Abstract
Background. Guidelines recommend that initiation of breast cancer screening (BCS) among women aged 40 to 49 years include a shared decision-making process. The objective of this study is to evaluate the effect of a breast cancer screening patient decision-aid (BCS-PtDA) on the strength of the relationship between individual risk and the decision to initiate BCS, knowledge, and decisional conflict. Methods. We conducted a randomized clinical trial of a BCS-PtDA that included individual risk estimates compared with usual care. Participants were women 39 to 48 years of age with no previous mammogram. Primary outcomes were strength of association between breast cancer risk and mammography uptake at 12 months, knowledge, and decisional conflict. Results. Of 204 participants, 65% were Black, the median age (interquartile range [IQR]) was 40.0 years (39.0–42.0), and median (IQR) breast cancer lifetime risk was 9.7% (9.2–11.1). Women who received mammography at 12 months had higher breast cancer lifetime risk than women who had not in both intervention (mean, 95% CI): 12.2% (10.8–13.6) versus 10.5% (9.8–11.2), P = 0.04, and control groups: 11.8% (10.4–13.1) versus 9.9% (9.2–10.6), P = 0.02. However, there was no difference between groups in the strength of association between mammography uptake and breast cancer risk (P = 0.87). Follow-up knowledge (0–5) was greater in the intervention versus control group (mean, 95% CI): 3.84 (3.5–4.2) versus 3.17 (2.8–3.5), P = 0.01. There was no change in decisional conflict score (1–100) between the intervention versus control group (mean, 95% CI): 24.8 (19.5–30.2) versus 32.4 (25.9–39.0), P = 0.07. Conclusions. The BCS-PtDA improved knowledge but did not affect risk-based decision making regarding age of initiation of BCS. These findings indicate the complexity of changing behaviors to incorporate objective risk in the medical decision-making process.
Keywords: Shared Decision Making, Breast Cancer Screening, Decision Aid, Mammography
Breast cancer screening (BCS) with mammography is an evidence-based intervention for women 40 to 74 years of age with established efficacy of breast cancer mortality reduction.1,2 However, the risk and benefit balance approaches a decision of equipoise for women 40 to 49 years of age given their relatively low risk of developing breast cancer and relatively high risk of experiencing a false positive screen.2 The process of shared decision making (SDM) involves communication of decision options, evidence-based outcome information, support for value elicitation, and discussion and decision making with the health care provider.3,4 Use of an SDM process is posited to improve decision quality outcomes, particularly in settings of decision equipoise.5,6 Decision aids are structured tools that facilitate these steps,7 seeking to improve the SDM outcomes of increased knowledge, decreased decisional conflict, value aligned decisions, and decreased decisional regret.8
Several decision aids have been developed for BCS, primarily focusing on women 40 to 49 years of age and women 75 years or older.9–13 A systematic review of breast cancer screening decision aids (BCS-DAs) on intentions to undergo mammography reports that BCS-DAs (v. usual care) in three randomized controlled trials resulted in increased decisions to delay screening (relative risk [RR] 1.48, 95% CI 1.04–2.13, P = 0.03), with a greater effect in younger women aged 38 to 50 years (RR 1.77, 95% CI 1.35–2.34), P < 0.001.14,15 In a pre-post intervention design reported by Eden and colleagues, a BCS-DA designed for women 40 to 49 years of age and evaluated among women enrolled in a rural clinical practice found a reduction in decisional conflict but no change in screening intentions.10 Similarly, Scariati and colleagues report a reduction in decisional conflict but no change in screening intentions from pre- to postintervention assessments in an evaluation of a BCS-DA among average risk women 38 to 48 years of age.9
Patient understanding and processing of risk information is a key construct in theoretical frameworks of behavioral change and in the development of DAs.16 The use of exemplars that place risk information in a story or narrative form may increase the salience of quantitative risk information.17 Risk perceptions are also influenced by presentation of comparator risk information.18,19 Although guidelines for BCS among women of average risk in their 50s vary in the recommended interval, they are consistent in recommending that women in this age group undergo BCS at a regular interval.1,2,20 Inclusion of information about breast cancer risk of women in their 50s, therefore, provides context for the interpretation of risk among younger women considering this decision.21,22
The objectives of this study are to evaluate whether use of a breast cancer screening patient decision aid (BCS-PtDA) that presents individual risk estimates in a robust way (including comparative risk information and exemplars), will increase the strength of the relationship between individual risk and the decision to initiate BCS, increase knowledge about BCS, and decrease decisional conflict regarding the age to initiate BCS.
Methods
Study Design
We conducted a randomized controlled trial (RCT) of a web-based BCS-PtDA compared with usual care using a parallel design. Inclusion criteria were women 39 to 48 years of age with no prior mammogram and enrolled in one of four primary care practices affiliated with an academic medical center in the Northeast United States. Exclusion criteria were cognitive impairment based on clinical history and inability to speak English. Primary outcomes were initiation of mammography screening within 12 months, knowledge about mammography screening, and decisional conflict regarding the age at which to initiate screening. Our primary hypotheses were that participants randomized to receive the BCS-PtDA versus usual care would demonstrate 1) a stronger association of individual breast cancer risk with mammography uptake within 12 months, 2) increased knowledge about mammography screening, and 3) decreased decisional conflict. Exploratory outcomes were 1) breast cancer worry, 2) anticipated regret, 3) accuracy of breast cancer risk perceptions, and 4) intentions regarding initiation of mammography screening. We also conducted an exploratory qualitative analysis of responses entered on the BCS-PtDA indicating the reasons for a participant’s decision regarding initiation of mammography screening to gain further insight regarding the impact of this intervention.23
Study Protocol
Participants were identified through the University of Pennsylvania Health Care System (UPHCS) electronic medical records based on age and gender criteria with no prior mammogram in the UPHCS. Patients who met these criteria were approached at a scheduled visit to ascertain interest in the study. Further screening questions confirmed eligibility criteria. Written informed consent was obtained prior to randomization. Randomization occurred by concealed assignment. Research assistants that were conducting the chart review to assess outcomes were blinded to group assignment. Educational sessions that presented the study design, format, and content of the BCS-PtDA and principles of SDM were held with clinicians and clinic staff of each participating site prior to the start of study enrollment.
Intervention and Control
Participants assigned to the intervention were offered assistance with log in to the BCS-PtDA using a laptop computer in the clinic and had the option of completing the program in the waiting room. Participants also received an email with a link to the BCS-PtDA so it could be completed at a later time. Summary sheets (see description of summary sheets below in Content and Format of the BCS-PtDA section) from the BCS-PtDA were printed by study staff, attached to the clinical electronic medical record, and available for clinician review. Participants randomized to the control group were asked to fill out a breast cancer risk assessment after randomization and then proceeded with usual care. All participants were notified that they would receive a 6-week follow-up survey. Participants received $20 in compensation on study enrollment.
