Abstract
Very little is known about the effects of whole body vibration on the supraspinal central nervous system. Though much clinical outcome data and mechanistic data about peripheral neural and musculoskeletal mechanisms have been explored, the lack of central understanding is a barrier to evidence-based, best practice guidelines in the use of vibrational therapy. Disparate methods of administration render study to study comparisons difficult. To address this lack of uniformity, we present the use of targeted subcutaneous vibration combined with simultaneous in vivo electrophysiological recordings as a method of exploring the central effects of peripheral vibration therapy. We used implanted motors driven by both Grass stimulators and programmed microcontrollers to vary frequency and location of stimulation in an anesthetized in vivo rat model while simultaneously recording firing rate from gamma-aminobutyric acid (GABA) neurons in the ventral tegmental area. We show that peripheral vibration can alter GABA neuron firing rate in a location- and frequency-dependent manner. We include detailed schematics and code to aid others in the replication of this technique. This method allows for control of previous weaknesses in the literature including variability in body position, vibrational intensity, node and anti-node interactions with areas of differing mechanoreceptor densities, and prefrontal cortex influence.
Keywords: vibration, GABA, mechanoreceptors, addiction, methods
Introduction
Vibrational therapy, through the use of whole body vibration (WBV) platforms or localized peripheral vibration (LPV), is increasing in popularity. Early investigations looked at vibration-induced enhancement of physical performance such as power and strength,1 and this body of literature is now extensive. Over the last 15 years, the vibration literature has rapidly expanded to include studies on various responses ranging from flexibility2,3 and balance,4 to bone metabolism,5 hormone release,6 and aging and loss of balance.7 The application of vibration stimulates mechanoreceptors in the skin, muscle, and joint, which influence monosynaptic and polysynaptic pathways in the peripheral nervous system and central nervous system (CNS). Much of the initial explanation regarding the functional efficacy of WBV or LPV has focused on peripheral neuromechanical alterations.
Early work using LPV directly to a tendon or muscle helped establish peripheral neuromechanical responses to vibration. Vibration can invoke the stretch reflex by exciting group Ia afferents,8 while secondary afferents and Golgi tendon organs (GTOs) show a decreased response to vibration input.9 However, the firing rate of both afferents and the GTO response increase with muscle contraction.9,10 Other studies suggest that both the H-reflex and stretch reflex are suppressed during vibration due to pre-synaptic inhibition11 and that this suppression is sustained following the application of WBV.12,13 Weight-bearing activities and exercise application of WBV on reflex responses appear more controversial with load-bearing movement and complexity of tasks possibly interfering with assumed neuromechanisms.12 More recently, however, the variability in results of similar studies and the broadening of vibration application for study has suggested a more important role of central or supraspinal influence. Reflex contraction appears dependent on efferent fusimotor input, which suggests supraspinal control is involved.14 Current research observing the effects of vibration on symptoms of various medical conditions including stroke15 and Parkinson disease,16 among others,17 suggests that WBV is a safe and beneficial method for improving these symptoms, as well as general movement patterns, gait, and balance. Evaluation of such conditions offers further evidence that supraspinal and central mechanisms are involved in the body’s responses to vibration input. Reports of enhanced corticospinal excitability concomitant with spinal inhibition18 and facilitated motor evoked potentials19 further highlight the enhanced role of the CNS in modulating responses to vibration. The attendant increased cortical excitability seems to compensate for lower excitability at the spinal level.20
Touch (both fine and crude) pathways such as the dorsal column/medial lemniscal (DCML) pathways and pain/temperature pathways (anterolateral columns) are well defined in both texts and research literature regarding their structures and regions crossed as information is relayed from a peripheral receptor to the somatosensory cortex. However, activation of specific mechanoreceptor responses within a defined area and their effects on central neuron activity remains largely unknown. Mechanical stimulation of the DCML pathway via mechanoacupuncture at the wrist has been shown to produce modulatory effects in gamma-aminobutyric acid (GABA)-containing neurons in the midbrain ventral tegmental area (VTA).21 Furthermore, these effects have been traced through synapses in the nucleus cuneatus and subsequent relays through the thalamus and lateral habenula before finally producing inhibition of VTA GABA neurons.21 Thus, groundwork has been laid demonstrating, indirectly, that peripheral mechanoreceptor activation affects higher order functions of the CNS (eg, limbic), beyond simple somatosensory processing. There is also compelling evidence demonstrating central changes in response to mechanical vibration as measured with fMRI, EEG, heart rate variability, and evoked potentials.18,22-24 Unfortunately, the lack of better detail to supraspinal responses is still an impediment to the development of evidence-based therapeutic guidelines and consensus on vibration’s therapeutic value and mechanism of action. The use of fMRI lacks temporal and spatial resolution and primarily gives regional specificity and indirect information about neuron activity. Conversely, the use of EEG offers improved temporal resolution but lacks regional specificity. Evoked potentials give information about a change in the facilitation/inhibition of the entire pathway with limitations similar to EEG. Heart rate variability also lacks spatiotemporal resolution and is, at best, a generalized indirect measure of autonomic nervous activity. These deficiencies underpin the need for novel methods of investigation to determine how peripheral mechanical vibration affects central activity. These previous methods leave a gap in the ability to understand how individual neurons within a targeted area respond to LPV.
