Abstract
Objective:
In acute ischemic stroke, early neurological deterioration (END) may occur in up to one-third of patients. However, there is still no satisfying or comprehensive predictive model for all the stroke subtypes. We propose a practical model to predict END using magnetic resonance imaging (MRI).
Method:
Patients with anterior circulation infarct were recruited and they underwent an MRI within 24 hours of stroke onset. END was defined as an elevation of ≥2 points on the National Institute of Health Stroke Scale (NIHSS) within 72 hours of stroke onset. We examined the relationships of END to individual END models, including: A, infarct swelling; B, small subcortical infarct; C, mis-match; and D, recurrence.
Results:
There were 163 patients recruited and 43 (26.4%) of them had END. The END models A, B and C significantly predicted END respectively after adjusting for confounding factors (p=0.022, p=0.007 and p<0.001 respectively). In END model D, we examined all imaging predictors of Recur-rence Risk Estimator (RRE) individually and only the “multiple acute infarcts” pattern was signifi-cantly associated with END (p=0.032). When applying END models A, B, C and D, they success-fully predicted END (p<0.001; odds ratio: 17.5[95% confidence interval: 5.1–60.8]), with 93.0% sensitivity, 60.0% specificity, 45.5% positive predictive value and 96.0% negative predictive value.
Conclusion:
The results demonstrate that the proposed model could predict END in all stroke sub-types of anterior circulation infarction. It provides a practical model for clinical physicians to select high-risk patients for more aggressive treatment to prevent END.
Keywords: Early Neurological Deterioration (END), acute ischemic stroke, MRI, perfusion, stroke, MR
1. INTRODUCTION
In acute ischemic stroke, a number of patients suffer from worsening neurological deficits, commonly called Early Neurological Deterioration (END). END occurs in up to one-third of patients and it varies due to different definitions of END by inconsistent scales or timeframes [1]. No matter what definition for END is used, it often leads to greater mortality and functional disability.
Several mechanisms have been proposed to explain END in acute ischemic stroke, including failure of collaterals, clot progression, recurrent stroke, cerebral edema, seizures, hemorrhagic transformation or re-occlusion of a recanalised artery [2]. Among these, hemodynamic factors and perfusion abnormalities are likely to play a critical role in END [3], leading to the infarct growth in the same vascular territory. It is referred to as “progressive stroke”, which is usually irreversible irrespective of whether medical treatments are applied [4]. Therefore, it is important to develop a practical guide to predict which patients are at risk of END. The improvement of neuroimaging helps to sheds light on the prediction of END after ischemic stroke. Magnetic Resonance Imaging (MRI) is widely used in clinical practice, providing information about the intracranial and extracranial vessels by Magnetic Resonance (MR) angiography, infarct volume by Diffusion Weighted Imaging (DWI), and hemodynamic status by perfusion imaging.
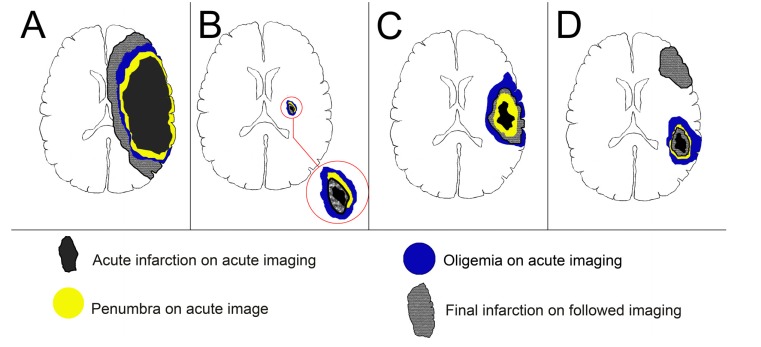
Based on the neuroimaging, Alawneh et al. proposed an operational classification of END after anterior circulation stroke by different mechanisms: 1. large infarct core, with further vasogenic edema to surrounding tissue; 2. large asymptomatic oligemia, with further transformation into infarction; 3. new ischemic event at previously healthy tissue in different vascular territory [3]. However, there was no practical definition for their classification. Moreover, the END classification by Alawneh et al. could not predict END in acute lacunar stroke because of small infarct and hypoperfused areas. Current studies have proved that infarct locations, Branch Atheromatous Disease (BAD) and perfusion abnormalities play important roles in the development of END in lacunar infarction [5-7]. Therefore, we introduce new neuroimaging END models A-D based on recent MRI studies, including infarct swelling, small subcortical infarct, mismatch and recurrence (Fig. 1). It represents the major underlying mechanisms of END, including cerebral edema, hemodynamic compromise and recurrent stroke.
Fig. (1).
Illustration of early neurological deterioration models: infarct swelling (A), small subcortical infarct (B), mismatch (C) and recurrence (D). END A represents brain swelling of a large acute infarction and the final infarction may exceed the area of oligemia due to edematous swelling of infarction tissue itself. END B shows small subcortical infarct and the END is due to the transformation of non-core hypoperfused area into infarction in the anatomy of corticospinal tracts. END C demonstrates the perfusion-diffusion mismatch concept and the END is related to the extension of infarction into a previously oligemic area. END D reveals a new ischemic area in a previously healthy area.
The concept of END model A, “large infarct core”, is derived from studies of malignant Middle Cerebral Artery (MCA) infarction (MMI), defined as a large infarction resulting in neurological deterioration due to edematous swelling of brain tissue with mass effect to the surrounding normal tissue. The most common predictor in Computed Tomography (CT) is the hypodense area beyond 50% of MCA territory. The DWI provides a more accurate estimation for core infarct volume and several cut points with an infarct volume over 78ml were associated with END and malignant MCA infarction (Table 1) [8-15]. The END model B, “small subcortical infarct”, is applied to evaluate END in small vessel disease, using specific infarction location and perfusion defect [5, 6]. The END model C, “mismatch”, uses the concept of perfusion-diffusion mismatch in MRI to predict END (Table 2) [16-18]. The END model D, “recurrence”, indicates a new ischemic event using imaging predictors of Recurrence Risk Estimator (RRE) score [19, 20].
