Abstract
Effective therapies are limited for pancreatic cancer, particularly for those with distant tumour metastases. Therefore, more individualised drug screening is urgently required. Next-generation sequencing (NGS) is a powerful tool to investigate the genomic landscape of patients and the mechanism of drug response, which may provide a broader vision for potential clinical drug screening. Patient-derived xenograft (PDX) models may have a significant advantage in predicting clinical treatment response. In our previous study, a PDX of pancreatic cancer bone metastasis was established, and NGS was conducted to investigate the molecular information. In the present study, these data were further analysed and fibroblast growth factor receptor 1 (FGFR1) amplification was identified in a panel of 416 cancer-associated genes. Thus, AZD4547, an inhibitor against FGFR, was selected as a potential therapy, and was evaluated using the PDX model. AZD4547 was shown to exhibit antitumor activity by reducing the expression of FGFR1 and its targets. The present study also demonstrated the high potential of the novel NGS/PDX-based drug screening platform to improve individualised cancer treatment.
Keywords: next-generation sequencing, patient-derived xenograft model, pancreatic cancer with bone metastasis, fibroblast growth factor receptor
Introduction
The efficacy of traditional chemoradiotherapies remains limited for pancreatic cancer (1–3), which is expected to be the second most lethal malignancy in the USA by 2020 (4,5). Distant tumour metastases, frequently in the liver or peritoneum and rarely in the bone, indicate a poor prognosis (6,7). Thus, effective targeted therapies are urgently warranted. Therefore, individualised drug screening is urgently required for the clinical treatment of pancreatic cancer patients, particularly those in advanced or metastatic disease stages (8–11).
Accumulating evidence indicates that patient-derived xenografts (PDXs) are reliable cancer research tools for personalised drug screening, and they have been increasingly used in various types of translational cancer research in recent years (12). These so-called Avatar models mimic the morphological and molecular characteristics of the tumour and predict clinical treatment response (13), as they are formed when tissue from a patient's tumour is grafted onto a mouse or other animal (14).
Adequate understanding of the genomic landscape of pancreatic cancer can be beneficial for drug screening (10,15–17), and the precise molecular profile of the tumour assists in predicting drug responses (18). Next-generation sequencing (NGS) is a powerful tool to investigate the genomic landscape of patient tumours and the mechanism of drug response, which may provide a broader vision for potential clinical drug screening (19–21). Therefore, NGS technologies are being used by pharmaceutical companies throughout the drug discovery process (22).
In our previous study, a PDX model was established from pancreatic cancer bone metastasis tumour tissue, and a 416-gene exon panel was sequenced to investigate the molecular characteristics of the tumour (23). In the present study, the NGS high-throughput information was further analysed to search for individualised therapy targets for pancreatic cancer patients with bone metastasis. Based on the sequencing results and associated literature, AZD4547, a potent inhibitor of fibroblast growth factor receptor (FGFR), was selected for evaluation in the pancreatic cancer PDX model and was examined as a potential therapy (24).
Materials and methods
Reagents and drugs
AZD4547 (cat. no. S2801) and capecitabine (cat. no. S1156) were purchased from Selleck Chemicals (Shanghai, China). The antibodies against FGFR1 (cat. no. ab63601), phosphorylated protein kinase B (p-Akt; phospho S473, cat. no. ab227748) and Ki-67 (OTI5D7; cat. no. ab156956) were purchased from Abcam (Shanghai, China).
Establishment of a PDX model and NGS
Pancreatic cancer bone metastasis (diagnosed as adenocarcinoma) tissues were used to establish a PDX model subsequent to being obtained at surgery from a 67-year-old female patient. Written consent was provided by the patient and ethical approval was obtained from the Ethics Committee of The First Affiliated Hospital, Zhejiang University School of Medicine (Hangzhou, China). In total, 50 (range, 4–6 weeks) female BALB/c nude mice (12–16 g) were purchased from Shanghai Laboratory Animal Center (Shanghai, China) for establishing PDX models. The mice were kept at 26–28°C, with 40–60% humidity at a 10 h/14 h light/dark cycle. Mice were kept in a SPF environment. Tumour tissues were harvested from PDX models for NGS investigation in a 416-gene exon panel, as conducted by Geneseeq Technology, Inc (Nanjing, China). The protocol for establishment of the PDX model and NGS was as previously described (23).
