Abstract
Two separate lines of research indicate (a) that prenatal stress is associated with heightened behavioral and physiological reactivity and (b) that these postnatal phenotypes are associated with increased susceptibility to both positive and negative developmental experiences. Therefore, prenatal stress may increase sensitivity to the rearing environment. We tested this hypothesis by manipulating prenatal stress and rearing-environment quality, using a cross-fostering paradigm, in prairie voles. Results showed that prenatally stressed voles, as adults, displayed the highest behavioral and physiological reactivity when cross-fostered to low-contact (i.e., low-quality) rearing but the lowest behavioral and physiological reactivity when cross-fostered to high-contact (i.e., high-quality) rearing; non-prenatally stressed voles showed no effect of rearing condition. Additionally, while neither prenatal stress nor rearing condition affected oxytocin receptor binding, prenatally stressed voles cross-fostered to high-contact rearing showed the highest vasopressin-1a receptor binding in the amygdala. Results indicate that prenatal stress induces greater environmental sensitivity, making it both a risk and an opportunity factor.
Keywords: plasticity, prenatal stress, early environment, anxiety, vasopressin
Extensive evidence indicates that prenatal stress is a risk factor for a variety of detrimental physical and mental health outcomes (for a review, see Van den Bergh, Mulder, Mennes, & Glover, 2005). Although such human evidence suggests that prenatal stress disrupts “optimal” development, we offer—and test using an animal model—a radically different interpretation. On the basis of research on human infants showing (a) that prenatal stress is associated with heightened negative emotionality and physiological reactivity and (b) that these postnatal phenotypes are associated with increased susceptibility to both positive and negative developmental experiences, we hypothesized that prenatal stress programs postnatal plasticity, making stressed voles most susceptible to effects of both positive and negative rearing.
Prenatal Stress and Behavioral-Physiological Dysregulation
Much research indicates that prenatal stress, measured in a variety of ways (e.g., maternal anxiety, cortisol), predicts greater behavioral and physiological dysregulation in infancy and childhood. Concerning behavioral dysregulation, prenatal stress is linked to increased displays of sadness, frustration, and fear, as well as a stable disposition of negative emotional reactivity (Gartstein & Rothbart, 2003; Glover, 2011). Maternal psychological stress during pregnancy is associated with increased behavioral reactivity of 4-month-olds (Davis et al., 2004), irregular sleeping and eating patterns of 6-month-olds, and heightened inhibition and negative emotionality of 5-year-olds (Martin, Noyes, Wisenbaker, & Huttenen, 1999). Relatedly, higher levels of cortisol in pregnant women forecast greater infant negativity at 7 weeks (de Weerth, van Hees, & Buitelaar, 2003) and 2 months of age, even when controlling for maternal postnatal psychological state (Davis et al., 2007).
Concerning physiological functioning, evidence indicates that prenatal stress is associated with dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis in infants and children (Davis, Glynn, Waffarn, & Sandman, 2011; Field et al., 2004), effects which extend to even the first day of school (Gutteling, de Weerth, & Buitelaar, 2005). Notably, a natural experiment revealed that pregnant mothers positioned near the New York City terrorist attacks on 9/11 who subsequently developed posttraumatic stress disorder had infants with dysregulated diurnal cortisol rhythms at 1 year of age compared with infants of other mothers (Maccari, Krugers, Morley-Fletcher, Szyf, & Brunton, 2014). Such findings are consistent with rodent experiments indicating that prenatal stress promotes higher baseline and reactive corticosterone levels in offspring (Yehuda et al., 2005).
Postnatal Developmental Plasticity
The research just summarized linking prenatal stress with early-life behavioral and physiological dysregulation becomes especially intriguing when juxtaposed with independent work showing that highly negatively emotional children are not only more adversely affected than other children by negative environmental exposures (e.g., poverty) and developmental experiences (e.g., harsh parenting), but also benefit more from supportive contextual conditions (e.g., sensitive-responsive parenting; Belsky, Bakermans-Kranenburg, & Van Ijzendoorn, 2007; Belsky & Pluess, 2009, 2013). In fact, a recent meta-analysis of observational studies found that early negative emotionality moderates effects of various environmental factors on a range of child-adjustment outcomes (e.g., social competence, cognitive development; Slagt, Dubas, Deković, & van Aken, 2016) in just such a “for-better-and-for-worse" manner (Belsky et al., 2007).
Furthermore, children with heightened physiological reactivity are more susceptible to environmental influences, again in a for-better-and-for-worse manner (Boyce & Ellis, 2005). For example, heightened physiological reactivity moderates the effects of marital conflict on externalizing problems (Obradović, Bush, & Boyce, 2011) and family adversity on school achievement (Obradović, Bush, Stamperdahl, Adler, & Boyce, 2010). Additionally, evaluations of experimental interventions (e.g., van den Berg & Bus, 2014) show that more negatively emotional or physiologically reactive children benefit more, sometimes exclusively, from such efforts than do other children. In summary, more physiologically/behaviorally reactive children prove most vulnerable to the negative effects of contextual adversity and most likely to benefit from environmental support.
Prenatal Programming of Postnatal Plasticity
Consideration of both sets of evidence summarized above—one indicating that prenatal stress is associated with elevated behavioral and physiological dysregulation and the other that such phenotypic functioning is associated with heightened susceptibility to positive and negative environmental influences—raises the intriguing hypothesis first advanced by Pluess and Belsky (2011) that prenatal stress fosters, promotes, or “programs” postnatal developmental plasticity. If true, this hypothesis could account for many of the adverse, later-developing phenotypes associated with prenatal-stress exposure. Perhaps the reason that prenatal stress is associated with problematic functioning in childhood and adolescence is because the very forces that engendered stress in pregnancy (e.g., poverty, marital conflict) continue postnatally for many whose prenatal experience fostered heightened developmental plasticity. Thus, when children are exposed, postnatally, to conditions of adversity that persist beyond pregnancy, they prove especially responsive to them.