Table 1.
MRI predictors for malignant middle cerebral artery infarction.
| MRI Predictor | Number | Image Time | Outcome |
Sensitivity/
Specificity/PPV/NPV |
Odds Ratio (95% Confidence Interval) P value | Study |
|---|---|---|---|---|---|---|
| DWI volume >78 ml DWI volume >78 ml + NIHSS≧22 after 24 h |
135 | <24 hours | MMI 2† | 59%/ 98%/ 89%/ 91% 79%/ 96%/ 94%/ 85% |
NA | Kruetzelmann et al. [11] |
| ADC<80% lesion volume >82 ml TTP lesion volume >162 ml |
37 | <6 hours | MMI 1* | 87%/ 91%/ 82%/ 92% 83%/ 75%/ 53%/ 86% |
NA | Thomalla et al. [15] |
| DWI volume >82 ml | 140 | <6 hours | MMI 2† | 52%/ 98%/ 88%/ 90% | 59.8 (12.2–292.8) p < 0.001 |
Thomalla et al. [14] |
| DWI volume >87 ml DWI volume >87 ml + Hemi-ICD |
116 | <6 hours | MMI 2† | 76%/ 93%/ 70%/ 95% 67%/ 99%/ 93% /93% |
p < 0.001 p=0.002 |
Beck et al. [9] |
| DWI volume >89 ml | 30 | <6 hours | Elevation of NIHSS≥4 within 48hours | 86%/ 96%/NA /NA | 11.5 (2.31–57.1) p=0.0028 |
Arenillas et al. [8] |
| DWI volume >102 m | 69 | <48 hours | MMI 3‡ | 85%/ 91%/ NA /NA | p <0.01 | Goto et al. [10] |
| DWI volume >145 ml | 61 | <14 hours | Herniation with deteriorated consciousness | 86%/ 88%/NA /NA | NA | Park et al. [13] |
| DWI volume >145 ml | 28 | <14 hours | MMI 4§ | 100%/ 94%/ 91%/ 100% | p <0.0001 | Oppenheim et al. [12] |
Abbreviations: ADC: APPARENT DIFFUSION COEFFICIENT; DWI: Diffusion Weighted Imaging; Hemi-ICD = Hemi-Intercaudate Distance; MMI: Malignant Middle Cerebral Artery (MCA) Infarction; NA: Not Available; NIHSS: National Institutes of Health Stroke Scale; TTP: Time to Peak; Tmax: Time to Maximum of Residual Tissue.
* MMI 1: Decline level of consciousness of ≥1 on item 1a of the NIHSS, and >2/3 MCA territory with compression of lateral ventricles or midline shift.
† MMI 2: MMI 1 + NIHSS˃18
‡ MMI 3: Decline in consciousness by Glasgow Coma Scale score and large space-occupying infarction with midline shift.
§ MMI 4: Deterioration of neurological and consciousness status with clinical signs of uncal herniation and mass effect.
Table 2.
Perfusion-diffusion mismatch in prediction of early neurological deterioration.
| Predictor | Number | Image Time | END |
Sensitivity/
Specificity/PPV/NPV |
Odds Ratio (95% Confidence Interval); P Value | Study |
|---|---|---|---|---|---|---|
| (Tmax>4s – DWI) >10ml | 137 | <24 hours | Any clinical deterioration | 77%/ 83%/ NA/ NA | NA | Asdaghi et al. [16] |
| (Tmax>6s/DWI) > 120% DWI < 100ml |
49 | <24 hours | NIHSS≧4 in 72 hours | 80%/ 79.5%/ NA/ NA | 17.0 (2.8~105.0); p<0.01 | Hsu et al. [17] |
| (Tmax>6s/DWI) > 120% + (Tmax>6s) > 10ml |
464 | <4.5 hours | NIHSS≧4 in 24 hours | 60%/ 67% / 9.4%/ 96.7% | NA | Simonsen et al. [18] |
| (Tmax>6s) > 35ml | 76%/ 70.4%/ 12.8%/ 98.1% | NA |
Abbreviations: DWI: Diffusion Weighted Imaging; END: Early Neurological Deterioration; NA: Not Available; NIHSS: National Institutes of Health Stroke Scale; NPV: Negative Predictive Value; PPV: Positive Predictive Value; Tmax: Time to Maximum of Residual Tissue.
In this study, we aim to investigate whether the END model can predict END occurrence within 72 hours after acute ischemic stroke.
2. MATERIALS AND METHODS
2.1. Patients
The clinical and imaging data were from two prospective studies using MRI to predict END at Chang Gung Memorial Hospital. In the prospective studies, patients were eligible to participate if they were 18 years of age or older, had a clinical diagnosis of ischemic stroke without thrombolytic therapy, and could undergo a complete MRI protocol within 24 hours after the onset of stroke, which was defined as the last time the patient was known to be without any neurological deficits. Patients with intravenous recombinant tissue plasminogen activator (rt-PA) or intra-arterial thrombectomy were excluded since these interventions will restore the compromised perfusion and significantly prevent END. The exclusion criteria in the prospective studies were patients: (1) with contraindications for MRI studies or gadolinium injections; (2) in whom DWI demonstrated no acute ischemic stroke; (3) with an acute ischemic stroke in the territories of posterior circulation. The prospective studies were approved by the Institutional Review Board of Chang Gung Memorial Hospital, and all examinations were performed after obtaining written informed consent from the patients or appropriate family members.
In the prospective studies, the neurological deficits were prospectively evaluated using the National Institute of Health Stroke Scale (NIHSS) on admission by a stroke neurologist or study nurse who was blinded to the patient’s MRI. Data on age, sex, cigarette smoking status, and a medical history of hypertension, diabetes mellitus, hypercholesterolemia, atrial fibrillation, prior coronary artery disease and prior cerebrovascular disease were recorded. Systolic and diastolic blood pressure values, blood biochemistry and cell counts were determined on admission. Complete surveys for stroke etiologies including electrocardiogram, carotid and transcranial Doppler and/or echocardiography were performed. END was defined as an elevation of ≧2 points on NIHSS within 72 hours of stroke onset [21-23]. Clinical outcomes at 3 months were evaluated using the modified Rankin Scale (mRS) by a study nurse who was blinded to the patient’s brain imaging. A good outcome was defined as a mRS score of 2 or less, and a favorable outcome was defined as a mRS score of 0 or 1. Mortality at 3 months was also recorded.
