Abstract
Background:
We report here an evaluation of a novel experimental system- cofactor-supplemented permeabilized cryopreserved human enterocytes (MetMax™ cryopreserved human entero-cytes (MMHE), patent pending) for applications in the evaluation of enteric drug metabolism. A major advantage of MMHE over Conventional Cryopreserved Human Enterocytes (CCHE) is the simplification of the use procedures including storage at -80°C instead of in liquid nitrogen, and use of the cells imme-diately after thawing without a need for centrifugation and microscopic evaluation of cell density and via-bility and cell density adjustment.
Methods:
In this study, we compared MMHE and CCHE in key phase 1 oxidation and phase 2 conjuga-tion Drug Metabolism Enzyme (DME) activities that we recently reported for cryopreserved human en-terocytes: CYP2C9 (diclofenac 4’- hydroxylation), CYP2C19 (s-mephenytoin hydroxylation), CYP3A4 (midazolam 1’-hydroxylation), CYP2J2 (astemizole O-demethylation), uridine 5'-diphospho-glucuronosyltransferase (UGT; 7-hydroxycoumarin glucuronidation), sulfotransferase (SULT; 7-hydroxycoumarin sulfation), N-acetyl transferase-1 (NAT-1; p-benzoic acid N-acetylation), and carboxy-esterase-2 (CES-2; hydrolysis of irinotecan to SN38). Both CCHE and MMHE were active in all the DME pathways evaluated, with specific activities of MMHE ranged from 142% (CYP2C9) to 1713% (UGT) of that for CCHE. β-hydroxylation and testosterone 6.
Result and Conclusion:
Our results suggest that the MMHE system represents a convenient and robust in vitro experimental system for the evaluation of enteric drug metabolism
Keywords: Permeabilized, cryopreserved human enterocytes, MMHE, CCHE, Drug metabolism enzyme (DME), enteric drug metabolism
1. Introduction
As oral administration represents the most preferred route of drug administration, assessment of oral bioavailability is a key activity in drug development. In vitro evaluation of oral availability is routinely performed to allow the selection of new chemical entities for further development. The most commonly used in vitro experimental models for the assessment of oral bioavailability include the Parallel Artificial Membrane Permeability Assay (PAMPA) [1] which models permeability across lipid bilayers, and the colon carcinoma cell line (Caco-2) [2, 3] transwell culture which models permeability, uptake transport, and efflux transport across a monolayer cell layer.
Besides permeability and transporter-mediated uptake and efflux, enteric drug metabolism is also known to be a major determinant of bioavailability [4-6]. Drugs with bioavailability affected by gut wall metabolism include m-octopamine [7], chlorpheniramine [8], terbutaline [9], cyclosporine [10], verapamil [11], tacrolimus [12, 13], midazolam [14], and rifampin [15]. The commonly used in vitro experimental systems such as PAMPA and Caco-2 systems lack enteric drug metabolism enzymes and therefore would not be useful in the assessment of the potential roles of enteric drug metabolism on the oral bioavailability.
In our laboratory, efforts have been initiated to develop physiologically relevant in vitro experimental systems for the evaluation of human enteric drug metabolism. Our research efforts led to the establishment of procedures for isolation and cryopreservation of enterocytes from human small intestine to retain viability as well as active drug metabolizing enzyme pathways including cytochromes P450 isoforms (CYP2C9, CYP2C19, CYP3A4, and CYP2J2), Uridine 5'-diphospho-Glucuronosyltransferase (UGT), Sulfotransferase (SULT), and Carboxylesterase (CES) [16]. We believe that cryopreserved enterocytes have the potential to be used for the assessment of enteric uptake, metabolism and efflux similar to the application of cryopreserved hepatocytes for the assessment of these key hepatic drug properties [17-20].
Another effort of our research activities is to develop experimental systems with intact cells to enhance the efficiency and robustness of the experimental systems. The use of intact cells such as cryopreserved hepatocytes and cryopreserved enterocytes in general require dedicated laboratories to include liquid nitrogen storage, centrifugation and microscopic equipment for quantification of cell number and viability, as well as personnel with expertise in cell handling to avoid complications of experimental results due to cellular damage during the performance of the study. Our research effort has led to the establishment of permeabilized, cofactor supplemented cryopreserved hepatocytes (MetMax™ cryopreserved hepatocytes) which have the advantages over conventional cryopreserved hepatocytes as they can be stored and use similar cell free fractions such as microsomes and S9, but with advantages over cell-free fractions in the full complement of phase I and II drug metabolizing enzyme pathways as that in conventional cryopreserved hepatocytes [21].
