Abstract
BACKGROUND
In non-alcoholic fatty liver disease (NAFLD), a high-fat or high-fructose diet increases intestinal permeability and promotes derangement of the gut-liver axis. We hypothesize that, diet could be able to modulate intestinal permeability in patients with NAFLD.
AIM
To detect diet-induced modification of intestinal permeability in patients with NAFLD undergoing a Mediterranean diet or a low-fat diet.
METHODS
The current study was a dietary intervention for non-diabetic, patients with biopsy-verified NAFLD and increased transaminases. A crossover design was employed: participants underwent 16 weeks of Mediterranean diet, 16 wk of free wash-out, and 16 weeks of low-fat diet. Both diets were hypocaloric and no consumption of supplements was allowed. All patients were followed bimonthly by a dietitian. Evaluations of clinical and metabolic parameters were completed at baseline and at the end of each dietary period. Intestinal permeability was assessed by chromium-51 ethylene diamine tetraacetate excretion testing (51Cr-EDTA).
RESULTS
Twenty Caucasian patients, 90% male, median age 43 years, body mass index (BMI) 30.9, with biopsy-verified NAFLD were enrolled. At the end of 16 weeks of a Mediterranean diet, a significant reduction in mean body weight (-5.3 ± 4.1 kg, P = 0.003), mean waist circumference (-7.9 ± 4.9 cm, P = 0.001), and mean transaminase levels [alanine aminotransferase (ALT) -28.3 ± 11.9 IU/L, P = 0.0001; aspartate aminotransferase (AST) -6.4 ± 56.3 IU/L, P = 0.01] were observed. These benefits were maintained after 16 wk of wash-out and also after 16 wk of low-fat diet, without further improvements. Fourteen of the 20 patients had intestinal permeability alteration at baseline (mean percentage retention of 51Cr-EDTA = 5.4%), but no significant changes in intestinal permeability were observed at the end of the 16 wk of the Mediterranean diet or 16 wk of the low-fat diet.
CONCLUSION
Mediterranean diet is an effective strategy for treating overweight, visceral obesity and serum transaminase in patients with NAFLD. If the Mediterranean diet can improve intestinal permeability in patients with NAFLD, it deserves further investigation.
Keywords: Liver steatosis, Gut-liver axis, Nutrition, Personalized medicine, Visceral obesity
Core tip: Diet, as well as intestinal microbiota, is a key regulator of intestinal permeability, the alteration of which is central in the derangement of the gut-liver axis. In patients with non-alcoholic fatty liver disease (NAFLD), intestinal permeability is increased, promoting translocation of bacteria-derived products into the portal circulation and increasing hepatic exposure to injurious substances that stimulate hepatic inflammation and fibrosis. In animal models, high-fat diet or high-fructose intake has been associated with increased gut permeability. The aim of this study was to detect diet-induced modification of intestinal permeability in non-diabetic patients with NAFLD undergoing a Mediterranean diet or a low-fat diet.
INTRODUCTION
In western countries, non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease and is closely associated with an unhealthy lifestyle. Patients with NAFLD are reported to engage in an excessive consumption of total energy, refined carbohydrates (including fructose), saturated fats and cholesterol with an insufficient intake of polyunsaturated fats, fibres and antioxidants (vitamin C and vitamin E)[1,2]. Because there is no pharmacotherapy currently available for patients with NAFLD, lifestyle modifications remain the cornerstone management[3,4]. In overweight/obese patients with NAFLD, calorie restriction drives weight loss, reducion of liver fat, and histological improvement of non-alcoholic steatohepatitis (NASH)[5,6] , but there are conflicting data on which hypocaloric dietary plan should be adopted according to the macronutrient composition[7,8].
Low-fat diets, popular in the treatment of obesity, are reported to be more effective than low-carbohydrate diets in reducing total cholesterol and low-density lipoprotein (LDL) cholesterol concentrations[9], whereas low-carbohydrate diets can be more effective than low-fat diets in increasing high-density lipoprotein (HDL) cholesterol concentrations and reducing triglyceride levels and transaminase levels through a greater reduction in the concentration of 24-h circulating insulin under both isocaloric and hypocaloric conditions[10-13].
The Mediterranean diet, first described in the 1960s by Ancel Keys, is a low-carbohydrate nutritional model inspired by the traditional diets of countries along the Mediterranean Sea Basin, an area of origin for the Olea Europaea plant[14,15]. European guidelines for management of NAFLD suggest the Mediterranean diet as a reference for macronutrient composition[16].
Diet, as well as intestinal microbiota, is a key regulator of intestinal permeability, the alteration of which is central in the derangement of the gut-liver axis[17]. In patients with NAFLD, intestinal permeability is increased, promoting translocation of bacteria-derived products into the portal circulation and increasing hepatic exposure to injurious substances that stimulate hepatic inflammation and fibrosis[18]. In animal models, adaptation of a high-fat diet or high-fructose intake has been associated with increased gut permeability and metabolic endotoxiemia[19-21]. Although diet can significantly influence intestinal permeability, clinical studies investigating the effects of dietary interventions on the intestinal permeability of NAFLD patients are lacking.
The aim of this study was to detect diet-induced modification of intestinal permeability in non-diabetic patients with NAFLD undergoing a Mediterranean diet or a low-fat diet in a crossover comparison. Secondary outcomes were modifications in transaminase levels, body weight and waist circumference.
MATERIALS AND METHODS
Patients
This single-centre prospective dietary intervention open-label trial was conducted in the outpatient clinic for Gastroenterology and Liver Disease at the Catholic University of the Sacred Heart of Rome and was approved by the local Ethics Committee. Every patient provided written informed consent before enrolment.
Adult patients with a histologically confirmed diagnosis of NAFLD and increased levels of alanine aminotransferase (ALT) > 1.5 time the upper normal limit (30 IU/L in males and 19 IU/L in females) were included[22]. We chose to include only patients with elevated transaminase levels at baseline because one of the secondary end-points of the study was a reduction in transaminases. All other causes of liver damage were ruled out. In particular, all patients had negative serological markers for hepatitis B and hepatitis C, an alcoholic consumption of < 30 g of ethanol for men and < 20 g for women, were not taking any hepatotoxic drug, did not have evidence of metabolic disease (Wilson's disease, hereditary haemochromatosis) or autoimmune disease (autoimmune hepatitis, primitive biliary cirrhosis, primitive sclerosing cholangitis). The main exclusion criteria were the presence of liver cirrhosis, diabetes, body mass index (BMI) ≥ 35 kg/m² or previous bariatric surgery, because these conditions already have well-established dietary recommendations.
