Abstract
Huntington's Disease-Like 2 (HDL2), caused by a CTG/CAG expansion in JPH3 on chromosome 16q24, is the most common Huntington's Disease (HD) phenocopy in populations with African ancestry. Qualitatively, brain MRIs of HDL2 patients have been indistinguishable from HD. To determine brain regions most affected in HDL2 a cross-sectional study using MRI brain volumetry was undertaken to compare the brains of nine HDL2, 11 HD and nine age matched control participants. Participants were ascertained from the region in South Africa with the world's highest HDL2 incidence. The HDL2 and HD patient groups showed no significant differences with respect to mean age at MRI, disease duration, abnormal triplet repeat length, or age at disease onset. Overall, intracerebral volumes were smaller in both affected groups compared to the control group. Comparing the HDL2 and HD groups across multiple covariates, cortical and subcortical volumes were similar with the exception that the HDL2 thalamic volumes were smaller. Consistent with other similarities between the two diseases, these results indicate a pattern of neurodegeneration in HDL2 that is remarkably similar to HD. However smaller thalamic volumes in HDL2 raises intriguing questions into the pathogenesis of both disorders, and how these volumetric differences relate to their respective phenotypes.
Keywords: Huntington's Disease-like 2, Huntington's Disease, HD-phenocopy, Magnetic Resonance Imaging, MRI brain volumetry
Highlights
-
•
HDL2 is the most common HD phenocopy in populations with African ancestry.
-
•
Qualitatively comparisons of HDL2 and HD MRIs show grey and white matter, and striatal atrophy that is distinguishable.
-
•
MRI volumetry,comparing both groups, showed similar volumes except thalamic volume were significantly smaller in HDL2.
1. Introduction
Huntington's disease like 2 (HDL2) is the most common Huntington's disease (HD) phenocopy in populations with an African ancestry and is emerging as the most common HD phenocopy in South America, the USA and parts of Europe (Margolis et al., 2004; Mariani et al., 2016; Walker et al., 2018). South Africa has the highest number of HDL2 cases described despite the under ascertainment of patients due to lack of resources in rural communities (Anderson et al., 2017b; Baine et al., 2016).
Huntington's Disease is caused by a CAG/CTG repeat expansion in exon 1 of the huntingtin gene on chromosome 4p16.3, while HDL2 is caused by a similarly sized CTG/CAG expansion in exon 2A of junctophilin-3 (JPH3) on chromosome 16q24.3 (Holmes et al., 2001). Both are autosomal dominant disorders characterized by psychiatric disease, movement disorder and cognitive dysfunction which progress and inevitably result in death approximately 15 to 20 years after clinical onset (Margolis et al., 2001). Observations of the HDL2 phenotype are from retrospective and anecdotal cases and reviews (Anderson et al., 2017b). Notwithstanding these genetic differences and the need for systematic study of the HDL2 clinical phenotype, HDL2 is the disease that has been described to most resemble HD (Walker et al., 2003).
Furthermore, qualitative MRI studies have not been able to differentiate between HDL2 and HD. To date these rare reports suggest similar patterns of atrophy between these two disorders (Anderson et al., 2017b). A systematic review of 69 published cases of HDL2 reported 20 MRI scans of HDL2 patients described as indistinguishable from HD (Anderson et al., 2017b). An exception was a patient with HDL2 who presented with parkinsonism and myoclonus. The MRI of this case showed typical cerebral atrophy but relative sparing of the caudate nucleus (Bardien et al., 2007). A report of a single case of HDL2 having two MRIs five years apart described progressive atrophy of the same areas as seen in HD (Rodrigues et al., 2008). Hyperintense putaminal rims on T2 imaging have been described in three cases with HDL2 from the same family (Schneider et al., 2012). The putaminal rim sign has also been detected in a number of other degenerative movement disorders including Multi-System Atrophy (MSA), Neuro-Acanthocytosis, Dentatorubral-Pallidoluysian Atrophy (DRPLA) and Spinocerebellar Ataxia Type 17 (SCA17).
Despite the fact that HD and HDL2 are caused by mutations in different genes, which are linked to different functions within the cell, the majority of the qualitative MRI reports, neuropathological analysis, and clinical phenotype, highlight the similarities between both disorders. We therefore hypothesized that quantitative MRI analysis of HDL2 patients would reveal similar findings to those detected in HD patients. To test this hypothesis, we performed a blinded systematic imaging study of matched HD and HDL2 patients with a normal control group, taking advantage of the relatively high number of HDL2 cases that have been reported in the region surrounding Johannesburg, South Africa (Anderson et al., 2017b; Krause et al., 2015).Comparing the brain MRIs of these clinically similar diseases has the potential to guide further investigation into the differences and similarities between HD and HDL2 and forms part of an ongoing systematic characterization of the phenotype of HDL2.
