of 51.9% was seen after a period of 14 months [13]. Other studies from Costa Rica, Colombia, and Zimbabwe found clearance rates of 67%, 77% and 73% within 12 months [12], [14], [15]. These different clearance rates highlight the need to further investigate the natural history of HPV and cervical cancer in various countries and settings.
This knowledge is of fundamental importance, since it greatly determines future screening strategies and algorithms of HPV-based screening for the prevention of cervical cancer, as is currently recommended by WHO [16]. We had an opportunity to compare genotype-specific HPV infections in a cohort of Ghanaian women. Our study presents some rare data on clearance and persistence of HPV infections found in a cohort of 104 women from the North Tongu District of Ghana over the period of four years.
2. Materials and methods
2.1. Initial screening study
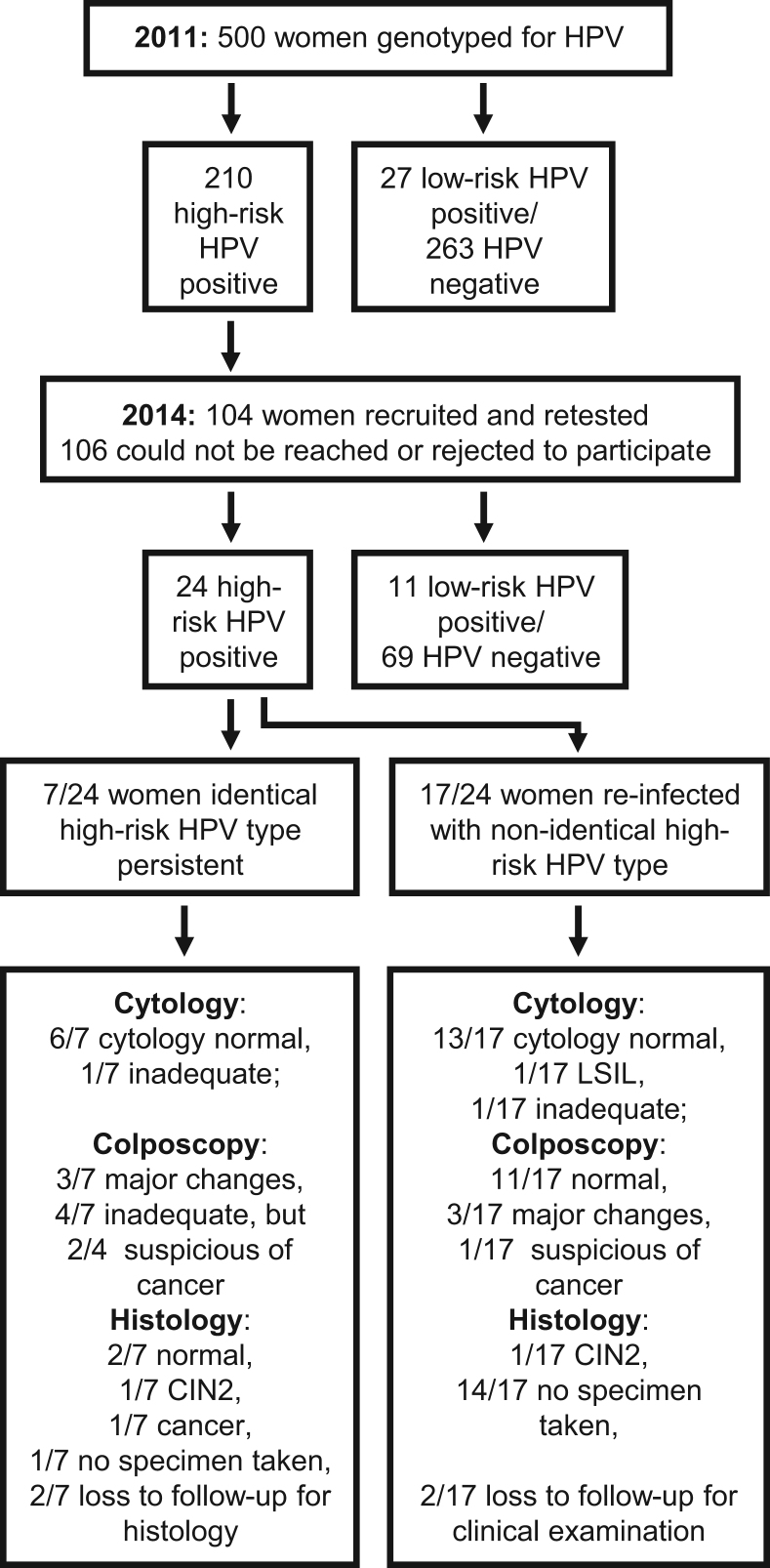
The recruitment strategy and patient flow for this study is presented in Fig. 1. As part of a cervical cancer screening project in 2010/2011, 3000 women attending the Battor Catholic Hospital in the North Tongu District, Volta Region in Ghana, were screened for cervical cancer and its precursor lesions. Women older than 15 years, with no history of cervical cancer and not pregnant were recruited into the study. Women who were unable to undergo speculum vaginal examination, including virgins, those who had undergone hysterectomy or conisation and women who were unable to give consent were excluded. Smear samples were collected using an Aylesbury spatula and sent to the Cytopathology Laboratory of the School of Allied Health Sciences, University of Ghana for cytological examination. Five hundred (500) of these 3000 women were randomly selected for HPV genotyping using nested multiplex PCR according to Sotlar et al. [17] at the Laboratory as part of a PhD project undertaken by GB and supervised by EKW. The following genotypes were tested for: 6/11, 16, 18, 31, 33, 35, 39, 42, 43, 44, 45, 51, 52, 56, 58, 59, 66, and 68. Ethical clearance for this study was provided by the University of Ghana School of Allied Health Sciences Ethics and Protocol Review Committee (Ref. No. SAHS ET/AA/24A/2010). Signed/thumb-printed written/translated informed consent was obtained from all women participating in the screening.