In this article, we lay out the application of localized subcutaneous vibration with simultaneous in-vivo single neuron electrophysiology. This technology has been a long-standing and commonly used technique to measure changes in neuron firing rate in response to various experimental conditions. However, to date, it has not been applied to the field of clinically applied vibration. The use of this technique can further our understanding of the non-canonical effects of LPV. Not only does this method give us the ability to understand the individual neuron response to LPV, but it also allows us to control the application of the peripheral vibration (region, frequency, and amplitude). To demonstrate the use of this technique, we aimed to test regional- and frequency-based differences in LPV on GABA neuron firing rate in the VTA. To test this, we implanted subcutaneous vibrating motors in 2 different regions (posterior neck C7-T1 and the biceps femoris of the posterior hindlimb) of wistar rats. Furthermore, we tested the difference between 50 and 115 Hz at the C7-T1 level of the spinal column. We hypothesized that regional- and frequency-based differences would produce disparate changes in GABA neuron firing rate due to differences in both mechanoreceptor density and specificity.
Materials and Methods
Vibrational Motors
The vibration excitation source used in this research was a 3 V, DC coin vibration motor (10 mm × 2.7 mm, DC 3 V/0.1 A; Uxcell, Hong Kong, CN). This motor induces vibration due to a rotating imbalance. The motor is encased in a small circular disc with a 10 mm diameter and 2.7 mm length. The weight of the motor was 6 grams. A photograph of the motor is shown in Figure 1.
Figure 1.

Vibrational motor.
To ensure proper excitation to the system through predictable output, the vibration output of the motor was evaluated. The procedure consisted of configuring the motor input voltage and then randomly selecting one of the predetermined frequencies. The motor output velocity was measured 10 times at each frequency and voltage setting using a laser Doppler vibrometer (LDV). The LDV provided a nonintrusive measurement without adding any mass to the motor. The displacement and acceleration were computed from the velocity measurements. Two input voltage settings of 1 and 2 V were used. The frequencies tested were at every 10 Hz ranging from 40 to 160 Hz.
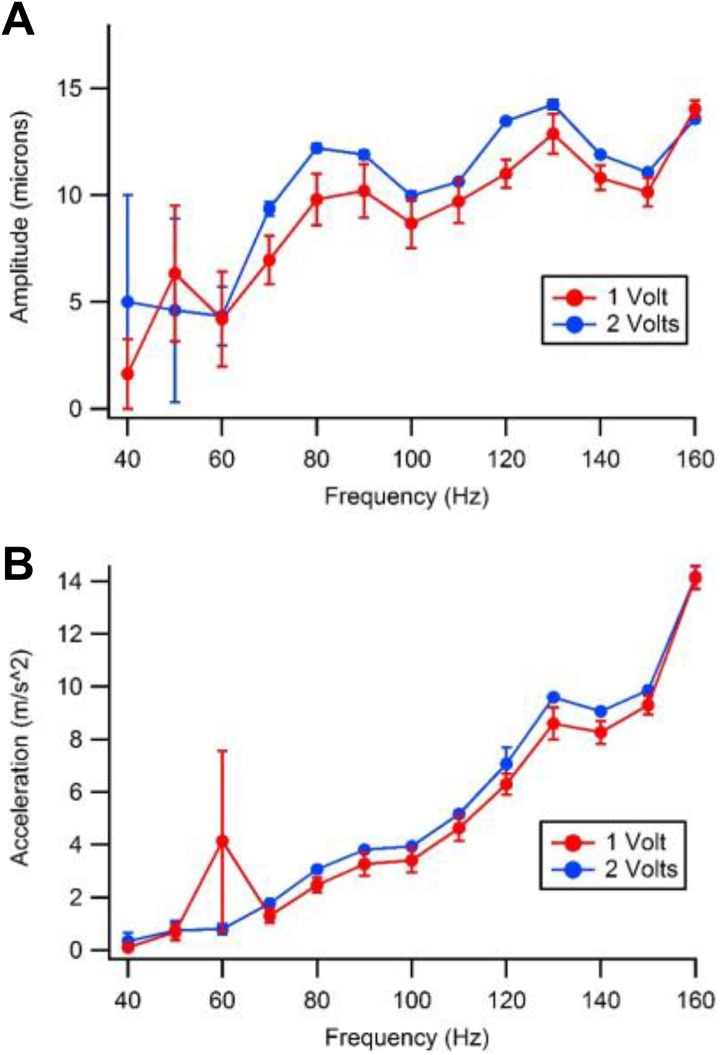
The motor characterization results are shown in Figure 2. Figure 2A shows the amplitude of the motor output displacement as a function of frequency. The displacement data show a relatively flat frequency response from 80 to 150 Hz. Figure 2B shows the acceleration versus frequency data for the motor. As expected, the acceleration output increases quadratically with frequency. From these data, it was determined that this motor had a fairly flat frequency response across the frequencies of interest and was sufficiently characterized to produce sufficiently repeatable results for the experiments described below.