The 6-week follow-up survey was delivered by mail and could be completed on paper and mailed back to the study team or online as a link was included in the mailed materials. Participants also received an email with a link to the follow-up survey that they could complete online. In order to optimize response rate, the protocol included three follow-up emails and one with a link to the follow-up survey and one follow-up telephone call. In order to improve follow-up, a $20 mailed incentive was added to the study protocol for completion of the survey.
Development of the Decision Aid
Theoretical Framework
The web-based BCS-PtDA was developed using a framework of SDM and incorporating exemplification theory. Exemplification theory posits that exemplars (example cases) can be used to shape an individual’s perceptions of a particular issue.17,24 The theory suggests that experienced and directly or indirectly witnessed occurrences affect subjective risk perceptions, support processing of risk information, and motivate behavioral change, and that persons use representative and availability heuristics in developing subjective risk perceptions.18,19 The exemplars were in the format of written narratives. The purpose, content, and evaluated valence of the narratives can be described using the framework developed by Shaffer and colleagues.25 The purpose of the narratives was to provide information, engage, model risk-based decision making, and persuade women to consider individual risk when making a decision about the age to initiate screening. The content of the narratives was primarily cognitive, presenting risk estimates that were low, medium, or high for women of the same age as participants and in comparison to women in their 50s. The content also modeled the process of decision making. Although women in the exemplars considered possible outcomes of mammography, the narratives were not designed to have a positive or negative valence.26 We developed three exemplars that were displayed to all users in a random order. For example, the low-risk exemplar described a woman with a 10-year breast cancer risk of 11 out of 1,000, stating that this risk is lower than the typical 50-year-old woman and lower than the average risk woman her age. In this exemplar, the woman decides to delay mammography until she is older. The medium and high-risk exemplars followed a similar approach of risk-based decision making and can be viewed on the BCS-PtDA (https://www.decide2screen.org/breast/study.aspx). As part of the development of these exemplars, earlier versions were evaluated in a study of an internet sample of women aged 35 to 49 and found to improve the accuracy of perceived risk among woman that had a 10-year breast cancer risk of <1.5%. In this pilot testing, tailoring the exemplars to women with respect to age, race, family history, and parity did not add to the overall effectiveness and therefore was not included in our DA.27
Content and Format of the BCS-PtDA
The BCS-PtDA included the following components: 1) ascertainment of breast cancer risk factors needed for the National Cancer Institute Breast Cancer Risk Assessment Tool (NCI-BCRAT)21,22,28; 2) an introduction to the decision problem (including a description of differing guidelines, overview of risks and benefits including a brief description of overdiagnosis, and the goals of the decision aid); 3) a table outlining United States Preventive Services Task Force and American Cancer Society guidelines; 4) comparison of mortality reduction attributed to mammography between women 40–49 and 50–59 of age; 5) pictographs depicting outcomes of mammography including cancer detection, interval cancers, false positive tests, and true negative tests shown for women aged 40 to 49 and compared with women aged 50 to 59 years of age; 6) pictographs comparing 10-year and lifetime risk for the individual woman to an average-risk woman the same age and 10-year risk compared with an average-risk 50-year-old woman based on the NCI-BCRAT; 7) exemplars that demonstrated women considering the impact of breast cancer risk on their decisions about when to initiate mammography; and 8) an interactive summary sheet where women state their intentions and complete a value clarification exercise where they list the factors most important to them in deciding when to have their first mammogram. The summary sheet also encouraged women to discuss this decision with their provider and could be printed or emailed and sent to a mobile device. Additional features were a secure sign in code and ability to review and modify risk assessment.
The BCS-PDA met 6 out of 6 qualifying criteria for a decision aid, as described in a Delphi consensus survey conducted by the International Patient Decision Aid Standards group.4 The full decision aid can be viewed at https://www.decide2screen.org/breast/study.aspx.
Baseline and Outcome Measures
Primary outcomes of the study were the association of risk with uptake of mammography at 12 months, knowledge about mammography, and decisional conflict. Chart review was conducted 12 months following the date of enrollment to evaluate mammography uptake. The chart review encompassed both entries in the health system radiology package and primary care progress notes to ascertain if mammography was obtained outside of the health care system. Knowledge about mammography screening and decisional conflict were ascertained on the 6-week follow-up survey. Data from the BCS-PtDA or a baseline breast cancer risk assessment form was used to calculated breast cancer risk using the NCI-BCRAT.28 Knowledge was assessed with the following five multiple-choice questions (correct answers): 1) Do all women who have an abnormal mammogram have breast cancer? (No), 2) Do mammograms detect every cancer? (No), 3) Which of the following age groups will have the most extra tests, biopsies, or procedures as a result of having mammograms? (40–49), 4) Can mammograms detect breast cancer when it is at an early stage? (Yes), and 5) For what age group are more deaths prevented by mammograms? (50–59). A sixth knowledge question was included in the survey but an a priori decision was made to exclude from the knowledge score as the stem “For which groups are mammograms most beneficial” was thought to be ambiguous. The knowledge scale was scored from 0 to 5 reflecting the number of correct responses. Decisional conflict was assessed with the 16-item Decisional Conflict Scale (DCS). This scale includes five subscale scores in the domains of Uncertainty, Feeling Informed, Clear Values, Support for Decision, and Effective Decision Making.29 The DCS is scored from 0 to 100, with scores <25 associated with increased adherence to decisions made.30
Breast cancer worry, anticipated regret, accuracy of risk perception, and breast cancer screening intentions were exploratory outcomes. Breast cancer worry was assessed with the 3-item Lerman Breast Cancer Worry Scale.31 Items were summed for a score of 1 to 13. Anticipated regret for decisions to delay or to initiate mammography were assessed on a 7-point Likert-type scale with response options ranging from 1 (strongly disagree) to 7 (strongly agree), with increasing values indicating greater anticipated regret. Perceived lifetime breast cancer risk was assessed with the question, “What do you think is your chance of developing breast cancer in your lifetime out of 1,000? Please choose a number between 0 and 1,000.” Objective breast cancer risk was assessed using the NCI-BCRAT.28 Decision intentions were assessed with the two questions: “At this point in time, have you made a decision about the age at which you will start having mammograms?” with response items of “yes” or “no.” Subjects were also asked “At what age do you plan to have your fist mammogram?” with the options to enter an age or respond “I don’t know” or “I never plan to have a mammogram” and provided a text field to list the most important reasons for their decision. Numeracy was assessed with the 4-item ability subscale of the subjective numeracy scale32 (see full survey in the Online Appendix).
Statistical Analysis
We used descriptive statistics to describe characteristics of our total population and those who responded to the 6-week survey. We used a logistic regression analysis to estimate the association between survey response and race, study arm, and the race by study arm interaction to evaluate whether there was differential nonresponse between the two study arms by race.