We have subsequently adopted the procedures for MetMax™ hepatocytes for the preparation of permeabilized, co-factor supplemented cryopreserved human enterocytes (MetMax™ cryopreserved human enterocytes; MMHE). We report here a comparison of Conventional Cryopreserved Human Enterocytes (CCHE) and MMHE in enteric drug metabolizing enzyme activities.
2. Materials and Methods
2.1. Chemicals
Astemizole, irinotecan hydrochloride, diclofenac sodium salt, 7-ethyl-10-hydroxycamptothecin (SN38), 4-hydroxydiclofenac, s-mephenytoin, and testosterone were purchased from Cayman Chemical (Ann Arbor, MI). 7-Hydroxycoumarin was purchased from Chem Service (West Chester, Pennsylvania). 7-Hydroxycoumarin sulfate potassium salt was obtained from Santa Cruz Biotechnology (Dallas, Texas). Coumarin, 6β-hydroxytestosterone, 7-hydroxycoumarin β-D-glucuronide sodium salt, (2S, 3S)-hydroxy were purchased from Sigma-Aldrich (St. Louis, MO). Midazolam, 1'-hydroxymidazolam, O-desmethyl astemizole, and 4-hydroxy-S-mephenytoin were obtained from Toronto Research Chemicals (Toronto, Canada).
2.2. Preparation of Pooled Donor Cryopreserved Human Enterocytes as CCHE and MMHE
Cryopreserved human enterocytes from 10 individual donors were recovered, pooled and divided into two groups, with one group re-cryopreserved using the conventional procedures as CCHH and stored in liquid nitrogen, and another group permeabilized with a proprietary procedure (patent pending) and cryopreserved as MMHE at 2 x 106 enterocytes/mL and stored at a -80°C freezer.
2.3. Incubation of Enterocytes with Drug Metabolizing Enzyme Substrates
DME substrate incubations were performed in a cell culture incubator maintained at 37°C with a humidified atmosphere of 5% CO2. For CCHE, the enterocytes were recovered as described above, with cell density adjusted to 3 x 106 cells/ml in HQMTM for subsequent incubation with the designated substrates. MMHE were thawed for used directly for substrate incubations, with a final cell density of 3 x 106 cells/ml. Incubations were performed by adding aliquots of 50 µl of the CCHE or MMHE cell suspensions to individual wells of a 96-well plate for the evaluation of drug metabolism activities. After cell addition, the 96-well plate was pre-warmed in the incubator for 15 min, followed by the addition of 50 µl of pre-warmed (37°C) HQMTM containing DME substrates at 2x final concentration and incubated for 1 hour. The final incubation mixture in each well, therefore, had a volume of 100 μl, with a cell density of 1.5 x 106 cells/ml for CCHE and 1.0 x 106 cells/ml for MMHE. Metabolism was terminated in each well by the addition of 100 µl acetonitrile. The final incubation samples were stored at -80°C for the subsequent LC/MS-MS analysis as previously reported [16]. The metabolism substrates used, identities of the metabolites quantified, and LC/MS-MS parameters are shown in Table 2.
Table 2.
Metabolic pathways, substrates, metabolites and LC/MS-MS parameters for the quantification of the drug metabolizing enzyme activities of cryopreserved human enterocytes. Tolbutamide was used as an internal standard with the MRM (Mass Transition Monitoring) at m/z 271.2 to 91.3 and m/z 269.1 to 105.9 for positive mode and negative mode, respectively. The concentrations used for each substrate is as indicated. The total incubation time was 1 hour.