Figure 2.
Motor displacement and acceleration by frequency. A, Displacement. B, Acceleration. The dots on the graphs represent an average of 10, test samples and the vertical lines at each data point represent one standard deviation above and below the indicated value.
Surgical Procedure and In Vivo Single Cell Electrophysiology
Wistar rats, weighing 250 to 320 g, from our breeding colony at Brigham Young University were used. Rats were housed in groups of 2 to 3 at a fixed temperature (21-23°C) and humidity (55-65%) on a reverse light/dark cycle with ad libitum food and water. Rats were anesthetized using isoflurane and placed in a stereotaxic apparatus. Anesthesia was then maintained at 1%. Body temperature was maintained at 37.4 ± 0.4°C by a feedback regulated heating pad. A 10 mm incision was made either at the C7-T1 levels posteriorly at midline or at the ipsilateral biceps femoris muscle. The Fascia was dissected and muscle tissue was left intact. A 10 mm × 2.7 mm, DC 3 V/0.1 A microcoin vibration motor (Uxcell, Hong Kong, China), was then implanted subcutaneously (to the right of midline for cervical implant) and the incision was closed adhesively. Extracellular potentials were recorded by a single 3.0 M KCl-filled micropipette (2-4 Mohms; 1-2 μm inside diameter). With the skull exposed, a hole was drilled for placement of the pipette which was driven into the VTA with a piezoelectric microdrive (EXFO Burleigh 8200 controller and Inchworm, Victor, New York) via stereotaxic coordinates (from bregma: 5.6-6.5 posterior (P), 0.5-1.0 lateral (L), 6.5 -7.8 ventral (V)). Potentials were displayed on a digital oscilloscope and amplified with an Axon Instruments Multiclamp 700A amplifier (Union City, California). Single-cell activity was filtered at 0.3-10 kHz (3 dB) with Multiclamp 700A and sampled at 20 kHz (12-bit resolution) with National Instruments acquisition boards. Extracellularly recorded action potentials were discriminated with a WPI, WP-121 Spike Discriminator (Sarasota, Florida). Single-unit potentials, discriminated spikes, and stimulation events were captured by National Instruments NB-MIO-16 digital I/O and counter/timer acquisition boards (Austin, Texas) in Mac computers.
Characterization of VTA GABA Neurons In Vivo and Recordings
The VTA GABA neurons were identified by previously established stereotaxic coordinates and by spontaneous and stimulus-evoked electrophysiological criteria. They included relatively fast firing rate (>10 Hz), ON-OFF phasic nonbursting activity, and spike duration less than 200 µs. We evaluated only those spikes that had greater than 5:1 signal-to-noise ratio. After positive GABA neuron identification, baseline firing rate was measured for 5 minutes to ensure stability prior to vibratory stimulus.
Grass Stimulator and Vibration Stimulation
Following measurement of the GABA neuron baseline firing rate, a 60-second vibration stimulus was introduced. The vibrating motor was connected to the S44 Grass Stimulator (Grass Medical Instruments, West Warwick, Rhode Island). For electrophysiological recordings, the stimulator was set at 2 V for 0.1ms duration and 0 ms delay. Pulses per second were varied to produce variations in vibrational frequency. All vibratory stimuli were 60 seconds in duration and followed by 15 minutes of recording the GABA neuron firing rate. Experimental groups included 50 Hz (n = 4) and 115 Hz (n = 4) given subcutaneously at the C7/T1 level and 50 Hz (n = 4) given subcutaneously at the right biceps femoris muscle.
Micro Controller Driver
In order to facilitate the replication of this technique, we include an alternative method for generating the vibratory stimulus using a PIC24F16KA301 microcontroller to generate a pulse width modulation (PWM) signal. This PWM can be modified to emulate multiple signals of varying frequency and amplitude. The PWM is then passed through a transistor that provides the needed current to the motor. The full circuit is pictured in Figure 3 and the schematic and code to drive the microcontroller can be found at http://github.com/steffensenlab.
Figure 3.