Our primary hypothesis was that individuals randomized to the intervention versus control group would demonstrate 1) a stronger association between individual risk and mammography uptake within 12 months, 2) increased knowledge about mammography screening, and 3) decreased decisional conflict regarding the age to initiate BCS with mammography. We used ANOVA to test the association between objective risk as determined by the NCI-BCRAT and receipt of mammography within 12 months by study arm as well as the difference in the strength of this association between study arms. Assuming 100 participants in each study arm and that 20% in each arm have received a mammogram at 1 year, the study had 80% power to detect an absolute difference of 3.27% between study arms in the difference in NCI-BCRAT determined risk between women receiving and not receiving a mammogram at the two-sided alpha = 0.05 level.
Exploratory analyses evaluated differences between intervention and control groups on anticipated regret, breast cancer worry, accuracy of the perceived risk of breast cancer, and intentions regarding age of initiation of BCS. Objective lifetime risk as determined by the NCI-BCRAT21,22 was described as a continuous variable (0% to 100%) and as determined by the following categories: low risk (<12%), medium (12% to <20%), and high (≥20%) risk. We estimated the means and 95% confidence intervals (CIs) for continuous primary and exploratory outcomes and compared these outcomes in the intervention and control groups using t tests. We summarized categorical outcomes using proportions and 95% CIs separately for the intervention and control groups and used chi-squared tests to evaluate differences between groups. We assessed the association between perceived and objective lifetime breast cancer risk using Spearman’s correlation, overall and stratified by study arm.
We entered qualitative data from the BCS-PtDA summary page into the NVivo v12 qualitative software package.33 Open coding was conducted and a coding scheme developed to categorize factors important to participants when deciding the age to initiate mammography. The data were independently coded by two coders (MS, AF) with differences resolved by a consensus process. The frequency of responses was stratified by the following screening intentions: 1) intend to initiate screening this year, 2) undecided, or 3) plan to wait until closer to age 50.
Results
We approached 382 persons to participate in the study. Of these, 100 were excluded due to having had a prior mammogram on further screening questions. Of the 282 eligible patients, 75 declined enrollment and 207 (73%) were enrolled. Those that declined enrollment were no different in age or race than those that participated. There were three participants who dropped out of the study after enrollment. This left 102 in the intervention and 102 in the control group for analysis. The response rate for the 6-week follow-up survey was 55%, resulting in 113 participants available for the analysis of knowledge, decisional conflict, and exploratory outcomes ascertained by the survey (Figure 1).
Figure 1.
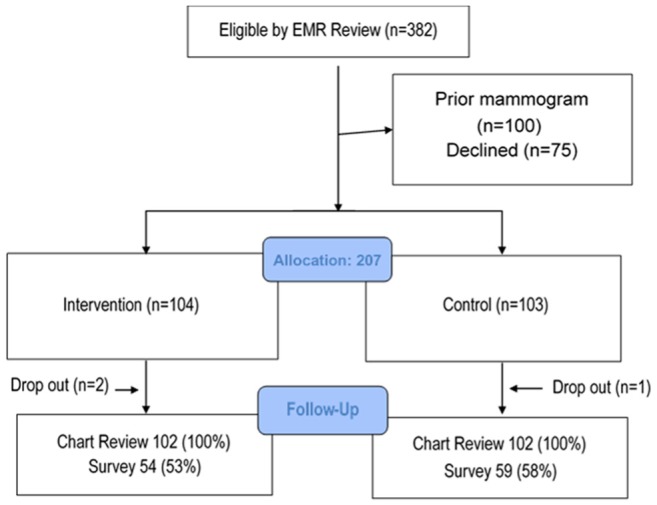
CONSORT diagram.
Study Population
Of the 204 participants, 65% were black and 27% white. The median age (interquartile range [IQR]) was 40.0 (39.0–42.0). Participants were recruited from Internal Medicine (47%), Family Medicine (26%), and Ob/Gyn (28%) practices. The median (IQR) lifetime risk in the cohort was 9.7% (9.2, 11.1) (Table 1). The response rate for the 6-week follow-up survey that included assessment of education and numeracy was 55%. Among survey respondents, 12% had up to a high school level education, 26% had some college or a technical degree, and 62% had a college degree. The subjective numeracy ability scale (score range 1–6) was (median, IQR) 4.8 (3.5, 5.5) (Table 2). Non-black women had higher survey response rates than black women (odds ratio [OR] 5.5, 95% CI 2.0, 18.0) but this association did not differ by study arm (OR 0.7 for interaction term, 95% CI 0.2, 2.7) (see Online Supplemental Table). One respondent in the control group did not have data available to determine objective breast cancer risk using the NCI-BCRAT.
Table 1.
Characteristics of All Study Participantsa
| Total (N = 204) | BCS-PtDA (n = 102) | Control (n = 102) | |
|---|---|---|---|
| Age (years), median (IQR) | 40.0 (39.0, 42.0) | 40.0 (39.0, 42.0) | 40.0 (39.0, 42.0) |
| Missing | 0 | 0 | 0 |
| Lifetime breast cancer risk (NCI-BCRAT) (%), median (IQR) | 9.7 (9.2, 11.1) | 9.7 (9.1, 12.1) | 9.6 (9.3, 10.7) |
| Missing | 1 | 0 | 1 |
| Lifetime breast cancer risk (NCI-BCRAT) categories, n (%) | |||
| ≤12 | 162 (79.8) | 76 (74.5) | 86 (85.1) |
| >12 to ≤20 | 34 (16.7) | 21 (20.6) | 13 (12.9) |
| >20 | 7 (3.4) | 5 (4.9) | 2 (2.0) |
| Missing | 1 | 0 | 1 |
| Race, n (%) | |||
| Black | 132 (64.7) | 60 (58.8) | 72 (70.6) |
| White | 55 (27.0) | 32 (31.4) | 23 (22.5) |
| Asian | 6 (2.9) | 4 (3.9) | 2 (2.0) |
| Hispanic | 3 (1.5) | 2 (2.0) | 1 (1.0) |
| Other/unknown | 8 (3.9) | 4 (3.9) | 4 (3.9) |
| Clinic affiliation, n (%) | |||
| Family practice | 52 (25.6) | 27 (26.5) | 25 (24.8) |
| Internal medicine | 95 (46.8) | 47 (46.1) | 48 (47.5) |
| Ob/Gyn | 56 (27.6) | 28 (27.5) | 28 (27.7) |
| Missing | 1 | 0 | 1 |
| Mammography within 1 year, n (%) | |||
| No | 163 (79.9) | 82 (80.4) | 81 (79.4) |
| Yes | 41 (20.1) | 20 (19.6) | 21 (20.6) |
BCS-PtDA, breast cancer screening patient decision-aid; IQR, interquartile range; NCI-BCRAT, National Cancer Institute Breast Cancer Risk Assessment Tool.