| Drug Metabolizing Enzymes | Substrate and Concentrations | Marker Metabolite Analyzed |
Ion Mode
Application |
Mass Transitions
Monitoring |
|---|---|---|---|---|
| CYP2C9 | Diclofenac (25 μM) | 4-Hydroxydiclofenac | Negative | m/z 309.8 to 265.9 |
| CYP2C19 | S-Mephenytoin (250 μM) | 4-Hydroxy-S-Mephenytoin | Positive | m/z 235.2 to 150.0 |
| CYP3A4-1 | Midazolam (20 μM) | 1’-Hydroxymidazolam | Positive | m/z 342.1 to 203.1 |
| CYP3A4-2 | Testosterone (200 μM) | 6β-Hydroxytestosterone | Positive | m/z 305.2 to 269.1 |
| CYP2J2 | Astemizole (50 μM) | O-Demethylastemizole | Positive | m/z 445.0 to 204.2 |
| UGT | 7-Hydroxycoumarin (100 μM) | 7-Hydroxycoumarin Glucuronide | Negative | m/z 336.9 to 160.9 |
| SULT | 7-Hydroxycoumarin (100 μM) | 7-Hydroxycoumarin Sulfate | Negative | m/z 240.9 to 161.0 |
| CES2 | Irinotecan (50 μM) | SN38 | Positive | m/z 393.0 to 349.3 |
• SULT: SULT activity was evaluated by quantifying the formation of 7-hydroxycoumarin sulfate from 7-hydroxycoumarin. The specific activity (pmole/min/106 cells) of MMHH was 13 ± 0.69 which was 179% of that of CCHE.
• CES-2: CES-2 activity was evaluated by quantifying the formation of SN38 from irinotecan. The specific activity (pmole/min/106 cells) of MMHH was 0.38 ± 0.27 which was similar (102%) to that of CCHE.
2.4. Data Analysis
Data are presented as mean and standard deviation of triplicate incubations derived using the Microsoft Excel 6.0 software. Statistical analysis was performed using student’s t-test with the Microsoft Excel 6.0 software, with the probability of p<0.05 to be considered statistically significant. Specific activity (pmole/min/million hepatocytes) of each drug metabolizing enzyme pathway was determined by dividing the total metabolite formed by the incubation time and normalized to cell concentration.
3. Results
3.1. Donor Demographics
The race, gender and age of the donors of the small intestines used for the generation of enterocytes for this study are shown in Table 1. There were 5 male and 5 female donors with ages ranged from 32 to 60 years old. There were 7 Caucasian, 2 African American, and 1 Hispanic donors.
Table 1.
Demographics of the human donors for the enterocytes used in the study. Each lot represent enterocytes isolated and cryopreserved from a single donor. These cryopreserved enterocyte lots were thawed, pooled, and re-cryopreserved as either conventional cryopreserved human enterocytes (CCHE) or as MetMax™ cryopreserved enterocytes (MMHE) for the study.
| Lot No. | Gender | Race | Age (Years) |
|---|---|---|---|
| HE3031 | F | C | 49 |
| HE3032 | F | C | 48 |
| HE3006 | F | C | 44 |
| HE3027 | F | C | 53 |
| HE3011 | F | C | 50 |
| HE3021 | M | AA | 60 |
| HE3019 | M | C | 61 |
| HE3028 | M | AA | 34 |
| HE3033 | M | H | 32 |
| HE3010 | M | C | 47 |
3.2. Drug Metabolizing Enzyme Activities
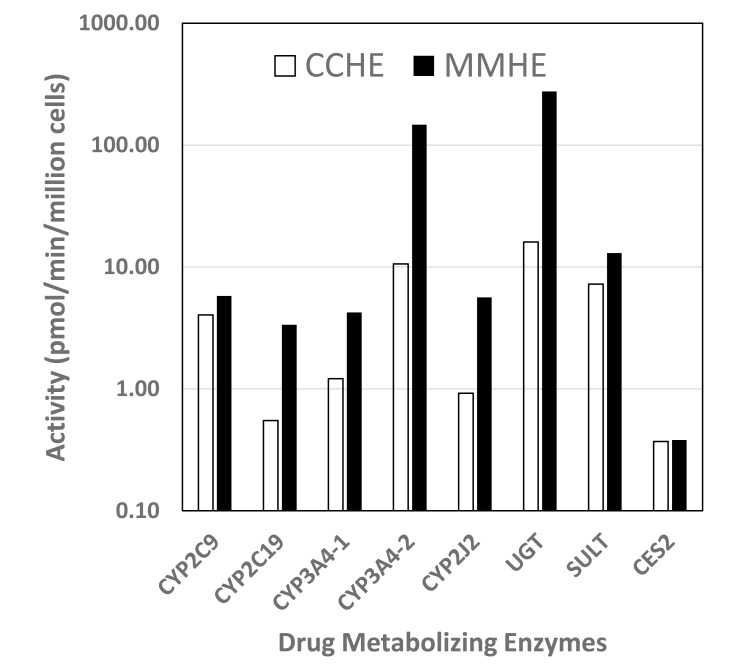
The activities of key enteric drug metabolizing enzymes: CYP2C9, CYP2C19, CYP3A4, CYP2J2, UGT, SULT, and CES2 were evaluated in MMHH and CCHH. Our results show that both experimental systems were active in the metabolism of the following pathway-selective substrates. A comparison of the specific metabolic activities of MMHH and CCHH for the metabolic pathways evaluated is shown in Fig. (1) and Table 3. Substrates for the multiple drug metabolism pathways evaluated are shown in Table 2. Results with are as follows:
Fig. (1).