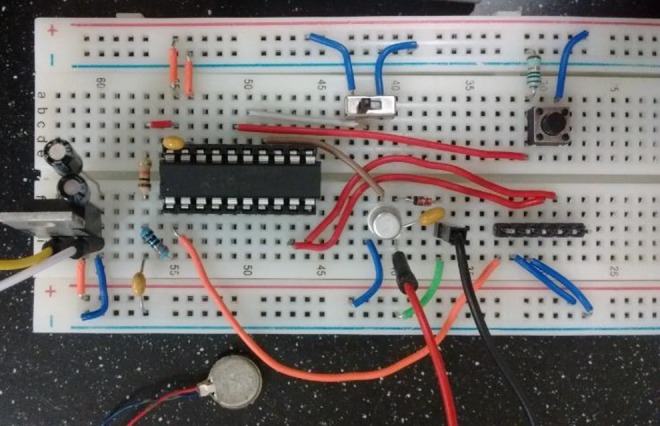
Circuit for microcontroller driving motor.
Statistical Analysis
Single-unit, discriminated spikes were processed with IGOR Pro software (Wavemetrics, Lake Oswego, Oregon). Extracellularly recorded single-unit action potentials were discriminated by a peak detector digital processing LabVIEW algorithm. After 5 minutes of recording, the final 60 seconds of firing rate data before vibrational stimulus were averaged to establish a baseline for comparison. The results for control, 50, and 115 Hz vibrational groups were derived from calculations performed on ratemeter records and expressed as mean (standard error of the mean [SEM]). All statistical tests were performed in JMP13. Data from each recording were normalized and combined and binned in 100 second intervals for comparison across time. All comparisons were initially made using a one-way analysis of variance (ANOVA). Following the ANOVA, all groups and bins were compared to corresponding controls using a Dunnett analysis. The control group was established by taking firing rate data from GABA neurons in rats that received no vibratory stimulation but did receive subcutaneous implants of vibrating motors which were placed near the cervical spine as described previously. Baseline recordings (n = 4) were normalized and combined prior to comparison with experimental groups. Figures were compiled using IGOR Pro Software.
Results
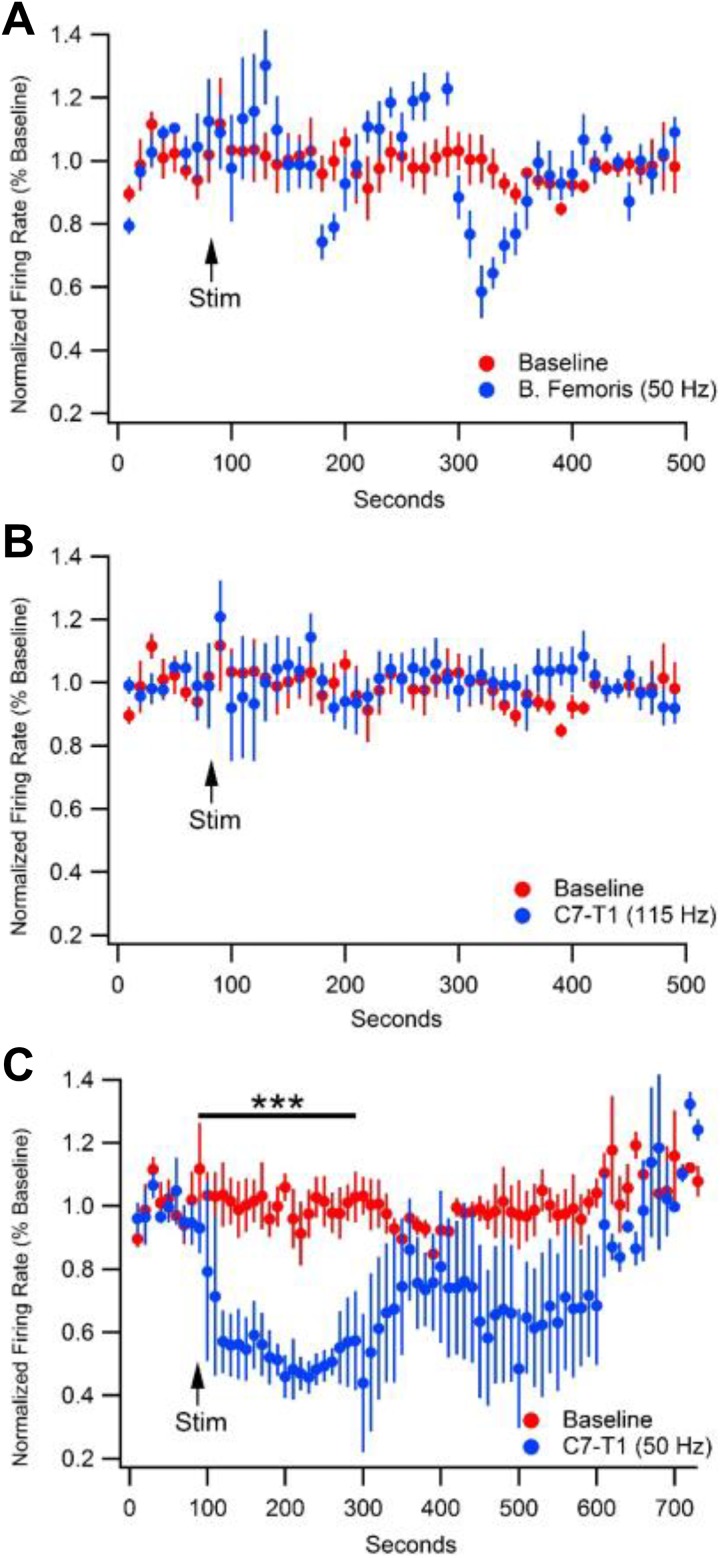
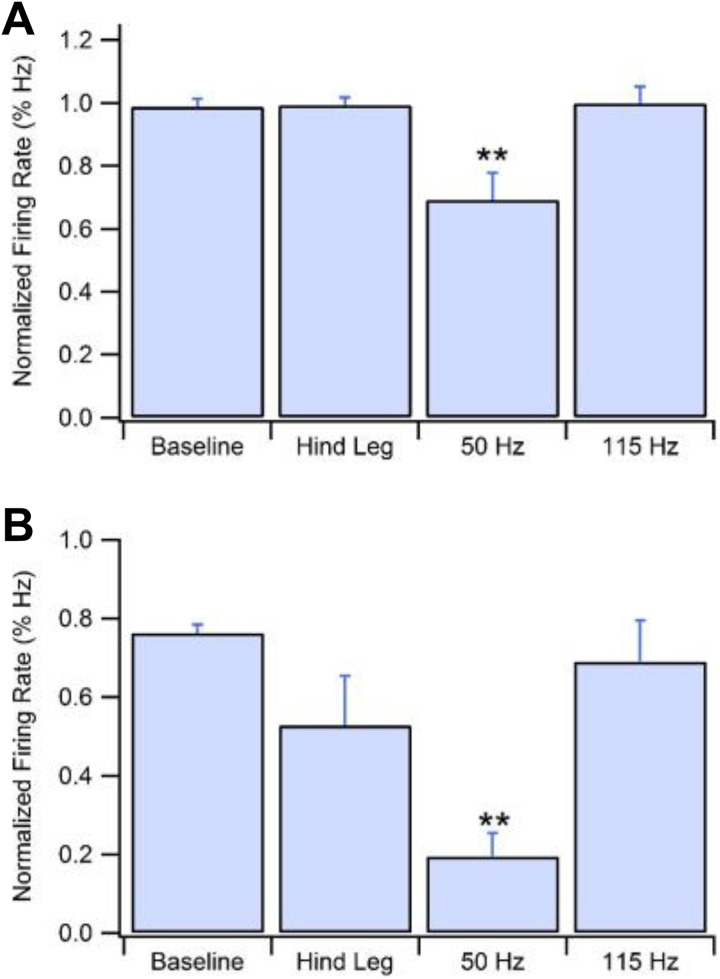
Figure 4 demonstrates the 3 LPV experimental conditions that were tested and compared to unstimulated baseline. Figure 4A shows stimulation at the right hind leg. A vibrational stimulus at this location of 50 Hz for 60 seconds showed no significant change in GABA firing rate over any time frame. Figure 4B demonstrates the effects of a 115 -Hz stimulation at C7-T1 for 60 seconds. As with stimulation to the