Table 2.
Characteristics of Follow-Up Survey Respondentsa
| Total (N = 113) | BCS-PtDA (n = 54) | Control (n = 59) | |
|---|---|---|---|
| Age (years), median (IQR) | 40.0 (39.0, 41.0) | 40.0 (39.0, 41.0) | 40.0 (39.0, 42.0) |
| Missing | 0 | 0 | 0 |
| Lifetime breast cancer risk (NCI-BCRAT) (%), median (IQR) | 9.7 (9.5, 12.2) | 10.2 (9.6, 13.2) | 9.6 (9.2, 11.1) |
| Missing | 0 | 0 | 0 |
| Lifetime breast cancer risk (NCI-BCRAT) categories, n (%) | |||
| ≤12 | 84 (74.3) | 35 (64.8) | 49 (83.1) |
| >12 to ≤20 | 24 (21.2) | 15 (27.8) | 9 (15.3) |
| >20 | 5 (4.4) | 4 (7.4) | 1 (1.7) |
| Missing | 0 | 0 | 0 |
| Perceived lifetime risk (per 1,000), median (IQR) | 67.5 (10.0, 300.0) | 25.0 (10.0, 185.0) | 100.0 (10.0, 425.0) |
| Missing | 7 | 3 | 4 |
| Perceived lifetime risk categories, n (%) | |||
| ≤12 | 71 (67.0) | 35 (68.6) | 36 (65.5) |
| >12 to ≤20 | 7 (6.6) | 5 (9.8) | 2 (3.6) |
| >20 | 28 (26.4) | 11 (21.6) | 17 (30.9) |
| Missing | 7 | 3 | 4 |
| Race, n (%) | |||
| Black | 57 (50.4) | 23 (42.6) | 34 (57.6) |
| White | 42 (37.2) | 22 (40.7) | 20 (33.9) |
| Asian | 6 (5.3) | 4 (7.4) | 2 (3.4) |
| Hispanic | 3 (2.7) | 2 (3.7) | 1 (1.7) |
| Other/unknown | 5 (4.4) | 3 (5.6) | 2 (3.4) |
| Clinic affiliation, n (%) | |||
| Family practice | 22 (19.6) | 9 (16.7) | 13 (22.4) |
| Internal medicine | 54 (48.2) | 27 (50.0) | 27 (46.6) |
| Ob/Gyn | 36 (32.1) | 18 (33.3) | 18 (31.0) |
| Missing | 1 | 0 | 1 |
| Education, n (%) | |||
| Less than high school | 3 (2.7) | 1 (1.9) | 2 (3.4) |
| High school graduate or GED | 10 (8.9) | 4 (7.4) | 6 (10.3) |
| Some college or technical school | 29 (25.9) | 12 (22.2) | 17 (29.3) |
| College graduate or beyond | 70 (62.5) | 37 (68.5) | 33 (56.9) |
| Missing | 1 | 0 | 1 |
| Numeracy (SNS) (1–6), median (IQR) | 4.8 (3.5, 5.5) | 4.4 (3.5, 5.6) | 4.8 (3.0, 5.4) |
| Missing | 1 | 0 | 1 |
| Mammography within 1 year, n (%) | |||
| No | 87 (77.0) | 41 (75.9) | 46 (78.0) |
| Yes | 26 (23.0) | 13 (24.1) | 13 (22.0) |
BCS-PtDA, breast cancer screening patient decision-aid; IQR, interquartile range; NCI-BCRAT, National Cancer Institute Breast Cancer Risk Assessment Tool.
Primary Outcomes
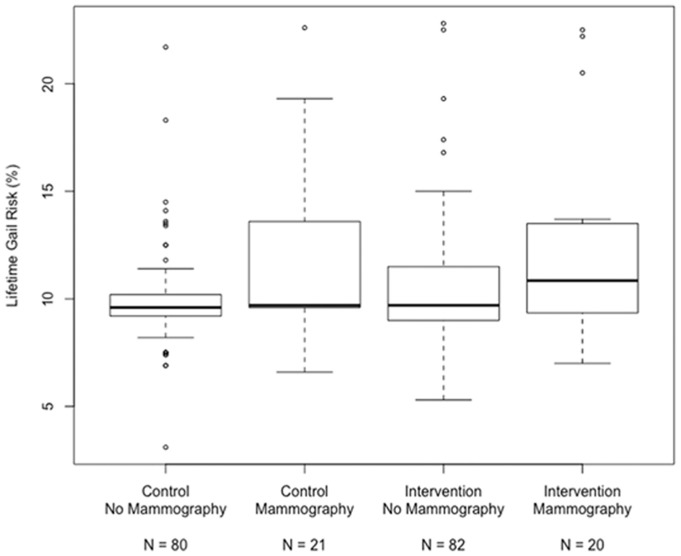
We report a positive association between NCI-BCRAT-determined lifetime breast cancer risk and initiation of mammography screening (mean, 95% CI) in both the intervention (No Mammography: 10.5 [9.8, 11.2]; Mammography: 12.2 [10.8, 13.6], P = 0.02) and control group (No Mammography: 9.9 [9.2, 10.6]; Mammography: 11.8 [10.4, 13.1], P = 0.02) (Table 3). However, there was no interaction between study arm and the strength of association between NCI-BCRAT-determined risk and initiation of screening (P = 0.87). The lack of an interaction between study arm and the strength of an association between risk and initiation of screening remained in an analysis of participants in the intervention group who had completed the BCS-PtDA (P = 0.74) (Figure 2). We further evaluated the strength of association between breast cancer risk and mammography uptake between study arms for women with higher (above median) versus lower numeracy and found similar results.
Table 3.
Lifetime Breast Cancer Risk Stratified by Study Arm and Receipt of Mammography Within 1 Yeara
| No Mammography, Mean (95% CI) | Mammography, Mean (95% CI) | Difference, Mean (95% CI) | P value | |
|---|---|---|---|---|
| Control | 9.9 (9.2, 10.6) | 11.8 (10.4, 13.1) | 1.8 (0.3, 3.4) | 0.02 |
| Intervention | 10.5 (9.8, 11.2) | 12.2 (10.8, 13.6) | 1.7 (0.1, 3.2) | 0.04 |
CI, confidence interval.
P values are for difference in mean risk between women receiving and not receiving mammography within each study arm based on ANOVA. The P value for the interaction between study arm and receipt of mammography was 0.87. Lifetime breast cancer risk determined by the National Cancer Institute Breast Cancer Risk Assessment Tool.21,22
Figure 2.
Lifetime breast cancer risk stratified by study arm and mammography uptake.
