Abstract
Pathogenic sepsis is not a monolithic condition. Three major types of sepsis exist within this category: bacterial, viral, and fungal, each with its own mechanism of action. While similar in symptoms, the etiologies and immune mechanisms of these types differ enough that a discrete patient base can be recognized for each one. Non-specific treatment, such as broad-spectrum antibiotics, without determination of sepsis origins may worsen sepsis symptoms and leads to increased morbidity and mortality in patients. However, recognition of current and historical patterns in likely patients for each sepsis type may aid in differentiation between pathogens prior to definitive blood testing. Clinicians may ultimately be able to diagnose and treat bacterial, viral, and fungal sepsis using analysis of previous patient patterns and circumstances in addition to standard care. This method is likely to decrease incidence of multidrug-resistant organisms, organ failure due to ineffective treatment, and turnaround time to the correct treatment for each sepsis patient. Ultimately, we aim to provide classification information on these patient populations and to suggest epidemiology-based screening methods that can be integrated into critical care medicine, specifically triage and treatment of sepsis.
Keywords: sepsis, intensive care, bacteremia, viremia, fungemia, inflammation, critical care
Introduction
Sepsis is a complex disease with highly varied presentation, depending in large part on the identity of the inciting pathogen, the method of disease contraction, and patient immune competence. Although sepsis mortality rates have decreased, the incidence of sepsis in the United States has risen, with nearly a 50% increase in sepsis hospitalizations between 2003 and 2009.1 In contrast, general hospitalizations for infection rose only 11.5% in that same time period.1 Between 2010 and 2015, the average mortality rate for patients hospitalized with sepsis decreased from 24.1% to 14.8%. However, 50% of sepsis mortality is due to hospital-acquired sepsis (HAS), which is often more severe and resistant to antibiotics than sepsis present upon admission.2,3 HAS case-fatality rates may reach 45%. This is often due to sepsis contracted by patients in the intensive care unit (ICU) and contributes substantially to the average mortality rate in developed countries, as opposed to the relatively lower mortality rates (15% as opposed to 45%) in cases of sepsis acquired outside the hospital.2–4
Over 5 000 000 deaths due to sepsis occur globally every year, with over 750 000 hospitalizations and 215 000 deaths in the United States alone.5,6 Similar proportions of sepsis deaths occur in other developed countries, such as the United Kingdom.5 Despite varied causes, standard treatment is aimed primarily at bacterial sepsis. First-line protocols often include empirical treatment with broad-spectrum antibiotics even before the acquisition of blood cultures.7
In many cases, treatment may cause more harm than it prevents. While bacterial infections remain the primary cause of pathogenic sepsis, viruses and fungi comprise a meaningful percentage of sepsis etiologies, especially among immunocompromised patients and those with other comorbidities; 17% of sepsis as assessed in US hospitals, for example, is attributable to Candida fungal species.3,8,9 Among the adult patient population, the elderly and patients suffering from human immunodeficiency virus (HIV)/acquired immune deficiency syndrome (AIDS), diabetes, cardiovascular disease, and other common comorbidities are especially prone to sepsis of heterogeneous origin and/or increased severity, while neonates are predisposed to similar but distinct subtypes.3,9–11 Antibiotics may be administered as part of the standard of care, even without a documented need. Antibacterial treatment has no effect on non-bacterial sepsis and is associated with increased mortality when mistakenly administered at high doses to treat fungal sepsis.12
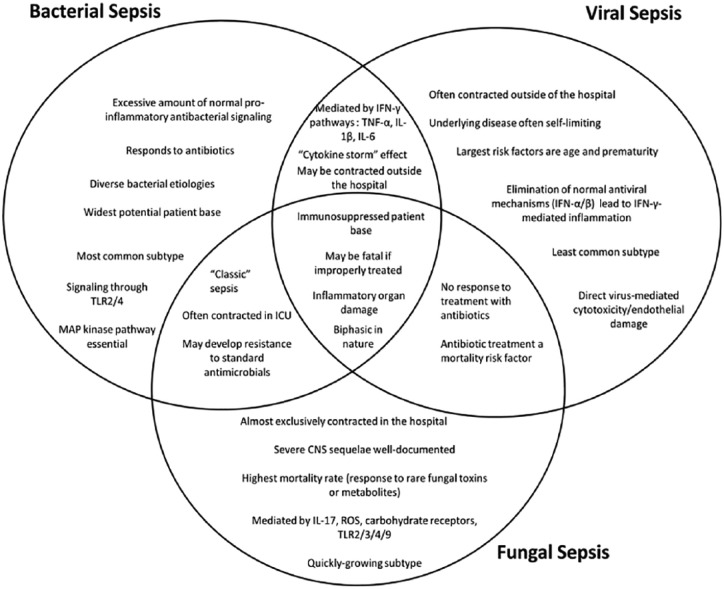
Common mechanisms unite the different states of pathogenic sepsis, but significant differences exist and contribute to unique complications. Each type of sepsis—bacterial, viral, and fungal—is associated with distinct groups of patients, comorbidities, and circumstances (Figure 1). The essential treatment for each type varies, and what works for one may be detrimental to the others. Therefore, it is essential that the medical and scientific communities know the different mechanisms, risks, and likelihood of presence of each of the three when a potential case of sepsis appears.