hind leg, 115 Hz did not show a change in GABA firing rate across any time. In contrast, Figure 4C reveals a depression in GABA neuron firing rate resultant from a 50 Hz vibration at C7-T1 for 60 seconds. Initial ANOVA revealed differences between bins at 101 to 200 seconds (F (3,11) = 16.7675, P = .0002) and 201 to 300 seconds (F (3,11) = 19.51, P = .0001). After 50 Hz stimulus was given at 100 seconds, from time 100 to 200 seconds, GABA neuron firing rate depressed to 48.3% (±3.2%, n = 4, P < .0001) of baseline and to 45.9% (±7.3%, n =4, P < .0001) of baseline from 200 to 300 seconds. No other time frame or experimental condition produced a significant change in firing rate. Figure 5A shows group data comparing firing rate averages of all locations and frequencies from time of vibrational stimulus to 400 seconds poststimulation. Analysis revealed significant differences between average vibration-induced firing rate depression between groups (F (3,11) = 7.14, P = .006). 50 Hz stimulation at the right C1-T1 laminae produced an average depression to 69.2% (±3.0%, n = 4, p = .0002) of baseline and was the only stimulus to produce a significant reduction in firing rate over time. Panel B compares the greatest firing rate depression produced in each experimental group. Because VTA GABA neurons exhibit a natural cyclical ebb and flow some variation is expected even within the unstimulated group. There was a significant difference between the minimum firing rates of the 4 experimental groups (F (3,11) = 7.8545, P = .0044). Unstimulated baseline recordings produce a maximal drop to 78.1% (±0.4%, n = 4) of averaged baseline. All other maximal depressions were compared to the variation in the baseline group. 50 Hz vibration to the cervical spine produced an average maximal drop in firing rate to 24.8% (±1.4%, n = 4, P = .003) of baseline. No other group produced a significant inhibition to firing rate.
