Abstract
In the field of stem cell therapy, research on the application of Mesenchymal Stem Cells (MSCs) has flourished because of the various functions. On the other hand, research on the method of cell transplantation has developed from the administration of cell suspensions to cell-sheet engineering and 3D technology. In the trend, a cell transplantation platform named CellSaic, which is a combination of xeno-free recombinant scaffolds in a cell aggregate-like shape, was developed. CellSaic is the cell trans-plantation platform that can prevent the central necrosis within cell aggregates by arranging the cells and petaloid pieces of Recombinant Peptide (RCP) in a mosaic. The prevention of central necrosis is the most significant advantage over other 3D culture systems. This review details the unique characteristics of CellSaic including safety examination results and describes its future application for MSC transplantation. Particularly, in the application of MSCs, it has been reported that the MSC CellSaics increased the effect on improving various symptoms compared with MSCs only in the application of the therapy to Inflamma-tory Bowel Disease (IBD), cerebral infarction, bone cartilage regeneration in joints, and islet transplanta-tion. In accordance with the “One Health” concept, it is anticipated that this technology is expected to con-tribute to companion animal therapy and human therapy in the future.
Keywords: MSC, Recombinant peptide (RCP), Cell transplantation, mesenchymal stem cells, inflammatory bowel disease (IBD), CellSaic
1. Introduction
Research on the use of protein scaffolds for cell transplantation and regenerative medicine has been in progress for many years, ever since the concept of tissue engineering was advocated by Langer and Vacanti in 1993 [1]. Traditionally, scaffolds derived from natural products such as animal-derived gelatin and collagen have been used for research, and it was reported that the presence of the scaffold enhanced cell function [2-5].
The issue of bovine spongiform encephalopathy in the early 2000s was the motivation for placing emphasis on the concept of xeno-free products that contain no animal-derived components as ingredients for medicinal products and medical devices. This created the demand for scaffold materials that are also xeno-free [6-8].
On the other hand, cell therapy research has developed mainly in the administration of cell suspensions. Within this field, research on the clinical application of Mesenchymal Stem Cells (MSCs) has flourished because of the various functions of these cells [9, 10]. These cells are implicated in a wide range of conditions including inflammatory bowel disease [11], orthopedics [12, 13], graft-versus-host disease [14-16], autoimmune diseases [9], cerebral infarction [17, 18], and myocardial infarction [19, 20]. Coupled with the recent concept of “One Health”, the target of this treatment has expanded to include companion animal therapy in addition to treatment of humans [21]. “One Health” is meant to improve the lives of all species by integrating human and veterinary medicine [22, 23].
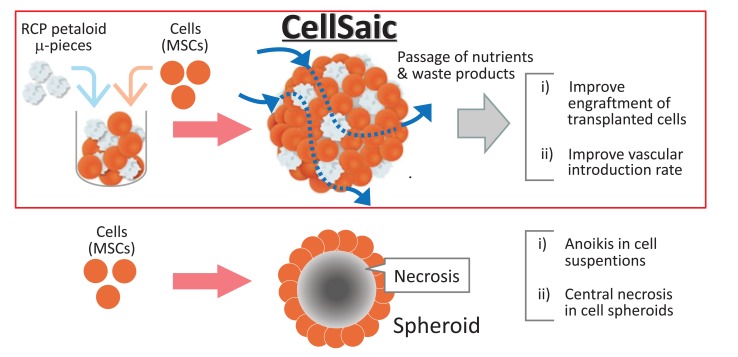
Research on cell transplantation has developed from the administration of simple cell suspensions to cell-sheet engineering and 3D technology [24-26]. Particularly, in recent years, there has been a large number of reports on cell aggregates known as three-dimensional spheroids [26-28]. We reported a cell transplantation platform named CellSaic, which is a combination of a xeno-free and recombinant scaffold in a cell aggregate-like shape [29, 30]. A Recombinant Peptide (RCP) petaloid μ-piece measuring a few dozen μm is used as the scaffold, with MSCs as the main cell source [29-31]. The obtained mosaic-like cell aggregates are referred to as CellSaic (cell- and scaffold-forming mosaic). This review details the unique characteristics of CellSaic and describes its future application for MSC transplantation.
2. Basic properties of CellSaic
2.1. Scaffold Shape: RCP Petaloid μ-piece
We focused on the functions exhibited when cells form aggregates while at the same time, we incorporated the minimum scaffold to resolve the issue of central necrosis that occurs as a consequence of aggregate formation (Fig. 1) [29, 30].
Fig. (1).
The design concept of CellSaic.