Participants had to have stable body weight (variation < 5% within the preceding 3-mo period) and maintain their usual level of physical activity for the duration of the intervention. The participants who were already taking medications had to be on a stable regimen for at least 6 months before study enrolment. The dose of these medications had to remain stable during the study. Participants were allowed to start a new medication only if it was medically necessary. No consumption of supplements (including vitamin E) was allowed during the study.
Design of the study
Patients underwent a dietary treatment via a crossover design lasting 48 wk: 16-wk of a Mediterranean diet (W1-W16), 16-wk of a free wash-out diet (W17-W32) and 16-wk of a low-fat diet (W33-W48).
Both the Mediterranean diet (40% carbohydrates, 40% fat with < 10% saturated fat, 20% protein) and low-fat diet (62% carbohydrates, 18% fat, 20% protein) were caloric-equivalent (1400 Kcal) and were processed by the dietary service of our Hospital in accordance with international standards (Supplementary Tables 1-3). We chose hypocaloric diets as opposed to isocaloric diets because the beneficial effect of caloric restriction on NAFLD has been well-documented in the literature, and it would have been unethical to not have provided patients with an indication recommended by all guidelines.
Before starting each diet and at the end of each dietary period, patients underwent a medical examination, provided and performed electrocardiogram and blood tests, including blood count, ALT, aspartate aminotransferase (AST), gamma glutamyl transpeptidase (GGT), alkaline phosphatase, bilirubin, blood glucose, basal insulinaemia, total cholesterol, HDL cholesterol, triglycerides, albumin, creatinine, sodium, potassium, and urine test. All blood tests were determined by standard methods in a certified clinical chemistry laboratory.
Anthropometric measurements were directly measured by the same dietitian at every visit: weight (light clothing, shoes off) to the nearest 0.1 kg, height (standing tall, feet hip-width apart and head level) to the nearest 1 cm, waist and hip circumference (tape lying flat and level, taut but not tight) to the nearest 1 cm.
All patients were re-evaluated bimonthly by the same dietitian for compliance to the prescribed diet and physical activity level. Dietary history was carried out by means of a 24-h recall[23], food frequency questionnaire (including alcohol intake)[24], and 10-point Mediterranean-diet Score[25], with subsequent calculation of caloric-nutritional intake. Physical activity was assessed at both baseline and at the end of each dietary period using the International Physical Activity Questionnaire (IPAQ) long form[26].
Evaluation of intestinal permeability
Intestinal permeability was evaluated at baseline and at the end of each diet period using the validated method of urinary excretion of chromium-51 ethylene diamine tetraacetate excretion testing (51Cr-EDTA). Many methods for measuring intestinal permeability in the clinical setting have been validated (e.g., lactulose/mannitol, sucralose, sucrose), but none has proven to be superior to others nor has gained a place in everyday clinical practice[17]. In our study we chose to use the 51Cr-EDTA test because it is poorly influenced by bacterial degradation in cases of small intestine bacterial overgrowth, sometimes associated with NAFLD, and it is easily repeatable and less time-consuming for the health workers compared to oligosaccharides-based tests[27] .
After an overnight fast, patients drank 1.85 MBq 51CrEDTA (Amersham Health, England) in 10 mL water, and urine was collected for the next 24 h. Two 3-mL samples of the collected urine were assessed with a gamma counter (LKB-Wallac 1282 Compugamma, Turku, Finland), as previously described[28]. 51Cr-EDTA clearance was calculated using the following formula: [(Mean urinary counts × Urinary volume) × (Standard counts × 50)]-1. Results were expressed as percentages of the ingested dose. In normal subjects, 1%-3% of an orally administered dose of 51Cr-EDTA is absorbed by the gastrointestinal tract.
Statistical analysis
The data were analysed using a descriptive statistical method (Statistical Package for Social Sciences 17.0), and the results were expressed as the means and standard deviations. Student’s t-tests were used to determine statistically significant differences (P < 0.05) between groups.
RESULTS
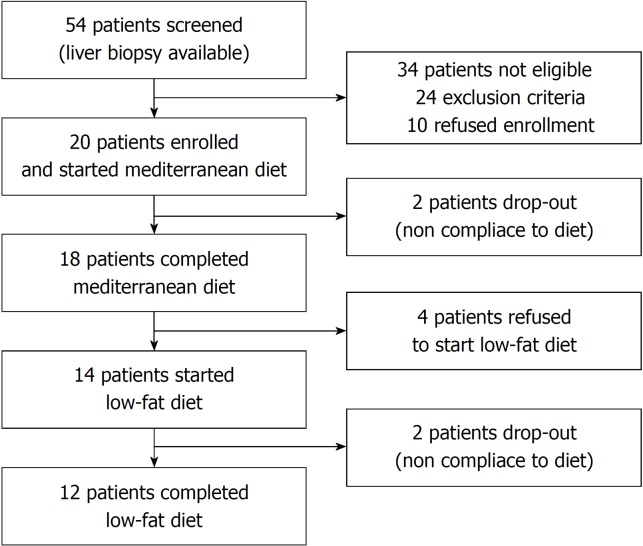
Fifty-four patients with NAFLD underwent liver biopsy at our centre during the two-year timeframe (Figure 1). Twenty-four patients were not eligible for the study because of one or more exclusion criteria (16 patients for ALT levels < 1.5 time the upper normal limit, 5 patients for diabetes, 3 patients for BMI ≥ 35 kg/m²). Ten patients refused to participate in the study (5 patients because of lack of motivation to start a diet program and 5 patients because of working reasons). Twenty patients were enrolled and started the Mediterranean diet. Eighteen patients completed the 16-wk Mediterranean diet (W1-W16), whereas 2 patients dropped out due to poor compliance to the diet. After 16 weeks of a free wash-out diet (W17-W32), 4 patients refused to continue the study. 14 patients started the 16 wk of the low-fat diet (W33-48), but 2 patients dropped out due to poor compliance to the diet. Twelve patients completed the entire protocol. According to the results of IPAQ long form, no patient significantly changed his or her level of physical activity during the study.
Figure 1.
Flow chart of study patients.