Figure 1.
A pictorial representation of the similarities and differences in mechanism, circumstances, and patient base between the three major types of pathogenic sepsis. The best-known mechanisms for each sepsis type are represented, although these molecules and mechanisms are not universal. CNS indicates central nervous system; IFN, interferon; IL, interleukin; ROS, reactive oxygen species; TLR, toll-like receptor; TNF, tumor necrosis factor.
Ultimately, the need for more effective triage methods in patients with suspected sepsis is highlighted not only by sepsis mortality and case-fatality rates, but by the fact that no triage system that differentiates sepsis patients from other at-risk patients has been implemented in US hospitals. Sepsis patients spend an average of 5 h in the emergency department after admission, the same length of time as patients deemed non-emergent by current cardiac triage methods.13 While attempts have been made to implement triage systems that more accurately take into account the likelihood of sepsis for patients in emergency departments, these screening tools primarily take symptoms, that is, sequential organ failure assessment (SOFA) criteria and other sepsis-identification methods, into account rather than epidemiology and potential pathogenic origin of the sepsis in question.14,15 We recognize that observation of patients and inclusion of patient history in sepsis diagnosis, while important, can and should be a part of standard care. However, the fact remains that these methods have thus far not been widely used in terms of differentiating between sepsis types. In this review of the typical situations leading to the three major types of pathogenic sepsis, we propose the use of thus far underutilized methods as an addition to standard care, for the purpose of such differentiation.
Common Mechanisms and Complications of Sepsis
Sepsis is defined as life-threatening organ damage resulting from a dysregulated host response to infection.16 The sepsis process creates a biphasic reaction in the host; immune hyperresponsiveness is followed by a hyporesponsive state due to immune exhaustion. The majority of sepsis deaths occur during this second stage, often from secondary infection.17 However, the coagulopathy, vascular endothelial leakage, cardiovascular strain, and massive release of pro-inflammatory cytokines characteristic of early sepsis comprise the major mechanism of organ damage.17-19 Standard treatments are thus often aimed at this stage.7
The common mechanism of the sepsis process, when caused by an invasive pathogen, typically involves inter- and intracellular signaling through cell-surface receptors that initiate the complex signal transduction mechanisms common to many types of sepsis. Excessive strain on the heart from septic activation of the autonomic nervous system creates a state of global cardiac hypokinesis, hypotension, and hypoperfusion. This further exacerbates ischemia from excessive coagulation within the organs and creates further tissue pathology.17,19 Ultimately, these converging mechanisms lead to a hypotensive, electrolyte-imbalanced patient, with organs failing at various rates due to ischemic injury, hypoxemia, and pro-inflammatory tissue damage.
This inflammatory stage of sepsis may last for hours to days, depending on the extent of damage. Early sepsis mortality peaks at approximately 3 to 5 days.20 If the patient survives the inflammatory stage, immunosuppression will set in over the next several days after the exhaustion of pro-inflammatory resources. At this point, signaling toward an anti-inflammatory phenotype begins.17,21 At 20 to 30 days following the onset of sepsis, deaths from secondary infection peak due to commensal flora or opportunistic bacteria that could not normally colonize a healthy patient.18
Reactivation of latent viruses is another common complication of late sepsis and may cause further morbidity and mortality, even if the sepsis itself is not viral. Viruses reactivated during this stage include cytomegalovirus, herpes simplex, and Epstein-Barr.18 These generally benign viruses have been found to increase 90-day mortality in sepsis patients. Increased viremia (detected in multiple bodily fluids) leads to a higher risk of morbidity or death.22
Pre-existing immunocompromise is perhaps the most important risk factor for sepsis patients. Although anyone can contract sepsis, regardless of health status, immunocompromised patients face a much more serious course of illness. For example, premature neonates are extremely susceptible to bacterial and fungal sepsis.9 Adult patients with pre-existing cardiovascular disease, diabetes, or HIV/AIDS are more susceptible to opportunistic sepsis, especially through antibiotic-resistant organisms.3,4 This is more common for HAS patients in the ICU than for community-acquired sepsis (CAS) patients, a dangerous factor considering the ambiguous state of ICU patients.3
The distinct stages of sepsis are common between the major etiological types (bacterial, viral, and fungal); it is primarily patient heterogeneity that determines the difference in morbidity, mortality, and complications. However, the mechanisms and etiologies of each type of sepsis differ enough that major patient populations can be extrapolated for each and possibly used as an effective triage tool with standard treatment.