2. Methods
Patients with HDL2 and HD were identified and diagnosed through the Division of Human Genetics, National Health Laboratory Service and the School of Pathology, The University of the Witwatersrand, in Johannesburg, South Africa. Controls were unrelated healthy acquaintances matched for gender, age and race. Participants were enrolled between January 2015 and June 2017 and all subjects had African or mixed ancestry including a significant African component. The Human Research Ethics Committee of The University of the Witwatersrand approved this cross-sectional study (M140872).
MRIs of subjects were performed at the University of the Witwatersrand Donald Gordon Medical Centre Radiology Department on a 1.5 T Philips Intera MR scanner (Philips Medical Systems, Eindhoven). There were no relevant scanner upgrades during the study. The MRI sequences used in this study are available in the supplement.
We used the Brain Research: Analysis of Images, Networks, and Systems (BRAINS) protocol, which has been validated and extensively used to measure intracerebral volumes for HD in the large PREDICT-HD study (Paulsen et al., 2008). Scans were processed by Brain Image Analysis, LLC using the cross-sectional analysis stream of NeuroAnalytica version 1.3.09, a commercial image analysis platform based on AutoWorkup (Pierson et al., 2011). A discriminant tissue classification was then performed (Harris et al., 1999). Artificial neural networks for each subcortical structure were applied (Powell et al., 2008). Additional information about this technique is available in the supplement.
Comparison of age at MRI, disease duration, abnormal repeat length, and age at onset among the groups was performed using a one-way ANOVA. Comparison of variables among the three groups, controlling for age at MRI and separately, for disease duration, expanded repeat length, and age of disease onset was performed using a General Linear Model. Post-hoc comparisons were performed using the Tukey-Kramer adjustment. The false discovery rate was controlled by the Benjamini-Hochberg procedure and significance was calculated at p < .0084. Data analysis was carried out using SAS version 9.4 for Windows.