Treatment protocol
From the 3rd mouse generation, PDX tumours were permitted to grow to a volume of 150–200 mm3, and then mice were randomised (6 mice with tumors were set per group and housed in per rearing cage). AZD4547 and capecitabine were administered daily for 4 weeks at the following doses: 50 mg/kg oral AZD4547, 1.0 mM/kg oral capecitabine (1 ml saline administered orally for control group). Mice were weighed for signs of toxicity and tumour size was evaluated once per week. Animals were monitored periodically for their weight with an electronic balance and tumour growth was measured with a Vernier caliper once per week. Tumour volume was calculated according to the formula V=LD × (SD)2/2, where V represents the tumour volume, and LD and SD are the longest and shortest tumour diameters, respectively. Relative tumour growth inhibition (TGI) (%) was calculated using the formula (1-T/C), where T represents the relative tumour volume of the treated mice and C represents the relative tumour volume of the control mice. Euthanasia was conducted on the mice prior to the single tumour volume reaching 1,500 mm3. The usage of experimental animals was according to the Principles of Laboratory Animal Care (NIH no. 85-23, 1985 version). All animal studies were according to the Institutional Animal Care and Use Committee of Zhejiang University and the approval ID was SYXK(ZHE)2005-0072.
Fluorescence immunohistochemistry
Mice with similar tumour sizes were anaesthetised with chloral hydrate (4%) at 300 mg/kg by intraperitoneal injection. The vasculature was perfused with 4% paraformaldehyde in 0.1 mol/l PBS by inserting an 18-gauge cannula into the left ventricle aorta. Next, the xenograft tumour was removed and stored in 4% paraformaldehyde in 0.1 mol/l PBS for 2 h at 4°C. Subsequent to a rinse in PBS, tumour tissues were incubated in 30% sucrose overnight at 4°C and frozen with liquid nitrogen for 1 min for cryostat sectioning (8–10 µm) after being embedded in optimal cutting temperature compound. Cryostat sections were fixed in acetone for ~10 min. The slides were allowed to air dry for 30 min and were washed 3 times for 5 min each in PBS. Samples were subsequently incubated in 5% bovine serum albumin (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) in PBS for 30 min at room temperature to block non-specific antibody binding. Following blocking with a non-specific antibody, the slides were incubated in two primary antibodies [FGFR1 (dilution, 1:100) and p-Akt (dilution, 1:25)] overnight at room temperature. The slides were then incubated for 1 h at 37°C with fluorescent (Cy3- or FITC-conjuncted) secondary antibodies (goat anti-rat; dilution, 1:50; cat. no. E670005; Sangon Biotech Co., Ltd., Shanghai, China). All slides were counterstained with DAPI (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at room temperature for 20 min. Tissue sections were imaged using an Olympus BX51 fluorescence microscope (magnification, ×200; Olympus Corporation, Tokyo, Japan).
Immunohistochemistry
Tumour specimens were fixed in 10% neutral formalin at 4°C for 6 h, then embedded in paraffin, sectioned (5-µm thick) and placed on slides for marker analysis. Sections were incubated with the primary antibody (Ki-67, dilution, 1:150) overnight at 4°C, after blocking for non-specific antibody (goat anti-rat, 1:50, Shanghai cat. no., C516337; Sangon Biotech Co., Ltd.) binding at 4°C overnight. The streptavidin-biotin-peroxidase complex method (Lab Vision, Fremont, CA) was used for immunohistochemistry (25). Images of the slides were captured using an Olympus BX60 (Olympus Corporation).
Statistical analysis
The results are presented as the mean ± standard deviation. Calculations and statistics were performed with Excel 2010 (Microsoft Corporation, Redmond, WA, USA) and GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA). One-way analysis of variance (ANOVA) was used to analyse the significance of differences among groups. Bonferroni's correction was the post hoc test used following one-way ANOVA. P<0.05 was considered to indicate a statistically significant difference.
Results
NGS of pancreatic cancer bone metastasis in a PDX model highlights FGFR1 as a potential therapeutic target
Based on a 416-gene exon NGS panel, our previous study focused on gene polymorphisms/mutations, while the present study focused on gene amplification (Table I). FGFR1, MYC, PIK3CA, RECQL4 and SOX2 genes were found to be amplified, among which FGFR1 was amplified with the most significant fold-change (3.1-fold), while the fold change for the remaining were the following: RECQL4 3.0, SOX2 2.7, MYC 2.3, and PIK3CA 2.2. Therefore, AZD4547, a potent inhibitor of FGFR, was selected as a potential therapy to be evaluated in our PDX model.