Figure 4.
Effects of variable frequency and locale subcutaneous vibrational stimulus on GABA neuron firing rate in the VTA. 50 Hz stimulus given at right hindleg (A) and 115 Hz stimulus given at cervical spine (B) show no effect on VTA GABA neuron firing rate. C, 50 Hz stimulus at cervical spine causes a 52.9% reduction in GABA firing rate from 100 to 300 seconds. GABA indicates gamma-aminobutyric acid; VTA, ventral tegmental area.
Figure 5.
Group data showing average GABA neuron firing rate depression from baseline and maximum depression. A, 50 Hz stimulus to the cervical spine produces an average depression to 69.2% of baseline. B, 50 Hz stimulus to the cervical spine produces an average maximal depression to 24.8% of baseline. GABA indicates gamma-aminobutyric acid.
Discussion
These findings are suggestive that LPV produces transient changes to cellular excitability in the VTA. Furthermore, that vibrational frequency and location of stimulus are important factors in peripheral vibration-induced changes. The sites of stimulation were chosen due to their disparate relative levels of mechanoreceptor expression in the subcutaneous tissue and adjacent joint capsules. In humans, high levels of cutaneous and joint mechanoreceptors have been identified in the intervertebral disc and facet joints of the spine, with greater relative levels in the cervical spine than thoracic or lumbar.25,26 The control stimulation was chosen due to its distance from joint mechanoreceptors. The motor implant site provided at least 20 mm of distance between the motor and the nearest joint. Further, there is a lack of cutaneous Pacinian corpuscle expression in rodents enhancing the effect of increased distance from joint.27 The 2 frequencies of vibration were chosen to more selectively activate either Meissner’s corpuscles or Pacinian corpuscles; the former having a lower intensity dynamic range of around 5 to 100 Hz and the latter a higher range of around 100 to 300 Hz.28-31 However, it is noted that these ranges are commonly thought to be approximate and some overlap certainly exists. A small population of lanceolate endings exist which have been shown to respond to vibration in a range around 5 to 200 Hz, although this population of endings has only been demonstrated in recordings of cat whisker hair.32 Other common cutaneous and joint mechanoreceptors are thought to be more specific to light touch (Merkel Cells) and stretch (Ruffini corpuscles) and thus are less likely to be significant in the presence of prolonged vibrational input.33 50 and 115 Hz vibrational frequencies were chosen to more selectively target Meissner and Pacinian corpuscles respectively.
VTA GABA neuron activity transiently and significantly decreased after targeted subcutaneous vibration at the C7-T1 level when stimulation occurred at 50 Hz. These changes were not present when stimulation was given at 115 Hz or when stimulation was given at the muscle belly of the biceps femoris muscle of the upper hind leg. Additionally, they suggest that targeted, anatomically specific and frequency-variable vibration coupled with single-unit extracellular electrophysiology is an effective method for measuring direct alterations in neuronal firing rate resultant from peripheral vibration. A previous study recording GABA neuron firing rate changes in the VTA due to acupuncture treatment suggested that receptor sensitivities might be responsible for the reported frequency dependent effects on these neurons.34 Gamma-aminobutyric acid neurons in the VTA were chosen due to internal preliminary data indicating their responsiveness to peripheral vibration and because of their relevance in addiction research.34-36 This method can be employed to further clarify regional- and frequency-based sensitivities, mechanoreceptor subtypes, and tractology involved in alterations of central neuronal function. To our knowledge, this method has not been applied to the field of therapeutic vibration. It can provide a framework for ongoing description of the central pathways involved in this emerging field of physical medicine.
Future Direction
It is unknown the extent to which higher order neuronal processing affects the currently observed effects of WBV such as improvement in gait, movement patterns, and balance. By extension, even less is understood about the changes that occur primarily in these higher circuits. This lack of understanding is a major impediment to our ability to develop optimized, evidence-based care protocols and could contribute to the mixed outcome data surrounding both WBV and LPV, and their therapeutic applications. The diversity of vibrational testing protocols divergent in frequency, duration, type, amplitude, resonant location, and subject positioning render inference about precise neural mechanisms difficult. The lack of homogenous testing protocols could contribute to the mixed reports of the efficacy of WBV in the treatment of various conditions.37,38 Indeed, it has been shown that slight variations in a subject’s posture or weight distribution can greatly affect the transmission of vibratory stimuli through the body.39 Future studies should focus on addressing these issues, specifically tracking the cellular and circuitry changes that contribute to current therapeutic applications and outcomes from WBV. Frequency and regional specificity along with duration or treatment must be addressed in order to maximize patient outcomes. These answers will be difficult to ascertain without directly measuring circuitry of interest in animal models and human subjects for each condition for which WBV is a potential therapy. An understanding of cellular and circuitry changes could provide insight into applications not currently being considered, including methods such as deep brain stimulation or genetic therapies. Localized subcutaneous vibration with simultaneous in vivo single-unit electrophysiology can provide heretofore lacking information about individual neuronal changes in the CNS. Filling this gap may help provide a more complete picture of the central effects of vibratory therapeutics and lead to improved continuity of application and outcome in the future.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by PHS NIH grants AA020919 and DA035958 to SCS.