Demographics
Clinical and laboratory features of the cohort are summarized in Table 1. Overall, 18 (90%) of the patients enrolled were male, with a mean age of 42.7 years. Thirteen (65%) patients had hypertension, 5 (25%) had hypercholesterolemia, and 13 (65%) met diagnostic criteria for metabolic syndrome according to ATP III[29] (Table 1). Based on liver biopsy results, 12 (60%) presented with a Kleiner score of 3-4 and 8 (40%) with a Kleiner score ≥ 5, indicative of NASH[30]. Mean BMI was 30.9 ± 3.6, mean waist circumference was 107.2 ± 9.0 cm, mean hip circumference was 107.5 ± 6.7 cm, mean waist/hip ratio was 0.99 ± 0.05, mean systolic blood pressure was 129.0 ± 14.3 mmHg, and mean diastolic blood pressure was 84.5 ± 11.4 mmHg. Mean fasting blood glucose was 93.8±13.1 mg/dL, mean HOMA-IR score[31] was 5.2, mean total cholesterol was 186.5 ± 45.1 mg/dL, mean LDL cholesterol was 112.9 ± 44.1 mg/dL, mean HDL cholesterol was 41.7 ± 6.3 mg/dL, mean triglycerides were 139.0 ± 90.4 mg/dL, mean ALT levels were 80.5 ± 41.6 IU/L, mean AST levels were 39.8 ± 15.1 IU/L, and mean GGT levels were 102.3 ± 123.5 IU/L.
Table 1.
Effects on clinical, metabolic and intestinal permeability paramethers after 16 wk of Mediterranean diet or low-fat diet in patients with non-alcoholic fatty liver disease
| Basal | After Mediterranean diet | P value (≥ 0.05 NS) | After wash-out | After low-fat diet | P value (≥ 0.05 NS) | |
| (W1) | (W16) | W1 vs W16 | (W32) | (W48) | W32 vs W48 | |
| Weight (kg) | 91.3 ± 10.9 | 86.0 ± 12.4 | 0.003 | 86.3 ± 8.4 | 85.7 ± 9.4 | NS |
| Body mass index | 30.9 ± 3.6 | 29.3 ± 4.1 | NS | 29.7 ± 2.9 | 29.3 ± 3.2 | NS |
| Waist (cm) | 107.2 ± 9.0 | 99.3 ± 11.5 | 0.001 | 102.0 ± 11.5 | 96.8 ± 11.5 | NS |
| Hip (cm) | 107.5 ± 6.7 | 103.9 ±6.9 | 0.001 | 103.7 ± 6.3 | 101.7 ± 11.1 | NS |
| Waist/hip ratio | 0.99 ± 0.05 | 0.96 ± 0.08 | NS | 0.97 ± 0.04 | 0.95 ± 0.06 | NS |
| Systolic blood pressure (mmHg) | 129.0 ± 14.3 | 130.5 ± 20.3 | NS | 130.0 ± 8.9 | 122.5 ± 17.5 | NS |
| Diastolic blood pressure (mmHg) | 84.5 ± 11.4 | 81.0 ± 14.7 | NS | 79.2 ± 9.2 | 75.8 ± 14.3 | NS |
| Glycemia (mg/dL) | 93.8 ± 13.1 | 92.9 ± 11.8 | NS | 95.5 ± 9.0 | 96.2 ± 7.3 | NS |
| HOMA-IR | 5.2 ± 2.5 | 3.5 ± 2.1 | NS | 4.3 ± 1.2 | 3.8 ± 1.5 | NS |
| Total Cholesterol (mg/dL) | 186.5 ± 45.1 | 176.9 ± 42.9 | NS | 186.3 ± 65.7 | 177.0 ± 50.5 | NS |
| LDL cholesterol (mg/dL) | 112.9 ± 44.1 | 113.7 ± 35.2 | NS | 119.7 ± 55.8 | 109.5 ± 44.6 | NS |
| HDL cholesterol (mg/dL) | 41.7 ± 6.3 | 42.1 ± 9.3 | NS | 44.7 ± 4.5 | 44.7 ± 4.7 | NS |
| Tryglicerid (mg/dL) | 139.0 ± 90.4 | 105.4 ± 45.4 | NS | 110.5 ± 48.7 | 105.8 ± 36.1 | NS |
| ALT (UI/L) | 80.5 ± 41.6 | 52.2 ± 32.3 | 0.0001 | 56.2 ± 33.8 | 58.3 ± 38.7 | NS |
| AST (UI/L) | 39.8 ± 15,1 | 33.4 ± 15.7 | 0.01 | 26.5 ± 10.4 | 29.0 ± 11.3 | NS |
| GGT (UI/L) | 102.3 ± 123.5 | 89.6 ± 129.5 | NS | 100.7 ± 128.3 | 70.7 ± 75.3 | NS |
| Albumin (g/dL) | 4.6 ± 0.3 | 4.5 ± 0.3 | NS | 4.6 ± 0.4 | 4.5 ± 0.4 | NS |
| Emoglobin (g/dL) | 15.1 ± 1.1 | 14.9 ± 1.1 | NS | 15.4 ± 1.3 | 15.3 ± 1.3 | NS |
| Creatinin (mg/dL) | 0.9 ± 0.1 | 0.9 ± 0.1 | NS | 0.9 ± 0.1 | 0.9 ± 0.1 | NS |
W1: Week 1; W16: Week 16; W32: Week 32; W48: Week 48; HOMA-IR: Homeostatic model assessment - insulin resistance; LDL: Low density lipoprotein; HDL: High density lipoprotein; ALT: Alanine transaminase; AST: Aspartate transaminase; GGT: Gamma glutamyl trasferase.
Nutritional assessment
At the baseline nutritional assessment, patients ingested a mean of 1798 kcal per day, divided as follows: 53.2% carbohydrates, 18.3% proteins and 28.4% lipids, of which 11.4 g were saturated fat, 4.5 g polyunsaturated fats, 29.7 g monounsaturated fatty acids and 248.2 g cholesterol (Table 2). Regarding micronutrients, patients ingested a mean of only 16.9 g of total daily fibre, 88.8 mcg of vitamin A, 68.3 g of vitamin C, 7.7 g of vitamin E and 9.5 g of total iron. Regarding the daily nutrient distribution, 22% of patients usually did not have breakfast; 45% were not used to having a full lunch and 75% did not have a mid-morning and afternoon snack. The results of the food frequency questionnaire administered at baseline are shown in the supplementary appendix. According to the 10-point Mediterranean-diet Score, 45% of patients obtained a score ≤ 3, indicative of a food pattern poorly adherent to the Mediterranean diet.
Table 2.