2.2. MRI Protocol and Image Analysis
All data were collected using a 3 Tesla Siemens Verio MRI system (Siemens Medical System, Erlangen, Germany) with a 16-channel head coil. The first MRI protocol included axial DWI, axial T1- and T2 images, MR angiography, fluid-attenuated inversion recovery (FLAIR) imaging and dynamic susceptibility contrast perfusion imaging. The follow-up protocol, including DWI, axial T1- and T2 images, MR angiography and FLAIR imaging, was followed on the 7th day after stroke onset. The detail MRI protocol and post-processing analysis were same to our previous study [24]. The parameters of time to maximum of residual tissue (Tmax) and Cerebral Blood Flow (CBF) were used to evaluate the hemodynamics. The imaging data were evaluated by two experienced stroke neurologists (Y.C.H. and Y.T.P.) and one neuroradiologist (Y.H.T.).
2.3. Definition of END Model
The END model includes 4 types (Fig. 1), with following definitions for positive END:
END A, infarct swelling: an acute infarction over 80ml in DWI.
END B, small subcortical infarct: a single subcortical infarction of less than 20mm in diameter in the territory of penetrating arteries, with an acute DWI lesion in the posterior limb of the internal capsule or posterior location of the centrum semiovale, combined with a visible perfusion defect in CBF.
END C, mismatch: (Tmax>6s volume)/(DWI infarct volume) ratio > 120% and a Tmax>6s volume>10ml.
END D, recurrence: imaging predictors in the RRE score, including multiple acute infarcts, simultaneous infarcts in different circulations, multiple infarcts of different ages and isolated cortical infarcts.
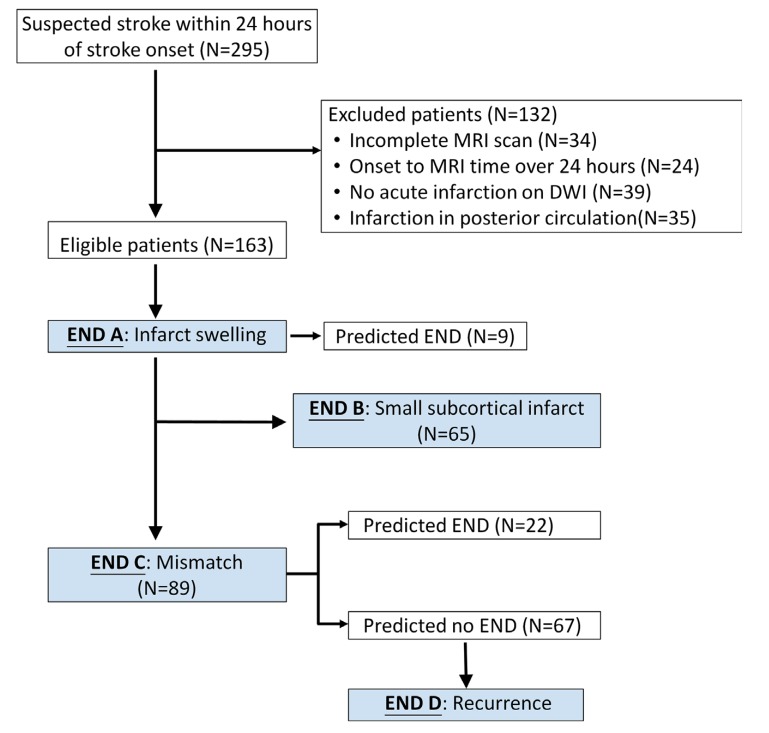
The selection flowchart for END models is shown in Fig. (2).
Fig. (2).
Flow diagram of study participants for END models. A total of 163 patients were eligible to enter this study. First, all patients were evaluated for END model A and 9 patients were stratified. The others were selected for evaluation of END model B when a single subcortical infarction of less than 20mm in diameter in the territory of penetrating arteries in DWI was present. After the exclusion of patients with positive model END A (N=9) and small subcortical infarction (N=65), the remaining patients (N=89) were evaluated for END model C. After excluding patients who were positive for END model C (N=22), the others with negative END model C (N=67) were surveyed for END model D.
2.4. Statistical Analysis
All statistical analyses were performed using the Statistical Program for Social Sciences (SPSS) statistical software (version 18, Chicago, IL, USA). Continuous variables were expressed as means ± standard deviations or median and interquartile range. The differences between the two groups were analyzed with the Mann-Whitney U test or Student t-test after testing for normality. Categorical data were analyzed using Fisher’s exact or Pearson’s Chi-Square test, as appropriate. Cohen’s Kappa coefficient was used to examine the reliability between the image readers. A multivariate logistic regression model was constructed to adjust for baseline variables when a p-value <0.1 was found in the univariate analysis in each END model in the prediction of END. All p values were two-tailed and a p value < 0.05 was considered to be statistically significant.
3. Results
A total of 295 patients with suspected stroke within 24 hours of the onset of symptoms were evaluated during the study period. The selection flowchart is shown in Fig. (2). A total of 163 eligible patients were recruited and the demographic data are shown in Table 3.
Table 3.