Initially, the spherical beads and plain shapes were tried as the minimum scaffold. However these scaffolds could not prevent the central necrosis within cell aggregates due to nutrient depletion, although that provided the adhesion surfaces for cells. Consequently, we designed a scaffold that is large enough to enable cell adhesion and selected a scaffold structure that produces gaps to allow passage of nutrients and waste products when cell aggregates are formed. As a result, as per our previous report, we discovered that a scaffold shape known as an RCP petaloid μ-piece, which is approximately 50–100 µm in size and is molded into a petaloid shape, is the optimal scaffold [29, 32].
The formation of this petaloid shape increases the cell adhesion surface area and guarantees gaps within the cell aggregate (CellSaic) because of its complex shape. We found that this increased the survival rate of transplanted cells in both in vitro and in vivo tests. Therefore the most significant difference of CellSaic from the other 3D culture systems is caused by the shape of the scaffolds producing the gaps within cell aggregates. The scaffold shape can also be prescribed numerically as parameters that express the complexity of the shape (tapped density and boundary length to square root of the area ratio) [29, 32].
2.2. Improved Engraftment of Transplanted Cells, Improved Vascular Introduction Rate, and Altered Cytokine Release
The basic characteristics of CellSaic have been reported as i) improved engraftment of transplanted cells, ii) improved vascular introduction rate, and iii) altered cytokine release [29, 32-34].
The main factors involved in the mechanism that causes improved engraftment of the transplanted cells are inhibition of cell anoikis through the presence of a scaffold and avoidance of central necrosis within cell aggregates. The latter in particular is reported to produce a significant difference depending on the shape of the scaffold and the presence/absence of a scaffold [29].
The two factors involved in the mechanism that causes improved vascular introduction rate are thought to be the scaffold structure that contains gaps within the graft and the ability to rearrange the cells and the μ-piece, making it easier for host-derived cells in the vascular system to migrate. In fact, in experiments with NOD/SCID mice, MSC CellSaics induced significantly greater introduction of blood vessels than did MSC spheroids [29].
The mechanisms involved in altered cytokine release have not yet been fully verified, but there were cases where differences were observed in the volume of cytokines released, unlike when there were only cells present. An example of this is the increased volume of TSG-6 released by MSCs. In this instance, we confirmed that MSCs formed a CellSaic shape and released a large volume of TSG-6, an anti-inflammatory cytokine [33, 34].
2.3. Safety Studies of MSC CellSaic
The results of tumorigenicity and general toxicity tests on NOG mice for canine Adipose-Derived Stem Cell (cADSC) CellSaics to confirm the safety of CellSaic have been reported [33]. In line with the philosophy of “One Health”, we evaluated the safety of canine ADSC CellSaics, envisioning that clinical research in companion animal therapy would lead to development of human therapy products. At the same time, the results of the evaluation indicated that there was no tumorigenicity, and there was also no significant toxicity found from the results of the general toxicity tests [33].
Specifically, the general toxicity and tumorigenicity were assessed between cADSC CellSaic group (107cells cADSC and 1.04 mg µ-pieces per mouse), saline control group and culture medium control group in NOG mice, the male (N=12) and female (N=12) mice, referring WHO guideline [35]. Nodules were not visible at day11. Histological evaluation of the administration sites, at 4 and 8 weeks, showed no cell division despite the presence of a remnant. As concers general toxicity, no significant intergroup difference was observed in the biochemistry test, hematological test, organ weight, food consumption, or body weight. No abnormalities were found during the pathological evaluation of the organs, in the cADSC CellSaic group, at 4 and 8 weeks [33].
3. Application of CellSaic
3.1. Cell Therapy Research Using MSC CellSaics
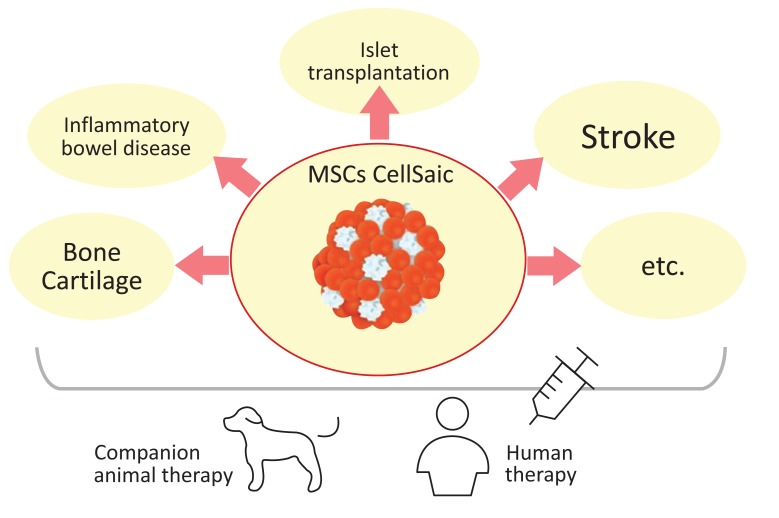
Research on disease treatment with MSC CellSaics has demonstrated the application of the therapy to IBD (inflammatory bowel disease), cerebral infarction, bone cartilage regeneration in joints, and islet transplantation. Research on these applications has shown that the use of MSC CellSaics increased the effect on improving various symptoms compared with the use of MSCs only.