Nutritional assessment
| Basal (W1) | After mediterranean diet (W16) | After wash-out (W32) | After low-fat diet (W48) | |
| Macronutrients | ||||
| kcal per day | 1798 | 1421 | 1853 | 1414 |
| Carbohydrates (%) | 53.2 | 40.5 | 55.0 | 62.1 |
| Proteins (%) | 18.3 | 21.1 | 16.5 | 20.2 |
| Lipids (%) | 28.4 | 38.4 | 28.5 | 17.7 |
| Saturated fat (g) | 11.4 | 7.2 | 9.0 | 5.5 |
| Polyunsaturated fats (g) | 4.5 | 3.5 | 4.2 | 2.6 |
| Monounsaturated fatty acids (g) | 29.7 | 27.2 | 25.0 | 14.4 |
| Cholesterol (g) | 248.2 | 157.5 | 258.2 | 153.3 |
| Micronutrients | ||||
| Total daily fiber (g) | 16.9 | 28.2 | 16.8 | 21.2 |
| Vitamin A (mcg) | 88.8 | 47.6 | 86.1 | 33.4 |
| Vitamin C (g) | 68.3 | 143.3 | 72.6 | 119.3 |
| Vitamin E (g) | 7.7 | 7.8 | 6.7 | 3.3 |
| Total iron (g) | 9.5 | 15.5 | 10.7 | 12.7 |
W1: Week 1; W16: Week 16; W32: Week 32; W48: Week 48.
Effects of nutritional intervention
An assessment of adherence to the two diet periods is shown in Table 2. A total of 18 patients out of 20 were judged to be adherent to the Mediterranean diet, and 12 out of 14 patients were judged to be adherent to the low-fat diet.
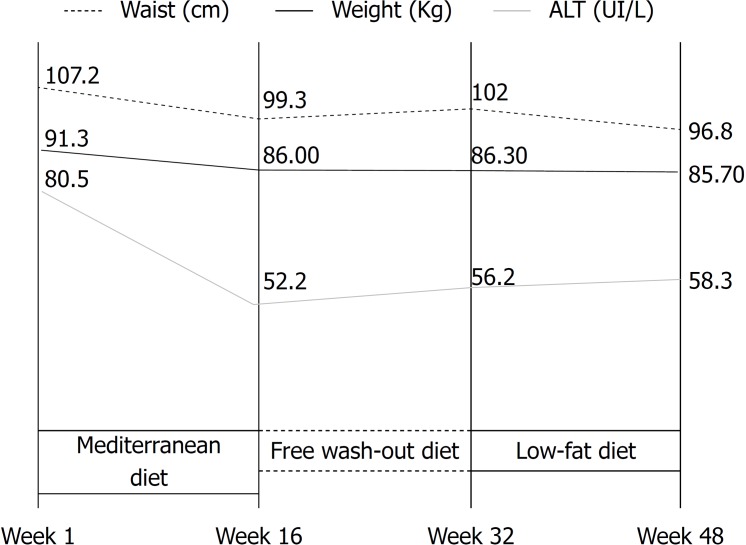
At the end of 16 wk of Mediterranean diet (W1-W16), were observed a significant reduction in mean body weight (-5.3 ± 4.1 kg, P = 0.003), mean waist circumference (-7.9 ± 4.9 cm, P = 0.001), mean hip circumference (-8.2 ± 8.6 cm, P = 0.001), and mean transaminase levels (ALT -28.3 ± 11.9 IU/L, P = 0.0001; AST -6.4 ± 56.3 IU/L, P = 0.01). (Table 1 and Figure 2).
Figure 2.
Evolution of the parameters body weight, waist circumference and level of transaminases during the study. ALT: Alanine transaminase.
At the end of 16 wk of the free wash-out diet (W16-W32), no significant variations were observed in anthropometric or laboratory parameters compared to results observed at the end of the Mediterranean diet period. At the end of the 16 wk of the low-fat diet (W32-W48), no significant variations were observed in any of the anthropometric or laboratory parameters analysed (Table 1 and Figure 2).
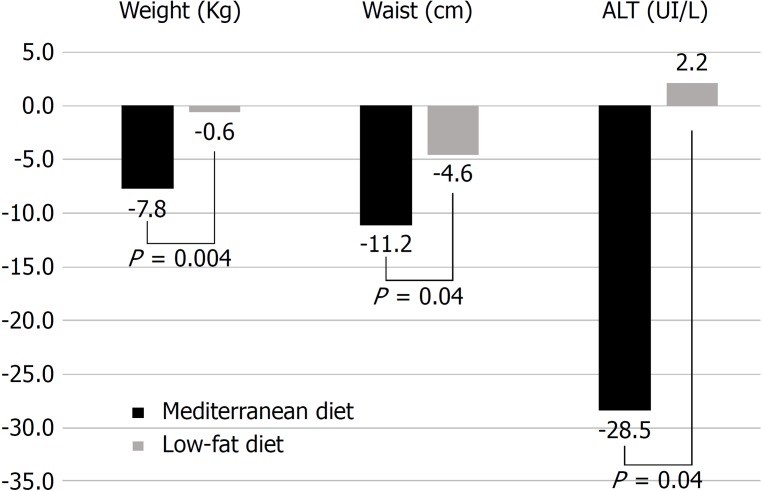
Analysing only patients who completed both diets (Figure 3), we observed that after 16-weeks of the Mediterranean diet, compared to 16-wk of the low-fat diet in the same patients, there was a significant reduction in mean body weight (-7.8 ± 2.3 kg vs 0.6 ± 2.4 kg, P = 0.004), mean waist circumference (-11.2 ± 2.6 cm vs 4.6 ± 7.6 cm, P = 0.04), and mean ALT levels (-28.5 ± 9.5 IU/L vs 2.2 ± 5.5 IU/L, P = 0.04).
Figure 3.
Comparison between variations obtained after 16 wk of mediterranean diet or low-fat diet. ALT: Alanine transaminase.
No adverse events related to either the Mediterranean diet or low-fat diet were recorded.