Bacterial Sepsis
Bacteria are the most common cause of sepsis, with 62.2% of patients with positive blood cultures harboring Gram-negative bacteria and 46.8% infected with Gram-positive bacteria.3 This overlap can be explained by polymicrobial sepsis, which is frequently simulated in mouse models.8,23 While Escherichia coli can be found in approximately 1 in 6 culture-positive patients, Gram-positive bacteria such as methicillin-resistant Staphylococcus aureus (MRSA) have made up an increasing percentage of sepsis with the advent of excessive antibiotic treatment.24 The lungs are the most frequent site of colonization; pneumonia averages 38% to 39% of all bacterial cases. However, abdominal, urinary tract, and wound-related sepsis make up significant percentages as well.3
The general mechanism of bacterial sepsis shares many conserved elements, no matter the type of bacteria involved. Bacterial surface toxins, such as lipopolysaccharide (LPS), or other secreted bacterial pathogen-associated molecular patterns (PAMPs), stimulate toll-like receptors (TLRs) and other cell-surface receptors on host cells.25 Intracellular signaling then initiates pro-inflammatory cascades and additional inflammatory cell recruitment. It is this process, not simply bacteremia, that causes the organ damage, coagulopathy, and obtunded state characteristic of severe sepsis.19
Bacterial sepsis acquired in the hospital, especially when a patient is already in the ICU, tends to follow a much more severe course than sepsis for which a patient is admitted to the hospital. HAS is thus approximately five times more expensive than CAS, and the mortality rate of HAS is approximately twice that of CAS, with calculated in-hospital mortality rate of 19.2% as opposed to 8.6%.2–4,26 The organisms responsible for HAS also differ from CAS; they are often opportunistic and resistant to some or all of the first-line antibiotics used to treat them. Whereas pathogenic Gram-negative bacteria are at least partially responsible for the majority of bacterial sepsis cases, a heterogeneous mixture predominates in HAS. A large number of cases, for example, can be attributed to drug-resistant Pseudomonas species.27 Other common Gram-negative species include E. coli as well as Klebsiella and Enterobacter.28 The majority of Gram-positive HAS, on the other hand, can be attributed to Staphylococcus species, especially methicillin-resistant Staphylococcus aureus (MRSA), a bacterium that is a growing concern among the lay and medical populations alike.28
An immunocompromised state is the unifying factor for those patients who are most susceptible to bacterial sepsis. Many conditions contribute to susceptibility, including cardiovascular disease, steroid treatment, organ transplantation, cancer, and chronic obstructive pulmonary disease (COPD).4,29 Age is also a contributing factor, not only from age-related immunocompromise, but because elderly patients present with a comorbid condition such as diabetes or cardiovascular disease in 70% of cases, as opposed to 56% of adult sepsis patients.30,31 HIV/AIDS patients are also an important sub-population. AIDS patients frequently contract septic infections when in the ICU, with an average case-fatality rate of 42% and increasing incidence of antibiotic resistance in the relevant pathogens.3 Sepsis in AIDS patients is now more often caused by mixed and/or opportunistic Gram-negative bacteria than by fungi, which have previously been collectively considered an AIDS-defining illness.3,32 This indicates possible problems with standard treatment of bacterial sepsis as much as inherent vulnerability of AIDS patients, as 57% of sepsis in HIV patients is contracted in the hospital.3
Treatment for bacterial sepsis involves early administration of broad-spectrum antibiotics, fluid replenishment, and close blood-pressure monitoring.7 Vasopressors may be administered as needed.33 These treatments focus on alleviating the symptoms of sepsis, not the basal inflammation; an exception is glucocorticoid treatment. However, the sepsis process is often resistant to this anti-inflammatory class of medications, and thus, glucocorticoids may be ineffective or harmful to the patient.34
Bacterial sepsis, on the whole, most closely fits the definition of classic sepsis in mechanism, course, and treatment. As in the other two major types, immunocompromised patients have the highest risk of sepsis and poor outcome; however, the variety of potential bacteria and universal nature of the pro-inflammatory response provide a large patient pool. Consequent overlap with potential fungal and viral sepsis patients underscores the importance of considering patient history and epidemiology of pathogens in sepsis assessment.
Viral Sepsis
The pro-inflammatory mechanisms of viral and bacterial sepsis are similar. Severe viral infections can cause septic syndromes that are functionally identical to bacterial sepsis.19,35 This typically occurs through viral knockdown of the initial antiviral response; however, a number of viruses, such as the common parechovirus, can also cause sepsis-like inflammatory syndromes and complications.36 While the host antiviral response differs from the antibacterial response, common pro-inflammatory mechanisms can lead to sepsis in certain populations.