An investigation into the application of this technology to IBD using a dextran sulfate sodium colitis mouse model found that administration of MSC CellSaics improved symptoms in models where sufficient improvement in symptoms could not be achieved with MSC only [33]. Specifically, in DSS-induced model mice of colitis, intraperitoneally administered MSC CellSaics significantly recover body weight and colon length compared with MSC only. Pathological evaluations of the depth and area of ulceration, edema, and infiltration of inflammatory cells showed that MSC CellSaics group improved the histological score and the edema and ulceration calmed down [33]. The reasons for this are listed in the discussion and include increased levels of residual MSCs in the body and increased release of anti-inflammatory cytokines.
Even in the cerebral infarction mouse model, local administration of MSC CellSaics was reported to contribute to improved motor function compared to local administration of MSCs only [36, 37].
Naritomi et al. reported a notable increase in differentiation and an increase in the amount of cartilage matrix produced when MSC CellSaics were used for cartilage differentiation compared to MSCs only [38]. Even in tests performed using a rabbit osteochondral defect model, the results showed accelerated bone cartilage regeneration with the use of MSC CellSaics compared to that shown with MSCs only [39].
In reports on co-transplantation of MSCs with islets, it was confirmed that the use of CellSaic increased islet engraftment and increased the blood sugar regulation effect in diabetes model mice [29].
All aforementioned reports compared and verified the use of MSCs only and MSC CellSaics in diseases where the efficacy of MSCs has been reported. These reports suggest that CellSaic may enhance the therapeutic effect of MSCs.
3.2. Use in In vivo Pancreatic Cancer Models
The use of MSC CellSaics has been reported for animal models in drug discovery research. In this report, it was disclosed that MSC CellSaics were combined with cancer cell transplantation to create a pancreatic cancer tumor-bearing animal model [40]. As a result, rich stromal tissues were formed around the pancreatic cancer cells. Conversely, no formation of this rich stromal tissue was observed with transplantation of cancer cells only. Pancreatic cancer animal models with rich stromal tissue presented similar morphology to that of pancreatic cancer pathology findings in humans. The reports indicated that this model may be useful as a drug-resistant pancreatic cancer model.
3.3. 3-D modeling Using CellSaics as Parts
The use of CellSaics as parts enables the creation of a variety of molded objects. WO/2017/057547 reported a method of producing a sheet-like cell structure with a thickness of 1 mm or more by utilizing CellSaic technology [41]. Even when it is difficult to create sheet-like structures with cells only, particularly with MSCs, the use of CellSaics enables the acquisition of a thick sheet-like cell structure.
Other reported methods of molding even more complex structures are those where CellSaic is arranged like a one-dot drawing and those where the structure is formed by packing CellSaics into a template [42, 43]. These methods are considered to be the technical concept of 3D printing with CellSaics (several hundred µm in size) composed of cells and scaffold as one dot. Because CellSaics contain cells, adjacent CellSaics will fuse to form large structures if left for some time. Therefore, adhesion bond is not required. Examples of tube structures created using CellSaics have also been shown, and the possibility of utilizing them for applications such as artificial blood vessels and other tubular structures has been reported.
Conclusion
CellSaic was developed as a novel cell transplantation platform using a recombinant scaffold.
The main functions of CellSaic are i) improving engraftment of transplanted cells, ii) improving vascular introduction rate, and iii) altering cytokine release. These functions have made significant contributions to improved engraftment of transplanted cells in particular.
Reports have indicated the effective use of CellSaic for MSC therapy based on these functions for conditions such as IBD, cerebral infarction, cartilage regeneration, and islet transplantation. The application target of course considers both human and animal therapy (Fig. 2). In accordance with the “One Health” concept, the use of MSC CellSaic in dogs was selected as the very first clinical application, and safety tests were implemented with canine MSC CellSaics.
Fig. (2).
The application target of MSC CellSaic. Both human and animal therapies are considered as target on the “One Health” concept.