Current Study
Because it is unethical to experimentally test the proposition that prenatal stress promotes developmental plasticity in humans, we conducted an animal study. We subjected pregnant prairie voles (Microtus ochrogaster) to either high or low levels of stress, using a social-stress paradigm, and then randomly cross-fostered their offspring to rearing parents known to have previously provided more or less supportive care (i.e., high- or low-contact rearing) to their own offspring (see Fig. 1 for study design). We predicted that, after birth, prenatally stressed pups would display the highest frequency of distress calls and the strongest rearing effects (i.e., the highest or lowest score, depending on condition) on anxietylike behavior, corticosterone reactivity, and vasopressin-1a receptor (V1aR) and oxytocin receptor (OTR) densities in the amygdala. We focused on these outcomes because (a) distress calls may be an early-life indicator of behavioral reactivity like negative emotionality in humans, (b) anxietylike behavior and corticosterone reactivity are well-studied indicators of dysregulated functioning that have been linked to prenatal stress and rearing condition (Glover, 2011; Maccari et al., 2003), and (c) OTR and V1aR density in the amygdala regulate social and anxiety behaviors (Carter, Grippo, Pournajafi-Nazarloo, Ruscio, & Porges, 2008).
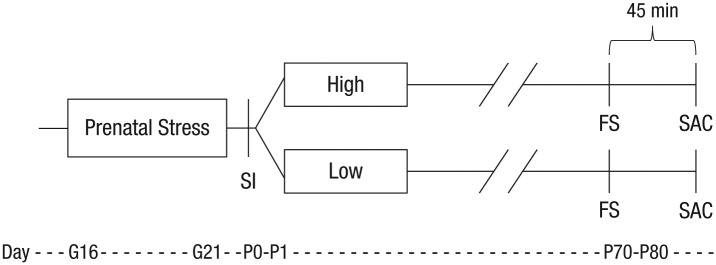
Fig. 1.
Experimental design. Pregnant voles assigned to the prenatal-stress condition were exposed to a social stressor 10 min per day for the last 5 days of gestation (G16–G21). Within 24 hr of birth (P0–P1), voles were subject to a social-isolation (SI) test and then cross-fostered to either high- or low-contact parents for the rearing condition. During adulthood (P70–80), subjects underwent a forced swim (FS) test to measure anxietylike behavior and were sacrificed (SAC) 45 min later to collect plasma and brains. The procedure for subjects in the control groups is not illustrated, but these subjects were similarly cross-fostered to either high- or low-contact parents and underwent all procedures (with the exception of prenatal stress) in the same timeline. G = gestational day; P = postnatal day; P0 = day of birth.
Prairie voles were studied because they, unlike rats and mice, display key characteristics of social monogamy and selective social behavior, including preference for a familiar partner, an emotional attachment to the pair-mate, and male care of offspring. Additionally, prairie voles naturally vary—in traitlike fashion across multiple litters—in the amount of care they display toward their pups (Perkeybile, Griffin, & Bales, 2013). Thus, prairie voles are an optimal animal to use in cross-fostering paradigms when testing hypotheses based on human studies.
Method
Subjects
Subjects were laboratory-bred prairie voles (N = 78), descendants of a stock originally wild-caught near Champaign, Illinois. Breeder pairs were housed in large polycarbonate cages (44 cm × 22 cm × 16 cm). Food (high-fiber Purina rabbit chow) and water were available ad libitum, and cotton squares were provided for nesting material. After weaning on postnatal day 20, weanlings were housed in same-sex pairs in small polycarbonate cages (27 cm × 16 cm × 16 cm). Animals were maintained on a 14:10 light-dark cycle. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the University of California, Davis.
Research design
Parental care
Prior to the start of the experiment, both mother and father parenting behaviors (e.g., nursing, contact, licking, and grooming) were recorded twice a day for 2 consecutive days after birth (4 total observations) in order to quantify each breeder pair’s natural level of parenting (Perkeybile et al., 2013). Mean durations of each behavior were computed across the four observations, and maternal and paternal means were summed to produce a total parental-behavior score for each breeder pair. Scores were then ranked into quartiles on the basis of the amount of total contact directed toward the pups. The top-ranked quartile became the high-contact breeder group, the bottom quartile became the low-contact breeder group, and those falling in between became the medium-contact breeder group. High- and low-contact breeders served as (postnatal) rearing parents, whereas medium-contact breeders were subject to variation in prenatal stress.
Prenatal stress
Medium-contact breeders were assigned to either a prenatal-stress or control condition to avoid any potential genetic confounds from high- or low-contact breeders. For the prenatal-stress condition, pregnant voles were transferred to a cage containing an unfamiliar and lactating—hence, aggressive—female that was separated by a divider for 10 min per day for 5 consecutive days during the last week of pregnancy. This paradigm is known to increase stress reactivity in offspring, both behaviorally and physiologically (Brunton & Russell, 2010). Breeders in the control condition were left undisturbed in their cages. After a washout period of one litter, treatments crossed over for a final litter so that medium breeders that received prenatal stress the first time did not receive prenatal stress the second time and vice versa.