Intestinal permeability
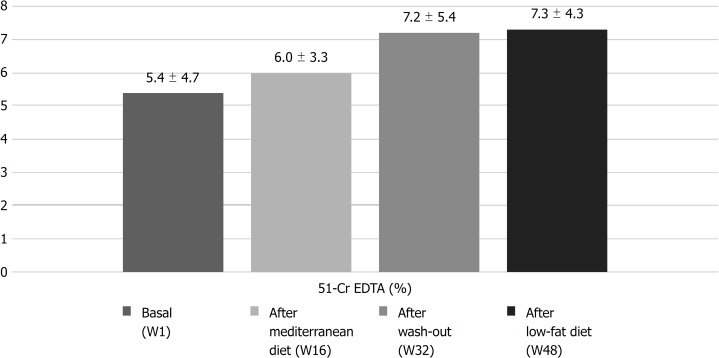
Seventy percent of patients had intestinal permeability alteration at baseline with a mean percentage retention of 51Cr-EDTA of 5.4% ± 4.7% (Figure 4). No significant changes in intestinal permeability were observed at the end of the Mediterranean diet period (6.0% ± 3.3%), after the wash-out period (7.2% ± 5.4%) or at the end of the low-fat diet period (7.3% ± 4.3%).
Figure 4.
Intestinal permeability. 51Cr-EDTA: Chromium-51 ethylene diamine tetraacetate excretion testing.
DISCUSSION
Our crossover study demonstrated a significant reduction in body weight, waist circumference and transaminase levels after 16 wk of Mediterranean diet and maintenance of these results following a low-fat diet period. The Mediterranean diet is a low-carbohydrate high-unsaturated fat nutritional model that appears to be an ideal diet for improving insulin sensitivity in NAFLD patients[32]. In a study conducted in Israel with 259 diabetic obese patients, a Mediterranean diet was superior to two types of low-fat diets for reducing serum transaminase levels at 6 and 12 mo[33]. A meta-analysis demonstrated that omega-3 fatty acids, found in the Mediterranean diet, were beneficial for reducing hepatic steatosis[34]. In a small pilot study conducted in Australia with 12 patients with NAFLD, 6 wk of ad libitum Mediterranean diet were more effective than a low-fat diet for improving insulin sensitivity and reducing the fat content in the liver, measured by magnetic resonance spectroscopy, even without a change in weight or serum transaminase levels[35]. A randomized trial of the Mediterranean diet vs a low-fat diet (MEDINA) is currently ongoing in Australia and New Zealand, with improvement of insulin sensitivity set as the primary end-point[36]. The Prevención con Dieta Mediterránea (PREDIMED) trial, conducted in Spain with over 7000 persons at high risk for cardiovascular disease, showed that a Mediterranean diet supplemented with extra-virgin olive oil or nuts reduced the incidence of major cardiovascular events over a control diet[37]. In a subsequent sub-analysis, it was observed that patients with lower adherence to the Mediterranean diet had higher values of serum transaminases[38]. In our study, we confirmed that 16 weeks of a Mediterranean diet was effective for improving serum transaminase levels in patients with NAFLD. Recently, a randomized 12-wk interventional study conducted in Australia with ad libitum isocaloric diets demonstrated that both a Mediterranean and a low-fat diet reduced hepatic steatosis and transaminases by a similar degree, but the Mediterranean diet also improved Framingham risk scores[39].
The choice of a crossover design for this study was based on our attempt to control confounding factors in the best possible way, as each subject served as their own control. However, it is very likely that there was an effect of the sequence of the diets, because the patients arrived at the end of a free wash-out diet (W32) with the same clinical characteristics that they had reached at the end of the Mediterranean diet (W16). In other words, despite a planned wash-out period, patients did not start the low-fat diet in the same condition in which they started the Mediterranean diet. We can therefore state that, in our study, 16 weeks of a low-fat diet was sufficient to maintain the benefits obtained after the Mediterranean diet period.
It is well-known that elevated concentrations of fructose favour pro-inflammatory microbiota, thereby producing endotoxins and suppressing production of short-chain fatty acids that are essential for intestinal barrier function[40]. Pro-inflammatory microbiota and their products recruit macrophages and bind to toll-like receptors, leading to the release of cytokines, such as tumour necrosis factor-α, causing mucosal inflammation[41]. Subsequently, inflammation decreases expression of tight junction proteins, resulting in higher permeability of the gut barrier[42]. Diet-induced increases in blood lipopolysaccharide levels are known as metabolic endotoxaemia and play an important role in the activation of Toll-like receptor-mediated low-grade liver inflammation, which are associated with NAFLD and NASH[43]. Current evidence from animal studies suggests that a high-fat diet or a high-fructose diet can induce metabolic endotoxaemia by altering the intestinal tight junction proteins, mainly zonula occludens-1 and occludin[44-47]. Our group has provided evidence that increased intestinal permeability in NAFLD patients is caused by disruption of intestinal tight junctions, as documented by decreased expression of zonula occludens 1 in the intestinal mucosa[48]. In NAFLD adolescents, postprandial endotoxin levels were increased compared to healthy subjects in response to fructose, but not glucose, beverages (consumed with meals) in a 24-hour feeding challenge[49]. On the other hand, there are currently no data concerning diet modulation of intestinal permeability in patients with NAFLD, and it is thought that a healthy diet can reduce intestinal permeability in patients with NAFLD by restoring the integrity of tight junctions. The Mediterranean diet contains a high intake of mono- and polyunsaturated fatty acids, fibres, polyphenols, antioxidants and phytochemicals; many of these components promote short-chain fatty acid-producing gut bacteria and have significant prebiotic effects[50]. As such, we hypothesized a therapeutic effect of the Mediterranean diet for reducing impaired intestinal permeability in patients with NAFLD. Under conditions of enhanced intestinal permeability, an increased fraction of orally administered 51Cr-EDTA crosses the intestinal epithelium along the paracellular pathway, enters the blood stream and is rapidly cleared through glomerular filtration[27]. Arslan et al[51] found that 51Cr-EDTA excretion is a good indicator of the severity of gut-mucosal inflammation and loss of tight junctions. In our study, the majority of patients presented at baseline, as expected, with high intestinal permeability evaluated according to 51Cr-EDTA, but neither 16 wk of a Mediterranean diet nor 16 wk of a low-fat diet were sufficient to modulate it.
In our view, the modulation of intestinal permeability in humans is much more difficult to obtain than in animal models. For example, probiotics cannot modify migraine-associated intestinal permeability changes[52] and a recent Finnish study demonstrated that nor probiotics nor long chain polyunsaturated fatty acid supplements can counteract intestinal permeability changes in pregnancy[53]. On the other hand, a potential improvement of intestinal permeability (assessed by an indirect test) induced by higher dietary fiber intake was observed after a longer dietary intervention[54]. We ask ourselves whether our result could be modified with a different duration of the observation and of the diet program.