First-line antiviral signaling occurs via activation of Type I α/β interferon (IFN), as well as IFN-γ, a type II IFN responsible for pro-inflammatory cytokine and chemokine signaling.37,38 These proteins then initiate pro-inflammatory reactions primarily through phosphorylation of proteins that then initiate production of more IFN.39 Although cytokine-dependent signaling also begins, the IFN system is the primary host antiviral mechanism. In fact, IFNs are protective against sepsis induced by bacterial endotoxins as well.39
IFNs do not, however, preclude sepsis. A number of viruses downregulate the interferon response, and as a result can induce a septic state identical to that caused by non-viral pathogens. The influenza virus, which is primarily a threat to children and the elderly, causes inflammation through upregulation of cytokines.20 The coagulopathies responsible for the symptoms of various hemorrhagic fevers are also based on the same mechanisms of vascular damage that create other types of sepsis.40,41 This is, in effect, identical to bacterial sepsis-induced loss of normal vascular fluid dynamics.41 As in bacterial sepsis, viral sepsis is largely mediated by pro-inflammatory cytokines; the subsequent mechanisms of tissue and vascular damage are functionally identical.37
Despite these conserved mechanisms, viral sepsis is uncommon in the general population. Organisms that are neither bacteria nor fungi are cultured in fewer than 5% of documented adult sepsis cases.3 The groups most at risk for sepsis of viral origin, despite the pro-inflammatory pathways common to all types, are children and the elderly. While the epidemiology and estimations for pure viral sepsis are difficult to describe and calculate, possibly due to septic reactivation of latent viruses and resulting complications, respiratory syncytial virus (RSV) is a major cause of systemic viral infection in vulnerable populations.18,22,42 Like other sepsis-related pathogens, its primary mechanism of mortality is through secondary infections caused by viral immune compromise; pre-existing immunocompromise and inadequate medical care are risk factors for RSV-related death.43 Viruses are by nature nonresponsive to antibiotics, so the major treatment for viral sepsis is supportive care, although experimental antiviral treatment has had some positive effect in mice and humans.44,45
Viral sepsis in healthy adults mainly occurs through virus-mediated inactivation of IFN signaling, as previously discussed. Despite its rarity in this population, it does have some historical precedent in previous influenza pandemics.46–48 These same mechanisms of cytokine storm-based inflammation are responsible for the severe sepsis symptoms seen in current viral hemorrhagic fever outbreaks.49 Despite these exceptions, however, the patient pool for viral sepsis is the same as those most prone to dying of seasonal influenza. This must be considered when treating sepsis patients within this group, as antibiotics may ultimately harm these and other hospital patients in general.
Fungal Sepsis
Fungal sepsis shares common mechanisms with bacterial sepsis, but in contrast, it is a fast-growing and often lethal subtype.50,51 Approximately 17% of sepsis can be attributed to Candida species, with 2% to 3% more caused by Aspergillus and others.3,52 Fungi make up part of the normal flora in many parts of the body.53 In an invasive situation, however, fungal sepsis can kill at a rate of 40% to 60%.11,52 This is far higher than the approximately 30% average case-fatality rate of bacterial or viral sepsis, and approaches or exceeds the upward of 45% case-fatality rate of antibiotic-resistant HAS.3,26
Much of this difference in morbidity and mortality is traceable to two factors: mechanism and mode of acquisition. While bacterial sepsis is often a result of an immune response to cell-surface antigens, fungal sepsis is often caused by reactions to fungus-specific toxins and byproducts.53 Gliotoxin, a fungal metabolite, induces sepsis-typical proteins in experimental cell models and may induce in vivo sepsis through destruction of gut tissue.53 Otherwise, neutral and/or unnoticed variations in innate immunocompetence are also thought to allow fungal overgrowth and treatment-resistant sepsis in otherwise healthy patients. This may partially explain the difference between mortality rates for bacterial and fungal sepsis.52
While bacterial and viral sepsis are mediated by the same mechanisms of antigen sensing and cytokine production, fungal sepsis occurs through different mechanisms. The sequence of classic sepsis is similar in bacterial and fungal sepsis, but fungal sepsis is largely perpetuated through signal transduction pathways that differ from those of the more common bacterial sepsis, such as interleukin (IL)-17.54 However, this molecule is not solely responsible for fungal sepsis and may not be a viable therapeutic target. Many diverse cell-surface receptors, including carbohydrate receptors, are involved in sensing fungal antigens, due to the fact that fungi by nature present carbohydrates on their surfaces rather than bacterial or viral cell-surface markers. These diverse sensing and signaling pathways can lead to similar symptoms, but different cytokine profiles.54–56 The same pathways also cause upregulation of anti-inflammatory cytokines such as IL-10, contributing to immunosuppression that may be partially responsible for the high mortality rate.55 Some signaling attributable only to fungal sepsis also heavily damages the kidneys, which may contribute to organ pathology.54 While no one cytokine is solely responsible for the symptoms of fungal or any other type of sepsis, the differences between the mechanisms of fungal versus other types of sepsis must be acknowledged.
The second factor is that fungal sepsis is almost exclusively acquired in a health care setting; 93% of bloodstream candidiasis is hospital-acquired; 80% or more of fungal sepsis patients have been exposed to empiric antibiotics, and although the mechanism for the increased likelihood of fungal sepsis is not fully known, it is a risk factor.57 Even neonates with fungal sepsis, often transferred in utero through maternal genital infection, only become seriously ill in the hospital sometime after they are born.55 Fungi enter the bloodstream upon prolonged exposure of the vasculature to skin flora, often through indwelling catheters, and sepsis can result from this fungal exposure.58
Fungal sepsis is made more dangerous by the fact that its complications are often more severe than those resulting from other types. While viral infection can cause encephalitis and meningitis, especially in children, these are often self-limiting conditions due to the nature of viral infections.37 In contrast, fungal sepsis can cause severe ascending pathology within the central nervous system. Fungal meningitis, especially with Cryptococcus species in HIV/AIDS patients, requires treatment with high doses of often toxic antifungal drugs, such as Amphotericin B.57 This drug is severely nephrotoxic, already a concern for patients with conditions such as diabetes.59 While this drug is no longer the first-line treatment for fungemia, azole drugs given orally or intravenously may be ineffective due to previous prophylactic exposure. This is itself a risk factor for medication-resistant fungal sepsis.60
Meningitis and meningoencephalitis are only two of the potential sequelae of fungal sepsis. Candida and Aspergillus species in particular can lead to large lung infiltrates that are disseminated and/or granulomatous and can worsen common pre-existing lung conditions such as asthma and COPD. Aspergillus can itself cause lung pathology and complications in the form of a severe allergic reaction.57 In those with such conditions, which may already have caused some vulnerability to the fungi, these infections are especially dangerous.