3. Results
Images of nine HDL2, 11 HD and nine normal control participants were included in the study. None of the HDL2 participants were related. Three of the HD pedigrees each contributed two participants to the study. There were no significant differences among the groups with respect to mean age at MRI (F [2,26] = 0.54; p = .59), disease duration (F [1,18] =0.57; p = .57), abnormal repeat length (F [1,18] =0.18; p = .18), or age at onset (F [1,18] =0.63; p = .63) (Table 1).
Table 1.
Table showing subject demographic data and MRI volumes. a. Subject ages, age of disease onset, duration of disease and triplet repeat length. b. Unadjusted volumetric analysis of selected subcortical structures, hippocampi and total intracranial volumes in cm3. HD = Huntington's Disease, HDL2 = Huntington's Disease like 2, n = number SD = Standard Deviation.
| 1a Demographics and Genetics | HDL2 | HD | Controls |
|---|---|---|---|
| Number of participants | 9 | 11 | 9 |
| Male: Female | 5:4 | 6:5 | 5:4 |
|
| |||
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Age at MRI (years) | 47.6 (9.1) | 46.0 (12.4) | 42.6 (9.1) |
| Abnormal repeat length (n) | 47.0 (3.5) | 44.9 (3.2) | – |
| Duration of disease (years) | 5.6 (3.0) | 6.6 (4.8) | – |
| Age at onset(years) | 42.0 (10.2) | 39.4 (13.1) | – |
|
| |||
| 1b Region Volume (cm3) | Mean (SD) | Mean (SD) | Mean (SD) |
| Left thalamus | 5.156 (0.671) | 6.453 (0.868) | 6.628 (0.698) |
| Right thalamus | 4.885 (0.805) | 6.232 (0.745) | 6.269 (0.643) |
| Left putamen | 2.147 (0.452) | 2.615 (0.233) | 4.771 (0.637) |
| Right putamen | 2.249 (0.389) | 2.698 (0.263) | 4.714 (0.502) |
| Left caudate | 1.010 (0.455) | 1.528 (0.485) | 3.359 (0.548) |
| Right caudate | 1.080 (0.475) | 1.667 (0.488) | 3.392 (0.629) |