Lifetime breast cancer risk determined by the National Cancer Institute Breast Cancer Risk Assessment Tool (NCI-BCRAT) based on the Gail model. Mammography uptake within 12 months of study enrollment determined by chart review.21,22
Participants randomized to the intervention versus control demonstrated higher knowledge about screening mammography on a scale of 0 to 5 (mean, 95% CI): 3.8 (3.5, 4.2) versus 3.2 (2.8, 3.5), respectively, P = 0.01. There was no difference in decisional conflict in the intervention versus control group (mean, 95% CI): 24.8 (19.5, 30.2) versus 32.4 (25.9, 39.0), P = 0.07 (Table 2).
Exploratory Outcomes
In exploratory analyses, there was no difference in breast cancer worry, anticipated regret, and the accuracy of risk perceptions or intentions regarding age of initiation of mammography (Table 4). The Spearman correlation between perceived and objective breast cancer lifetime risk was 0.13 (Figure 3a) and did not differ between the study arms (P = 0.26). When excluding the four outliers who had a perceived breast cancer risk of >60%, the correlation between perceived an objective breast cancer risk increased to 0.16 (Figure 3b). Of the 67% of women who had decided when to start screening at the 6-week follow-up assessment there was no difference between the intervention and control groups in the intended age of a first mammogram (mean, 95% CI): 42.7 (414, 44.0) versus 42.4 (41.2, 43.70), respectively, P = 0.75) (Table 4).
Table 4.
Primary and Exploratory Outcomesa
| BCS-PtDA Intervention, Mean or % (95% CI) | Control, Mean or % (95% CI) | Difference in Mean or % (95% CI) | P Value | |
|---|---|---|---|---|
| Primary outcomes | ||||
| Mammography uptake at 12 months (%) | 19.6 (11.9,27.3) | 20.6 (12.7,28.4) | −1.0 (−12.0, 10.0) | 1.00 |
| Total knowledge score (0–5) | 3.8 (3.5, 4.2) | 3.2 (2.8, 3.5) | 0.7 (0.2, 1.2) | 0.01 |
| Decisional conflict total score (0–100) | 24.8 (19.5, 30.2) | 32.4 (25.9, 39.0) | −7.6 (−15.9, 0.7) | 0.07 |
| Uncertainty | 27.0 (20.4, 33.6) | 31.3 (24.2, 38.4) | −4.3 (−13.8, 5.2) | 0.37 |
| Informed | 29.9 (23.2, 36.7) | 35.9 (28.7, 43.2) | −6.0 (−15.7, 3.7) | 0.22 |
| Values | 27.0 (21.0, 33.0) | 36.1 (28.9, 43.2) | −9.1 (−18.2, 0.1) | 0.05 |
| Support | 20.8 (16.1, 25.5) | 28.3 (21.6, 35.0) | −7.5 (−15.5, 0.4) | 0.06 |
| Effective decision making | 23.4 (17.2, 29.6) | 29.2 (22.8, 35.6) | −5.8 (−14.5, 2.9) | 0.19 |
| Exploratory outcomes | ||||
| Anticipated regret: Delay mammogram (1–7) | 5.4 (4.8, 5.9) | 5.7 (5.3, 6.1) | −0.4 (−1.0, 0.3) | 0.30 |
| Anticipated regret: Have mammogram (1–7) | 3.5 (2.9, 4.0) | 3.3 (2.8, 3.8) | 0.2 (−0.6, 0.9) | 0.67 |
| Breast cancer worry (1–13) | 5.4 (4.9, 6.0) | 5.0 (4.5, 5.5) | 0.4 (−0.3, 1.1) | 0.28 |
| Accuracy of lifetime breast cancer risk perception: Difference between perceived risk and risk determined by the NCI-BCRAT28 | 3.3 (−2.7, 9.3) | 9.3 (2.3, 16.3) | −6.0 (−15.0, 3.1) | 0.20 |
| Accuracy of lifetime breast cancer risk perception: Low, medium, or high category (%) | 66.7 (57.5, 75.8) | 54.5 (44.9, 64.2) | 12.1 (−1.2, 25.4) | 0.28 |
| Have made a decision about the age at which to start having mammograms (%) | 35.2 (25.9, 44.5) | 26.3 (17.8, 34.9) | 8.9 (−3.7, 21.5) | 0.42 |
| Intended age of first mammogram (years) | 42.7 (41.4, 44.0) | 42.4 (41.2, 43.7) | 0.3 (−1.4, 2.0) | 0.75 |
BCS-PtDA, breast cancer screening patient decision-aid; CI, confidence interval; NCI-BCRAT, National Cancer Institute Breast Cancer Risk Assessment Tool.
Difference column reports difference in mean or percentage between study arms and the 95% CI for the difference. Anticipated regret of delay in having a mammogram was in response to the following question: “If I do not have a mammogram in my 40s, and, at a later date, breast cancer is detected, I will regret not having a mammogram.” Anticipated regret of having a mammogram was in response to the question” “If I have a mammogram in my 40s and have unnecessary follow-up tests or procedures, I will regret having mammograms”. The questions were answered on a scale of 1 (strongly disagree) to 7 (strongly agree).
Figure 3a.
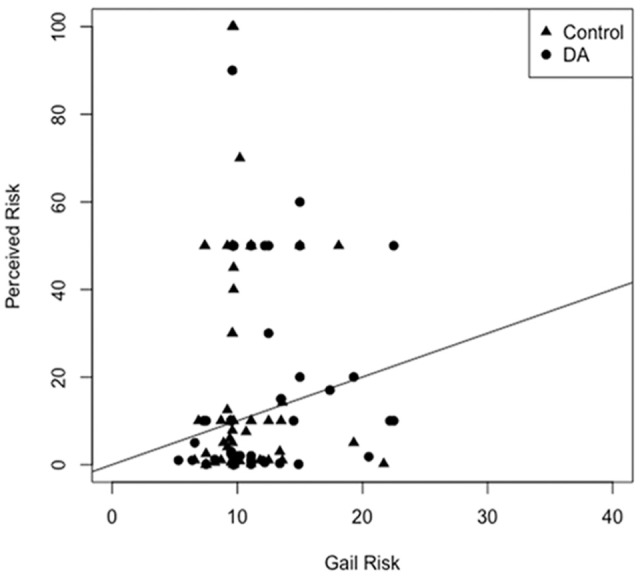
Correlation of perceived and objective lifetime breast cancer risk among study participants.
Objective risk determined by the National Cancer Institute Breast Cancer Risk Assessment Tool (NCI-BCRAT) and based on the Gail model.21,22 The diagonal line represents concordance between perceived and objective risk. The Spearman correlation coefficient is 0.13. There was no difference in correlation between study arms (P = 0.26).
Figure 3b.