It may appear at first glance that the patient pools for bacterial and fungal sepsis should be similar, given the similar mechanisms of action that sometimes group the two into the definition of classic sepsis.50 However, the vastly disparate sequelae of bacterial and fungal sepsis reveal the differences in mechanism between these two types of organism, despite illness similarity. Review of the circumstances of each type of sepsis also shows differences in patient populations. Fungal sepsis is rarely found outside of the hospital, specifically the ICU, while bacterial sepsis is often found in patients admitted with symptoms.2–4,26,57 The patterns of organ-specific symptoms may also enable health care workers to decide on treatment before definitive blood cultures are returned, possibly leading to better patient outcomes.59
The Need for Better Triage Methods
The ultimate goal in treating sepsis patients should involve making the hospital safe for future patients as well as decreasing morbidity and mortality. Infections are often worsened by antibiotic resistance, which creates a problem when antibiotics are used as the first-line treatment for suspected sepsis.7 This may also impact other ICU patients, as the isolated nature of intensive care and movement of clinicians from room to room makes the transfer of medication-resistant infections more likely. The immediate severity of infections contracted in the ICU corroborates this hypothesis.2–4,26
Extreme measures to reduce antibiotic resistance, however necessary they are, must be tempered by the fact that early antibiotic therapy in bacterial sepsis patients has been shown to decrease in-hospital adjusted mortality rates of 20% to 25%, as opposed to an adjusted mortality rate of 25% to 30% when antibiotics are given after 12 h (60). Standard care for bacterial sepsis, the most common subtype, must not be eliminated, but rather added to with methods that take epidemiological patterns into consideration. This could be implemented in the emergency department, for cases of CAS, or in the ICU for cases of HAS.
Previously attempted sepsis triage methods have, with some success, been used to reduce symptoms and overall case-fatality rate, but these are based on symptomatic assessment of organ failure. Triage methods for other conditions that are based on patient history and current symptoms, however, are largely successful. For example, triage for cardiovascular events such as myocardial infarctions is based on recent patient history, physical symptoms, general affect and appearance, and the presence or absence of several key biomarkers.61 This has been shown to be largely effective in early diagnosis and treatment of severe cardiovascular events. Similar questions and training to recognize sepsis symptoms could potentially be implemented in both the emergency department and the ICU, with the possible addition of biomarker tests if they can be developed as suggested.62 An option for ICU triage is computer flagging of patients who have not responded to standard treatment, using algorithms based on whether the patient’s vital signs have significantly improved from the baseline, with the goal of re-evaluation by the treatment team. Evidence that early pinpointed therapy improves sepsis patient outcomes provides some support for this approach.60
It is true that in the initial effort to treat a patient at risk for severe sepsis or septic shock, identification of the specific pathogen prior to treatment is understandably not a major priority. However, observing patterns of patient populations that most closely correlate to each major sepsis type will likely allow better classification of sepsis without a full pathogen profile. This may save not only time and money for hospitals and health care professionals, but also the lives, and quality of life, of future patients that would otherwise encounter medication-resistant organisms.
The patterns of typical patients for bacterial, viral, and fungal sepsis all contain populations with some degree of immunocompromise. However, knowledge of the situations that lead to each type of sepsis may assign these patients to discrete groups. Bacterial sepsis is the broadest group, with patients of all ages represented. Nevertheless, these patients are more likely to be admitted with sepsis than to contract it in the hospital, which narrows down the subtypes among admitted patients.26 These patients are also more likely to have been exposed to opportunistic bacteria in situations such as prolonged ventilation, without concomitant trauma. This has been the case for decades.63 Finally, pre-existing comorbid conditions make this subtype most likely.3,4,31,32 While antibiotics are effective for this sepsis subtype, susceptibility testing should be done as quickly as possible, as broad-spectrum administration leads to resistance.28
We recognize that the methods we propose are based primarily on observation, and that viral sepsis is most commonly found in very young children and the elderly, and is overwhelmingly respiratory or neurological in origin, save for the rarer and less discriminating viruses that directly attack the vasculature. This is the rarest type, but it cannot be treated with antibiotics and such treatment risks creation of drug-resistant organisms.45 Thus, triage to rule out this subtype should be geared toward those of the relevant age groups who are already ill, and treatment should be primarily supportive care, with the exception of antiviral treatments that have already been successfully used.43,44 In addition, the existence of separate emergency departments and intensive care wards for pediatric and adult patients may allow better discrimination between sepsis types primarily in pediatric medicine.