Table I.
Gene amplification identified by next-generation sequencing.
| Gene | Fold-change |
|---|---|
| FGFR1 | 3.1 |
| MYC | 2.3 |
| PIK3CA | 2.2 |
| RECQL4 | 3.0 |
| SOX2 | 2.7 |
FGFR1, fibroblast growth factor receptor 1; MYC, MYC proto-oncogene; PIK3CA, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit α; RECQL4, RecQ-like helicase 4; SOX2, SRY-box 2.
Growth of pancreatic cancer bone metastasis in a PDX model is lower in AZD4547-treated mice
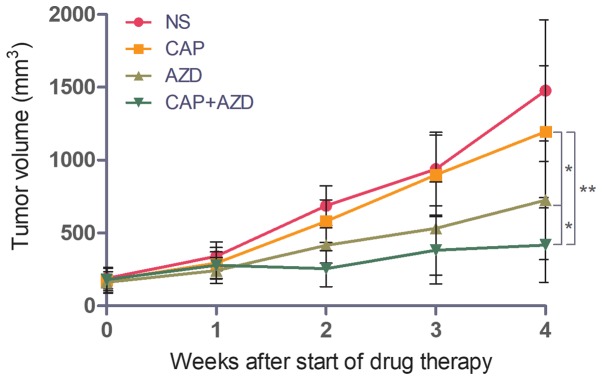
To test whether the PDX model of pancreatic cancer bone metastasis was sensitive to FGFR inhibition, the ability of AZD4547 to inhibit tumour growth was evaluated. Capecitabine, a chemotherapy drug, was used as a positive control. When the tumour volume reached 150–200 mm3, saline, AZD4547 (50 mg/kg), capecitabine (1.0 mM/kg) or the two drugs together was administered orally once per day for 28 days. The mice were sacrificed and excised tumours were measured. Mice treated with AZD4547 alone exhibited increased tumour growth inhibition (TGI, 43.1%) compared with those treated with capecitabine alone (TGI, 12.9%), while the combination of the two demonstrated a synergistic effect, with a TGI of 70.5% (Fig. 1).
Figure 1.
AZD4547 inhibits tumour growth in a patient-derived xenograft model of pancreatic cancer bone metastasis. *P<0.05 and **P<0.01. Error bars indicate the standard deviation. NS, saline; CAP, capecitabine; AZD, AZD4547.
Expression of FGFR1 and downstream targets is reduced in pancreatic cancer bone metastasis in a PDX model in AZD4547-treated mice
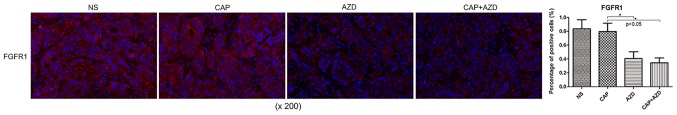
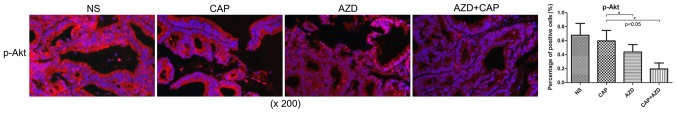
Prior to drug treatment, PDX tumour tissue was identified by fluorescence immunohistochemistry to exhibit high expression of FGFR1, which was significantly suppressed following AZD4547 treatment (Fig. 2). AKT pathway has been reported to be regulated by upstream FGFR1 (26). Using immunohistochemistry, it was found that Ki-67, a cell proliferation marker, and p-Akt were significantly reduced in the AZD4547-treated groups (Figs. 3 and 4). Therefore, AZD4547 may be effective at reducing tumour growth in this pancreatic cancer PDX model by inhibiting the function of FGFR1.
Figure 2.
Fluorescence immunohistochemical expression of FGFR1 in a patient-derived xenograft model of pancreatic cancer bone metastasis following AZD4547 treatment. *P<0.05. Error bars indicate the standard deviation. FGFR1, phosphorylated fibroblast growth factor receptor 1; NS, saline; CAP, capecitabine; AZD, AZD4547.