Fig. 1.
Flow chart of HPV screening studies in 2011 and 2014 presenting inclusion of 104 women by HPV test outcome and clinical diagnosis for persistence analysis. Abbreviations: LSIL – low-grade squamous intraepithelial lesion; CIN2 – cervical intraepithelial lesion grade 2.
All the cytology reports were released to the Battor Hospital for management and follow up. However, the HPV testing was started in 2011 and completed in 2013.
2.2. ACCESSING study – Follow-up
In 2013 collaboration between the Catholic Hospital Battor, Ghana and the Charité University Hospital Berlin, Germany started with the topic “Adequate Cervical cancer Capacity Building, Education and Screening with new Scientific Instruments in Ghana”, namely the ACCESSING study. This study was independent from the previous study within a new collaboration but concentrating on the prevalence of HPV and cervical cancer in the same geographical area. Ethical clearance for this study was given by the Ghana Health Service Ethical Review Committee (Ref. No. GHS-ERC: 05/05/13) in October 2013.
Recruitment for an initial pilot study started in March 2014 in order to validate sample collection, logistics and diagnostic testing. For this pilot study 150 women between the ages of 18–65 years with a previous history of HPV infection or abnormal cytological result but currently not pregnant were recruited at the gynecological clinic of the Catholic Hospital Battor. Women who had remained untreated since 2011 and fulfilled the criteria of this study were re-called and asked to participate again for final follow-up as part of this pilot. The participants were recruited on a convenience approach. Potential differences in age and HPV type positivity between women agreeing to and declining recruitment in 2014 were assessed using chi-square test of independence. Samples were collected for cytology and HPV genotype testing. Cytological examination was done at the Department of Pathology, University of Cape Coast, Ghana. HPV genotyping was done on cytobrush samples by BSGP5+/6+ PCR followed by Luminex-MPG read-out detecting the genotypes 6, 11, 16, 18, 26, 31, 33, 35, 39, 42, 43, 45, 51, 52, 53, 54, 56, 57, 58, 59, 66, 68a, 68b, 70, 72, 73, 82, and 90 at Charité Universitätsmedizin Berlin, Germany [18]. Differences in age between women with cleared or persistent high-risk HPV infection as well as newly infected women were tested using chi-square test. Those who tested positive for high-risk HPV were recalled for colposcopy and if indicated by colposcopy LEEP was performed. Based on the histology results from the LEEP specimen additional treatment was provided if needed. Every woman screened in 2014 filled out a questionnaire asking for general demographic data (e.g. age, education, and income level per month) as well as specific risk factors such as age at first intercourse, number of sexual partners, etc.
For reasons of consistency the HPV high-risk classification of WHO was used, classifying the HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59 as carcinogenic HPV genotypes. Additionally, we consider HPV 66 and 68 as high-risk. The remaining types are defined as potentially carcinogenic or low-risk types [19]. It is important to note that all carcinogenic high-risk types are detected with both assays, however some low-risk types are not detected consistently with both assays. The nested PCR according to Sotlar et al., as performed in Accra, Ghana, included HPV 44, which was not tested with the BSGP5+/6+ PCR according to Schmitt et al. in Berlin, Germany, while HPV types 26, 53, 54, 57, 70, 72, 73, 82, and 90 were not tested by the nested PCR method used in Accra. In the following analysis clearance and persistence of low-risk types will therefore only refer to the HPV types 6, 11, 42, and 43 included in both assays.
3. Results
3.1. Characteristics of study participants
Attempt was made to contact all the women who tested high-risk HPV positive in the Accra study in 2010/2011 and re-call them but only 104 out of the initial 210 women could be reached and agreed to participate in the ACCESSING pilot study in 2014, and were re-screened for HPV.
Comparing the age and HPV type positivity between the women who agreed to participate and those who were not re-screened, we observed only small differences. The median age of women re-screened was 34 years in 2010/2011 and of those not re-screened 35 years. This difference was not significant (chi-square test; p-value = 0.336). When tested to see if the initial positivity for the individual 14 high-risk HPV types in 2010/2011 differed between the two groups, we found that less women re-screened were initially HPV52 positive compared to those not re-screened (chi-square test; p-value = 0.001) and more of those re-screened were initially positive for HPV68 (chi-square test; p-value = 0.028).
Among the women re-screened in 2014 almost one third (30.8%) of the women had no formal education, 21.2% completed elementary school, 47.1% completed secondary school and 1% continued with post-secondary school education at the time of re-screening. More than half (64.4%) of the women screened were married and the majority of women (73.1%) had two to three sexual partners, as shown in Table 1. The results from the questionnaire showed that more than two thirds of the women (68.3%) did not use contraceptives.