A comparison of MetMax™ cryopreserved human enterocytes (MMHE; filled bars) and conventional cryopreserved human enterocytes (CCHE; open bars) in drug metabolizing enzyme activities.
Table 3.
A comparison of conventional cryopreserved human enterocytes (CCHE) and MetMax™ cryopreserved human enterocytes (MMHE) in the activities of various drug metabolizing enzyme pathways. CYP3A4 activity was evaluated using midazolam (CYP3A4-1) and testosterone as (CYP3A4-2) substrates. Mean and standard deviations (sd) of triplicate samples are shown. *: activity observed with MMHE that are statistically significant (p<0.05) to be different from that for CCHE. MMHE/CCHE ratio was calculated using the following equation: Activity (MMHE)/Activity (CCHE) x 100%.
| Drug Metabolism Enzyme | Substrate | Marker Metabolite | Metabolite Activity (pmole/106/min) | ||
|---|---|---|---|---|---|
| CCHE | MMHE |
MMHE/CCHE
Ratio (%) |
|||
| CYP2C9 | Diclofenac | 4-OH Diclofenac | 4.05 ± 0.16 | 5.78 ± 1.13* | 142% |
| CYP2C19 | S-Mephenytoin | 4-OH S-Mephenytoin | 0.55 ± 0.03 | 3.36 ± 0.32* | 610% |
| CYP3A4-1 | Midazolam | 1-OH-midazolam | 1.21 ± 0.03 | 4.23 ± 1.22* | 349% |
| CYP3A4-2 | Testosterone | 6βOH-testosterone | 10.6 ± 3.3 | 147 ± 14.5* | 1386% |
| CYP2J2 | Astemizole | O-Demethyl Astemizole | 0.92 ± 0.43 | 5.63 ± 1.53* | 614% |
| UGT | 7-OH-Coumarin | 7-Hydroxycoumarin Glucuronide | 16.05 ± 0.32 | 275 ± 79.5* | 1713% |
| SULT | 7-OH-Coumarin | 7-Hydroxycoumarin Sulfate | 7.24 ± 0.34 | 13 ± 0.69* | 179% |
| CES2 | Irinotecan | SN38 | 0.37 ± 0.14 | 0.38 ± 0.27 | 102% |
CYP2C9: CYP2C9 activity was evaluated by quantifying the formation of 4-OH diclofenac from diclofenac. The specific activity (pmole/min/106 cells) of MMHH was 5.78 ± 1.13 which was 142% of that of CCHE.
CYP2C19: CYP2C19 activity was evaluated by quantifying the formation of 4-OH S-mephenytoin from S-mephenytoin. The specific activity (pmole/min/106 cells) of MMHH was 3.36 ± 0.32which was 612% of that of CCHE.
CYP3A4: CYP3A4 activity was evaluated by quantifying the formation of 1-OH-midazolam from midazolam and 6β-OH-testosterone from testosterone. The specific activity (pmole/min/106 cells) of MMHH was 4.23 ± 1.22 for midazolam 1-hydroxylation and 147 ± 14.5 for testosterone 6β-hydroxylation, which were 349% and 1386%, respectively of that of CCHE.
CYP2J2: CYP2J2 activity was evaluated by quantifying the formation of O-demethyl astemizole from astemizole. The specific activity (pmole/min/106 cells) of MMHH was 5.63 ± 1.53 which was 614% of that of CCHE.