A limitation of our study was the difficulty with recruitment because many patients refused to enrol for personal reasons. This limitation reflects the difficulty that patients experience adhering to a lifestyle intervention, which is far more compelling than taking medication. Larger longer-term studies are needed to confirm the durability of the benefits of a Mediterranean diet.
In conclusion, the Mediterranean diet is a safe and effective strategy for treating overweight, visceral obesity and serum transaminase in patients with NAFLD, thereby confirming that it should be the first step in a treatment program for patient with NAFLD. If the Mediterranean diet can improve intestinal permeability in patients with NAFLD, it deserves further investigation.
ARTICLE HIGHLIGHTS
Research background
In patients with non-alcoholic fatty liver disease (NAFLD), intestinal permeability is increased, promoting translocation of bacteria-derived products into the portal circulation and increasing hepatic exposure to injurious substances that stimulate hepatic inflammation and fibrosis. In animal models, adaptation of a high-fat diet or high-fructose intake has been associated with increased gut permeability and metabolic endotoxiemia
Research motivation
Although diet can significantly influence intestinal permeability, clinical studies investigating the effects of dietary interventions on the intestinal permeability of NAFLD patients are lacking. The Mediterranean diet contains a high intake of mono- and polyunsaturated fatty acids, fibres, polyphenols, antioxidants and phytochemicals; many of these components promote short-chain fatty acid-producing gut bacteria and have significant prebiotic effects. As such, we hypothesized a therapeutic effect of the Mediterranean diet for reducing impaired intestinal permeability in patients with NAFLD.
Research objectives
Aim of the study is to detect diet-induced modification of intestinal permeability in patients with NAFLD undergoing a Mediterranean diet or a low-fat diet.
Research methods
Patients underwent a dietary treatment via a crossover design lasting 48 wk: 16-wk of a Mediterranean diet, 16-wk of a free wash-out diet and 16-wk of a low-fat diet. Intestinal permeability was evaluated at baseline and at the end of each diet period using the validated method of urinary excretion of chromium-51 ethylene diamine tetraacetate excretion testing (51Cr-EDTA). 51Cr-EDTA excretion is a good indicator of the severity of gut-mucosal inflammation and loss of tight junctions.
Research results
Patients with NAFLD experienced a significant reduction in body weight, waist circumference and transaminase levels after 16 wk of a Mediterranean diet. Seventy percent of patients had intestinal permeability alteration at baseline, but no significant changes in intestinal permeability were observed at the end of the Mediterranean diet period or at the end of the low-fat diet period.
Research conclusions
The Mediterranean diet is a safe and effective strategy for treating overweight, visceral obesity and serum transaminase in patients with NAFLD, thereby confirming that it should be the first step in a treatment program. If the Mediterranean diet can improve intestinal permeability in patients with NAFLD, it deserves further investigation.
Research perspectives
In our view, the modulation of intestinal permeability in humans is much more difficult to obtain than in animal models. We ask ourselves whether our result could be modified with a different duration of the observation and of the diet program.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was reviewed and approved by the Ethics Committee of Catholic University of Sacred Heart (Rome, Italy).
Informed consent statement: All study participants provided informed written consent prior to study enrollment.
Conflict-of-interest statement: There are no conflicts of interest to report.
STROBE statement: The authors have checked the manuscript according to STROBE checklist.
Peer-review started: November 5, 2018
First decision: December 20, 2018
Article in press: January 18, 2019
P- Reviewer: Gong ZJ, Seta WK S- Editor: Ma RY L- Editor: A E- Editor: Yin SY
Contributor Information
Marco Biolato, Department of Gastroenterological, Endocrine-Metabolic and Nefro-Urological Sciences, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome 00168, Italy. marco.biolato@policlinicogemelli.it.
Fiorella Manca, Institute of Special Medical Pathology and Medical Semeiotics, Università Cattolica del Sacro Cuore, Rome 00168, Italy.
Giuseppe Marrone, Department of Gastroenterological, Endocrine-Metabolic and Nefro-Urological Sciences, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome 00168, Italy.
Consuelo Cefalo, Institute of Internal Medicine and Geriatrics, Università Cattolica del Sacro Cuore, Rome 00168, Italy.
Simona Racco, Department of Gastroenterological, Endocrine-Metabolic and Nefro-Urological Sciences, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome 00168, Italy.
Giacinto A Miggiano, Department of Gastroenterological, Endocrine-Metabolic and Nefro-Urological Sciences, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome 00168, Italy; Institute of Special Medical Pathology and Medical Semeiotics, Università Cattolica del Sacro Cuore, Rome 00168, Italy.
Venanzio Valenza, Department of Image Diagnostics, Oncological Radiotherapy and Hematology Sciences, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome 00168, Italy; Nuclear Medicine Institute, Università Cattolica del Sacro Cuore, Rome 00168, Italy.
Antonio Gasbarrini, Department of Gastroenterological, Endocrine-Metabolic and Nefro-Urological Sciences, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome 00168, Italy; Institute of Special Medical Pathology and Medical Semeiotics, Università Cattolica del Sacro Cuore, Rome 00168, Italy.
Luca Miele, Department of Gastroenterological, Endocrine-Metabolic and Nefro-Urological Sciences, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome 00168, Italy; Institute of Special Medical Pathology and Medical Semeiotics, Università Cattolica del Sacro Cuore, Rome 00168, Italy.
Antonio Grieco, Department of Gastroenterological, Endocrine-Metabolic and Nefro-Urological Sciences, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome 00168, Italy; Institute of Internal Medicine and Geriatrics, Università Cattolica del Sacro Cuore, Rome 00168, Italy.