Table 1.
Sociodemographic and behavioral characteristics of women included in HPV persistence analysis at time point of inclusion in 2014 (n = 104).
| n | % | |||
|---|---|---|---|---|
| Age | ||||
| Mean | 39.39 yrs | |||
| 20–30 | 27 | 25.96 | ||
| 31–40 | 35 | 33.65 | ||
| 41–50 | 22 | 21.15 | ||
| 51–60 | 16 | 15.38 | ||
| 60 + | 4 | 3.85 | ||
| Education | ||||
| None | 32 | 30.77 | ||
| Primary | 22 | 21.15 | ||
| Junior High School | 35 | 33.65 | ||
| Secondary | 14 | 13.46 | ||
| Post Secondary | 1 | 0.96 | ||
| Income level per month | ||||
| <100 GH¢ | 65 | 62.50 | ||
| 100–250 GH¢ | 16 | 15.38 | ||
| 251–500 GH¢ | 4 | 3.85 | ||
| >500 GH¢ | 2 | 1.92 | ||
| missing data | 17 | 16.35 | ||
| Occupation | ||||
| Farmer/Trader | 70 | 67.31 | ||
| Food Vendor | 4 | 3.85 | ||
| Hairdressing | 5 | 4.81 | ||
| Nurse | 2 | 1.92 | ||
| Seamstress | 6 | 5.77 | ||
| Unemployed | 2 | 1.92 | ||
| Other (Baker, Caterer, Student, etc.) | 10 | 9.62 | ||
| missing data | 5 | 4.81 | ||
| Marital status | ||||
| Single | 6 | 5.77 | ||
| Have a steady partner | 9 | 8.65 | ||
| Living with someone (unmarried) | 6 | 5.77 | ||
| Married | 67 | 64.42 | ||
| Divorced | 7 | 6.73 | ||
| Widowed | 9 | 8.65 | ||
| # of sexual partners | ||||
| 1 | 19 | 18.27 | ||
| 2–3 | 76 | 73.07 | ||
| >3 | 9 | 8.65 | ||
| # of children | ||||
| None | 9 | 8.65 | ||
| 1–2 | 25 | 24.04 | ||
| 3–4 | 36 | 34.62 | ||
| 5–6 | 16 | 15.38 | ||
| >6 | 17 | 16.35 | ||
| missing data | 1 | 0.96 | ||
| Age at first intercourse | ||||
| <15 | 3 | 2.88 | ||
| 15–18 | 45 | 43.27 | ||
| 19–22 | 36 | 34.62 | ||
| >22 | 8 | 7.69 | ||
| N/A | 12 | 11.54 | ||
| Contraceptive use | ||||
| None | 71 | 68.27 | ||
| Abstinence | 11 | 10.58 | ||
| Injectable | 13 | 12.50 | ||
| Norplant/Jadelle | 1 | 0.96 | ||
| Pill | 8 | 7.69 | ||
| Current smoking | ||||
| Yes | 3 | 2.88 | ||
| No | 101 | 97.12 | ||
Abbreviations: GH¢ - Ghana Cedi (local currency); N/A - no answer.
3.2. Results initial screening study
Among the 104 high-risk HPV-positive women in the 2010/2011 study, 56 had single infections and 48 multiple infections and the mean number of high-risk HPV types was 1.6. The most prevalent high-risk HPV types were HPV 18 (26.0%), 58 (23.1%), 52 (22.1%), 68 (20.1%), and 66 (14.4%). HPV 16 was detected in one sample (1.0%), the only squamous cell carcinoma detected. HPV 43 was the most prevalent low-risk type with 19.2%.
3.3. Results ACCESSING study
In 2014 no high-risk HPV infection was proven in 76.9% (80/104) after approximately four years of follow up, with 73.1% (76/104) being completely HPV negative while 3.8% (4/104) were positive for low-risk HPV (6, 11, 42, or 43) only. Twenty-four (23.1%) women were high-risk HPV positive. Altogether, 45 high-risk HPV infections and five low-risk infections were found. Of the 24 high-risk positive women, 13 had single and 11 multiple infections with a mean number of 1.9 high-risk HPV types (Range: 1–5). The most prevalent high-risk HPV genotypes found were HPV 16 and 52 (8.7% each), 39 (4.8%) and 45 and 51 (3.8% each) among the 104 women. HPV 18 was diagnosed in none of the samples. HPV 70 was the most prevalent low-risk type found in 4.8% (5/104) of the patients. This low-risk type had not been tested for in the 2011 study. HPV 43 was found in only one of the patients.
3.4. Longitudinal comparison
Comparing the HPV genotyping results from 2010/2011 with the results from 2014, 6.7% (7/104; 95% CI: 2.7–13.4%) women had ≥1 persistent high-risk HPV type (Table 2). None of the low-risk HPV types persisted. Persistent HPV types found were HPV 16, 35, 39, 51, 52 and 68. HPV 52 persisted in three women, while the other HPV types were found persistent in only one woman, respectively. One woman had two persistent HPV types (HPV 51 and 52). Based on the comparison of type-specific infections, the clearance rate found within four years was 93.3% (97/104; 95% CI: 86.6–97.3%) and rate of reinfection with a new high-risk HPV type was 21.2% (22/104; 95% CI: 13.8–30.3%).