ORCID iD: Kyle B. Bills  https://orcid.org/0000-0001-9742-3728
https://orcid.org/0000-0001-9742-3728
References
- 1. Bosco C, Cardinale M, Tsarpela O. Influence of vibration on mechanical power and electromyogram activity in human arm flexor muscles. Eur J Appl Physiol Occup Physiol. 1999;79(4):306–311. [DOI] [PubMed] [Google Scholar]
- 2. Feland JB, Hawks M, Hopkins JT, Hunter I, Johnson AW, Eggett DL. Whole body vibration as an adjunct to static stretching. Int J Sports Med. 2010;31(8):584–589. [DOI] [PubMed] [Google Scholar]
- 3. Houston MN, Hodson VE, Adams KK, Hoch JM. The effectiveness of whole-body-vibration training in improving hamstring flexibility in physically active adults. J Sport Rehabil. 2015;24(1):77–82. [DOI] [PubMed] [Google Scholar]
- 4. Tseng SY, Hsu PS, Lai CL, Liao WC, Lee MC, Wang CH. Effect of two frequencies of whole-body vibration training on balance and flexibility of the elderly: a randomized controlled trial. Am J Phys Med Rehabil. 2016;95(10):730–737. [DOI] [PubMed] [Google Scholar]
- 5. Dionello CF, Sa-Caputo D, Pereira HV, et al. Effects of whole body vibration exercises on bone mineral density of women with postmenopausal osteoporosis without medications: novel findings and literature review. J Musculoskelet Neuronal Interact. 2016;16(3):193–203. [PMC free article] [PubMed] [Google Scholar]
- 6. Paineiras-Domingos LL, Sa-Caputo DDC, Moreira-Marconi E, et al. Can whole body vibration exercises affect growth hormone concentration? A systematic review. Growth Factors. 2017;35(4-5):189–200. [DOI] [PubMed] [Google Scholar]
- 7. Jepsen DB, Thomsen K, Hansen S, Jorgensen NR, Masud T, Ryg J. Effect of whole-body vibration exercise in preventing falls and fractures: a systematic review and meta-analysis. BMJ Open. 2017;7(12):e018342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Matthews PB. Reflex activation of the soleus muscle of the decerebrate cat by vibration. Nature. 1966;209(5019):204–205. [DOI] [PubMed] [Google Scholar]
- 9. Brown MC, Engberg I, Matthews PB. The relative sensitivity to vibration of muscle receptors of the cat. J Physiol. 1967;192(3):773–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burke D, Hagbarth KE, Lofstedt L, Wallin BG. The responses of human muscle spindle endings to vibration during isometric contraction. J Physiol. 1976;261(3):695–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gillies JD, Lance JW, Neilson PD, Tassinari CA. Presynaptic inhibition of the monosynaptic reflex by vibration. J Physiol. 1969;205(2):329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ritzmann R, Kramer A, Gollhofer A, Taube W. The effect of whole body vibration on the H-reflex, the stretch reflex, and the short-latency response during hopping. Scand J Med Sci Sports. 2013;23(3):331–339. [DOI] [PubMed] [Google Scholar]
- 13. Karacan I, Cidem M, Yilmaz G, Sebik O, Cakar HI, Turker KS. Tendon reflex is suppressed during whole-body vibration. J Electromyogr Kinesiol. 2016;30:191–195. [DOI] [PubMed] [Google Scholar]
- 14. Rittweger J. Vibration as an exercise modality: how it may work, and what its potential might be. Eur J Appl Physiol. 2010;108(5):877–904. [DOI] [PubMed] [Google Scholar]
- 15. Park YJ, Park SW, Lee HS. Comparison of the effectiveness of whole body vibration in stroke patients: a meta-analysis. Biomed Res Int. 2018;2018:5083634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sharififar S, Coronado RA, Romero S, Azari H, Thigpen M. The effects of whole body vibration on mobility and balance in Parkinson disease: a systematic review. Iran J Med Sci. 2014;39(4):318–326. [PMC free article] [PubMed] [Google Scholar]
- 17. Dionello CF, de Souza PL, Sa-Caputo D, et al. Do whole body vibration exercises affect lower limbs neuromuscular activity in populations with a medical condition? A systematic review. Restor Neurol Neurosci. 2017;35(6):667–681. [DOI] [PubMed] [Google Scholar]
- 18. Krause A, Gollhofer A, Freyler K, Jablonka L, Ritzmann R. Acute corticospinal and spinal modulation after whole body vibration. J Musculoskelet Neuronal Interact. 2016;16(4):327–338. [PMC free article] [PubMed] [Google Scholar]