References
- 1.Mouzaki M, Allard JP. The role of nutrients in the development, progression, and treatment of nonalcoholic fatty liver disease. J Clin Gastroenterol. 2012;46:457–467. doi: 10.1097/MCG.0b013e31824cf51e. [DOI] [PubMed] [Google Scholar]
- 2.Zelber-Sagi S, Ivancovsky-Wajcman D, Fliss Isakov N, Webb M, Orenstein D, Shibolet O, Kariv R. High red and processed meat consumption is associated with non-alcoholic fatty liver disease and insulin resistance. J Hepatol. 2018;68:1239–1246. doi: 10.1016/j.jhep.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 3.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 4.George ES, Forsyth A, Itsiopoulos C, Nicoll AJ, Ryan M, Sood S, Roberts SK, Tierney AC. Practical Dietary Recommendations for the Prevention and Management of Nonalcoholic Fatty Liver Disease in Adults. Adv Nutr. 2018;9:30–40. doi: 10.1093/advances/nmx007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, Fava JL, Wing RR. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121–129. doi: 10.1002/hep.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, Friedman SL, Diago M, Romero-Gomez M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology. 2015;149:367–78.e5; quiz e14-5. doi: 10.1053/j.gastro.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Thoma C, Day CP, Trenell MI. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: a systematic review. J Hepatol. 2012;56:255–266. doi: 10.1016/j.jhep.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Boden G. High- or low-carbohydrate diets: which is better for weight loss, insulin resistance, and fatty livers? Gastroenterology. 2009;136:1490–1492. doi: 10.1053/j.gastro.2009.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nordmann AJ, Nordmann A, Briel M, Keller U, Yancy WS, Jr, Brehm BJ, Bucher HC. Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: a meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166:285–293. doi: 10.1001/archinte.166.3.285. [DOI] [PubMed] [Google Scholar]
- 10.Kirk E, Reeds DN, Finck BN, Mayurranjan SM, Patterson BW, Klein S. Dietary fat and carbohydrates differentially alter insulin sensitivity during caloric restriction. Gastroenterology. 2009;136:1552–1560. doi: 10.1053/j.gastro.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLaughlin T, Carter S, Lamendola C, Abbasi F, Yee G, Schaaf P, Basina M, Reaven G. Effects of moderate variations in macronutrient composition on weight loss and reduction in cardiovascular disease risk in obese, insulin-resistant adults. Am J Clin Nutr. 2006;84:813–821. doi: 10.1093/ajcn/84.4.813. [DOI] [PubMed] [Google Scholar]
- 12.Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, Szapary PO, Rader DJ, Edman JS, Klein S. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;348:2082–2090. doi: 10.1056/NEJMoa022207. [DOI] [PubMed] [Google Scholar]
- 13.Ryan MC, Abbasi F, Lamendola C, Carter S, McLaughlin TL. Serum alanine aminotransferase levels decrease further with carbohydrate than fat restriction in insulin-resistant adults. Diabetes Care. 2007;30:1075–1080. doi: 10.2337/dc06-2169. [DOI] [PubMed] [Google Scholar]
- 14.Keys A, Aravanis C, Blackburn HW, Van Buchem FS, Buzina R, Djordjević BD, Dontas AS, Fidanza F, Karvonen MJ, Kimura N, Lekos D, Monti M, Puddu V, Taylor HL. Epidemiological studies related to coronary heart disease: characteristics of men aged 40-59 in seven countries. Acta Med Scand Suppl. 1966;460:1–392. [PubMed] [Google Scholar]
- 15.Hu FB. The Mediterranean diet and mortality--olive oil and beyond. N Engl J Med. 2003;348:2595–2596. doi: 10.1056/NEJMp030069. [DOI] [PubMed] [Google Scholar]
- 16.European Association for the Study of the Liver; European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO) EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, Serino M, Tilg H, Watson A, Wells JM. Intestinal permeability--a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189. doi: 10.1186/s12876-014-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leung C, Rivera L, Furness JB, Angus PW. The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol Hepatol. 2016;13:412–425. doi: 10.1038/nrgastro.2016.85. [DOI] [PubMed] [Google Scholar]
- 19.Serino M, Luche E, Gres S, Baylac A, Bergé M, Cenac C, Waget A, Klopp P, Iacovoni J, Klopp C, Mariette J, Bouchez O, Lluch J, Ouarné F, Monsan P, Valet P, Roques C, Amar J, Bouloumié A, Théodorou V, Burcelin R. Metabolic adaptation to a high-fat diet is associated with a change in the gut microbiota. Gut. 2012;61:543–553. doi: 10.1136/gutjnl-2011-301012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spruss A, Bergheim I. Dietary fructose and intestinal barrier: potential risk factor in the pathogenesis of nonalcoholic fatty liver disease. J Nutr Biochem. 2009;20:657–662. doi: 10.1016/j.jnutbio.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Spruss A, Kanuri G, Wagnerberger S, Haub S, Bischoff SC, Bergheim I. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology. 2009;50:1094–1104. doi: 10.1002/hep.23122. [DOI] [PubMed] [Google Scholar]
- 22.Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, Del Vecchio E, Vianello L, Zanuso F, Mozzi F, Milani S, Conte D, Colombo M, Sirchia G. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1–10. doi: 10.7326/0003-4819-137-1-200207020-00006. [DOI] [PubMed] [Google Scholar]
- 23.Buzzard IM. 24-hour dietary recall and food records methods. In: Willett WC, editor. Nutritional Epidemiology. NY: Oxford University Press; 2002. pp. 50–73. [Google Scholar]
- 24.Cade JE, Burley VJ, Warm DL, Thompson RL, Margetts BM. Food-frequency questionnaires: a review of their design, validation and utilisation. Nutr Res Rev. 2004;17:5–22. doi: 10.1079/NRR200370. [DOI] [PubMed] [Google Scholar]
- 25.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348:2599–2608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- 26.Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 27.Johnston SD, Smye M, Watson RP. Intestinal permeability tests in coeliac disease. Clin Lab. 2001;47:143–150. [PubMed] [Google Scholar]
- 28.Rossi M, Sganga G, Mazzone M, Valenza V, Guarneri S, Portale G, Carbone L, Gatta L, Pioli C, Sanguinetti M, Montalto M, Glieca F, Fadda G, Schiavello R, Silveri NG. Cardiopulmonary bypass in man: role of the intestine in a self-limiting inflammatory response with demonstrable bacterial translocation. Ann Thorac Surg. 2004;77:612–618. doi: 10.1016/S0003-4975(03)01520-0. [DOI] [PubMed] [Google Scholar]