Table 2.
Prevalence in 2011 and 2014 and Persistence/Clearance/Reinfection of high-risk HPV by type among study participants (n = 104).
| HPV Type |
Prevalence of high-risk HPV in 2011 |
Prevalence of high-risk HPV in 2014 |
Persistence |
Clearance |
Reinfection | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | |
| HPV 16 | 1 | 1.0 | 9 | 8.7 | 1 | 100.0 | 0 | 0.0 | 8 |
| HPV 18 | 27 | 26.0 | 0 | 0.0 | 0 | 0.0 | 27 | 100.0 | 0 |
| HPV 31 | 7 | 6.7 | 2 | 1.9 | 0 | 0.0 | 7 | 100.0 | 2 |
| HPV 33 | 7 | 6.7 | 0 | 0.0 | 0 | 0.0 | 7 | 100.0 | 0 |
| HPV 35 | 11 | 10.6 | 2 | 1.9 | 1 | 9.1 | 10 | 90.9 | 1 |
| HPV 39 | 6 | 5.8 | 5 | 4.8 | 1 | 16.7 | 5 | 83.3 | 4 |
| HPV 45 | 1 | 1.0 | 4 | 3.8 | 0 | 0.0 | 1 | 100.0 | 4 |
| HPV 51 | 13 | 12.5 | 4 | 3.8 | 1 | 7.7 | 12 | 92.3 | 3 |
| HPV 52 | 23 | 22.1 | 9 | 8.7 | 3 | 13.0 | 20 | 87.0 | 6 |
| HPV 56 | 8 | 7.7 | 1 | 1.0 | 0 | 0.0 | 8 | 100.0 | 1 |
| HPV 58 | 24 | 23.1 | 1 | 1.0 | 0 | 0.0 | 24 | 100.0 | 1 |
| HPV 59 | 1 | 1.0 | 3 | 2.9 | 0 | 0.0 | 1 | 100.0 | 3 |
| HPV 66 | 15 | 14.4 | 2 | 1.9 | 0 | 0.0 | 15 | 100.0 | 2 |
| HPV 68 | 21 | 20.2 | 3 | 2.9 | 1 | 4.8 | 20 | 95.2 | 2 |
| All HPV | 165 | 45 | 8/165 | 4.8 | 157/165 | 95.2 | 37 | ||
| (95% CI: 2.1–9.3%) | (95% CI: 90.7–97.9%) | ||||||||
| In 104 women | 104 | 24 | 7/104 | 6.7 | 97/104 | 93.3 | 21.2% (22/104) | ||
| (95% CI: 2.7–13.4%) | (95% CI: 86.6–97.3%) | (95% CI: 13.8–30.3%) | |||||||
Abbreviation: CI - Confidence interval.
Clearance rates of HPV remained high throughout all age groups. Proportions of women with reinfection with other high-risk HPV types was 8.0% and 20.0% in the age groups 20–29 and 30–39 years, while it was more than double in relative terms (30.8% and 33.3%) in the age groups 40–49 and 50–59 years (Table 3). Most persistent cases could be seen in the younger age groups (20–29 years with 2/7 cases and 30–39 years with 3/7 cases) compared to the remaining two cases across the older age groups (40–49 years with 1/7 cases and 50–59 years with 1/7 cases).
Table 3.
Persistence, Clearance, and Reinfection between 2011 and 2014 by age group (n = 104).
| Age group |
Persistence |
Clearance |
Reinfection | ||||
|---|---|---|---|---|---|---|---|
| n | n | % | n | % | n | % | |
| 20–29 | 25 | 2 | 8.0 | 23 | 92.0 | 2 | 8.0 |
| 30–39 | 30 | 3 | 10.0 | 27 | 90.0 | 6 | 20.0 |
| 40–49 | 26 | 1 | 3.8 | 25 | 96.2 | 8 | 30.8 |
| 50–59 | 15 | 1 | 6.7 | 14 | 93.3 | 5 | 33.3 |
| 60+ | 6 | 0 | 0.0 | 6 | 100.0 | 1 | 16.7 |
| Unknown | 2 | 0 | 0.0 | 2 | 100.0 | 0 | 0.0 |
| Total | 104 | 7 | 6.7 | 97 | 93.3 | 22 | 21.2 |
| 95% CI | 2.7–13.4% | 86.6–97.3% | 13.8–30.3% | ||||
| chi-square test | 1.03 | 1.03 | 4.67 | ||||
| p-value | 0.795 | 0.795 | 0.197 | ||||
Abbreviation: CI - Confidence interval.
When testing the association between persistence, clearance, and reinfection rates across the different age groups with chi-square test no significant association was seen.
3.5. Clinical outcome
Women testing positive for high-risk HPV in 2014 underwent colposcopic examination and a smear was taken for cytology. If a lesion was suspected, women underwent LEEP for further histological confirmation and decision on further treatment was based on these results. Out of the seven women who were positive for the same HPV type as in 2011, six women had normal cytology results. One cytology was unsatisfactory and the woman was recalled for a repeat smear but was lost to follow up. Her colposcopic examination had been inadequate due to the lack of visibility of the transformation zone, with no visible changes. She had persistent HPV 52 infection.