UGT: UGT activity was evaluated by quantifying the formation of 7-hydroxycoumarin glucuronide from 7-hydroxycoumarin. The specific activity (pmole/min/106 cells) of MMHH was 275 ± 79.5 which was 1713% of that of CCHE.
The results show that MMHE were active in all drug metabolizing enzymes evaluated, with activities similar (CES-2) or higher (CYP2C9, CYP2C19, CYP3A4, CYP2J2, UGT, SULT) than that for CCHE.
4. Discussion
We recently reported a novel experimental system in which human hepatocytes are permeabilized followed by cryopreservation (MetMax™ cryopreserved human hepatocytes (MMHH); patent pending). MMHH was found to have similar drug metabolizing enzyme activities as conventional cryopreserved human hepatocytes, but with advantages in the simplified use procedures including storage at -80 deg., and use directly after thawing without centrifugation and microscopic examination. We report here the adaptation of this approach towards cryopreserved human enterocytes to allow similar advantages in the use of cryopreserved human enterocytes in the evaluation of enteric drug metabolism.
A comparison of MMHE and CCHE in the activities of key drug metabolizing enzyme pathways showed no apparent deficiencies in these activities in MMHE. Both experimental systems were competent in the phase 1 oxidation and phase 2 conjugation drug metabolizing enzyme pathways evaluated, with most activities observed in MMHE higher than that for CCHE. The following is a summary of the activities in MMHE as percentages of that for CCHE, in descending order:
UGT (1713%), CYP3A4 (testosterone 6b-hydroxylation; 1386%), CYP2J2 (614%), CYP2C19 (610%), CYP3A4 (Midazolam 1’-hydroxylation; 349%), SULT (179%), CYP2C9 (142%), and CES2 (102%).
The results, therefore, suggest that MMHE represent a robust experimental system for the evaluation of enteric drug metabolism. As MMHE were derived from the CCHE, thereby containing the same enterocytes, the differences are attributed to the cofactors that were supplemented. In our laboratory, efforts are underway to determine whether the comparative relevance of the drug metabolism enzyme activities of CCHE and MMHE activity to in vivo enteric drug metabolism.
Our experimental findings suggest that MMHE may represent a convenient enterocyte-based experimental system that can be used for the evaluation of enteric drug metabolism. An initial study using MMHE in the evaluation of metabolic stability and metabolite profiling showed promising results with drugs known to be subjected to a variety of DME [22].
As described previously for MetMax™ cryopreserved human hepatocytes [21], ease of use is a major advantage for MMHE over CCHE, especially for analytical chemists as experimental procedures such as storage in liquid nitrogen, centrifugation for cell recovery after thawing, and microscopic examination for cell number and viability quantification are not required. The strengths of MMHE as an in vitro experimental system, therefore, include the convenience of use and the robust drug metabolizing enzyme activities. However, intact enterocytes remain valuable for the evaluation of drug properties that require intact plasma membranes and function gene expression and protein synthesis such as uptake and efflux transport, enzyme induction, and enterotoxicity. We have recently developed a Cryopreserved Human Intestinal Mucosa (CHIM) system which can be used to evaluate most, if not all of the above-mentioned enteric drug properties [23]. The MMHE system reported here should complement the intact cryopreserved enterocytes [16] and the Cryopreserved Intestinal Mucosa (CHIM) [23] as in vitro experimental systems for investigations on enteric drug properties.
Acknowledgements
Declared none.
Ethics Approval and Consent to Participate
Not applicable.