Fungal sepsis is phenotypically similar to bacterial sepsis and can occur in similar situations, but signs and symptoms as well as patient risk factors differ enough that effective triage and type identification is possible. For example, systemic fungal infection often shows unique symptoms at the origin point, such as distinctive rash and exudate.59 The rarity of fungal sepsis outside the hospital also helps to rule it out when a patient arrives with symptoms of sepsis; conversely, the predominance of bacteria-derived sepsis in common immobility situations, such as position-induced aspiration pneumonia, may aid in distinction even within the ICU.57,63 The presence of HIV/AIDS or premature birth status in a patient with meningitis may make fungal sepsis part of the differential diagnosis, and imaging the lungs will likely be helpful if quickly obtained.57
The differences in these patient groups are subtle, but observation of sepsis patients has created evidence for discrete grouping in the literature as a whole. If such observations, questions, and standard time-based re-assessment of potentially septic patients are implemented as part of standard triage for patients with symptoms of sepsis, hospitals would likely benefit from lack of wasted resources and decreased antimicrobial resistance. This would be especially helpful in the first 72 h after initiation of antibiotic treatment, when the multidisciplinary treatment team has had an opportunity to evaluate and re-evaluate the patient. This would thereby provide a cohesive approach by which to continue or alter the treatment regimen.
Conclusions
Pathogenic sepsis is not a single disease, nor is it solely the domain of bacteria, despite common misconceptions. While bacteria are the most common cause of sepsis, they are not the only cause. Consequent overuse or misuse of antibiotics leads to antibiotic resistance in the hospital and complications for future patients. Non-bacterial sepsis in these cases may arise from resistant organisms, and previous exposure to antibiotics is likely to induce more severe sepsis. We encourage our colleagues to be vigilant in their selection and use of antibiotics as well as cognizant of the non-bacterial causes of sepsis and the conditions, situations, and populations in which they may occur. Furthermore, the importance of the multidisciplinary approach to the septic patient cannot be strongly emphasized enough.
The implementation of novel methods of triage, namely using pattern recognition to more accurately estimate the cause of pathogenic sepsis before administration of treatment, may be beneficial. It is not meant to delay initial treatment of the septic patient. Education of health care professionals on similarities and differences in typical patients for each sepsis subtype may lead to faster and more accurate diagnosis, or at least provide a rational basis for a pivot in clinical treatment if a patient responds poorly. However, this approach would have to be supported by clinical studies and would be in addition to, not instead of, current triage and treatment protocols. Taking into account subtle differences in symptoms as well as pattern recognition of likely demographics, health care workers may be able to more quickly ascertain and begin the proper treatment while at the same time adding to the body of scientific knowledge regarding the approach to sepsis.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: HD conducted the literature review and wrote the manuscript. TP provided input into critical care medicine. XC and ZP corrected the manuscript from a scientific perspective.
References
- 1. Walkey AJ, Lagu T, Lindenauer PK. Trends in sepsis and infection sources in the United States. A population-based study. Ann ATS. 2015;12:216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Meyer MD, Harhay MO, Small DS, et al. Temporal trends in incidence, sepsis-related mortality, and hospital-based acute care after sepsis. Crit Care Med. 2018;46:354–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence. 2014;5:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. López-Mestanza C, Andaluz-Ojeda D, Gómez-López JR, Bermejo-Martín JF. Clinical factors influencing mortality risk in hospital-acquired sepsis. J Hosp Infect. 2018;98:194–120. [DOI] [PubMed] [Google Scholar]
- 5. Brent AJ. Sepsis. Medicine. 2018;45:649–653. [Google Scholar]
- 6. Moore JX, Donnelly JP, Griffin R, Howard G, Safford MM, Wang HE. Defining sepsis mortality clusters in the United States. Crit Care Med. 2016;44:1380–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. [DOI] [PubMed] [Google Scholar]
- 8. Alberti C, Brun-Buisson C, Burchardi H, et al. Epidemiology of sepsis and infection in ICU patients from an international multicenter cohort study. Intens Care Med. 2002;28:108–121. [DOI] [PubMed] [Google Scholar]
- 9. Chavez-Bueno S, McCulloh RJ. Current trends in epidemiology and antimicrobial resistance in neonatal sepsis. Ann Upd Int Care Emerg Med. 2018;39–51. [Google Scholar]
- 10. Palella FJ, Baker RK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43:27–34. [DOI] [PubMed] [Google Scholar]