One of the six women with normal cytology had normal colposcopy results, major changes were seen in three women, and two women were suspected to have cervical cancer. Due to these abnormal findings during colposcopy, four women underwent LEEP surgery, which showed no lesion to be present in two women but revealed one case each of CIN2 and microinvasive cervical cancer (Fig. 1). One of the two women with suspected cervical cancer from colposcopy did not return to the clinic for histological confirmation and potential treatment. She had persistent HPV 51 and 52 infections. The case of CIN2 was a woman with persistent infection with HPV 68 and the case of microinvasive cancer was caused by persistent infection with HPV 16. The other two women having HPV 35 or 52 persistent HPV infections had no lesions. Unfortunately, two of these seven women were lost to follow up despite several attempts to convince them for triage and treatment.
Out of the 17 women who were re-infected with high-risk HPV genotypes 13 had normal cytology, one woman was diagnosed with LSIL, for one woman the Pap-smear was unsatisfactory and two women were lost to follow-up without cytology results. On colposcopic examination of the 15 triaged women 11 women were diagnosed as normal, three women showed major colposcopic changes, of whom one was cytologically confirmed LSIL, and one woman was suspicious for cancer. The woman suspicious of cancer had normal cytology results and a LEEP biopsy specimen revealed a CIN2 lesion on histological examination. No further histological confirmation was obtained for any of the other women.
4. Discussion
This comparative analysis of two independent HPV genotyping projects in smears from the same group of women sampled years apart was used to determine the persistence and frequency of resolution of prevalent HPV infections.
The most remarkable result emerging from this study is that after an exceptionally long follow-up period of four years, high-risk HPV infection persisted in only 6.7% (95% CI: 2.7–13.4%) of the women. Almost three quarters of the women had completely cleared their high-risk HPV infection and were HPV-negative at the time of follow up, without receiving any form of treatment in the intervening period. It is important to note that a total of 21.2% (95% CI: 13.8–30.3%) had been re-infected with new high-risk HPV types. Such infections become only apparent as new and non-persistent by genotyping HPV assays and could have been mistaken as persistent high-risk HPV infections. Persistent infections would be classified with a higher risk of malignant transformation compared to those with a type change or clearance [11].
The clearance and persistence rates found are concordant with other studies having such a long follow-up period. In a study from Columbia among women with a median age of 29 years a clearance rate of 93% was found after five years of follow up [14]. Even at shorter follow-up periods remarkably high clearance rates can be found. At six months for example clearance of 55% and at 12 months of 67% is reported for oncogenic HPV in a study population in Costa Rica [12]. In South Korea 77% of high-risk HPV cleared within 18 months [20]. The median time to clearance reported by Giuliano et al. is 9.8 months for oncogenic types and 4.3 month for non-oncogenic types [21]. Muñoz et al. report a similar trend in which oncogenic types have longer time to clearance compared to non-oncogenic types and especially HPV 16 infections clear after significantly longer time intervals compared to low-risk HPV and other high-risk non α-9 types [6].
There was no evidence for an association between clearance or persistence rates and age. This could be due to the low number of women who were diagnosed with the same HPV type after four years. There was also no association between reinfection and the different age groups. Several studies have shown though that women at younger age and after initiation of sexual activity tend to have high prevalence rates but that infections clear quickly, in contrast to women at older ages, which tend to have lower prevalence rates, resulting rather from persistent infections [22].
With the switch of many national cervical cancer screening programs to HPV-testing based screening these results may support the available evidence on clearance that is needed to decide on the duration between screening visits.
WHO recommends to initiate screening programs based on HPV testing instead of cytology in countries that have no program in place [16].
Interestingly, none of the low-risk HPV types detected in 2011 persisted. This is also consistent with findings from other studies. In Columbia, low-risk HPV types were found to have a lower persistence rate compared to HPV 16 [14]. In a study from Zimbabwe persistence of low-risk HPV types was 21.9% over a median period of 21 months, which was significantly lower compared to high-risk HPV types with 37.3% persistence [15].
Different oncogenic HPV types have different duration until clearance and different carcinogenic potential. Within our study population we found the HPV types 16, 35, 39, 51, 52 and 68 to be persistent. Among these HPV 16 and 68 caused confirmed invasive cervical cancer and CIN2 within the follow-up period, respectively. The woman found with persistent HPV 16 infection was diagnosed with invasive cervical cancer after an abnormal colposcopic finding and LEEP biopsy. It was remarkable that she presented with a normal cytological result of NILM “Negative for Intraepithelial Lesion and Malignancy” in 2014. The histologically examined LEEP specimen indicated micro-invasive cervical cancer, upon which a radical hysterectomy was performed.
In comparison, among the 17 women who were found newly infected with high-risk HPV during the second testing round in 2014 only one case of CIN2 was detected. Cytology detected one woman with LSIL, from whom no biopsy could be taken.
While HPV 16 clearly is the main driver of cervical cancer and can be found in most cases of cervical cancer worldwide [9], HPV 68 is not very common in high-grade lesions [23]. Only in South Africa has HPV 68 been found to be among the “Top 10″ cervical cancer-causing HPV types [7].
HPV 16 has been reported in the literature as the HPV type that persists significantly longer or in other words has lower clearance rates compared to other HPV types. Bulkman et al. showed in a population-based cervical screening cohort with 44.102 women that the type-specific clearance rates of high-risk HPV differ with HPV 16 and HPV 31 having significantly lower clearance rates in women with normal cytology results at baseline [24]. Moreover, HPV 16 is also the type with the highest detection rate (12%) of CIN3+ within 18 months after normal cytology at baseline [24].