Human and Animal Rights
No Animals/Humans were used for studies that are the basis of this research.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Seo P.R., Teksin Z.S., Kao J.P., Polli J.E. Lipid composition effect on permeability across PAMPA. Eur. J. Pharm. Sci. 2006;29(3-4):259–268. doi: 10.1016/j.ejps.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 2.Dannenfelser R.M., He H., Joshi Y., Bateman S., Serajuddin A.T. Development of clinical dosage forms for a poorly water soluble drug I: Application of polyethylene glycol-polysorbate 80 solid dispersion carrier system. J. Pharm. Sci. 2004;93(5):1165–1175. doi: 10.1002/jps.20044. [DOI] [PubMed] [Google Scholar]
- 3.Sun D., Yu L.X., Hussain M.A., Wall D.A., Smith R.L., Amidon G.L. In vitro testing of drug absorption for drug ‘developability’ assessment: Forming an interface between in vitro preclinical data and clinical outcome. Curr. Opin. Drug Discov. Devel. 2004;7(1):75–85. [PubMed] [Google Scholar]
- 4.Benet L.Z., Izumi T., Zhang Y., Silverman J.A., Wacher V.J. Intestinal MDR transport proteins and P-450 enzymes as barriers to oral drug delivery. J. Control. Release. 1999;62(1-2):25–31. doi: 10.1016/s0168-3659(99)00034-6. [DOI] [PubMed] [Google Scholar]
- 5.Thummel K.E. Gut instincts: CYP3A4 and intestinal drug metabolism. J. Clin. Invest. 2007;117(11):3173–3176. doi: 10.1172/JCI34007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galetin A., Hinton L.K., Burt H., Obach R.S., Houston J.B. Maximal inhibition of intestinal first-pass metabolism as a pragmatic indicator of intestinal contribution to the drug-drug interactions for CYP3A4 cleared drugs. Curr. Drug Metab. 2007;8(7):685–693. doi: 10.2174/138920007782109805. [DOI] [PubMed] [Google Scholar]
- 7.Hengstmann J.H., Konen W., Konen C., Eichelbaum M., Dengler H.J. Bioavailability of m-octopamine in man related to its metabolism. Eur. J. Clin. Pharmacol. 1975;8(1):33–39. doi: 10.1007/BF00616412. [DOI] [PubMed] [Google Scholar]
- 8.Huang S.M., Athanikar N.K., Sridhar K., Huang Y.C., Chiou W.L. Pharmacokinetics of chlorpheniramine after intravenous and oral administration in normal adults. Eur. J. Clin. Pharmacol. 1982;22(4):359–365. doi: 10.1007/BF00548406. [DOI] [PubMed] [Google Scholar]
- 9.Nyberg L. Pharmacokinetic parameters of terbutaline in healthy man. An overview. Eur. J. Respir. Dis. Suppl. 1984;134:149–160. [PubMed] [Google Scholar]
- 10.Kolars J.C., Awni W.M., Merion R.M., Watkins P.B. First-pass metabolism of cyclosporin by the gut. Lancet. 1991;338(8781):1488–1490. doi: 10.1016/0140-6736(91)92302-i. [DOI] [PubMed] [Google Scholar]
- 11.Fromm M.F., Busse D., Kroemer H.K., Eichelbaum M. Differential induction of prehepatic and hepatic metabolism of verapamil by rifampin. Hepatology. 1996;24(4):796–801. doi: 10.1002/hep.510240407. [DOI] [PubMed] [Google Scholar]
- 12.Floren L.C., Bekersky I., Benet L.Z., Mekki Q., Dressler D., Lee J.W., Roberts J.P., Hebert M.F. Tacrolimus oral bioavailability doubles with coadministration of ketoconazole. Clin. Pharmacol. Ther. 1997;62(1):41–49. doi: 10.1016/S0009-9236(97)90150-8. [DOI] [PubMed] [Google Scholar]
- 13.Tuteja S., Alloway R.R., Johnson J.A., Gaber A.O. The effect of gut metabolism on tacrolimus bioavailability in renal transplant recipients. Transplantation. 2001;71(9):1303–1307. doi: 10.1097/00007890-200105150-00021. [DOI] [PubMed] [Google Scholar]
- 14.Gorski J.C., Jones D.R., Haehner-Daniels B.D., Hamman M.A., O’Mara E.M., Hall S.D. The contribution of intestinal and hepatic CYP3A to the interaction between midazolam and clarithromycin. Clin. Pharmacol. Ther. 1998;64(2):133–143. doi: 10.1016/S0009-9236(98)90146-1. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J.N., Liu X.G., Zhu M., Chiu F.C., Li R.C. Assessment of presystemic factors on the oral bioavailability of rifampicin following multiple dosing. J. Chemother. 1998;10(5):354–359. doi: 10.1179/joc.1998.10.5.354. [DOI] [PubMed] [Google Scholar]