Baseline characteristics, imaging findings and outcomes in patients with and without END.
|
END
N=43 |
No END
N=120 |
p | |
|---|---|---|---|
| Age (year) | 72.6±14.6 | 70.6±10.9 | p=0.061 |
| Sex (Female) | 18(41.9%) | 55(45.8%) | p=0.653 |
| BMI | 24.3±3.9 | 24.7±3.9 | p=0.491 |
| Atrial fibrillation | 13(30.2%) | 29(24.2%) | p=0.435 |
| Diabetes mellitus | 21(48.8%) | 53(44.2%) | p=0.598 |
| Hypertension | 36(83.7%) | 95(79.2%) | p=0.519 |
| Hyperlipidemia | 32(74.4%) | 85(70.8%) | p=0.654 |
| Coronary artery disease | 4(9.3%) | 7(5.8%) | p=0.483 |
| Old stroke | 15(34.9%) | 26(21.7%) | p=0.087 |
| Smoking | 8(18.6%) | 31(25.8%) | p=0.340 |
| Systolic blood pressure (mmHg) | 176.7±39.0 | 169.5±34.0 | p=0.397 |
| Diastolic blood pressure (mmHg) | 99.9±20.6 | 95.7±17.5 | p=0.390 |
| Sugar (mg/dL) | 151.3±73.5 | 149.0±64.0 | p=0.997 |
| Onset-MRI duration (hour) | 12.8±7.1 | 14.5±6.9 | p=0.202 |
| NIHSS baseline | 11.0±8.6 | 6.7±5.8 | p=0.003 |
| NIHSS on 3rd day | 14.0±9.6 | 5.0±5.4 | p<0.001 |
| Core infarct volume (ml) | 45.0±87.6 | 8.1±18.2 | p=0.012 |
| Tmax >6s volume (ml) | 73.1±118.3 | 6.6±21.3 | p<0.001 |
| Final infarct volume (ml) | 74.3±120.6 | 11.4±24.8 | p<0.001 |
| Infarct growth (ml) | 26.9±48.9 | 3.3±10.7 | p<0.001 |
| mRS at 3-months | 3.7±1.7 | 1.5±1.8 | p<0.001 |
| Favorable outcome at 3-months | 5(11.6%) | 73(60.8%) | p<0.001 |
| Good outcome at 3-months | 10(23.3%) | 86(71.7%) | p<0.001 |
| Mortality at 3-months | 6(14.0%) | 2(1.7%) | p=0.001 |
All data was expressed as mean ± standard deviation or presented as counts and percentages.
Abbreviations:
BMI: Body Mass Index; END: Early Neurological Deterioration; MRI: Magnetic Resonance Imaging; NIHSS: National Institute of Health Stroke Scale; mRS: Modified Rankin Scale; Tmax: Time to Maximum of Residual Tissue.
Among the 163 patients, there were 43 patients (26.4%) who had END within 72 hours after stroke onset. There were no differences in age, sex ratio, rate of stroke risk factors, blood pressure or sugar at arrival, and duration of onset to MRI between patients with or without END. Compared to patients without END, those with END had higher initial NIHSS (11.0 vs. 6.7, p=0.003), higher NIHSS on third day (14.0 vs. 5.0, p<0.001), larger initial infarction volume (45.0ml vs. 8.1ml, p=0.012), larger initial perfusion defect volume (Tmax >6s: 73.1ml vs. 6.6ml, p<0.001), larger final infarction volume (74.3ml vs. 11.4ml; p<0.001), less good and favorable outcomes (11.6% vs. 60.8%, p<0.001; 23.3% vs. 71.7%, p<0.001), and higher mortality rate (14.0% vs. 1.7%, p=0.001). The Cohen’s Kappa coefficient was 0.816 to predict END model B between 2 image readers.
Table 4 shows the results of each END model in the prediction of END. The END models A, B and C significantly predicted END respectively after adjusting for confounding factors (p=0.022, odds ratio: 15.8; p=0.007, odds ratio: 23.6; p<0.001, odds ratio: 12.8). In END model C, 22 patients were predicted as positive END and 67 patients were predicted as negative END. The 67 patients were selected for END model D. We examined each imaging predictor of REE (Supplementary Table 1 (112.3KB, pdf) ) and only the “multiple acute infarcts” pattern was significantly associated with END (p=0.028). Therefore, “multiple acute infarcts” pattern was selected to define END model D and it significantly predicted END after adjusting for confounding factors (p=0.032; odds ratio: 7.2).
Table 4.
END model in prediction of early neurological deterioration.
| END Model | Total Number | All END Number (%) | Predicted END Number | Successfully Predicted END Number | Parameters | p |
Odds Ratio
(95% Confidence Interval) |
Sensitivity/
Specificity/PPV/NPV |
|---|---|---|---|---|---|---|---|---|
| A | 163 | 43 (26.1%) | 9 | 8 | DWI > 80ml | 0.003 0.022* |
25.6(3.1 – 211.8) 15.8(1.5 – 166.0)* |
18.6%/ 99.2%/ 88.9%/ 77.3% |
| B | 65 | 12 (18.5%) | 30 | 11 | Perfusion defect in CBF maps + infarct location | 0.006 0.007† |
19.7(2.4 – 164) 23.6(2.4 – 233.5)† |
91.7%/ 64.2%/ 36.7%/ 97.1% |
| C | 89 | 23 (25.8%) | 22 | 14 | (Tmax>6s/DWI) > 120% + (Tmax>6s) > 10ml | <0.001 <0.001‡ |
11.3(3.7 – 34.5) 12.8(3.8 – 42.7) ‡ |
60.9%/ 87.9%/ 63.6%/ 86.6% |
| D | 67 | 9 (14.3%) | 28 | 7 | Multiple acute infarcts | 0.032 0.032‡ |
6.2(1.2 – 32.4) 7.2 (1.2 – 43.1)‡ |
77.8%/ 63.8%/ 25.0%/ 94.9% |
| A+B+C | 163 | 43 (26.1%) | 61 | 33 | Models A+B+C | <0.001 <0.001* |
10.8(4.8 – 24.7) 9.3(4.0 – 21.6) * |
76.7%/ 76.7%/ 54.1%/ 90.2% |
| A+B+C+D | 163 | 43 (26.1%) | 89 | 40 | Models A+B+C+D | <0.001 <0.001* |
20.0(5.9 – 68.3) 17.5(5.1 – 60.8)* |
93.0%/ 60.0%/ 45.5%/ 96.0% |
Abbreviations:
DWI: Diffusion Weighted Imaging; END: Early Neurological Deterioration; NPV: Negative Predictive Value; PPV: Positive Predictive Value; Tmax: Time to Maximum of Residual Tissue.