Cross-fostering
Within 24 hr of birth, infants were briefly removed from the nest and were weighed, sexes were checked, and a social-isolation task was performed to assess negative emotionality (see below). If necessary, litters were culled to four pups. Final litter size ranged from two to four pups. Infants were then cross-fostered to an unrelated breeder pair previously characterized as providing high- or low-quality parental care. Both control and prenatally stressed pups were handled for equal amounts of time. For the prenatal-stress condition, 16 prenatally stressed voles were cross-fostered to high-contact breeders (8 males, 8 females) and 23 to low-contact breeders (11 males, 12 females). In the control group, 20 voles were cross-fostered to high-contact breeders (10 males, 10 females) and 19 to low-contact breeders (10 males, 9 females). Previous studies, using a cross-fostering design, document significant rearing effects on developmental outcomes based on similar sample sizes (e.g., Perkeybile et al., 2013).
Outcome measurements
To avoid litter effects, we used only one male and one female from each litter in subsequent measurements. Subjects were assessed during two developmental time points: immediately following birth and during adulthood. All measurements taken during adulthood were past the age of sexual maturity for prairie voles, which is 30 to 40 days for females and 35 to 45 days for males (Stalling, 1990). In addition to the measures highlighted for purposes of testing the hypothesis under consideration, other behavioral measures were collected for other reasons and thus are not reported.
Distress calls
During cross fostering (offspring postnatal days 0–1), subjects underwent a social-isolation task in which they were removed from their home cage, one at a time, and placed in an empty polycarbonate cage for 5 min. Social isolation has been shown to be an effective stressor because maternal separation induces a stress response, especially in highly social animals like prairie voles. We recorded ultrasonic vocalizations in response to social isolation as a behavioral measure of negative emotionality. Neonatal rodents vocalize under stressful situations, and in prairie voles, ultrasonic vocalizations and corticosterone are correlated (Shapiro & Insel, 1990). Temperature was maintained throughout the task using heat lamps to prevent artifacts of thermal stress.
Forced swim test
As adults (offspring postnatal days 70–80), subjects underwent a forced swim test, which is a moderate physical stressor. The swim stressor consisted of 3 min of swimming in lukewarm water in a large plastic cage (20 cm × 25 cm × 45 cm). The subjects could neither touch the bottom nor climb out of the container. Duration of swimming and of struggling behaviors (i.e., thrashing, displaying upward-directed movement of the forepaws against the side of the container) were recorded to assess anxietylike behavior. Previous work indicates that more struggling behavior and less swimming during a forced swim is associated with a more anxious phenotype (Varadarajulu et al., 2011). An anxietylike behavior composite measure was created by subtracting mean duration of swimming from struggling and was transformed to reflect positive values; hence, higher scores indicate greater anxietylike behavior.
Corticosterone reactivity
Following swimming, animals normally show approximately a doubling of corticosterone, with a maximum increase within approximately 45 min (Taymans et al., 1997), and levels remain elevated for several hours. Thus to assess stress reactivity, we collected blood 45 min following the forced swim test to assay using radioimmunoassay for corticosterone. All samples were assayed during the same session in duplicate, and the intra-assay coefficient of variation was 3.21%.
OTR and V1aR autoradiography
Immediately after blood collection, we deeply anesthetized voles using isoflurane gas and then euthanized them by cervical dislocation and rapid decapitation. Brains were removed, flash frozen, and stored at −80° C. Brains were sectioned at 20 µm into six series, mounted onto Super-frost slides, and stored at −80° C until assayed. Slides were allowed to thaw at room temperature and were then fixed in 0.1% paraformaldehyde (7.4 pH) and rinsed twice in 50 mM Tris-HCl buffer solution (7.4 pH), followed by incubation in the tracer buffer at room temperature for 60 min. Tracer buffer consisted of 50 mM Tris-HCl buffer (7.4 pH) with 10 mM MgCl2, 0.1% bovine serum albumin, and 50 pM of radiotracer. For OTR binding, [125I]-ornithine vasotocin analog [(125I)OVTA] [vasotocin, d(CH2)5[Tyr(Me)2, Thr4, Orn8, (125I)Tyr9-NH2]; 2200 Ci/mmol] was used (PerkinElmer, Waltham, MA). For V1aR binding, 125I-lin-vasopressin [125I-phenylacetyl-D-Tyr(ME)-Phe-Gln-Asn-Arg-Pro-Arg-Tyr-NH2] (PerkinElmer) was used. Following the incubation period, unbound radioligand was removed by 4 washes in 50 mM Tris buffer and 2% MgCl2, pH 7.4, and then dipped in dH20 and air dried. Once dry, the slides were exposed to Kodak BioMaxMR film (Kodak, Rochester, NY), with 125I microscale standards (American Radiolabeled Chemicals, St., Louis, MO) for 96 hr.
The optical binding density (OBD) was quantified using MCID Core Digital Densitometry system (Cambridge, United Kingdom). OBD values from a set of 10 autoradiography standards (American Radiolabeled Chemicals) were loaded into the software and used to generate a standard curve from which OBD values for the regions of interest (ROIs) were extrapolated. For each specimen, OBD values were calculated for each ROI, as well as one background area where no binding was detected. Three separate measurements for each ROI were taken per specimen and averaged. For each specimen, the average background binding value was subtracted from each average ROI measurement to yield normalized OBDs across specimens.