- 16.Ho M.D., Ring N., Amaral K., Doshi U., Li A.P. Human enterocytes as an in vitro model for the evaluation of intestinal drug metabolism: Characterization of drug-metabolizing enzyme activities of cryopreserved human enterocytes from twenty-four donors. Drug Metab. Dispos. 2017;45(6):686–691. doi: 10.1124/dmd.116.074377. [DOI] [PubMed] [Google Scholar]
- 17.Godoy P., Hewitt N.J., Albrecht U., Andersen M.E., Ansari N., Bhattacharya S., Bode J.G., Bolleyn J., Borner C., Böttger J., Braeuning A., Budinsky R.A., Burkhardt B., Cameron N.R., Camussi G., Cho C.S., Choi Y.J., Craig R.J., Dahmen U., Damm G., Dirsch O., Donato M.T., Dong J., Dooley S., Drasdo D., Eakins R., Ferreira K.S., Fonsato V., Fraczek J., Gebhardt R., Gibson A., Glanemann M., Goldring C.E., Gómez-Lechón M.J., Groothuis G.M., Gustavsson L., Guyot C., Hallifax D., Hammad S., Hayward A., Häussinger D., Hellerbrand C., Hewitt P., Hoehme S., Holzhütter H.G., Houston J.B., Hrach J., Ito K., Jaeschke H., Keitel V., Kelm J.M., Kevin P.B., Kordes C., Kullak-Ublick G.A., LeCluyse E.L., Lu P., Luebke-Wheeler J., Lutz A., Maltman D.J., Matz-Soja M., McMullen P., Merfort I., Messner S., Meyer C., Mwinyi J., Naisbitt D.J., Nussler A.K., Olinga P., Pampaloni F., Pi J., Pluta L., Przyborski S.A., Ramachandran A., Rogiers V., Rowe C., Schelcher C., Schmich K., Schwarz M., Singh B., Stelzer E.H., Stieger B., Stöber R., Sugiyama Y., Tetta C., Thasler W.E., Vanhaecke T., Vinken M., Weiss T.S., Widera A., Woods C.G., Xu J.J., Yarborough K.M., Hengstler J.G. Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch. Toxicol. 2013;87(8):1315–1530. doi: 10.1007/s00204-013-1078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wood F.L., Houston J.B., Hallifax D. Clearance prediction methodology needs fundamental improvement: Trends common to rat and human hepatocytes/microsomes and implications for experimental methodology. Drug Metab. Dispos. 2017;45(11):1178–1188. doi: 10.1124/dmd.117.077040. [DOI] [PubMed] [Google Scholar]
- 19.Li A.P. Human hepatocytes: Isolation, cryopreservation and applications in drug development. Chem. Biol. Interact. 2007;168(1):16–29. doi: 10.1016/j.cbi.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Jones B.C., Srivastava A., Colclough N., Wilson J., Reddy V.P., Amberntsson S., Li D. An Investigation into the prediction of in vivo clearance for a range of flavin-containing monooxygenase substrates. Drug Metab. Dispos. 2017;45(10):1060–1067. doi: 10.1124/dmd.117.077396. [DOI] [PubMed] [Google Scholar]
- 21.Li A.P., Ho M.D., Amaral K., Loretz C. A novel in vitro experimental system for the evaluation of drug metabolism: Cofactor-supplemented permeabilized cryopreserved human hepatocytes (MetMax Cryopreserved Human Hepatocytes). Drug Metab. Dispos. 2018 doi: 10.1124/dmd.117.079657. [DOI] [PubMed] [Google Scholar]
- 22.Yan Z., Wong S., Kelly J., Le H., Liu N., Kosaka M., Kenny J.R., Li A.P., Yan Z. Utility of pooled cryopreserved human enterocytes as an in vitro model for assessing intestinal clearance and drug-drug interactions. Drug Metab. Lett. 2017;12(1):3–13. doi: 10.2174/1872312812666171213114422. [DOI] [PubMed] [Google Scholar]
- 23.Li A.P., Alam N., Ho M.C., Loretz C., Mitchell W., Yang Q. Cryopreserved human intestinal mucosal epithelium: A novel in vitro experimental system for the evaluation of enteric drug metabolism, P450 induction, and enterotoxicity. Drug Metab. Dispos. 2018 doi: 10.1124/dmd.118.082875. [Epub ahead of print]. [1]. [DOI] [PubMed] [Google Scholar]