A multivariate logistic regression model was constructed to adjust for baseline variables when a p-value <0.1 was found in the univariate analysis:
* Adjusted for old stroke and baseline NIHSS.
† Adjusted for old stroke and sex.
‡ Adjusted for smoking and sex.
END model A had the highest specificity and positive predictive value (PPV), whereas END model B had the highest sensitivity and negative predictive value (NPV). When combined, END models A, B and C could predict END (p<0.001, odds ratio: 9.3) with 76.7% sensitivity, 76.7% specificity, 54.1% PPV and 90.2% NPV. When using END models A, B, C and D, END could be predicted (p<0.001; odds ratio: 17.5[95% confidence interval: 5.1–60.8]), with 93.0% sensitivity, 60.0% specificity, 45.5% PPV and 96.0% NPV.
4. Discussion
In this study, we propose a practical neuroimaging model to predict END in acute ischemic stroke, including infarction swelling, small subcortical infarct, mismatch and recurrence. The results demonstrate that the proposed model can predict END in all stroke subtypes of anterior circulation infarction. It provides a practical way for clinical physicians to select high-risk patients for more aggressive treatment to prevent END.
The END model A was well documented in MMI and early infarction volumes measured by DWI with cut points over 78ml all have high sensitivity and specificity to predict END [8-15]. END in the MMI trial was usually defined as the deterioration of consciousness with evidence of brain swelling. In addition, the early decompressive hemicraniectomy within 24 hours of stroke onset in patients with large MCA infarction was proved to reduce mortality and improve functional outcomes [25]. Furthermore, a large infarct core >100ml was associated with a worse outcome after reperfusion therapy due to brain swelling and hemorrhage transformation [26]. Therefore, for patients with END model A, early decompressive hemicraniectomy should be considered in selected patients but reperfused therapy should be prudently selected.
END model B, small subcortical infarct, is applied to evaluate END in small vessel disease. Several imaging predictors have been proposed to predict END including BAD [7], posterior location of centrum semiovale [5] and perfusion defect [6, 27, 28]. In this study, positive END model B was defined as an acute infarction in the posterior limb of the internal capsule or posterior location of the centrum semiovale, combined with a perfusion defect in CBF. It successfully predicts END with high sensitivity (91.7%) but the specificity (64.2%) and PPV (36.7%) were not satisfactory. Some patients with perfusion defects did not suffer from progressive motor deficits most likely because the perfusion defect and infarct growth did not involve the corticospinal tract. Nevertheless, given the high sensitivity in predicting END and less hemorrhagic complications to antithrombotic agents [29], the selected patients in END model B may merit careful consideration for aggressive treatment such as dual antiplatelet [30-32], statin [33] or short-term anticoagulant [34].
END model C used perfusion-diffusion mismatch to predict END. In acute ischemic stroke, the threshold of a 6s delay in Tmax is well adopted to salvage the penumbral tissue in reperfusion therapy [35-37] and was also proved effective to predict END (Table 2) [16-18]. Several reasons may help explain these paradoxical findings. The most likely reason is that there is no absolute cut point between penumbra and oligemia because the ischemic tolerance of the neurons may be different [38]. Furthermore, the “benign oligemia” is not always benign, and it still runs the risk of transforming into an infarction [39]. Therefore, the threshold of Tmax˃6s may contain both penumbra and oligemia, resulting in END model C being highly associated with END. However, the sensitivity and PPV in this model were not satisfactory. Some patients with positive END model C did not suffer from END probably due to the further restoration of perfection defects. Additionally, some infarct growth may not be manifested in NIHSS, and this may be the reason for the relatively low PPV. END model C did not predict 9 END patients but 7 of them were classified as positive END model D. This may suggest that additional arterial emboli may account for this END. The impaired clearance of emboli may increase the risk of END despite the perfusion compromise which doesn’t meet the criteria of the 6s lag in Tmax [40]. Further studies are needed to determine the optimal criteria, including Tmax threshold, hypoperfused volume or hypoperfused/infarct ratio. END model C provides an opportunity to select high-risk patients for more aggressive medical treatment or further endovascular thrombectomy.
END type D refers a recurrence of stroke, especially from cardioembolism or artery-to-artery embolism. The RRE score had been confirmed useful in prediction of early recurrent stroke within 7 days or within 90 days after stroke onset [19, 20]. The imaging predictor of “multiple acute infarcts” significantly predicts END, and is more likely to result from artery-to-artery emboli and hemodynamic compromise in large artery atherosclerosis [41-43]. The other predictors of “simultaneous infarcts in different circulations” and “isolated cortical infarcts” are associated to cardioembolism [44, 45] but they did not significantly predict END, probably due to a low recurrence rate in the acute stage. Therefore, the criteria of “multiple acute infarcts” in END type D focus on the large artery atherosclerosis. In these patients with a high risk of END, aggressive medical treatment is necessary for intracranial stenosis, whereas aggressive medical treatment followed by early endarterectomy is warranted in instances of internal carotid stenosis with minor stroke [46, 47].
Our study has certain limitations. First, the models in our study may not be extended to patients with posterior circulation infarction or with reperfusion therapy. Second, the timeframe from stroke onset to MRI scan was up to 24 hours and the optimal time in the prevention of END may gradually pass. However, the perfusion-diffusion mismatch was proved up to 48 hours in nonhuman primates [48] and more than half of END developed after 24 hours following stroke onset. Therefore, substantial salvageable tissue may still exist in this time window to prevent END. Third, the patient number in our study is relatively small. Further studies are necessary to validate and modify our model. Furthermore, attempting to predict END with a single MRI scan may be oversimplifying the underlying pathophysiology since the hemodynamic change after a stroke is a dynamic process.
Conclusion
The new practical END models successfully predict END within 72 hours after stroke onset in patients with anterior circulation infarction. Although not satisfactory, the proposed models are the most comprehensive imaging predictors of END in ischemic stroke and provide clinical physicians opportunities to select patients for clinical trials in the prevention END.
Ethics Approval and Consent to Participate
The prospective studies were approved by the Institutional Review Board of Chang Gung Memorial Hospital.