Data analysis
For all outcomes, we anticipated needing a minimum of 15 animals per group to detect the expected interactive effects. Power analysis for the 2 × 2 interaction with α = .05, a moderate effect size, and a power of 0.8, yields a sample size of 60 (15 per prenatal stressor per cross-fostering environment). Estimating a moderate effect size is reasonable on the basis of previous studies of developmental effects in voles. Notably, research using prairie voles has detected early-rearing effects using sample sizes smaller than ours (e.g., Perkeybile, Delaney-Busch, Hartman, Grimm, & Bales, 2015).
Prior to carrying out primary analyses, we evaluated effects of litter, detecting only one significant effect—for anxietylike behavior (p = .038). Importantly, controlling for this main effect left the two-way interaction central to this investigation unchanged; in fact, the statistical significance of the two-way interaction actually increased (from p = .037 to p = .019). All results are thus reported without consideration of litter.
The first step in the primary analyses evaluated, by means of an independent-samples t test, whether prenatal-stress and control-group pups differed on distress calls. For all other outcomes, we ran a 2 × 2 analysis of variance to test for main and interactive effects of prenatal stress and rearing condition. A three-way-interaction between prenatal stress, rearing, and sex was entered into the model but subsequently dropped because of a lack of significance. All tests were two-tailed, with significance levels set at p < .05. Lastly, because of an inability to obtain adequate samples or degradation of tissue, some subjects were dropped from the corticosterone (n = 18), OTR (n = 6), and V1aR (n = 5) analyses because of this random error.
Results
Distress vocalizations
The independent-samples t test failed to reveal a significant difference between prenatally stressed and control pups on frequency of ultrasonic vocalizations during social isolation, t(76) = −0.10, η2 < .001, 95% confidence interval (CI) = [−124.97, 113.02], p = .92.
Anxietylike behavior
Although neither the main effect of prenatal stress, F(1, 74) = 0.002, η2 < .001, 95% CI = [−10.88, 10.38], p = .966, nor of rearing, F(1, 74) = 1.50, η2 = .019, 95% CI = [−4.11, 17.15], p = .225, was significant, the interaction of these two experimental factors was significant, F(1, 74) = 4.53, η2 = .057, 95% CI = [0.73, 21.99], p = .037 (see Fig. 2). For prenatally stressed voles, rearing condition significantly predicted anxietylike behavior, t(37) = 2.28, p = .028; stressed voles cross-fostered to high-contact breeders displayed the least anxietylike behavior of all animals, M = 43.94, SE = 11.68, whereas stressed voles cross-fostered to low-contact breeders displayed the most anxietylike behavior, M = 79.70, SE = 9.74. For voles in the control group, rearing condition proved unrelated to anxietylike behavior, t(37) = −0.72, p = .477, although, as already implied, these voles scored in between the two groups of prenatally stressed voles.
Fig. 2.

Results of anxietylike behavior for prenatal stress by rearing conditions. There was a significant effect of rearing for voles that were prenatally stressed; prenatally-stressed voles cross-fostered to high-contact parents displayed the least anxietylike behavior but the most when cross-fostered to low-contact parents. There was no effect of high- or low-contact parents for voles in the control condition. Error bars represent standard errors of the mean. The asterisk indicates a significant difference between rearing conditions (p < .05).
Corticosterone reactivity
The prenatal-stress condition proved unrelated to corticosterone, F(1, 56) = 0.86, η2 = .013, 95% CI = [−118.73, 323.21], p = .358. Although the main effect of rearing on corticosterone was significant, F(1, 56) = 5.58, η2 = .083, 95% CI = [39.71, 481.66], p = .022, with voles cross-fostered to the low-contact condition evincing greater corticosterone reactivity, this main effect was qualified by a significant interaction between prenatal stress and rearing, F(1, 56) = 5.00, η2 = .074, 95% CI = [25.73, 467.68], p = .029 (see Fig. 3); and once again, rearing predicted corticosterone levels, but only for prenatally stressed voles, t(27) = 3.18, p = .004. Specifically, prenatally stressed voles showed the least corticosterone reactivity of all animals when cross-fostered to high-contact breeders, M = 1,751.9, SE = 235.65, but the most corticosterone reactivity when cross-fostered to low-contact breeders, M = 2,766.7, SE = 212.41. For voles in the control group, rearing did not predict corticosterone reactivity, t(29) = 0.09, p = .927, and once again, their scores fell between those of the two groups of prenatally stressed voles.
Fig. 3.
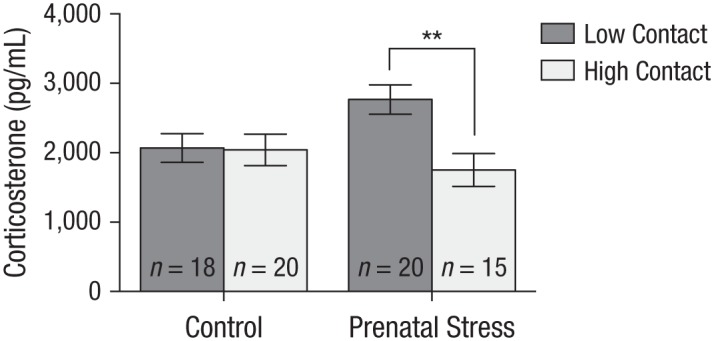
Results of corticosterone reactivity for prenatal stress by rearing conditions. There was a significant effect of rearing for voles that were prenatally stressed; prenatally stressed voles cross-fostered to high-contact parents displayed the least corticosterone reactivity but the most when cross-fostered to low-contact parents. There was no effect of either high- or low-contact parents for voles in the control condition. Error bars represent standard errors of the mean. The asterisks indicate a significant difference between rearing conditions (p < .01).