As a result, clinical research has begun in dogs. This technology is expected to contribute to scientific development, companion animal therapy and human therapy in the future.
Acknowledgements
The author would like to thank members of the technical staff of Bioscience & Technology Development Center, FUJIFILM Corporation, whose opinions and knowledge were very helpful throughout the completion of this work.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Langer R., Vacanti J.P. Tissue engineering. Science. 1993;260(5110):920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 2.Nerem R.M., Sambanis A. Tissue engineering: from biology to biological substitutes. Tissue Eng. 1995;1(1):3–13. doi: 10.1089/ten.1995.1.3. [DOI] [PubMed] [Google Scholar]
- 3.Peter S.J., Miller M.J., Yasko A.W., Yaszemski M.J., Mikos A.G. Polymer concepts in tissue engineering. J. Biomed. Mater. Res. 1998;43(4):422–427. doi: 10.1002/(sici)1097-4636(199824)43:4<422::aid-jbm9>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 4.Shoichet M.S., Hubbell J.A. Polymers for tissue engineering. J Biomater Sci. 1998;9(5):405–406. [Google Scholar]
- 5.Chen G., Ushida T., Tateishi T. Scaffold design for tissue engineering. Macromol. Biosci. 2002;2(2):67–77. [Google Scholar]
- 6.Halme D.G., Kessler D.A. FDA regulation of stem-cell-based therapies. N. Engl. J. Med. 2006;355(16):1730–1735. doi: 10.1056/NEJMhpr063086. [DOI] [PubMed] [Google Scholar]
- 7.Cimino M., Goncalves R.M., Barrias C.C., Martins M.C.L. Xeno-free strategies for safe human mesenchymal stem/stromal cell expansion: supplements and coatings. Stem Cells Int. 2017;6597815:1–13. doi: 10.1155/2017/6597815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richards M., Fong C.Y., Tan S., Chan W.K., Bongso A. An efficient and safe xeno‐free cryopreservation method for the storage of human embryonic stem cells. Nature. 1953;171(4356):737–738. doi: 10.1634/stemcells.22-5-779. [DOI] [PubMed] [Google Scholar]
- 9.Trounson A., Thakar R.G., Lomax G., Gibbons D. Clinical trials for stem cell therapies. BMC Med. 2011;9(52):1–7. doi: 10.1186/1741-7015-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galipeau J., Sensebe L. Mesenchymal stromal cells: Clinical challenges and taherapeutic opportunities. Cell Stem Cell. 2018;22(6):824–833. doi: 10.1016/j.stem.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ko I.K., Kim B.G., Awadallah A., et al. Targeting improves MSC treatment of inflammatory bowel disease. Mol. Ther. 2010;18(7):1365–1372. doi: 10.1038/mt.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caplan A.I. Review: Mesenchymal stem cells: Cell based reconstructive therapy in orthopedics. Tissue Eng. 2005;11(7-8):1198–1211. doi: 10.1089/ten.2005.11.1198. [DOI] [PubMed] [Google Scholar]
- 13.Nitkin C.R., Bonfield T.L. Concise review: Mesenchymal stem cell therapy for pediatric disease: Perspectives on success and potential improvements. Stem Cells Transl. Med. 2017;6(2):539–565. doi: 10.5966/sctm.2015-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jurado M., Gil A.C., Mata C., et al. A multicenter randomized clinical trial evaluating the safety and feasibility of the treatment of GvHD with allogenic Mesenchymal Stem Cells (MSC) from adipose tissue. Cytotherapy. 2017;19(5):S11. [Google Scholar]
- 15.Ringdén O., Uzunel M., Rasmusson I., et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81(10):1390–1397. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 16.Bahr L., Sundberg B., Lönnies L., et al. Long-term complications, immunologic effects, and role of passage for outcome in mesenchymal stromal cell therapy. Biol. Blood Marrow Transplant. 2012;18(4):557–564. doi: 10.1016/j.bbmt.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 17.Bhasin A., Srivastava M.V., Mohanty S., et al. Stem cell therapy: A clinical trial of stroke. Clin. Neurol. Neurosurg. 2013;115(7):1003–1008. doi: 10.1016/j.clineuro.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 18.Wechsler L.R., Bates D., Stroemer P., Andrews-Zwilling Y.S., Aizman I. Cell therapy for chronic stroke. Stroke. 2018;49(5):1066–1074. doi: 10.1161/STROKEAHA.117.018290. [DOI] [PubMed] [Google Scholar]