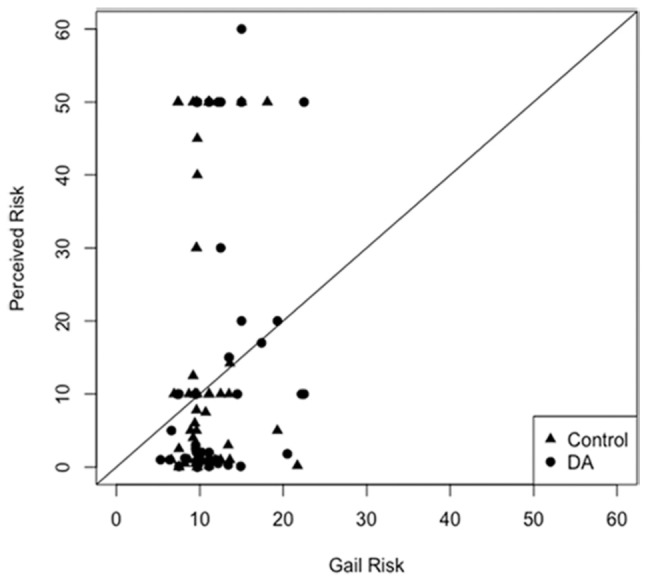
Correlation of perceived and objective lifetime breast cancer risk among study participants excluding outliers.
Correlation of perceived and objective lifetime breast cancer risk among study participants excluding four outliers with perceived risk >60%. The Spearman correlation after excluding outliers is 0.16.
Decision Aid Experience
Of the 102 people randomized to the intervention, 72 logged on to the BCS-PtDA. The mean (IQR) duration of time between logging in and logging out of the BCS-PtDA after excluding three outliers (>19 hours) was 10.2 minutes (2.1 to 120). Of those who used the BCS-PtDA, 90% viewed the narratives presented and 89% completed the summary sheet at the end of the DA. The primary categories of factors important to participants when deciding to initiate mammography that emerged from this analyses were 1) weighing the benefits and harms including perceptions of personal risk, family history, and age; false positive tests, mortality, and the efficacy of mammography; 2) beliefs about screening and affective factors including belief in the value of early detection, reassurance of negative screening tests, anticipated regret, and the value of a baseline mammography; and 3) guidelines and provider recommendations including reference to existing screening guidelines or plans to obtain a provider recommendation (Table 5).
Table 5.
Decision Aid Users: Reasons for Intended Time to Initiate Mammographya
| Most Important Reason for Decision | Intensions for Initiating
Mammography |
||
|---|---|---|---|
| This Year, n = 34 | Undecided, n = 19 | Wait: Closer to Age 50, n = 10 | |
| Weighing benefits and harms | |||
| Perceived high risk due to family history, age, or other risk factors | 7 | 0 | 0 |
| Perceived low risk due to family history, age, or other risk factors | 0 | 3 | 6 |
| Considered false positive tests | 2 | 6 | 3 |
| Mortality benefit is small | 0 | 0 | 1 |
| Mammography not effective | 0 | 0 | 1 |
| Beliefs and affective factors | |||
| Belief in screening and early detection | 6 | 1 | 1 |
| Reassurance | 7 | 0 | 1 |
| Anticipated regret | 1 | 0 | 0 |
| Wanting a baseline mammogram | 2 | 1 | 0 |
| Guidelines and provider recommendations | |||
| Plan to discuss with a provider | 3 | 6 | 1 |
| Guidelines or recommendations | 2 | 0 | 1 |
| Additional reasons | |||
| Mammography is painful | 0 | 1 | 0 |
| Competing comorbidities | 0 | 1 | 0 |
The table summarizes reasons provided for the decision to have a mammogram this year, still undecided, or to wait until closer to age 50 to initiate mammography. Coding conducted independently by two coders with differences resolved through a consensus process.
Discussion
We conducted an RCT of a risk based BCS-PtDA for women 39 to 48 years of age regarding the decision of age of initiation of BCS. We enrolled women with no previous mammogram in the setting of their primary care practice and randomized them to use of our web-based BCS-PtDA versus usual care. Outcomes included mammography uptake by 12 months and psychosocial and behavioral factors. Our decision aid was unique in the presentation of individual risk estimates, comparative risk estimates, and use of exemplars to increase the impact of individual risk information. We report that those randomized to the BCS-PtDA demonstrated increased knowledge about mammography screening. However, there was no difference in the strength of association between individual breast cancer risk and uptake of mammography or in decisional conflict regarding age of initiation of mammography.
As in previous BCS decision aid studies, the BCS-PtDA increased knowledge,14 a key component of a high-quality decision-making process in SDM frameworks.3,5 In the current study, our knowledge questions focused on conceptual aspects of BCS and comparative outcomes between women in their 40s and women 50 years of age or more. This finding indicates that the BCS-PtDA was successful in improving women’s understanding of the age-specific differences in BCS outcomes. Previous RCTs of BCS-DAs have focused on knowledge outcomes relating to both conceptual and numeric domains and understanding of overdiagnosis with knowledge scales developed to reflect content of the BCS-DA.34,35 Our results provide further evidence that DAs are effective in addressing a knowledge gap pertaining to treatment and screening decisions.36
The DCS measures constructs relevant to an informed and SDM process including subjective feelings of uncertainty, being informed, clarity of values, being supported, and being effective pertaining to the decision at hand. A recent meta-analysis of patient DAs in treatment and screening decisions reported strong evidence that patient DAs decrease indecision in the domain of personal values.36 In contrast, our study demonstrates no difference in decisional conflict in the BCS-PtDA versus control groups.
Ours is the first RCT of a BCS DA to examine uptake of mammography, as compared to behavioral intentions, as a primary outcome. Previous studies of DAs for women in their 40s9,35 or older than 7011,34 reported decreased behavioral intentions to have BCS but did not assess mammography uptake. Intentions are an accepted indicator of future behavior in established models of health behavioral change,37 but have been found to have only a moderate association with uptake of mammography.38 Our findings are consistent with the recent systematic review of treatment and screening DAs, which except in the case of prostate cancer screening found little evidence for the impact of DAs on decisions pertaining to diagnostic tests and screening.36
Given the emergence of precision medicine and personalized approaches to cancer screening, we further sought to evaluate whether an intervention that communicates individualized risk estimates effectively increased the correlation between individual breast cancer risk and mammography uptake. We evaluated subjective lifetime breast cancer risk perception both as a continuous variable (from 0 to 1,000) and by grouping subjective estimates into categories of low, medium, and high risk. Participants in both study arms overestimated lifetime breast cancer risk; by an absolute 3.3% and 9.3% in the intervention and control groups, respectively. Furthermore, the correlation between objective and subjective risk remained low in our study and did not differ between study arms. Despite the specific focus of the BCS-PtDA on increasing understanding and salience of individual risk information, including the use of personal narratives based on exemplification theory, the relatively modest correlation of objective breast cancer risk and mammography uptake seen in previous studies was not observed in our study. Our findings are in contrast to the recent meta-analysis of DAs for treatment and screening decisions that indicate improvement in accuracy of risk perceptions after use of a DA.36 To the degree that the implementation of precision medicine depends on patients incorporating personal risk information into their health care decisions, these results emphasize that such implementation may be challenging to accomplish.