This has also been presented in other studies. Wheeler et al. for example showed that the 2-year cumulative risk of developing CIN3+ for women with equivocal or mild cervical cytological abnormalities is different depending on the HPV type present. For HPV 16 the risk was 39.1%, while for the other high-risk types the cumulative risk was only 7.9% [25]. This trend can also be seen at 10 years in a study conducted by Khan et al. There, the cumulative incidence rate observed for CIN3+ among women with normal cytology at baseline was 20.7% for HPV 16+, and 17.7% for HPV 18+ women. In contrast, it was 1.5% for women who were non-HPV 16/18 high-risk positive [26]. A recent study conducted by Smelov et al. reported on the cumulative risk for CIN3+ from a 14-year follow-up study. The risk when HPV 16-positive at baseline was 34.5 as compared to 20.7 when positive for any high-risk type or 0.9 when HPV-negative [10]. Additionally, the analysis mentioned above by Elfgren et al. showed that all 40 women followed with HPV genotyping and persistent identical HPV type infection developed CIN2+ lesions within six years of persistence irrespective of the type the women remained positive for [11].
Based on the knowledge gained about type-specific clearance rates and risks for the development of CIN3+, repeat genotype testing within the regular screening algorithm for cervical cancer prevention should be considered and could greatly influence follow-up of HPV positive women [27]. This is of particular importance, considering that cytology-based screening algorithms have been shown to be inadequate in many settings for reasons such as limited sensitivity, limited human and financial resources, and poorly developed healthcare services and access to primary healthcare facilities [28]. Therefore, HPV testing is recommended for cervical cancer screening in low-resource settings by WHO since 2013 [16]. It is important though to consider the high sensitivity of HPV testing and the low positive predictive value for detection of cervical cancer of the HPV tests currently available for screening. It helps in finding more CIN2+ cases, compared to cytology, that are in need for treatment but at the same time may lead to a very high referral rate of HPV positive patients for follow-up and highlights the need for further triage strategies of HPV positive patients [29], [30]. High referral rates will result in a burden for the already low-staffed health care systems in LMICs. While persistence of high-risk HPV with the identical type according to Elfgren et al. should be recognized as a leading risk factor for the development of cervical lesions and if possible included in the screening algorithms [11], they also highlight the difficulties in managing follow-up for persistence patients.
Although the risk of developing lesions from persistent HPV infections is increased, we found five patients who had not developed any lesions, even after the extended time of persistent infection of four years. Castle et al. have described this phenomenon in a group of patients from Guanacaste, Costa Rica, who had persistent high-risk HPV infection without developing lesions, even after a mean follow-up period of 6.5 years. He suggests that an unmeasured susceptibility factor may hinder women from clearing their HPV infections but not necessarily result in the development of cervical lesions [31].
Bearing in mind the fact that in 2014 23% of the women were high-risk HPV positive, yet direct comparison of the HPV types revealed persistent infection in only 6.7% of the women, the importance of HPV genotyping tests is highlighted compared to group tests for high-risk HPV types. Especially when using persistent HPV infection as the basis for clinical follow-up, additional 16.3% (17/104) of the women would have been classified high-risk HPV persistently positive with a generic group test while they actually had cleared the original HPV types and were re-infected with a new high-risk type. Khan et al. suggested at least HPV 16 and 18 genotyping to increase the positive predictive value of HPV testing and potentially even create screening algorithms that immediately refer HPV 16/18 positive patients for colposcopy [26]. This has also been evaluated in the ATHENA study and confirmed the effectiveness of direct referral of HPV 16 or 18 positive women with NILM cytology for colposcopy as it is currently recommended in the 2006 American Society of Colposcopy and Cervical Pathology guidelines [32]. Genotyping was also shown to provide high sensitivity and specificity for the possible recurrence of disease after CIN treatment, as a ‘test of cure’ [27], [33]. Yet most of the currently available results are focused on partial genotyping only. Therefore, larger prospective cohort studies investigating the type-specific clearance, especially of non-HPV 16/18 types, such as HPV 31, 33 and 45 are needed.
Within our study we did not analyse potential risk factors for persistence of infection due to the small study group. However, in the literature, aspects such as age, cigarette smoking, parity, and oral contraceptive usage are described as characteristics associated with persistence of HPV infection [14] and could be included into risk assessment for HPV genotype persistence. We did not find an association between persistence and age, most likely due to the small study group with persistent infection, as mentioned above.
Despite the fact that this study reveals some important insight into the long-term persistence of high-risk HPV infected women with a time period of four years before follow-up, there are limitations due to the opportunistic character of this analysis. The study follow-up and longitudinal analysis was not intended from the beginning. The greatest limitation is that HPV genotyping was performed with two different HPV genotyping tests in 2011 and 2014. This may lead to misclassification of persistent infections due to the different targets of the tests (E6 and E7 vs L1) as well as the potentially different sensitivities and specificities and thus the results need to be interpreted with caution. HPV 18 was seen very frequently during the screening round in 2010/2011, yet no case of HPV 18 was seen in 2014 anymore. Beside from the clearance of HPV 18, this could be due to different sensitivities of the tests for HPV 18. Furthermore, the genotyping tests were also performed in different laboratories (Accra, Ghana and Berlin, Germany) resulting further in potential inter-laboratory differences, which limits the reliable comparison of HPV types found and, therefore, the classification of persistent infection.