- 19.Amado L.C., Saliaris A.P., Schuleri K.H., et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc. Natl. Acad. Sci. USA. 2005;102(32):11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin-Rendon E., Gyongyosi M. Mesenchymal stromal cell therapy as treatment for ischemic heart failure: the MSC-HF study. Cardiovasc. Diagn. Ther. 2017;7(Suppl. 2):S69–S72. doi: 10.21037/cdt.2016.11.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Volk S.W., Theoret C. Translating stem cell therapies: the role of companion animals in regenerative medicine. Wound Repair Regen. 2013;21(3):382–394. doi: 10.1111/wrr.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kahn L.H., Kaplan B., Monath T.P., Steele J.H. Teaching “one medicine, one health”. Am. J. Med. 2008;121(3):169–170. doi: 10.1016/j.amjmed.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.One Health Initiative 2012 www.onehealthinitiative.com
- 24.Yamato M., Okano T. Cell sheet engineering. Mater. Today. 2004;7(5):42–47. [Google Scholar]
- 25.Baksh N., Gallant N.D., Toomey R.G. Cell sheet engineering for integrating functional tissue in vivo: Successes and challenges. MRS Bull. 2017;42(5):350–355. [Google Scholar]
- 26.Baraniak P.R., McDevitt T.C. Scaffold-free culture of mesenchymal stem cell spheroids in suspension preserves multilineage potential. Cell Tissue Res. 2012;347(3):701–711. doi: 10.1007/s00441-011-1215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ylostalo J.H., Bazhanov Mohammadipoor N., Bartosh T.J. Production and administration of therapeutic mesenchymal stem/stromal cell (msc) spheroids primed in 3-D cultures under Xeno-free conditions. J. Vis. Exp. 2017;18(121) doi: 10.3791/55126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yanagihara K., Uchida S., Ohba S., Kataoka K., Itaka K. Treatment of Bone Defects by Transplantation of Genetically Modified Mesenchymal Stem Cell Spheroids. Mol. Ther. Methods Clin. Dev. 2018;9(15):358–366. doi: 10.1016/j.omtm.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakamura K., Iwazawa R., Yoshioka Y. Introduction to a new cell transplantation platform via recombinant peptide petaloid pieces and its application to islet transplantation with mesenchymal stem cells. Transpl. Int. 2016;29(9):1039–1050. doi: 10.1111/tri.12810. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura K. Cell construct comprising polymer blocks having biocompatibility and cells. US Patent . 2010.
- 31.Nakamura K., Tabata Y. A new fluorescent imaging of renal inflammation with RCP. J. Control. Release. 2010;148(3):351–358. doi: 10.1016/j.jconrel.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura K., Iwazawa R., Miyoshi H., et al. Cell structure for cell transplantation, biocompatible polymer block, and methods for producing same. 2013.
- 33.Iwazawa R., Kozakai S., Kitahashi T., Nakamura K., Hata K. The therapeutic effects of adipose-derived stem cells and recombinant peptide pieces on mouse model of DSS colitis. Cell Transplant. 2018 doi: 10.1177/0963689718782442. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kozakai S., Nakamura K. Trophic factor release agent and inflammatory disease treatment agent. 2016.
- 35. Recommendations for the evaluation of animal cell cultures as substrates for the manufacture of biological medicinal products and for the characterization of cell banks. . WHO technical report series, No 978 Annex 3. 2013.
- 36.Nakamura K. Cell structure for use in treatment of brain injury, method for producing same, and therapeutic agent for brain injury. 2014.
- 37.Miyamoto M., Nakamura K., Shichinohe H., et al. Human Recombinant Peptide Sponge Enables Novel, Less Invasive Cell Therapy for Ischemic Stroke. Stem Cells Int. 2018;4829534:1–8. doi: 10.1155/2018/4829534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naritomi M., Mizuno M., Katano H., et al. Petaloid recombinant peptide enhances in vitro cartilage formation by synovial mesenchymal stem cells. J. Orthop. Res. doi: 10.1002/jor.24042. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakamura K., Miyoshi H., Hada S., Watanabe M. Cartilage-regenerating material.International patent publication No. 2015; WO2016/148245. 2015.
- 40.Nakamura K., Mima S., Kitahashi T., Kakimuma C. Cell structure, non-human mode animal, method for producing non-human model animal, and method for evaluating test substance. 2017.
- 41.Nakamura K. Method for manufacturing sheet-shaped cell structure, and sheet-shaped cell structure. 2017.
- 42.Nakamura K. Tublar structure, device for producing tubular structure, and method for producing tubular structure. 2016.
- 43.Nakamura K, Kuchiishi K, Kizawa H, Aburaya A. Tubular structure, method for producing cell structure, and method for producing tubular structure. 2015.