The qualitative comments provided by users of the BCS-PtDA in this study provide some insight regarding the importance of individual risk when deciding about when to initiate mammography. Some participants cited risk-based considerations, noting either low or high perceived risks of breast cancer, or concern about false positive tests. Others cited general values and beliefs about cancer screening and anticipated emotions they would experience based on their decision. Although the qualitative analysis of comments made in the summary sheet are exploratory, the statements illustrate the range of factors considered in this decision making process.
We explored the constructs of anticipated regret and breast cancer worry as outcomes of our intervention. We assessed both anticipatory regret for a positive action (screening) and a negative action (delay in screening). Findings indicate higher levels of anticipated regret for consequences of delaying screening than initiating screening. However, anticipated regret was similar across study arms. Recent literature suggests that anticipated regret may be a motivating factor in daily decisions and in cancer screening decisions specifically and an important construct to include in future studies of patient decision aids.39–43
This study has some limitations. First, the RCT was conducted in a single health care system. However, we included multiple clinic sites and primary care specialties within the health care system and our patient population was diverse in background. Second, the relatively low response rate for the 6-week follow-up survey could limit the internal validity of findings pertaining to outcomes collected by survey. Participants that did not have a computer or smartphone may have had less opportunity to use the BCS-PtDA or to complete the electronic version of the follow-up survey. However, respondents and nonrespondents did not differ in age, breast cancer risk, or specialty type of clinic and the lower rate of completion by black women did not differ between study arms. Third, the follow-up period to assess mammography uptake was limited to 12 months. Women may have obtained a mammogram outside of this time frame. In addition, chart review may have missed cases when the mammogram was obtained elsewhere. However, our chart review included both a review of radiology system records and clinic notes with efforts made to identify any reference to a mammogram obtained within or outside of the health care system. Finally, the description of overdiagnosis in our BCS-PtDA was brief and did not include quantitative estimates of its occurrence. Overdiagnosis is a complex concept and further research is needed regarding how best to present the risk and outcomes of this potential harm in cancer screening decision aids.
In conclusion, we report that a web-based BCS-PtDA integrated in the primary care practice setting was effective in improving knowledge about age-based BCS. However, there was no impact of the BCS-PtDA on the strength of association between objective breast cancer risk and initiation of BCS within 12 months. Therefore, our hypothesis that the BCS-PtDA would change behavior toward risk-based uptake of initiation of BCS was not supported. These findings indicate the complexity of changing behaviors to incorporate objective risk in the medical decision-making process. Future work is needed to elucidate the role of providing tailored risk information in quantitative and narrative formats as part of a shared decision-making process for decisions of equipoise.
Supplemental Material
Supplemental material, DS_10.1177_2381468318812889 for The Impact of a Risk-Based Breast Cancer Screening Decision Aid on Initiation of Mammography Among Younger Women: Report of a Randomized Trial by Marilyn M. Schapira, Rebecca A. Hubbard, Holli H. Seitz, Emily F. Conant, Mitchell Schnall, Joseph N. Cappella, Tory Harrington, Carrie Inge and Katrina Armstrong in MDM Policy & Practice
Acknowledgments
The authors would like to acknowledge the help of Robert Hornik, PhD, for his thoughtful contributions in the development and successful completion of this work. We also acknowledge the help of Arshia Faghri in coding the qualitative data.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Financial support for this study was provided the National Cancer Institute–funded consortium, Population-based Research Optimizing Screening through Personalized Regimens (PROSPR) U54CA 163313. The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report.
Supplemental Material: The online supplementary materials for this article are available on the Medical Decision Making Policy & Practice website at http://journals.sagepub.com/home/mpp.
Contributor Information
Marilyn M. Schapira, Department of Medicine, Perelman School of Medicine; Center for Health Equity Research and Promotion, Philadelphia VA Medical Center, Philadelphia, Pennsylvania.
Rebecca A. Hubbard, Center for Clinical Epidemiology and Biostatistics and Department of Biostatistics, University of Pennsylvania, Philadelphia, Pennsylvania
Holli H. Seitz, Department of Communication, Social Science Research Center, Mississippi State University, Starkville, Mississippi
Emily F. Conant, Department of Radiology, Perelman School of Medicine
Mitchell Schnall, Department of Radiology, Perelman School of Medicine.
Joseph N. Cappella, Annenberg School of Communication, University of Pennsylvania, Philadelphia, Pennsylvania
Tory Harrington, Department of Medicine, Perelman School of Medicine.
Carrie Inge, Department of Medicine, Perelman School of Medicine.
Katrina Armstrong, Department of Medicine, Massachusetts General Hospital, Boston, Massachusetts.