- 19. Mileva KN, Bowtell JL, Kossev AR. Effects of low-frequency whole-body vibration on motor-evoked potentials in healthy men. Exp Physiol. 2009;94(1):103–116. [DOI] [PubMed] [Google Scholar]
- 20. Souron R, Besson T, McNeil CJ, Lapole T, Millet GY. An acute exposure to muscle vibration decreases knee extensors force production and modulates associated central nervous system excitability. Front Hum Neurosci. 2017;11:519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chang S, Ryu Y, Gwak YS, et al. Spinal pathways involved in somatosensory inhibition of the psychomotor actions of cocaine. Sci Rep. 2017;7(1):5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang N, Fard M, Bhuiyan MHU, Verhagen D, Azari MF, Robinson SR. The effects of physical vibration on heart rate variability as a measure of drowsiness. Ergonomics. 2018;61(9):1–19. [DOI] [PubMed] [Google Scholar]
- 23. Kaut O, Becker B, Schneider C, et al. Stochastic resonance therapy induces increased movement related caudate nucleus activity. J Rehabil Med. 2016;48(9):815–818. [DOI] [PubMed] [Google Scholar]
- 24. Satou Y, Ishitake T, Ando H, et al. Effect of short-term exposure to whole body vibration in humans: relationship between wakefulness level and vibration frequencies. Kurume Med J. 2009;56(1-2):17–23. [DOI] [PubMed] [Google Scholar]
- 25. McLain RF, Raiszadeh K. Mechanoreceptor endings of the cervical, thoracic, and lumbar spine. Iowa Orthop J. 1995;15:147–155. [PMC free article] [PubMed] [Google Scholar]
- 26. McLain RF. Mechanoreceptor endings in human cervical facet joints. Spine (Phila Pa 1976). 1994;19(5):495–501. [DOI] [PubMed] [Google Scholar]
- 27. Zelena J. The development of Pacinian corpuscles. J Neurocytol. 1978;7(1):71–91. [DOI] [PubMed] [Google Scholar]
- 28. Macefield VG. Physiological characteristics of low-threshold mechanoreceptors in joints, muscle and skin in human subjects. Clin Exp Pharmacol Physiol. 2005;32(1-2):135–144. [DOI] [PubMed] [Google Scholar]
- 29. Rowe MJ, Tracey DJ, Mahns DA, Sahai V, Ivanusic JJ. Mechanosensory perception: are there contributions from bone-associated receptors? Clin Exp Pharmacol Physiol. 2005;32(1-2):100–108. [DOI] [PubMed] [Google Scholar]
- 30. S Coren LW, Enns JT. Sensation and Perception. 5th ed San Diego, CA: Harcourt Brace and Co; 1999. [Google Scholar]
- 31. Biswas A, Manivannan M, Srinivasan MA. Vibrotactile sensitivity threshold: nonlinear stochastic mechanotransduction model of the Pacinian Corpuscle. IEEE Trans Haptics. 2015;8(1):102–113. [DOI] [PubMed] [Google Scholar]
- 32. Gottschaldt KM, Iggo A, Young DW. Functional characteristics of mechanoreceptors in sinus hair follicles of the cat. J Physiol. 1973;235(2):287–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fleming MS, Luo W. The anatomy, function, and development of mammalian Abeta low-threshold mechanoreceptors. Front Biol (Beijing). 2013;8(4). DOI: 10.1007/s11515-013-1271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim SA, Lee BH, Bae JH, et al. Peripheral afferent mechanisms underlying acupuncture inhibition of cocaine behavioral effects in rats. PLoS One. 2013;8(11):e81018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jin W, Kim MS, Jang EY, et al. Acupuncture reduces relapse to cocaine-seeking behavior via activation of GABA neurons in the ventral tegmental area. Addict Biol. 2017;23(1):165–181. [DOI] [PubMed] [Google Scholar]
- 36. Yang CH, Yoon SS, Hansen DM, et al. Acupuncture inhibits GABA neuron activity in the ventral tegmental area and reduces ethanol self-administration. Alcohol Clin Exp Res. 2010;34(12):2137–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lam FM, Lau RW, Chung RC, Pang MY. The effect of whole body vibration on balance, mobility and falls in older adults: a systematic review and meta-analysis. Maturitas. 2012;72(3):206–213. [DOI] [PubMed] [Google Scholar]
- 38. Orr R. The effect of whole body vibration exposure on balance and functional mobility in older adults: a systematic review and meta-analysis. Maturitas. 2015;80(4):342–358. [DOI] [PubMed] [Google Scholar]
- 39. Rohlmann A, Schmidt H, Gast U, Kutzner I, Damm P, Bergmann G. In vivo measurements of the effect of whole body vibration on spinal loads. Eur Spine J. 2014;23(3):666–672. [DOI] [PMC free article] [PubMed] [Google Scholar]