- 29.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 30.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 31.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 32.Zelber-Sagi S, Salomone F, Mlynarsky L. The Mediterranean dietary pattern as the diet of choice for non-alcoholic fatty liver disease: Evidence and plausible mechanisms. Liver Int. 2017;37:936–949. doi: 10.1111/liv.13435. [DOI] [PubMed] [Google Scholar]
- 33.Fraser A, Abel R, Lawlor DA, Fraser D, Elhayany A. A modified Mediterranean diet is associated with the greatest reduction in alanine aminotransferase levels in obese type 2 diabetes patients: results of a quasi-randomised controlled trial. Diabetologia. 2008;51:1616–1622. doi: 10.1007/s00125-008-1049-1. [DOI] [PubMed] [Google Scholar]
- 34.Parker HM, Johnson NA, Burdon CA, Cohn JS, O'Connor HT, George J. Omega-3 supplementation and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;56:944–951. doi: 10.1016/j.jhep.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 35.Ryan MC, Itsiopoulos C, Thodis T, Ward G, Trost N, Hofferberth S, O'Dea K, Desmond PV, Johnson NA, Wilson AM. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol. 2013;59:138–143. doi: 10.1016/j.jhep.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 36.Papamiltiadous ES, Roberts SK, Nicoll AJ, Ryan MC, Itsiopoulos C, Salim A, Tierney AC. A randomised controlled trial of a Mediterranean Dietary Intervention for Adults with Non Alcoholic Fatty Liver Disease (MEDINA): study protocol. BMC Gastroenterol. 2016;16:14. doi: 10.1186/s12876-016-0426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J, Lamuela-Raventos RM, Serra-Majem L, Pintó X, Basora J, Muñoz MA, Sorlí JV, Martínez JA, Fitó M, Gea A, Hernán MA, Martínez-González MA PREDIMED Study Investigators. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N Engl J Med. 2018;378:e34. doi: 10.1056/NEJMoa1800389. [DOI] [PubMed] [Google Scholar]
- 38.Cantero I, Abete I, Babio N, Arós F, Corella D, Estruch R, Fitó M, Hebert JR, Martínez-González MÁ, Pintó X, Portillo MP, Ruiz-Canela M, Shivappa N, Wärnberg J, Gómez-Gracia E, Tur JA, Salas-Salvadó J, Zulet MA, Martínez JA. Dietary Inflammatory Index and liver status in subjects with different adiposity levels within the PREDIMED trial. Clin Nutr. 2018;37:1736–1743. doi: 10.1016/j.clnu.2017.06.027. [DOI] [PubMed] [Google Scholar]
- 39.Properzi C, O'Sullivan TA, Sherriff JL, Ching HL, Jeffrey GP, Buckley RF, Tibballs J, MacQuillan GC, Garas G, Adams LA. Ad Libitum Mediterranean and Low-Fat Diets Both Significantly Reduce Hepatic Steatosis: A Randomized Controlled Trial. Hepatology. 2018;68:1741–1754. doi: 10.1002/hep.30076. [DOI] [PubMed] [Google Scholar]
- 40.Lambertz J, Weiskirchen S, Landert S, Weiskirchen R. Fructose: A Dietary Sugar in Crosstalk with Microbiota Contributing to the Development and Progression of Non-Alcoholic Liver Disease. Front Immunol. 2017;8:1159. doi: 10.3389/fimmu.2017.01159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ray K. NAFLD. Leaky guts: intestinal permeability and NASH. Nat Rev Gastroenterol Hepatol. 2015;12:123. doi: 10.1038/nrgastro.2015.15. [DOI] [PubMed] [Google Scholar]
- 42.Miele L, Marrone G, Lauritano C, Cefalo C, Gasbarrini A, Day C, Grieco A. Gut-liver axis and microbiota in NAFLD: insight pathophysiology for novel therapeutic target. Curr Pharm Des. 2013;19:5314–5324. [PubMed] [Google Scholar]
- 43.Kirpich IA, Marsano LS, McClain CJ. Gut-liver axis, nutrition, and non-alcoholic fatty liver disease. Clin Biochem. 2015;48:923–930. doi: 10.1016/j.clinbiochem.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 45.Brun P, Castagliuolo I, Di Leo V, Buda A, Pinzani M, Palù G, Martines D. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G518–G525. doi: 10.1152/ajpgi.00024.2006. [DOI] [PubMed] [Google Scholar]
- 46.Ding S, Chi MM, Scull BP, Rigby R, Schwerbrock NM, Magness S, Jobin C, Lund PK. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS One. 2010;5:e12191. doi: 10.1371/journal.pone.0012191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kavanagh K, Wylie AT, Tucker KL, Hamp TJ, Gharaibeh RZ, Fodor AA, Cullen JM. Dietary fructose induces endotoxemia and hepatic injury in calorically controlled primates. Am J Clin Nutr. 2013;98:349–357. doi: 10.3945/ajcn.112.057331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, Mascianà R, Forgione A, Gabrieli ML, Perotti G, Vecchio FM, Rapaccini G, Gasbarrini G, Day CP, Grieco A. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 49.Jin R, Willment A, Patel SS, Sun X, Song M, Mannery YO, Kosters A, McClain CJ, Vos MB. Fructose induced endotoxemia in pediatric nonalcoholic Fatty liver disease. Int J Hepatol. 2014;2014:560620. doi: 10.1155/2014/560620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bifulco M. Mediterranean diet: the missing link between gut microbiota and inflammatory diseases. Eur J Clin Nutr. 2015;69:1078. doi: 10.1038/ejcn.2015.81. [DOI] [PubMed] [Google Scholar]
- 51.Arslan G, Atasever T, Cindoruk M, Yildirim IS. (51)CrEDTA colonic permeability and therapy response in patients with ulcerative colitis. Nucl Med Commun. 2001;22:997–1001. doi: 10.1097/00006231-200109000-00009. [DOI] [PubMed] [Google Scholar]
- 52.de Roos NM, van Hemert S, Rovers JMP, Smits MG, Witteman BJM. The effects of a multispecies probiotic on migraine and markers of intestinal permeability-results of a randomized placebo-controlled study. Eur J Clin Nutr. 2017;71:1455–1462. doi: 10.1038/ejcn.2017.57. [DOI] [PubMed] [Google Scholar]
- 53.Mokkala K, Pussinen P, Houttu N, Koivuniemi E, Vahlberg T, Laitinen K. The impact of probiotics and n-3 long-chain polyunsaturated fatty acids on intestinal permeability in pregnancy: a randomised clinical trial. Benef Microbes. 2018;9:199–208. doi: 10.3920/BM2017.0072. [DOI] [PubMed] [Google Scholar]
- 54.Krawczyk M, Maciejewska D, Ryterska K, Czerwińka-Rogowska M, Jamioł-Milc D, Skonieczna-Żydecka K, Milkiewicz P, Raszeja-Wyszomirska J, Stachowska E. Gut Permeability Might be Improved by Dietary Fiber in Individuals with Nonalcoholic Fatty Liver Disease (NAFLD) Undergoing Weight Reduction. Nutrients. 2018;10 doi: 10.3390/nu10111793. [DOI] [PMC free article] [PubMed] [Google Scholar]