Figure 3.
Immunohistochemical expression of Ki-67 in a patient-derived xenograft model of pancreatic cancer bone metastasis following AZD4547 treatment. *P<0.05. Error bars indicate the standard deviation. Ki-67, marker of proliferation; NS, saline; CAP, capecitabine; AZD, AZD4547.
Figure 4.
Fluorescence immunohistochemical expression of p-Akt in a patient-derived xenograft model of pancreatic cancer bone metastasis following AZD4547 treatment. *P<0.05. Error bars indicate the standard deviation. p-Akt, phosphorylated protein kinase B; NS, saline; CAP, capecitabine; AZD, AZD4547.
Discussion
Multiple clinical studies have shown that NGS and PDX may replace or compliment personalised medicine in identifying novel therapeutic targets and biomarkers (27). Our previous study described a PDX model of pancreatic cancer bone metastasis which was confirmed presenting with clinical patients stable tumor characteristics (23). Based on these previous sequencing results, the FGFR1 gene was found to be amplified by 3.1-fold compared with RECQL4, SOX2, MYC, and PIK3CA. The FGFR family of receptor tyrosine kinases have been implicated in tumour progression and metastasis in human pancreatic cancer (28). In the present study, four other genes, including RECQL,4 were also revealed to be amplified; however, inhibitors for these proteins were not readily available. Therefore, AZD4547, a novel selective small-molecule inhibitor of FGFR (29), was selected as a potential therapy to be evaluated in the PDX model.
Several clinical trials of AZD4547 in the treatment of bladder cancer, gliomas, myeloma and lung cancer have been recently registered in clinicaltrials.gov, including NCT02546661, NCT02824133, NCT02465060, NCT02664935, NCT02965378 and NCT02154490 (updated to December 12, 2017). However, to the best of our knowledge, there are no studies evaluating the effect of AZD4547 in pancreatic cancer at a preclinical or clinical stage. In the present study, the antitumour efficacy of AZD4547 in a bone metastatic pancreatic cancer was demonstrated.
In the present study, the PDX model of pancreatic cancer bone metastasis was confirmed to exhibit high expression of FGFR1 prior to drug evaluation. It was demonstrated that AZD4547 exhibited higher efficacy in reducing growth than capecitabine, a chemotherapy drug. The combination of the two exhibited a significant synergistic effect, with a TGI of 70.5%. Furthermore, it was found that AZD4547 inhibited tumour cell proliferation and reduced the expression of FGFR1 targets, such as p-Akt (29). As amplification of FGFR1 has been identified in 2.6% of pancreatic ductal adenocarcinoma patients (30), AZD4547 may provide targeted treatment in this subpopulation of pancreatic cancer patients. However, as western blot analysis of total and FGFR1 and Akt in PDX tumour tissues was not performed in the present study, further investigation is required to confirm the mechanism of action of AZD4547 and to clarify whether resistance to the drug develops over time. Future studies will focus on monitoring the changes of associated signalling pathways during drug resistance in order to find targets for reversing drug resistance.
In conclusion, in the present study, AZD4547, a FGFR inhibitor, was revealed to supress proliferation and reduce expression of FGFR1 targets in an FGFR1-amplified pancreatic cancer PDX model. This inhibitor may prove to be an effective treatment in patients with FGFR1-amplified pancreatic cancer. In addition, it was successfully demonstrated that PDX-NGS-based drug screening is a novel, promising tool for individualised drug screening to improve the clinical treatment of pancreatic cancer patients.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural Science Foundation of China (grant nos. 81772537 and 81374014), the Zhejiang Provincial Science and Technology Projects of Traditional Chinese Medicine (grant nos. 2017ZB089 and 2016ZA128) and the Zhejiang Provincial Science and Technology Projects (grant nos. LGF18H160041, 2017C33212, 2017C33213 and 2015C33264).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
KJ and XW contributed to the design of the study. ZG contributed to main experiments and manuscript preparation. DS contributed to literature research, revision of manuscript, language retouching, and data collection. HL contributed to the data collection and processing.
Ethics approval and consent to participate
Written consent was provided by the patient and ethical approval was obtained from the Ethics Committee of The First Affiliated Hospital, Zhejiang University School of Medicine (Hangzhou, China). All animal studies were according to the Institutional Animal Care and Use Committee of Zhejiang University (approval no., SYXK(ZHE)2005-0072).