OTR and V1aR density in the amygdala
Neither prenatal stress, F(1, 68) = 0.46, η2 = .007, 95% CI = [−177.84, 360.16], p = .501; rearing, F(1, 68) = 0.26, η2 = .006, 95% CI = [−199.70, 338.30], p = .609; nor their interaction, F(1, 68) = 0.67, η2 = .006, 95% CI = [−379.70, 158.30], p = .414, was associated with OTR density in the amygdala. As for V1aR, no significant main effects of rearing, F(1, 69) = 2.31, η2 = .030, 95% CI = [−479.92, 64.77], p = .133, or prenatal stress, F(1, 69) = 1.58, η2 = .021, 95% CI = [−100.53, 444.16], p = .212, emerged. However, the interaction between rearing and prenatal stress was significant, F(1, 69) = 5.65, η2 = .073, 95% CI = [−596.85, −52.16], p = .020 (see Fig. 4), but in a manner somewhat different from the previously detected prenatal-stress-by-rearing effects. Prenatally stressed voles cross-fostered to high-contact breeders had greater V1aR density in the amygdala, M = 7,210.5, SE = 306.75, but did not exhibit lower V1aR density if cross-fostered to low-contact breeders, M = 6,146.3, SE = 239.32, t(35) = −2.83, p = .008. Once again, no such rearing effect emerging in the case of voles who were not stressed prenatally, t(34) = 0.59, p = .559. Thus, prenatally stressed voles were only more susceptible to the positive rearing condition (i.e., high contact), resulting in them having the highest V1aR density.
Fig. 4.
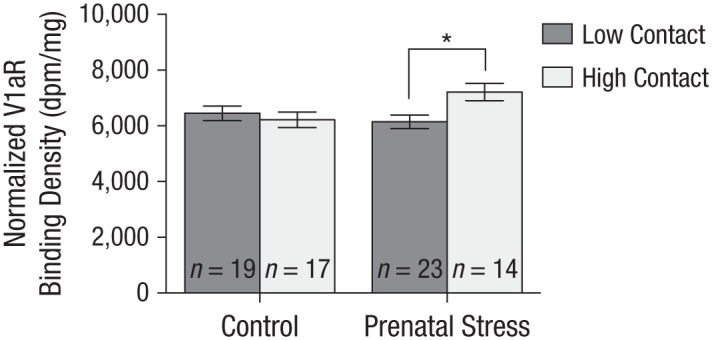
Results of vasopressin-1a receptor (V1aR) density in the amygdala for prenatal stress by rearing conditions. There was a significant effect of rearing for voles that were prenatally stressed; prenatally stressed voles cross-fostered to high-contact parents displayed the greatest V1aR density in the amygdala, but voles cross-fostered to low-contact parents did not have the lowest values. There was no effect of high- or low-contact parents for voles in the control condition. Error bars represent standard errors of the mean. The asterisk indicates a significant difference between rearing conditions (p < .05).
Secondary analyses
In an effort to illuminate possible physiological mechanisms responsible for observed anxietylike behavior, we performed two secondary analyses. Specifically, we controlled for the putative mechanism—corticosterone reactivity or V1aR density in the amygdala (in two separate analyses)—when predicting anxious behavior. Even with corticosterone reactivity taken into account, the interaction between prenatal stress and rearing remained significant, F(1, 55) = 4.22, η2 = .093, 95% CI = [2.63, 29.77], p = .020. When, however, V1aR density in the amygdala was controlled, the interaction between prenatal stress and rearing in predicting anxietylike behavior was reduced to a nonsignificant trend, F(1, 58) = 3.52, η2 = .054, 95% CI = [−0.82, 25.37], p = .066, thus providing suggestive evidence for a plausible mediating mechanism responsible for the heightened postnatal plasticity discerned in the case of prenatally stressed voles.
Discussion
The current experiment tested the hypothesis that prenatal stress promotes—or “programs”—heightened developmental plasticity (Pluess & Belsky, 2011). This hypothesis was based on prior human studies indicating (a) that prenatal stress is linked to greater behavioral and physiological reactivity during early life and (b) that both of these phenotypes are associated with increased responsiveness to both supportive and unsupportive rearing experiences (Belsky & Pluess, 2009, 2013). Some evidence emerged consistent with the hypothesis that prenatally stressed voles would show the strongest postnatal-rearing effects, scoring the highest and lowest (depending on rearing condition) on outcomes, while control voles would show the least.
Distress vocalizations
Given evidence linking prenatal stress with heightened negative emotionality in humans, we expected prenatally stressed voles to manifest the most vocal distress. However, this was not the case, perhaps because we measured frequency of ultrasonic vocalizations in pups only for a brief period of time and under highly stressful conditions (i.e., social isolation). This approach contrasts with measurements used in human studies that focus on a cross-situational, traitlike negative emotionality (Slagt et al., 2016). Thus, future research should test a more stable trait of negative emotionality in pups, possibly by assessing ultrasonic vocalizations or corticosterone levels under less stressful conditions.
Behavioral and physiological reactivity
Despite the distress vocalization findings, many other results proved consistent with the prenatal-programming hypothesis. Recall that prenatally stressed voles cross-fostered to high-contact breeders displayed the lowest anxietylike behavior and corticosterone reactivity to a stressor, while prenatally stressed voles cross-fostered to low-contact breeders displayed the highest. For voles in the control group, rearing condition proved unrelated to anxietylike behavior or corticosterone reactivity. These findings are consistent with those in the human literature showing that anxiety (e.g., Slagt et al., 2016) and physiological functioning (e.g., Dich, Doan, & Evans, 2015) are both subject to for-better-and-for-worse effects of environmental quality.