- 11. Delaloye J, Calandra T. Invasive candidiasis as a cause of sepsis in the critically ill patient. Virulence. 2014;5:161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burchard KW, Minor LB, Slotman GJ. Fungal sepsis in surgical patients. JAMA. 1983;118:217–221. [DOI] [PubMed] [Google Scholar]
- 13. Wang HE, Shapiro NI, Angus DC, Yealy DM. National estimates of severe sepsis in United States emergency departments. Crit Care Med. 2007;35:1928–1936. [DOI] [PubMed] [Google Scholar]
- 14. Moore LJ, Jones SL, Kreiner LA, et al. Validation of a screening tool for the early identification of sepsis. J Traum. 2009;66:1539–1547. [DOI] [PubMed] [Google Scholar]
- 15. Jouffroy R, Saade A, Carpentier A, et al. Triage of septic patients using qSOFA criteria at the SAMU regulation: a retrospective analysis. Prehosp Emerg Care. 2018;22:84–90. [DOI] [PubMed] [Google Scholar]
- 16. Singer M, Deutschman C, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rittirsch D, Flierl MA, Ward PA. Harmful molecular mechanisms in sepsis. Nat Rev Immunol. 2008;8:776–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koch RM, Kox M, de Jonge MI, van der Hoven JG, Ferwerda G, Picckers P. Patterns in bacterial- and viral-induced immunosuppression and secondary infections in the ICU. Shock. 2017;47:5–12. [DOI] [PubMed] [Google Scholar]
- 19. Evans T. Diagnosis and management of sepsis. Clin Med. 2018;18:146–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Florescu DF, Kalil AC. The complex link between influenza and severe sepsis. Virulence. 2014;5:137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Delano MJ, Ward PA. Sepsis-induced immune dysfunction: can immune therapies reduce mortality? J Clin Invest. 2016;126:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Walton AH, Muenzer JT, Rasche D, et al. Reactivation of multiple viruses in patients with sepsis. PLoS ONE. 2014;9:e98819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dejager L, Pinheiro I, Dejonckheere E, Libert C. Cecal ligation and puncture: the gold standard model for polymicrobial sepsis? Trends Microbiol. 2011;19:198–208. [DOI] [PubMed] [Google Scholar]
- 24. Saner FH. Sepsis and infection. Liver Anesth Crit Care Med. 2018;455–468. [Google Scholar]
- 25. McCulloh RM, Opal SM. Sepsis management: importance of the pathogen. In: Wiersinga WJ, Seymour CW. (eds) Handbook of Sepsis. Cham: Springer International Publishing; 2018:159–184. [Google Scholar]
- 26. Page DB, Donnelly JP, Wang HE. Community-, healthcare-, and hospital-acquired severe sepsis hospitalizations in the university healthsystem consortium. Crit Care Med. 2015;43:1945–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Palavutitotai N, Jitmuang A, Tongsai S, Kiratisin P, Angkasekwinai N. Epidemiology and risk factors of extensively drug-resistant Pseudomonas aeruginosa infections. PLoS ONE. 2018;13:e0193431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thaden JT, Li Y, Ruffin F, et al. Increased costs associated with bloodstream infections caused by multidrug-resistant gram-negative bacteria are due primarily to patients with hospital-acquired infections. Antimicrob Agents Chemother. 2017;61:e01709–e01716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ani C, Farshidpanah S, Bellinghausen Stewart A, Nguyen BH. Variations in organism-specific severe sepsis mortality in the United States: 1999-2008. Crit Care Med. 2015;43:65–77. [DOI] [PubMed] [Google Scholar]
- 30. Kalil AC, Opal SM. Sepsis in the severely immunocompromised patient. Curr Infect Dis Rep. 2002;17:487. [DOI] [PubMed] [Google Scholar]
- 31. Carbajal-Guerrero J, Cayuela-Domínguez A, Fernández-García E, et al. Epidemiology and long-term outcome of sepsis in elderly patients. Med Intensiva. 2014;38:21–32. [DOI] [PubMed] [Google Scholar]
- 32. Rosenberg AL, Seneff MG, Atiyeh L, Wagner R, Bojanowski L, Zimmerman JE. The importance of bacterial sepsis in intensive care unit patients with acquired immunodeficiency syndrome: implications for future care in the age of increasing antiretroviral resistance. Crit Care Med. 2001;29:548–556. [DOI] [PubMed] [Google Scholar]
- 33. Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of sepsis and septic shock. NEJM. 2001;345:1368–1377. [DOI] [PubMed] [Google Scholar]
- 34. Dendoncker K, Libert C. Glucocorticoid resistance as a major drive in sepsis pathology. Cytok Growth Factor Rev. 2017;35:85–96. [DOI] [PubMed] [Google Scholar]
- 35. Pinto AK, Ramos HJ, Wu X, et al. Deficient IFN signaling by myeloid cells leads to MAVS-dependent virus-induced sepsis. PLoS Pathog. 2014;10:e1004086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wolthers KJ, Benschop KSM, Schinkel J, et al. Human parechovirus as an important viral cause of sepsis-like illness and meningitis in young children. Clin Infect Dis. 2008;47:358–363. [DOI] [PubMed] [Google Scholar]
- 37. Kelly-Scumpia KM, Scumpia PO, Delano MJ, et al. Type I interferon signaling in hematopoietic cells is required for survival in mouse polymicrobial sepsis by regulating CXCL10. J Exp Med. 2010;207:319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Levy DE, García-Sastre A. The virus battles: IFN induction of the antiviral state and mechanisms of viral evasion. Cytok Growth Factor Rev. 2001;12:143–156. [DOI] [PubMed] [Google Scholar]