HPV testing was performed only at two time points within the follow-up period despite the recommendations proposed by Muñoz and Koshiol [5], [6]. Especially Muñoz suggested that only incident infections lasting longer than the median duration of infection should be considered persistent. A common problem is though that this recommendation is not yet widely used. As a consequence, this study cannot provide additional information on the time to clearance except from this point prevalence.
In addition, the quality of cytology performed on the samples from 2014 may not be perfectly adequate. At least one confirmed case of invasive cervical cancer was classified with normal cytological findings and two additional histologically confirmed cases of CIN2 were missed. Furthermore, cytology and colposcopy was only performed on women tested high-risk HPV positive in 2014 and not on those women tested HPV negative.
5. Conclusion
The main finding that only 6.7% of high-risk HPV types persisted after four years is of significant importance and should be considered for future prospective studies evaluating potential screening algorithms. Especially at a time of paradigm shift from cytology to HPV testing for the prevention of cervical cancer, further research on the significance and feasibility of genotyping for screening is urgently needed. This longitudinal observational data suggest that single HPV screening rounds may lead to over-referral. Including type-specific HPV re-testing or additional triage methods could help reduce follow-up rates. Despite the limitations mentioned but also due to the highly interesting and important results seen in this pilot trial the performance of further prospective long-term cohort studies investigating clearance and persistence of individual HPV genotypes especially in high prevalence populations in LMIC is warranted.
Acknowledgement
We gratefully acknowledge the support of all partners involved at the Department of Obstetrics and Gynaecology, Catholic Hospital Battor, at the School of Biomedical and Allied Health Sciences, University of Ghana and at the Clinic for Gynecology, Charité Universitätsmedizin Berlin for their substantial and continuous contribution to these two screening studies. We acknowledge support from the German Research Foundation (DFG) and the Open Access Pulication Fund of Charité - Universitätsmedizin Berlin.
Acknowledgments
Authors' contribution
All authors gave input or revised the final manuscript critically. All authors read and approved the final manuscript and take public responsibility for its content.
Additional contribution by authors:
Conceptualization: GB, RHA, EKW, AMK
Data curation: AK, GB, PD
Formal analysis: AK, GB, JEA, EKW, AMK
Funding acquisition: EKW, AMK
Investigation: AK, GB, PD, RAA, LA, DON, CMW, BTH, IG
Methodology: AK, EKW, JEA, RHA, AMK
Project administration: AK, GB
Supervision: RHA, EKW, AMK
Validation: AK, GB
Visualization: AK
Writing - original draft: AK
Writing - review & editing: AK, GB, JEA, EKW, AMK
Funding
The 2010/2011 study was supported by a grant to EKW from ORID, University of Ghana (URF/3/003/2010-2011) under the 3rd Call and a donation of €1000 by Sister Dr. Edgitha Gorges of Battor Hospital.
The ACCESSING study was supported by a grant from the German Corporation for International Development (GIZ) as part of the ESTHER Network for clinical partnerships (81163705) and a financial support by the German Rotary Voluntary Doctors (GRVD).
Declarations of interest
None of the authors have any conflict of interest to declare.
Contributor Information
Amrei Krings, Email: amrei.krings@charite.de.
Rashid A. Adams, Email: raadams@chs.edu.gh.
Richard H. Asmah, Email: rhasmah@chs.edu.gh.
Edwin K. Wiredu, Email: ekwiredu@chs.edu.gh.
Andreas M. Kaufmann, Email: andreas.kaufmann@charite.de.
References
- 1.L. Bruni, et al., ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in the World. Summary Report 27 July 2017.
- 2.Walboomers J.M. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999;189(1):12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 3.Ostor A.G. Natural history of cervical intraepithelial neoplasia: a critical review. Int J. Gynecol. Pathol. 1993;12(2):186–192. [PubMed] [Google Scholar]
- 4.McCredie M.R. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. 2008;9(5):425–434. doi: 10.1016/S1470-2045(08)70103-7. [DOI] [PubMed] [Google Scholar]
- 5.Koshiol J. Persistent human papillomavirus infection and cervical neoplasia: a systematic review and meta-analysis. Am. J. Epidemiol. 2008;168(2):123–137. doi: 10.1093/aje/kwn036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munoz N. Persistence of HPV infection and risk of high-grade cervical intraepithelial neoplasia in a cohort of Colombian women. Br. J. Cancer. 2009;100(7):1184–1190. doi: 10.1038/sj.bjc.6604972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.L. Bruni, et al., ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in Africa. Summary Report 27 July 2017.