Neuroendocrine functioning
With respect to neuroendocrine functioning, we found that prenatal stress resulted in a detectable rearing-group effect on V1aR density, but not OTR density, in the amygdala, an area critical for detecting and processing emotional salience. Once again, no such rearing effect appeared for control voles. Although oxytocin and vasopressin are closely related peptides that often interact, each has distinct effects on the development of complex social behavior (Carter et al., 2008). Specifically, vasopressin plays a critical role in regulating the HPA axis. For example, rats treated with V1aR antagonists show prolonged stress responses (Gray, Innala, & Viau, 2012). Further, a study using rats bred for high and low anxiety found that vasopressin messenger RNA expression, but not oxytocin, played a central role in regulating anxious and depressive behavior (Wigger et al., 2004). Given our results concerning anxietylike behavior and stress reactivity, it is consistent that V1aR, but not OTR, was affected by rearing in the case of prenatally stressed voles.
Additionally, some evidence suggests that the vasopressin system may be more sensitive than the oxytocin system to prenatal-stress effects. Consider research indicating (a) that effects of prenatal stress on social memory in rats were mediated by V1aR mRNA expression but not OTR (Grundwald, Benítez, & Brunton, 2016) and (b) that prenatal exposure to vasopressin or caffeine, but not oxytocin, altered learning in female rats (Swenson, Beckwith, Lamberty, Krebs, & Tinius, 1990). Thus, the vasopressin system may be more affected by prenatal or perinatal conditions, whereas the oxytocin system may be more subject to factors in the early postnatal environment. Future studies should investigate this possibility.
Also worth considering is that prenatal-stress effects on V1aR may be mediated through increases in fetal androgen exposure. The vasopressin system is sexually dimorphic and highly steroid responsive. For example, castration results in a significant decrease of vasopressin expression while testosterone replacement ameliorates such effects (DeVries, Buijs, Van Leeuwen, Caffé, & Swaab, 1985). In humans, prenatal stress is tied to higher fetal cortisol and, unlike in adults, fetal cortisol and testosterone are positively correlated (Gitau, Adams, Fisk, & Glover, 2005). Likewise, multiple studies document effects of prenatal stress on masculinization of brain and behavior, especially in females (Anderson, Rhees, & Fleming, 1985). Although no sex differences emerged in our work, further research may illuminate whether fetal testosterone plays a role in programming postnatal plasticity.
Potential mechanisms
We examined V1aR density and corticosterone reactivity as potential mechanisms responsible for the prenatal-stress-induced rearing effects on anxietylike behavior. Some evidence showed that differences in V1aR, but not corticosterone reactivity, may help explain how prenatal stress promoted postnatal plasticity. Yet it remains unclear whether variation in V1aR density causes differences in anxietylike behavior or if the experience of anxiety alters V1aR density. To address this issue, future work could measure differences in undisturbed animals’ V1aR density or administer a V1aR antagonist pretesting to potentially eliminate observed effects. Other work by Hartman and Belsky (in press) provide a more detailed consideration of potential biological mechanisms instantiating the prenatal programming of postnatal plasticity.
Conclusion
In summary, the research presented here provides novel evidence that prenatal stress fosters developmental plasticity. Indeed, our results indicating that prenatal stress operates as a risk factor in the face of adversity (i.e., low-contact rearing) and an opportunity factor in a supportive context (i.e., high-contact rearing) challenge the long-standing notion that prenatal stress adversely affects development. And, in so doing, these results may help account for why prenatal stress is related to so many “negative” outcomes: Because prenatally stressed fetuses are programmed to be highly responsive to postnatal rearing, they are especially vulnerable when stress continues postnatally.
To the extent that this speculative analysis is sound, it raises the provocative possibility that one way to foster developmental plasticity is to stress human fetuses! But this would only prove sensible—and humane—if the postnatal environment is likely to be nurturing and supportive. Needless to say, before any such steps are taken, far more work—with humans and animals—is called for. Special emphasis should be placed on illuminating how prenatal stress gets biologically embedded (e.g., epigenetics) to promote responsivity to the postnatal environment.
Acknowledgments
We thank Tiffany Shem, Kevin Su, Michelle Chen, Laura Tran, You You Tan, Adele Seelke, Trent Simmons, Cindy Clayton, and Jacob Hansen for assisting in data collection.
Footnotes
Action Editor: Steven W. Gangestad served as action editor for this article.
Author Contributions: S. Hartman, K. L. Bales, and J. Belsky designed and developed this study. S. Hartman carried out the behavior and corticosterone experiments, and S. Hartman and S. M. Freeman performed brain assays, quantification, and analysis. All authors contributed to writing and editing of the manuscript.
Declaration of Conflicting Interests: The author(s) declared that there were no conflicts of interest with respect to the authorship or the publication of this article.
Open Practices: The data that support the findings of this study are available from the corresponding author on reasonable request.