- 39. Baccala R, Welch MJ, Gonzalez-Quintial R, et al. Type I interferon is a therapeutic target for virus-induced lethal vascular damage. PNAS. 2014;111:8925–8930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Steinberg BE, Goldenberg NM, Lee WL. Do viral infections mimic bacterial sepsis? The role of microvascular permeability: a review of mechanisms and methods. Antivir Res. 2012;93:2–15. [DOI] [PubMed] [Google Scholar]
- 41. Kilani RA. Respiratory syncytial virus (RSV) outbreak in the NICU: description of eight cases. J Trop Ped. 2002;48:118–122. [DOI] [PubMed] [Google Scholar]
- 42. Caballero MT, Polack FP. Respiratory syncytial virus is an “opportunistic” killer. Pediatr Pulmonol. 2018;53:664–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rodriguez WJ, Gruber WC, Groothuis JR, et al. Respiratory syncytial virus immune globulin treatment of RSV lower respiratory tract infection in previously healthy children. Pediatrics. 1997;100:937–942. [DOI] [PubMed] [Google Scholar]
- 44. Shi W, Jiang Z, He H, et al. Discovery of 3,3′-Spiro(Azetidine)-2-oxo-indoline derivatives as fusion inhibitors for treatment of RSV infection. ACS Med Chem Lett. 2018;9:94–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Keipp Talbot H, Belongia EA, Walsh EE, Schaffner W. Respiratory syncytial virus in older adults: a hidden annual epidemic. Inf Dis Clin Pract. 2016;24:295–302. [Google Scholar]
- 46. Writing Committee of the WHO Consultation on Clinical Aspects of Pandemic (H1N1) 2009 Influenza. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. NEJM. 2010;362:1708–1719. [DOI] [PubMed] [Google Scholar]
- 47. London NR, Zhu W, Bozza FA, et al. Targeting Robo4-dependent Slit signaling to survive the cytokine storm in sepsis and influenza. Sci Transl Med. 2010;2:23ra19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lai KY, Ng WYG, Cheng FF. The W-shaped mortality-age distribution of novel H1N1 influenza virus helps reconstruct the second wave of pandemic 1918 Spanish flu. J Pulm Respir Med. 2015;5:245. [Google Scholar]
- 49. Hellman J. Addressing the complications of Ebola and other viral hemorrhagic fever infections: using insights from bacterial and fungal sepsis. PLoS Pathog. 2015;11:e1005088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bassetti M, Garnacho-Montero J, Calandra T, et al. Intensive care medicine research agenda on invasive fungal infection in critically ill patients. Intens Care Med. 2017;43:1225–1238. [DOI] [PubMed] [Google Scholar]
- 51. Spec A, Shindo Y, Burnham CD, et al. T cells from patients with Candida sepsis display a suppressive immunophenotype. Crit Care. 2016;20:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Upperman JS, Potoka DA, Zhang X, Wong K, Zamora R, Ford HR. Mechanism of intestinal-derived fungal sepsis by gliotoxin, a fungal metabolite. J Ped Surg. 2003;38:966–970. [DOI] [PubMed] [Google Scholar]
- 53. Huang J, Meng S, Hong S, Lin X, Jin W, Dong C. IL-17C is required for lethal inflammation during systemic fungal infection. Cell Mol Immunol. 2016;13:474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Netea MG, Joosten LAB, van der Meer JWM, Kullberg B, van der Veerdonk FL. Immune defense against Candida fungal infections. Nat Rev Immunol. 2015;15:630–642. [DOI] [PubMed] [Google Scholar]
- 55. Taylor PR, Roy S, Leal SM, et al. Activation of neutrophils by autocrine IL-17A-IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORγt and dectin-2. Nat Immunol. 2014;15:143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Denning DW, Perlin DS, Muldoon EG, et al. Delivering on antimicrobial resistance agenda not possible without improving fungal diagnostic capabilities. Emerg Infect Dis. 2017;23:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hassan M, Yasmeen BHN, Begum N. Fungal sepsis and Indications of antifungal prophylaxis and treatment in neonatal intensive care units: a review. N Int Med Coll J. 2014;6:6–8. [Google Scholar]
- 58. Pathak A, Pien FD, Carvalho L. Amphotericin B use in a community hospital, with special emphasis on side effects. Clin Infect Dis. 1998;26:334–338. [DOI] [PubMed] [Google Scholar]
- 59. Antinori S, Milazzo L, Sollima S, Galli M, Corbellino M. Candidemia and invasive candidiasis in adults: a narrative review. Eur J Int Med. 2016;34:21–28. [DOI] [PubMed] [Google Scholar]
- 60. Seymour CW, Gesten F, Prescott HC, et al. Time to treatment and mortality during mandated emergency care for sepsis. NEJM. 2017;376:2235–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Arslanian-Engorien C. Explicating nurses’ cardiac triage decisions. J Cardiovasc Nurs. 2009;24:50–57. [DOI] [PubMed] [Google Scholar]
- 62. Dolin HH, Papadimos TJ, Stepkowski S, et al. A novel combination of biomarkers to herald the onset of sepsis prior to the manifestation of symptoms. Shock. 2018;49:364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Trouillet J, Chastre J, Vuagnat A, et al. Ventilator-associated pneumonia caused by potentially drug-resistant bacteria. AJRCCM. 1998;157:531–539. [DOI] [PubMed] [Google Scholar]