- 8.Awua A.K. Prevalence of human papillomavirus genotypes among women with cervical cancer in Ghana. Infect. Agent Cancer. 2016;11:4. doi: 10.1186/s13027-016-0050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Sanjose S. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11(11):1048–1056. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 10.Smelov V. Long-term HPV type-specific risks of high-grade cervical intraepithelial lesions: a 14-year follow-up of a randomized primary HPV screening trial. Int. J. Cancer. 2015;136(5):1171–1180. doi: 10.1002/ijc.29085. [DOI] [PubMed] [Google Scholar]
- 11.Elfgren K. Management of women with human papillomavirus persistence: long-term follow-up of a randomized clinical trial. Am. J. Obstet. Gynecol. 2017;216(3):264 e1–264 e7. doi: 10.1016/j.ajog.2016.10.042. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez A.C. Rapid clearance of human papillomavirus and implications for clinical focus on persistent infections. J. Natl. Cancer Inst. 2008;100(7):513–517. doi: 10.1093/jnci/djn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sellors J.W. Incidence, clearance and predictors of human papillomavirus infection in women. CMAJ. 2003;168(4):421–425. [PMC free article] [PubMed] [Google Scholar]
- 14.Molano M. Determinants of clearance of human papillomavirus infections in Colombian women with normal cytology: a population-based, 5-year follow-up study. Am. J. Epidemiol. 2003;158(5):486–494. doi: 10.1093/aje/kwg171. [DOI] [PubMed] [Google Scholar]
- 15.Fukuchi E. Cervical human papillomavirus incidence and persistence in a cohort of HIV-negative women in Zimbabwe. Sex. Transm. Dis. 2009;36(5):305–311. doi: 10.1097/OLQ.0b013e318194eb76. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization . WHO Guidelines; 2013. Guidelines for Screening and Treatment of Precancerous Lesions for Cervical Cancer Prevention. [PubMed] [Google Scholar]
- 17.Sotlar K. Detection and typing of human papillomavirus by e6 nested multiplex PCR. J. Clin. Microbiol. 2004;42(7):3176–3184. doi: 10.1128/JCM.42.7.3176-3184.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmitt M. Homogeneous amplification of genital human alpha papillomaviruses by PCR using novel broad-spectrum GP5+ and GP6+ primers. J. Clin. Microbiol. 2008;46(3):1050–1059. doi: 10.1128/JCM.02227-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.International Agency for Research on Cancer Biological agents. A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum. 2012;100 B [Google Scholar]
- 20.Oh J.K. Acquisition of new infection and clearance of type-specific human papillomavirus infections in female students in Busan, South Korea: a follow-up study. BMC Infect. Dis. 2008;8:13. doi: 10.1186/1471-2334-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giuliano A.R. Incidence, prevalence, and clearance of type-specific human papillomavirus infections: the Young Women's Health Study. J. Infect. Dis. 2002;186(4):462–469. doi: 10.1086/341782. [DOI] [PubMed] [Google Scholar]
- 22.Moscicki A.B. Updating the natural history of human papillomavirus and anogenital cancers. Vaccine. 2012;30(Suppl 5):F24–F33. doi: 10.1016/j.vaccine.2012.05.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bosch F.X. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine. 2008;26(Suppl 10):K1–K16. doi: 10.1016/j.vaccine.2008.05.064. [DOI] [PubMed] [Google Scholar]
- 24.Bulkmans N.W. High-risk HPV type-specific clearance rates in cervical screening. Br. J. Cancer. 2007;96(9):1419–1424. doi: 10.1038/sj.bjc.6603653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wheeler C.M. Human papillomavirus genotypes and the cumulative 2-year risk of cervical precancer. J. Infect. Dis. 2006;194(9):1291–1299. doi: 10.1086/507909. [DOI] [PubMed] [Google Scholar]
- 26.Khan M.J. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J. Natl. Cancer Inst. 2005;97(14):1072–1079. doi: 10.1093/jnci/dji187. [DOI] [PubMed] [Google Scholar]
- 27.Choi Y.J., Park J.S. Clinical significance of human papillomavirus genotyping. J. Gynecol. Oncol. 2016;27(2):e21. doi: 10.3802/jgo.2016.27.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denny L., Quinn M., Sankaranarayanan R. Chapter 8: screening for cervical cancer in developing countries. Vaccine. 2006;24(Suppl 3):S3/71–S3/77. doi: 10.1016/j.vaccine.2006.05.121. [DOI] [PubMed] [Google Scholar]
- 29.Catarino R. Cervical cancer screening in developing countries at a crossroad: emerging technologies and policy choices. World J. Clin. Oncol. 2015;6(6):281–290. doi: 10.5306/wjco.v6.i6.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gustavsson I. Randomised study shows that repeated self-sampling and HPV test has more than two-fold higher detection rate of women with CIN2+ histology than Pap smear cytology. Br. J. Cancer. 2018;118(6):896–904. doi: 10.1038/bjc.2017.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castle P.E. Long-term persistence of prevalently detected human papillomavirus infections in the absence of detectable cervical precancer and cancer. J. Infect. Dis. 2011;203(6):814–822. doi: 10.1093/infdis/jiq116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright T.C., Jr. Evaluation of HPV-16 and HPV-18 genotyping for the triage of women with high-risk HPV+ cytology-negative results. Am. J. Clin. Pathol. 2011;136(4):578–586. doi: 10.1309/AJCPTUS5EXAS6DKZ. [DOI] [PubMed] [Google Scholar]
- 33.Jones J. Human Papillomavirus genotype testing combined with cytology as a ‘test of cure’ post treatment: the importance of a persistent viral infection. J. Clin. Virol. 2011;52(2):88–92. doi: 10.1016/j.jcv.2011.06.021. [DOI] [PubMed] [Google Scholar]