References
- Anderson D. K., Rhees R. W., Fleming D. E. (1985). Effects of prenatal stress on differentiation of the sexually dimorphic nucleus of the preoptic area (SDN-POA) of the rat brain. Brain Research, 332, 113–118. doi: 10.1016/0006-8993(85)90394-4 [DOI] [PubMed] [Google Scholar]
- Belsky J., Bakermans-Kranenburg M. J., Van Ijzendoorn M. H. (2007). For better and for worse: Differential susceptibility to environmental influences. Current Directions in Psychological Science, 16, 300–304. doi: 10.1111/j.1467-8721.2007.00525.x [DOI] [Google Scholar]
- Belsky J., Pluess M. (2009). Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin, 135, 885–908. doi: 10.1037/a0017376 [DOI] [PubMed] [Google Scholar]
- Belsky J., Pluess M. (2013). Beyond risk, resilience, and dysregulation: Phenotypic plasticity and human development. Development and Psychopathology, 25, 1243–1261. doi: 10.1017/s095457941300059x [DOI] [PubMed] [Google Scholar]
- Boyce W. T., Ellis B. J. (2005). Biological sensitivity to context: I. An evolutionary–developmental theory of the origins and functions of stress reactivity. Development and Psychopathology, 17, 271–301. doi: 10.1017/s0954579405050145 [DOI] [PubMed] [Google Scholar]
- Brunton P. J., Russell J. A. (2010). Prenatal social stress in the rat programmes neuroendocrine and behavioural responses to stress in the adult offspring: Sex-specific effects. Journal of Neuroendocrinology, 22, 258–271. doi: 10.1111/j.1365-2826.2010.01969.x [DOI] [PubMed] [Google Scholar]
- Carter C. S., Grippo A. J., Pournajafi-Nazarloo H., Ruscio M. G., Porges S. W. (2008). Oxytocin, vasopressin and sociality. Progress in Brain Research, 170, 331–336. doi: 10.1016/s0079-6123(08)00427-5 [DOI] [PubMed] [Google Scholar]
- Davis E. P., Glynn L. M., Schetter C. D., Hobel C., Chicz-Demet A., Sandman C. A. (2007). Prenatal exposure to maternal depression and cortisol influences infant temperament. Journal of the American Academy of Child & Adolescent Psychiatry, 46, 737–746. doi: 10.1097/chi.0b013e318047b775 [DOI] [PubMed] [Google Scholar]
- Davis E. P., Glynn L. M., Waffarn F., Sandman C. A. (2011). Prenatal maternal stress programs infant stress regulation. Journal of Child Psychology and Psychiatry, 52, 119–129. doi: 10.1111/j.1469-7610.2010.02314.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis E. P., Snidman N., Wadhwa P. D., Glynn L. M., Schetter C. D., Sandman C. A. (2004). Prenatal maternal anxiety and depression predict negative behavioral reactivity in infancy. Infancy, 6, 319–331. doi: 10.1207/s15327078in0603_1 [DOI] [Google Scholar]
- DeVries G. J., Buijs R. M., Van Leeuwen F. W., Caffé A. R., Swaab D. F. (1985). The vasopressinergic innervation of the brain in normal and castrated rats. Journal of Comparative Neurology, 233, 236–254. doi: 10.1002/cne.902330206 [DOI] [PubMed] [Google Scholar]
- de Weerth C., van Hees Y., Buitelaar J. K. (2003). Prenatal maternal cortisol levels and infant behavior during the first 5 months. Early Human Development, 74, 139–151. doi: 10.1016/s0378-3782(03)00088-4 [DOI] [PubMed] [Google Scholar]
- Dich N., Doan S. N., Evans G. W. (2015). Children’s emotionality moderates the association between maternal responsiveness and allostatic load: Investigation into differential susceptibility. Child Development, 86, 936–944. doi: 10.1111/cdev.12346 [DOI] [PubMed] [Google Scholar]
- Field T., Diego M., Dieter J., Hernandez-Reif M., Schanberg S., Kuhn C., , . . . Bendell D. (2004). Prenatal depression effects on the fetus and the newborn. Infant Behavior & Development, 27, 216–229. doi: 10.1016/s0163-6383(04)00012-8 [DOI] [Google Scholar]
- Gartstein M. A., Rothbart M. K. (2003). Studying infant temperament via the Revised Infant Behavior Questionnaire. Infant Behavior & Development, 26, 64–86. doi: 10.1016/s0163-6383(02)00169-8 [DOI] [Google Scholar]
- Gitau R., Adams D., Fisk N. M., Glover V. (2005). Fetal plasma testosterone correlates positively with cortisol. Archives of Disease in Childhood: Fetal & Neonatal Edition, 90, F166–F169. doi: 10.1136/adc.2004.049320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover V. (2011). Annual research review: Prenatal stress and the origins of psychopathology: An evolutionary perspective. Journal of Child Psychology and Psychiatry, 52, 356–367. doi: 10.1111/j.1469-7610.2011.02371.x [DOI] [PubMed] [Google Scholar]
- Gray M., Innala L., Viau V. (2012). Central vasopressin V1A receptor blockade impedes hypothalamic–pituitary–adrenal habituation to repeated restraint stress exposure in adult male rats. Neuropsychopharmacology, 37, 2712–2719. doi: 10.1038/npp.2012.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundwald N. J., Benítez D. P., Brunton P. J. (2016). Sex-dependent effects of prenatal stress on social memory in rats: A role for differential expression of central vasopressin-1a receptors. Journal of Neuroendocrinology, 28. doi: 10.1111/jne.12343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteling B. M., de Weerth C., Buitelaar J. K. (2005). Prenatal stress and children’s cortisol reaction to the first day of school. Psychoneuroendocrinology, 30, 541–549. doi: 10.1016/j.psyneuen.2005.01.002 [DOI] [PubMed] [Google Scholar]