Human and Animal Rights
No Animals were used for studies that are base of this research. All the humans used were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013 (http://ethics.iit.edu/ecodes/node/3931).
Consent for Publication
All participants or their guardians gave their informed consent.
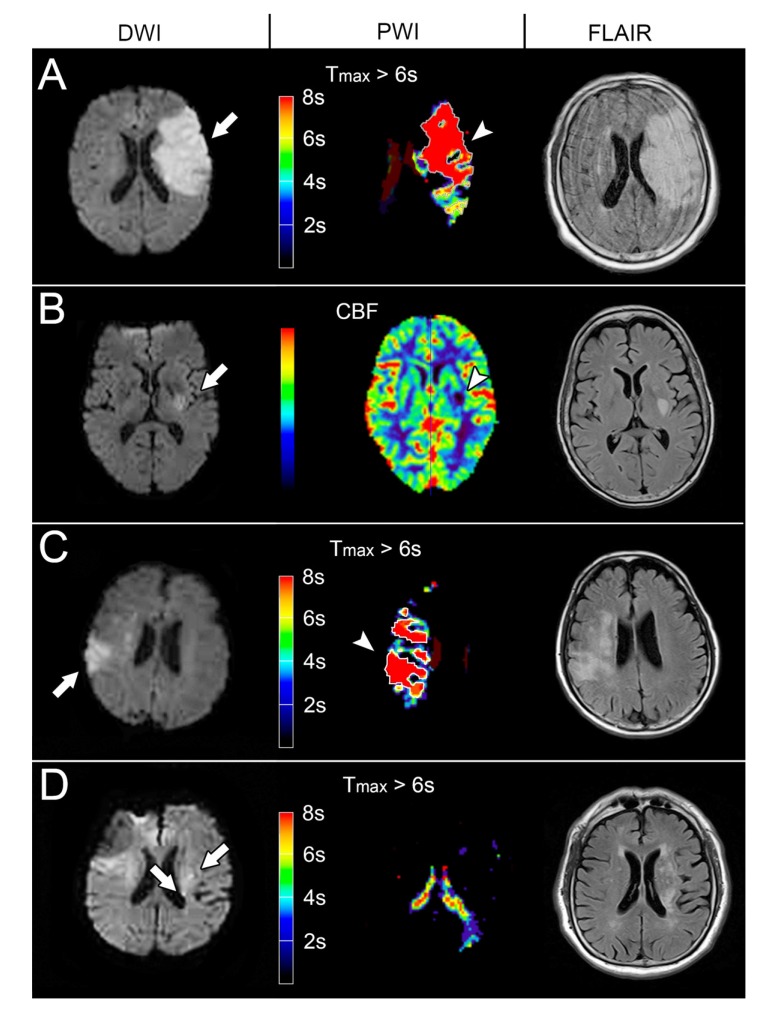
Fig. (3).
The END models of the representative patients with Diffusion Weighted Imaging (DWI), Perfusion-Weighted Image (PWI) and Fluid-Attenuated Inversion Recovery (FLAIR) imaging. (A) The DWI at 19.7 hours after stroke onset showed an acute infarct in the left Middle Cerebral Artery (MCA) territory (97.2ml, arrow), indicating positive infarct swelling model. The follow-up FLAIR imaging showed progressed infarct and brain swelling (119.8ml). (B) The DWI at 7.6 hours after stroke onset showed an acute infarct in the posterior limb of the left internal capsule (3.5ml, arrow) and a visible perfusion defect in the CBF maps (arrowhead), representing positive small subcortical infarct model. The follow-up FLAIR imaging showed infarct growth (9.3ml). (C) The DWI at 8.2 hours after stroke onset showed an infarction in the right MCA territory (22.6ml, arrow) and larger perfusion defect (Tmax > 6 seconds: 37.3 ml; arrowhead). The Tmax > 6s /infarct core ratio was 1.65 suggesting a mismatch model. The follow-up FLAIR imaging showed infarct growth (31.5 ml). (D) The DWI at 9.0 hours showed multiple acute infarction (0.6 ml, arrow) in the left centrum semiovale without perfusion defect, suggesting a recurrence model. The follow-up FLAIR imaging showed infarct growth (9.0 ml).
ACKNOWLEDGEMENTS
We thank Yi-Chen Kuo for assisting us with this study.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s web site along with the published article.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
Study funding
Supported by Chang Gung Memorial Hospital research grants (CMRPG6F0481, CORPG6D0132 and CORPG6D 0133).