References
- 1. Oeffinger KC, Fontham ET, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siu AL; US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164(4):279–96. [DOI] [PubMed] [Google Scholar]
- 3. Stiggelbout AM, Van der Weijden T, De Wit MP, et al. Shared decision making: really putting patients at the centre of healthcare. BMJ. 2012;344:e256. [DOI] [PubMed] [Google Scholar]
- 4. Joseph-Williams N, Newcombe R, Politi M, et al. Toward minimum standards for certifying patient decision aids: a modified Delphi consensus process. Med Decis Making. 2014;34(6):699–710. [DOI] [PubMed] [Google Scholar]
- 5. Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Educ Couns. 2006;60(3):301–12. [DOI] [PubMed] [Google Scholar]
- 6. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27(10):1361–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Durand MA, Witt J, Joseph-Williams N, et al. Minimum standards for the certification of patient decision support interventions: feasibility and application. Patient Educ Couns. 2015;98(4):462–8. [DOI] [PubMed] [Google Scholar]
- 8. O’Connor AM, Llewellyn-Thomas HA, Flood AB. Modifying unwarranted variations in health care: shared decision making using patient decision aids. Health Aff (Millwood). 2004;(Suppl Variation):VAR63–VAR72. [DOI] [PubMed] [Google Scholar]
- 9. Scariati P, Nelson L, Watson L, Bedrick S, Eden KB. Impact of a decision aid on reducing uncertainty: pilot study of women in their 40s and screening mammography. BMC Med Inform Decis Mak. 2015;15:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eden KB, Scariati P, Klein K, et al. Mammography decision aid reduces decisional conflict for women in their forties considering screening. J Womens Health (Larchmt). 2015;24(12):1013–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schonberg MA, Hamel MB, Davis RB, et al. Development and evaluation of a decision aid on mammography screening for women 75 years and older. JAMA Intern Med. 2014;174(3):417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schonberg MA, Kistler CE, Nekhlyudov L, et al. Evaluation of a mammography screening decision aid for women aged 75 and older: protocol for a cluster-randomized controlled trial. J Clin Trials. 2014;4:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tisnado DM, Moore AA, Levin JR, Rosen S. Developing and testing a decision aid for use by providers in making recommendations: about mammography screening in older women. J Appl Gerontol. 2015;34(3):343–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ivlev I, Hickman EN, McDonagh MS, Eden KB. Use of patient decision aids increased younger women’s reluctance to begin screening mammography: a systematic review and meta-analysis. J Gen Intern Med. 2017;32(7):803–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mathieu E, Barratt AL, McGeechan K, Davey HM, Howard K, Houssami N. Helping women make choices about mammography screening: an online randomized trial of a decision aid for 40-year-old women. Patient Educ Couns. 2010;81(1):63–72. [DOI] [PubMed] [Google Scholar]
- 16. Fishbein M. An Integrative Model for Behavioral Prediction and Its Application to Health Promotion. San Francisco: Jossey-Bass; 2009. [Google Scholar]
- 17. Zillmann D. Exemplification effects in the promotion of safety and health. J Commun. 2006;56(Suppl. 1):S221–S237. [Google Scholar]
- 18. Tversky A, Kahneman D. Judgment under uncertainty: heuristics and biases. Science. 1974;185(4157):1124–31. [DOI] [PubMed] [Google Scholar]
- 19. Tversky A, Kahneman D. The framing of decisions and the psychology of choice. Science. 1981;211(4481):453–8. [DOI] [PubMed] [Google Scholar]
- 20. Bevers TB, Anderson BO, Bonaccio E, et al. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis. J Natl Compr Canc Netw. 2009;7(10):1060–96. [DOI] [PubMed] [Google Scholar]
- 21. Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81(24):1879–86. [DOI] [PubMed] [Google Scholar]
- 22. Gail MH, Costantino JP, Pee D, et al. Projecting individualized absolute invasive breast cancer risk in African American women. J Natl Cancer Inst. 2007;99(23):1782–92. [DOI] [PubMed] [Google Scholar]
- 23. NIH Office of Behavioral and Social Sciences. Best Pratices for Mixed Methods Research in the Health Sciences. 2nd ed. Bethesda: National Institutes of Health; 2018. [Google Scholar]
- 24. Mundrof N, Zillman D. Effects of story sequencing on affective reactions to broadcast news. J Broadcasting Electronic Media. 1991;35(2):197–211. [Google Scholar]
- 25. Shaffer VA, Owens J, Zikmund-Fisher BJ. The effect of patient narratives on information search in a web-based breast cancer decision aid: an eye-tracking study. J Med Internet Res. 2013;15(12):e273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shaffer VA, Zikmund-Fisher BJ. All stories are not alike: a purpose-, content-, and valence-based taxonomy of patient narratives in decision aids. Med Decis Making. 2013;33(1):4–13. [DOI] [PubMed] [Google Scholar]
- 27. Seitz HH, Gibson L, Skubisz C, et al. Effects of a risk-based online mammography intervention on accuracy of perceived risk and mammography intentions. Patient Educ Couns. 2016;99(10):1647–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rockhill B, Spiegelman D, Byrne C, Hunter DJ, Colditz GA. Validation of the Gail et al. model of breast cancer risk prediction and implications for chemoprevention. J Natl Cancer Inst. 2001;93(5):358–66. [DOI] [PubMed] [Google Scholar]
- 29. O’Connor AM. Validation of a decisional conflict scale. Med Decis Making. 1995;15(1):25–30. [DOI] [PubMed] [Google Scholar]
- 30. O’Connor AM. User manual-decisional conflict scale. Available from: https://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Decisional_Conflict.pdf
- 31. Lerman C, Trock B, Rimer BK, Jepson C, Brody D, Boyce A. Psychological side effects of breast cancer screening. Health Psychol. 1991;10(4):259–67. [DOI] [PubMed] [Google Scholar]
- 32. Zikmund-Fisher BJ, Smith DM, Ubel PA, Fagerlin A. Validation of the Subjective Numeracy Scale: effects of low numeracy on comprehension of risk communications and utility elicitations. Med Decis Making. 2007;27(5):663–71. [DOI] [PubMed] [Google Scholar]
- 33. QSR International Pty Ltd. NVivo qualitative data analysis software. Available from: https://www.qsrinternational.com/nvivo/home
- 34. Mathieu E, Barratt A, Davey HM, McGeechan K, Howard K, Houssami N. Informed choice in mammography screening: a randomized trial of a decision aid for 70-year-old women. Arch Intern Med. 2007;167(19):2039–46. [DOI] [PubMed] [Google Scholar]
- 35. Hersch J, Barratt A, Jansen J, et al. Use of a decision aid including information on overdetection to support informed choice about breast cancer screening: a randomised controlled trial. Lancet. 2015;385(9978):1642–52. [DOI] [PubMed] [Google Scholar]
- 36. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;(4):CD001431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Frosch DL, Legare F, Fishbein M, Elwyn G. Adjuncts or adversaries to shared decision-making? Applying the integrative model of behavior to the role and design of decision support interventions in healthcare interactions. Implement Sci. 2009;4:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cooke R, French DP. How well do the theory of reasoned action and theory of planned behaviour predict intentions and attendance at screening programmes? A meta-analysis. Psychol Health. 2008;23(7):745–65. [DOI] [PubMed] [Google Scholar]
- 39. Bjälkebring P, Vastfjäll D, Svenson O, Slovic P. Regulation of experienced and anticipated regret in daily decision making. Emotion. 2016;16(3):381–6. [DOI] [PubMed] [Google Scholar]
- 40. Sandberg T, Conner M. Anticipated regret as an additional predictor in the theory of planned behaviour: a meta-analysis. Br J Soc Psychol. 2008;47(Pt. 4):589–606. [DOI] [PubMed] [Google Scholar]
- 41. Sandberg T, Conner M. A mere measurement effect for anticipated regret: impacts on cervical screening attendance. Br J Soc Psychol. 2009;48(Pt. 2):221–36. [DOI] [PubMed] [Google Scholar]
- 42. Brewer NT, DeFrank JT, Gilkey MB. Anticipated regret and health behavior: a meta-analysis. Health Psychol. 2016;35(11):1264–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Speck RM, Neuman MD, Resnick KS, Mellers BA, Fleisher LA. Anticipated regret in shared decision-making: a randomized experimental study. Perioper Med (Lond). 2016;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_2381468318812889 for The Impact of a Risk-Based Breast Cancer Screening Decision Aid on Initiation of Mammography Among Younger Women: Report of a Randomized Trial by Marilyn M. Schapira, Rebecca A. Hubbard, Holli H. Seitz, Emily F. Conant, Mitchell Schnall, Joseph N. Cappella, Tory Harrington, Carrie Inge and Katrina Armstrong in MDM Policy & Practice