- Hartman S., Belsky J. (in press). Prenatal programming of postnatal plasticity revisited—and extended. Development and Psychopathology. [DOI] [PubMed] [Google Scholar]
- Maccari S., Darnaudery M., Morley-Fletcher S., Zuena A. R., Cinque C., Van Reeth O. (2003). Prenatal stress and long-term consequences: Implications of glucocorticoid hormones. Neuroscience and Biobehavioral Reviews, 27, 119–127. doi: 10.1016/s0149-7634(03)00014-9 [DOI] [PubMed] [Google Scholar]
- Maccari S., Krugers H. J., Morley-Fletcher S., Szyf M., Brunton P. J. (2014). The consequences of early-life adversity: Neurobiological, behavioural and epigenetic adaptations. Journal of Neuroendocrinology, 26, 707–723. doi: 10.1111/jne.12175 [DOI] [PubMed] [Google Scholar]
- Martin R. P., Noyes J., Wisenbaker J., Huttenen M. O. (1999). Prediction of early childhood negative emotionality and inhibition from maternal distress during pregnancy. Merrill-Palmer Quarterly, 45, 370–391. doi: 10.1353/mpq.2008.0013 [DOI] [Google Scholar]
- Obradović J., Bush N. R., Boyce W. T. (2011). The interactive effect of marital conflict and stress reactivity on externalizing and internalizing symptoms: The role of laboratory stressors. Development and Psychopathology, 23, 101–114. doi: 10.1017/s0954579410000672 [DOI] [PubMed] [Google Scholar]
- Obradović J., Bush N. R., Stamperdahl J., Adler N. E., Boyce W. T. (2010). Biological sensitivity to context: The interactive effects of stress reactivity and family adversity on socioemotional behavior and school readiness. Child Development, 81, 270–289. doi: 10.1111/j.1467-8624.2009.01394.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkeybile A. M., Delaney-Busch N., Hartman S., Grimm K. J., Bales K. L. (2015). Intergenerational transmission of alloparental behavior and oxytocin and vasopressin receptor distribution in the prairie vole. Frontiers in Behavioral Neuroscience, 9, Article 191. doi: 10.3389/fnbeh.2015.00191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkeybile A. M., Griffin L. L., Bales K. L. (2013). Natural variation in early parental care correlates with social behaviors in adolescent prairie voles ( Microtus ochrogaster). Frontiers in Behavioral Neuroscience, 7, Article 21. doi: 10.3389/fnbeh.2013.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluess M., Belsky J. (2011). Prenatal programming of postnatal plasticity? Development and Psychopathology, 23, 29–38. doi: 10.1017/s0954579410000623 [DOI] [PubMed] [Google Scholar]
- Shapiro L. E., Insel T. R. (1990). Infant’s response to social separation reflects adult differences in affiliative behavior: A comparative developmental study in prairie and montane voles. Developmental Psychobiology, 23, 375–393. doi: 10.1002/dev.420230502 [DOI] [PubMed] [Google Scholar]
- Slagt M., Dubas J. S., Deković M., van Aken M. A. (2016). Differences in sensitivity to parenting depending on child temperament: A meta-analysis. Psychological Bulletin, 142, 1068–1110. doi: 10.1037/bul0000061 [DOI] [PubMed] [Google Scholar]
- Stalling D. T. (1990). Microtus ochrogaster. Mammalian Species, 355, 1–9. doi: 10.2307/3504103 [DOI] [Google Scholar]
- Swenson R. R., Beckwith B. E., Lamberty K. J., Krebs S. J., Tinius T. P. (1990). Prenatal exposure to AVP or caffeine but not oxytocin alters learning in female rats. Peptides, 11, 927–932. doi: 10.1016/0196-9781(90)90011-s [DOI] [PubMed] [Google Scholar]
- Taymans S. E., DeVries A. C., DeVries M. B., Nelson R. J., Friedman T. C., Castro M., , . . . Chrousos G. P. (1997). The hypothalamic–pituitary–adrenal axis of prairie voles (Microtus ochrogaster): Evidence for target tissue glucocorticoid resistance. General and Comparative Endocrinology, 106, 48–61. doi: 10.1006/gcen.1996.6849 [DOI] [PubMed] [Google Scholar]
- van den Berg H., Bus A. G. (2014). Beneficial effects of BookStart in temperamentally highly reactive infants. Learning and Individual Differences, 36, 69–75. doi: 10.1016/j.lindif.2014.10.008 [DOI] [Google Scholar]
- Van den Bergh B. R., Mulder E. J., Mennes M., Glover V. (2005). Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: Links and possible mechanisms. A review. Neuroscience & Biobehavioral Reviews, 29, 237–258. doi: 10.1016/j.neubiorev.2004.10.007 [DOI] [PubMed] [Google Scholar]
- Varadarajulu J., Lebar M., Krishnamoorthy G., Habelt S., Lu J., Bernard Weinstein I., , . . . Touma C. (2011). Increased anxiety-related behaviour in Hint1 knockout mice. Behavioural Brain Research, 220, 305–311. doi: 10.1016/j.bbr.2011.02.012 [DOI] [PubMed] [Google Scholar]
- Wigger A., Sánchez M. M., Mathys K. C., Ebner K., Frank E., Liu D., , . . . Landgraf R. (2004). Alterations in central neuropeptide expression, release, and receptor binding in rats bred for high anxiety: Critical role of vasopressin. Neuropsychopharmacology, 29, 1–14. doi: 10.1038/sj.npp.1300290 [DOI] [PubMed] [Google Scholar]
- Yehuda R., Engel S. M., Brand S. R., Seckl J., Marcus S. M., Berkowitz G. S. (2005). Transgenerational effects of posttraumatic stress disorder in babies of mothers exposed to the World Trade Center attacks during pregnancy. The Journal of Clinical Endocrinology & Metabolism, 90, 4115–4118. doi: 10.1210/jc.2005-0550 [DOI] [PubMed] [Google Scholar]