ReferenceS
- 1.Sedlaczek O., Caplan L., Hennerici M. Impaired washout--embolism and ischemic stroke: further examples and proof of concept. Cerebrovasc. Dis. 2005;19(6):396–401. doi: 10.1159/000085831. [DOI] [PubMed] [Google Scholar]
- 2.Thanvi B., Treadwell S., Robinson T. Early neurological deterioration in acute ischaemic stroke: predictors, mechanisms and management. Postgrad. Med. J. 2008;84(994):412–417. doi: 10.1136/pgmj.2007.066118. [DOI] [PubMed] [Google Scholar]
- 3.Alawneh J.A., Moustafa R.R., Baron J.C. Hemodynamic factors and perfusion abnormalities in early neurological deterioration. Stroke. 2009;40(6):e443–e450. doi: 10.1161/STROKEAHA.108.532465. [DOI] [PubMed] [Google Scholar]
- 4.Siegler J.E., Boehme A.K., Albright K.C., et al. A proposal for the classification of etiologies of neurologic deterioration after acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 2013;22(8):e549–e556. doi: 10.1016/j.jstrokecerebrovasdis.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohara T., Yamamoto Y., Tamura A., et al. The infarct location predicts progressive motor deficits in patients with acute lacunar infarction in the lenticulostriate artery territory. J. Neurol. Sci. 2010;293(1-2):87–91. doi: 10.1016/j.jns.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 6.Huang Y.C., Tsai Y.H., Lee J.D., et al. Hemodynamic factors may play a critical role in neurological deterioration occurring within 72 hrs after lacunar stroke. PLoS One. 2014;9(10):e108395. doi: 10.1371/journal.pone.0108395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamamoto Y., Ohara T., Hamanaka M., et al. Characteristics of intracranial branch atheromatous disease and its association with progressive motor deficits. J. Neurol. Sci. 2011;304(1-2):78–82. doi: 10.1016/j.jns.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Arenillas J.F., Rovira A., Molina C.A., et al. Prediction of early neurological deterioration using diffusion- and perfusion-weighted imaging in hyperacute middle cerebral artery ischemic stroke * editorial comment. Stroke. 2002;33(9):2197–2205. doi: 10.1161/01.str.0000027861.75884.df. [DOI] [PubMed] [Google Scholar]
- 9.Beck C., Kruetzelmann A., Forkert N.D., et al. A simple brain atrophy measure improves the prediction of malignant middle cerebral artery infarction by acute DWI lesion volume. J. Neurol. 2014;261(6):1097–1103. doi: 10.1007/s00415-014-7324-9. [DOI] [PubMed] [Google Scholar]
- 10.Goto Y., Kumura E., Watabe T., et al. Prediction of malignant middle cerebral artery infarction in elderly patients. J. Stroke Cerebrovasc. Dis. 2016;25(6):1389–1395. doi: 10.1016/j.jstrokecerebrovasdis.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 11.Kruetzelmann A., Hartmann F., Beck C., et al. Combining magnetic resonance imaging within six-hours of symptom onset with clinical follow-up at 24 h improves prediction of ‘malignant’ middle cerebral artery infarction. Int. J. Stroke. 2014;9(2):210–214. doi: 10.1111/ijs.12060. [DOI] [PubMed] [Google Scholar]
- 12.Oppenheim C., Samson Y., Manai R., et al. Prediction of malignant middle cerebral artery infarction by diffusion-weighted imaging. Stroke. 2000;31(9):2175–2181. doi: 10.1161/01.str.31.9.2175. [DOI] [PubMed] [Google Scholar]
- 13.Park J., Goh D.H., Sung J.K., et al. Timely assessment of infarct volume and brain atrophy in acute hemispheric infarction for early surgical decompression: strict cutoff criteria with high specificity. Acta Neurochir. (Wien) 2012;154(1):79–85. doi: 10.1007/s00701-011-1178-z. [DOI] [PubMed] [Google Scholar]
- 14.Thomalla G., Hartmann F., Juettler E., et al. Prediction of malignant middle cerebral artery infarction by magnetic resonance imaging within 6 hours of symptom onset: A prospective multicenter observational study. Ann. Neurol. 2010;68(4):435–445. doi: 10.1002/ana.22125. [DOI] [PubMed] [Google Scholar]
- 15.Thomalla G.J., Kucinski T., Schoder V., et al. Prediction of malignant middle cerebral artery infarction by early perfusion- and diffusion-weighted magnetic resonance imaging. Stroke. 2003;34(8):1892–1899. doi: 10.1161/01.STR.0000081985.44625.B6. [DOI] [PubMed] [Google Scholar]
- 16.Asdaghi N., Hill M.D., Coulter J.I., et al. Perfusion MR predicts outcome in high-risk transient ischemic attack/minor stroke: A derivation-validation study. Stroke. 2013;44(9):2486–2492. doi: 10.1161/STROKEAHA.111.000208. [DOI] [PubMed] [Google Scholar]
- 17.Hsu C.Y., Cheng C.Y., Tsai Y.H., et al. Perfusion-diffusion mismatch predicts early neurological deterioration in anterior circulation infarction without thrombolysis. Curr. Neurovasc. Res. 2015;12(3):277–282. doi: 10.2174/1567202612666150605122536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simonsen C.Z., Schmitz M.L., Madsen M.H., et al. Early neurological deterioration after thrombolysis: Clinical and imaging predictors. Int. J. Stroke. 2016;11(7):776–782. doi: 10.1177/1747493016650454. [DOI] [PubMed] [Google Scholar]
- 19.Arsava E.M., Kim G.M., Oliveira-Filho J., et al. Prediction of Early Recurrence After Acute Ischemic Stroke. JAMA Neurol. 2016;73(4):396–401. doi: 10.1001/jamaneurol.2015.4949. [DOI] [PubMed] [Google Scholar]
- 20.Maier I.L., Bauerle M., Kermer P., et al. Risk prediction of very early recurrence, death and progression after acute ischaemic stroke. Eur. J. Neurol. 2013;20(4):599–604. doi: 10.1111/ene.12037. [DOI] [PubMed] [Google Scholar]
- 21.Siegler J.E., Martin-Schild S. Early Neurological Deterioration (END) after stroke: The END depends on the definition. Int. J. Stroke. 2011;6(3):211–212. doi: 10.1111/j.1747-4949.2011.00596.x. [DOI] [PubMed] [Google Scholar]
- 22.Chen Z., Li W., Sun W., et al. Correlation study between small vessel disease and early neurological deterioration in patients with mild/moderate acute ischemic stroke. Int. J. Neurosci. 2016;•••:1–7. doi: 10.1080/00207454.2016.1214825. [DOI] [PubMed] [Google Scholar]
- 23.Siegler J.E., Boehme A.K., Kumar A.D., et al. What change in the National Institutes of Health Stroke Scale should define neurologic deterioration in acute ischemic stroke? J. Stroke Cerebrovasc. Dis. 2013;22(5):675–682. doi: 10.1016/j.jstrokecerebrovasdis.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin C.H., Tsai Y.H., Lee J.D., et al. Magnetic resonance perfusion imaging provides a significant tool for the identification of cardioembolic stroke. Curr. Neurovasc. Res. 2016;13(4):271–276. doi: 10.2174/1567202613666160901143040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heiss W.D. Malignant MCA Infarction: Pathophysiology and imaging for early diagnosis and management decisions. Cerebrovasc. Dis. 2016;41(1-2):1–7. doi: 10.1159/000441627. [DOI] [PubMed] [Google Scholar]