Patient consent for publication
The patient involved in this study confirmed that all medical records could be used for medical research and for publication in any formal printed or online open accessed journals.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Mattie M, Christensen A, Chang MS, Yeh W, Said S, Shostak Y, Capo L, Verlinsky A, An Z, Joseph I, et al. Molecular characterization of patient-derived human pancreatic tumor xenograft models for preclinical and translational development of cancer therapeutic. Neoplasia. 2013;15:1138–1150. doi: 10.1593/neo.13922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sohal DP, Mangu PB, Khorana AA, Shah MA, Philip PA, O'Reilly EM, Uronis HE, Ramanathan RK, Crane CH, Engebretson A, et al. Metastatic pancreatic cancer: American society of clinical oncology clinical practice guideline. J Clin Oncol. 2016;34:2784–2796. doi: 10.1200/JCO.2016.67.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative -intent resection of pancreatic cancer: A randomized controlled trial. JAMA. 2007;297:267–277. doi: 10.1001/jama.297.23.2582-a. [DOI] [PubMed] [Google Scholar]
- 5.Huang L, Holtzinger A, Jagan I, BeGora M, Lohse I, Ngai N, Nostro C, Wang R, Muthuswamy LB, Crawford HC, et al. Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids. Nat Med. 2015;21:1364–1371. doi: 10.1038/nm.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iguchi H, Yasuda M, Matsuo T, Sumii T, Funakoshi A. Clinical features and management of pancreatic cancer with bone metastases. Nippon Shokakibyo Gakkai Zasshi. 2004;101:872–878. (In Japanese) [PubMed] [Google Scholar]
- 7.Pneumaticos SG, Savidou C, Korres DS, Chatziioannou SN. Pancreatic cancer's initial presentation: Back pain due to osteoblastic bone metastasis. Eur J Cancer Care (Engl) 2010;19:137–140. doi: 10.1111/j.1365-2354.2007.00920.x. [DOI] [PubMed] [Google Scholar]
- 8.Garrido-Laguna I, Hidalgo M. Pancreatic cancer: From state-of-the-art treatments to promising novel therapies. Nat Rev Clin Oncol. 2015;12:319–334. doi: 10.1038/nrclinonc.2015.53. [DOI] [PubMed] [Google Scholar]
- 9.Boj SF, Hwang CI, Baker LA, Chio II, Engle DD, Corbo V, Jager M, Ponz-Sarvise M, Tiriac H, Spector MS, et al. Organoid models of human and mouse ductal pancreatic cancer. Cell. 2015;160:324–338. doi: 10.1016/j.cell.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heestand GM, Kurzrock R. Molecular landscape of pancreatic cancer: Implications for current clinical trials. Oncotarget. 2015;6:4553–4561. doi: 10.18632/oncotarget.2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The Lancet Oncology: Pancreatic cancer: Cause for optimism? Lancet Oncol. 2016;17:845. doi: 10.1016/S1470-2045(16)30234-0. [DOI] [PubMed] [Google Scholar]
- 12.Aparicio S, Hidalgo M, Kung AL. Examining the utility of patient-derived xenograft mouse models. Nat Rev Cancer. 2015;15:311–316. doi: 10.1038/nrc3944. [DOI] [PubMed] [Google Scholar]
- 13.Johnson JI, Decker S, Zaharevitz D, Rubinstein LV, Venditti JM, Schepartz S, Kalyandrug S, Christian M, Arbuck S, Hollingshead M, Sausville EA. Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br J Cancer. 2001;84:1424–1431. doi: 10.1054/bjoc.2001.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hidalgo M, Bruckheimer E, Rajeshkumar NV, Garrido-Laguna I, De Oliveira E, Rubio-Viqueira B, Strawn S, Wick MJ, Martell J, Sidransky D. A pilot clinical study of treatment guided by personalized tumorgrafts in patients with advanced cancer. Mol Cancer Ther. 2011;10:1311–1316. doi: 10.1158/1535-7163.MCT-11-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stanley RH. Molecular pathology. Mol Oncol. 2012;6:177–181. doi: 10.1016/j.molonc.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verweij J, de Jonge M, Eskens F, Sleijfer S. Moving molecular targeted drug therapy towards personalized medicine: Issues related to clinical trial design. Mol Oncol. 2012;6:196–203. doi: 10.1016/j.molonc.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garay JP, Gray JW. Omics and therapy-A basis for precision medicine. Mol Oncol. 2012;6:128–139. doi: 10.1016/j.molonc.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chakradhar S. Colorectal cancer: 5 big questions. Nature. 2015;521:S16. doi: 10.1038/521S16a. [DOI] [PubMed] [Google Scholar]