- 26.Albers G.W., Thijs V.N., Wechsler L., et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann. Neurol. 2006;60(5):508–517. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- 27.Poppe A.Y., Coutts S.B., Kosior J., et al. Normal magnetic resonance perfusion-weighted imaging in lacunar infarcts predicts a low risk of early deterioration. Cerebrovasc. Dis. 2009;28(2):151–156. doi: 10.1159/000225908. [DOI] [PubMed] [Google Scholar]
- 28.Yamada M., Yoshimura S., Kaku Y., et al. Prediction of neurologic deterioration in patients with lacunar infarction in the territory of the lenticulostriate artery using perfusion CT. AJNR Am. J. Neuroradiol. 2004;25(3):402–408. [PMC free article] [PubMed] [Google Scholar]
- 29.Pan Y.T., Lee J.D., Lin Y.H., et al. Comparisons of outcomes in stroke subtypes after intravenous thrombolysis. Springerplus. 2016;5:47. doi: 10.1186/s40064-016-1666-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kimura T., Tucker A., Sugimura T., et al. Ultra-early combination antiplatelet therapy with cilostazol for the prevention of branch atheromatous disease: A Multicenter Prospective Study. Cerebrovasc. Dis. Extra. 2016;6(3):84–95. doi: 10.1159/000450835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang C., Yi X., Zhang B., et al. Clopidogrel plus aspirin prevents early neurologic deterioration and improves 6-month outcome in patients with acute large artery atherosclerosis stroke. Clin. Appl. Thromb. Hemost. 2015;21(5):453–461. doi: 10.1177/1076029614551823. [DOI] [PubMed] [Google Scholar]
- 32.Wong K.S., Wang Y., Leng X., et al. Early dual versus mono antiplatelet therapy for acute non-cardioembolic ischemic stroke or transient ischemic attack: An updated systematic review and meta-analysis. Circulation. 2013;128(15):1656–1666. doi: 10.1161/CIRCULATIONAHA.113.003187. [DOI] [PubMed] [Google Scholar]
- 33.Fang J.X., Wang E.Q., Wang W., et al. The efficacy and safety of high-dose statins in acute phase of ischemic stroke and transient ischemic attack: A systematic review. Intern. Emerg. Med. 2017;12(5):679–687. doi: 10.1007/s11739-017-1650-8. [DOI] [PubMed] [Google Scholar]
- 34.Yi X., Chi W., Wang C., et al. Low-molecular-weight heparin or dual antiplatelet therapy is more effective than aspirin alone in preventing early neurological deterioration and improving the 6-month outcome in ischemic stroke patients. J. Clin. Neurol. 2015;11(1):57–65. doi: 10.3988/jcn.2015.11.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campbell B.C., Mitchell P.J., Kleinig T.J., et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N. Engl. J. Med. 2015;372(11):1009–1018. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 36.Lansberg M.G., Straka M., Kemp S., et al. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): A prospective cohort study. Lancet Neurol. 2012;11(10):860–867. doi: 10.1016/S1474-4422(12)70203-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saver J.L., Goyal M., Bonafe A., et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N. Engl. J. Med. 2015;372(24):2285–2295. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 38.Zemke D., Smith J.L., Reeves M.J., et al. Ischemia and ischemic tolerance in the brain: An overview. Neurotoxicology. 2004;25(6):895–904. doi: 10.1016/j.neuro.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 39.Alawneh J.A., Jones P.S., Mikkelsen I.K., et al. Infarction of ‘non-core-non-penumbral’ tissue after stroke: Multivariate modelling of clinical impact. Brain. 2011;134(Pt 6):1765–1776. doi: 10.1093/brain/awr100. [DOI] [PubMed] [Google Scholar]
- 40.Caplan L.R., Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch. Neurol. 1998;55(11):1475–1482. doi: 10.1001/archneur.55.11.1475. [DOI] [PubMed] [Google Scholar]
- 41.Bang O.Y., Lee P.H., Heo K.G., et al. Specific DWI lesion patterns predict prognosis after acute ischaemic stroke within the MCA territory. J. Neurol. Neurosurg. Psychiatry. 2005;76(9):1222–1228. doi: 10.1136/jnnp.2004.059998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee D.K., Kim J.S., Kwon S.U., et al. Lesion patterns and stroke mechanism in atherosclerotic middle cerebral artery disease: Early diffusion-weighted imaging study. Stroke. 2005;36(12):2583–2588. doi: 10.1161/01.STR.0000189999.19948.14. [DOI] [PubMed] [Google Scholar]
- 43.Lopez-Cancio E., Matheus M.G., Romano J.G., et al. Infarct patterns, collaterals and likely causative mechanisms of stroke in symptomatic intracranial atherosclerosis. Cerebrovasc. Dis. 2014;37(6):417–422. doi: 10.1159/000362922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kang D.W., Chalela J.A., Ezzeddine M.A., et al. Association of ischemic lesion patterns on early diffusion-weighted imaging with TOAST stroke subtypes. Arch. Neurol. 2003;60(12):1730–1734. doi: 10.1001/archneur.60.12.1730. [DOI] [PubMed] [Google Scholar]
- 45.Rovira A., Grive E., Rovira A., et al. Distribution territories and causative mechanisms of ischemic stroke. Eur. Radiol. 2005;15(3):416–426. doi: 10.1007/s00330-004-2633-5. [DOI] [PubMed] [Google Scholar]
- 46.Shahidi S., Owen-Falkenberg A., Gottschalksen B., et al. Risk of early recurrent stroke in symptomatic carotid stenosis after best medical therapy and before endarterectomy. Int. J. Stroke. 2016;11(1):41–51. doi: 10.1177/1747493015609777. [DOI] [PubMed] [Google Scholar]
- 47.Tsantilas P., Kuehnl A., Konig T., et al. Short time interval between neurologic event and carotid surgery is not associated with an increased procedural risk. Stroke. 2016;47(11):2783–2790. doi: 10.1161/STROKEAHA.116.014058. [DOI] [PubMed] [Google Scholar]
- 48.Zhang X., Tong F., Li C.X., et al. Temporal evolution of ischemic lesions in nonhuman primates: A diffusion and perfusion MRI study. PLoS One. 2015;10(2):e0117290. doi: 10.1371/journal.pone.0117290. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publisher’s web site along with the published article.