- 19.Macconaill LE, Garraway LA. Clinical implications of the cancer genome. J Clin Oncol. 2010;28:5219–2528. doi: 10.1200/JCO.2009.27.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belchis DA, Tseng LH, Gniadek T, Haley L, Lokhandwala P, Illei P, Gocke CD, Forde P, Brahmer J, Askin FB, et al. Heterogeneity of resistance mutations detectable by next-generation sequencing in TKI-treated lung adenocarcinoma. Oncotarget. 2016;7:45237–45248. doi: 10.18632/oncotarget.9931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jee J, Rasouly A, Shamovsky I, Akivis Y, Steinman SR, Mishra B, Nudler E. Rates and mechanisms of bacterial mutagenesis from maximum-depth sequencing. Nature. 2016;534:693–696. doi: 10.1038/nature18313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woollard PM, Mehta NA, Vamathevan JJ, Van Horn S, Bonde BK, Dow DJ. The application of next-generation sequencing technologies to drug discovery and development. Drug Discov Today. 2011;16:512–519. doi: 10.1016/j.drudis.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Guan Z, Lan H, Chen X, Jiang X, Wang X, Jin K. Individualized drug screening based on next generation sequencing and patient derived xenograft model for pancreatic cancer with bone metastasis. Mol Med Rep. 2017;16:4784–4790. doi: 10.3892/mmr.2017.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J, Zhang L, Su X, Li M, Xie L, Malchers F, Fan S, Yin X, Xu Y, Liu K, et al. Translating the therapeutic potential of AZD4547 in FGFR1-amplified non-small cell lung cancer through the use of patient-derived tumor xenograft modes. Clin Cancer Res. 2012;18:6658–6667. doi: 10.1158/1078-0432.CCR-12-2694. [DOI] [PubMed] [Google Scholar]
- 25.Shteyngart B, Chaiwiriyakul S, Wong J, Cantor JO. Preferential binding of lysozyme to elastic fibres in pulmonary emphysema. Thorax. 1998;53:193–196. doi: 10.1136/thx.53.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J, Zhu LX, Cheng X, Lin Y, Yan P, Peng B. Promotion of dental pulp cell migration and pulp repair by a bioceramic putty involving FGFR-mediated signaling pathways. J Dent Res. 2015;94:853–862. doi: 10.1177/0022034515572020. [DOI] [PubMed] [Google Scholar]
- 27.Garralda E, Paz K, López-Casas PP, Jones S, Katz A, Kann LM, López-Rios F, Sarno F, Al-Shahrour F, Vasquez D, et al. Integrated next-generation sequencing and avatar mouse models for personalized cancer treatment. Clin Cancer Res. 2014;20:2476–2484. doi: 10.1158/1078-0432.CCR-13-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katoh M, Nakagama H. FGF receptors: Cancer biology and therapeutics. Med Res Rev. 2014;34:280–300. doi: 10.1002/med.21288. [DOI] [PubMed] [Google Scholar]
- 29.Gavine PR, Mooney L, Kilgour E, Thomas AP, Al-Kadhimi K, Beck S, Rooney C, Coleman T, Baker D, Mellor MJ, et al. AZD4547: An orally bioavailable, potent, and selective inhibitor of the fibroblast growth factor receptor tyrosine kinase family. Cancer Res. 2012;72:2045–2056. doi: 10.1158/0008-5472.CAN-11-3034. [DOI] [PubMed] [Google Scholar]
- 30.Lehnen NC, von Mässenhausen A, Kalthoff H, Zhou H, Glowka T, Schütte U, Höller T, Riesner K, Boehm D, Merkelbach-Bruse S, et al. Fibroblast growth factor receptor 1 gene amplification in pancreatic ductal adenocarcinoma. Histopathology. 2013;63:157–166. doi: 10.1111/his.12115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.