| Left globus pallidus | 0.812 (0.190) | 0.749 (0.103) | 1.297 (0.311) |
| Right globus pallidus | 0.772 (0.150) | 0.766 (0.101) | 1.371 (0.323) |
| Left amygdala | 0.747 (0.230) | 0.743 (0.143) | 0.841 (0.171) |
| Right amygdala | 0.665 (0.170) | 0.755 (0.141) | 0.834 (0.266) |
| Left nucleus accumbens | 0.159 (0.050) | 0.174 (0.043) | 0.266 (0.075) |
| Right nucleus accumbens | 0.179 (0.067) | 0.232 (0.068) | 0.289 (0.059) |
| Intracranial volume | 1437.9 (112.7) | 1424.1 (118.5) | 1424.6 (156.5) |
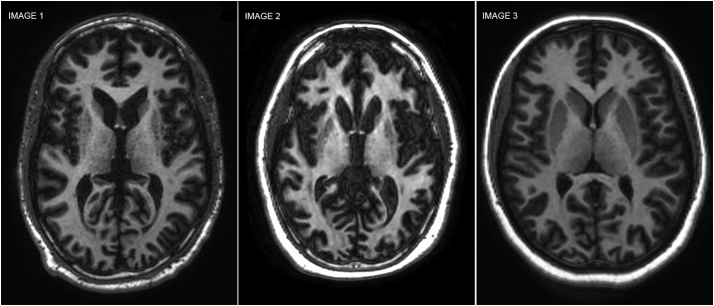
The main aim of the study was to contrast HD and HDL2 MRIs. Initially a qualitative comparison of MRIs could not distinguish between both pathologies (see Fig. 1.) The quantitative comparison was conducted using BRAINS. We conducted an initial comparison between HD and healthy controls in order to ensure comparability with previous studies and validation of the present methodology. Subsequently, the HDL2 group was compared to controls with the objective of providing the first MRI brain volumetry characterization of HDL2. Lastly, HD and HDL2 MRIs were compared.
Fig. 1.
Qualitative MRI Comparison. Three T1 axial 1.5 T MRIs comparing HDL2, HD and a normal control case. Image 1: 35-year-old HD subject with abnormal HTT allele = 49 triplet repeats. Image 2: 32-year-old HDL2 subject with abnormal JPH3 = 53 triplet repeats. Image 3: 32-year-old normal control subject. The HDL2 and HD subjects show qualitatively similar grey and white matter atrophy with caudate nuclei and putaminal volume loss compared to the normal control.
3.1. Comparison between patients with HD and normal controls
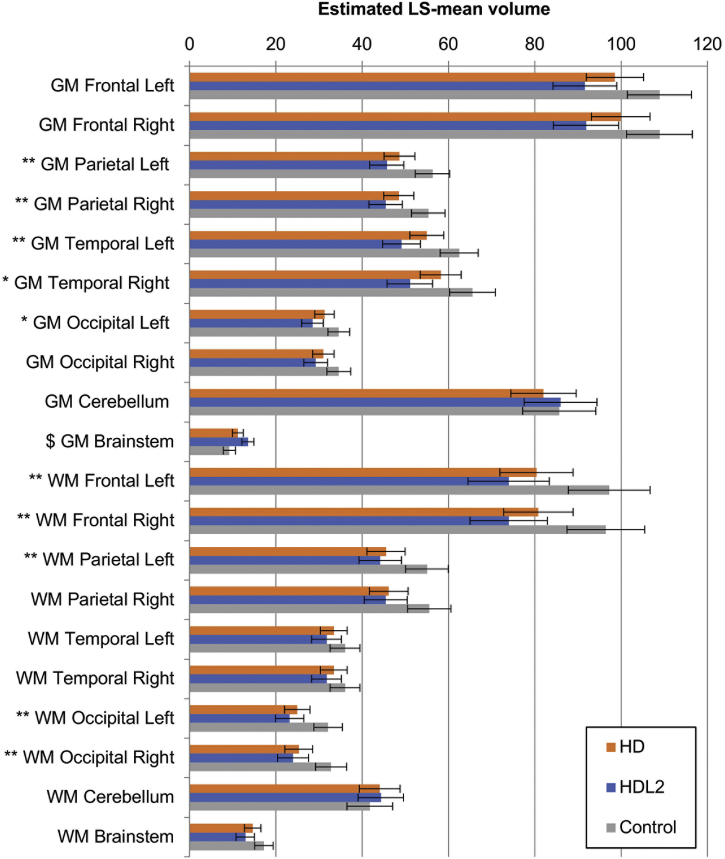
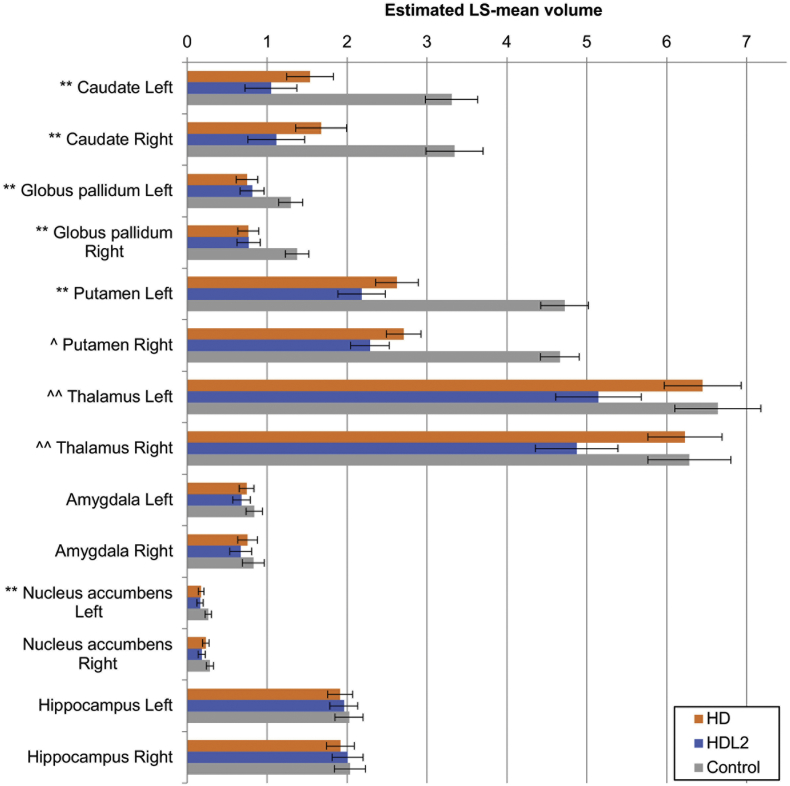
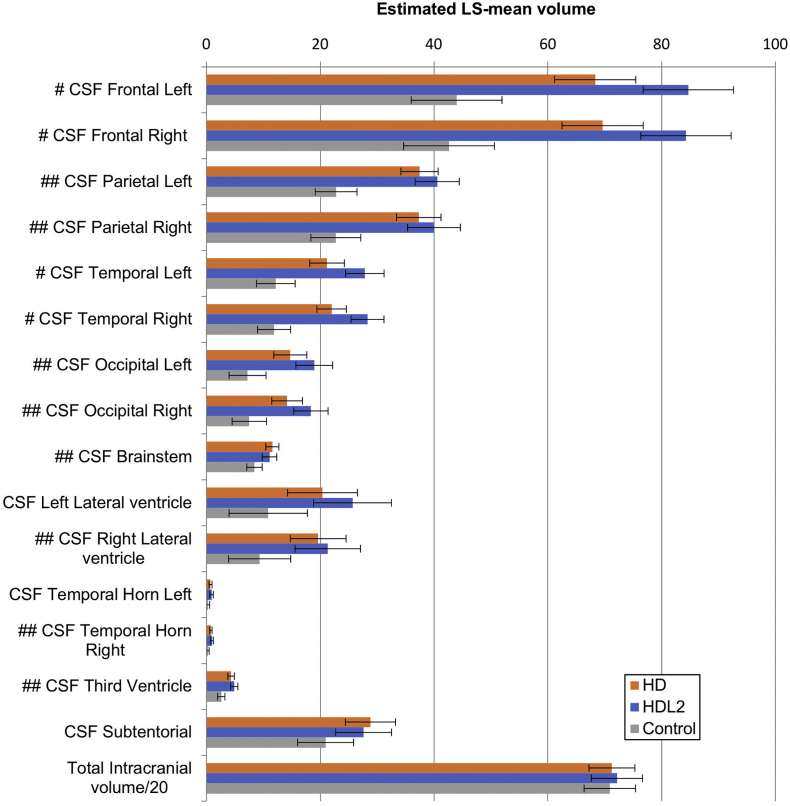
When compared to the normal control group and controlling for the age at the time of the MRI, the HD group showed significant less grey matter volume in both of the parietal and left temporal lobes with significantly smaller caudate nuclei, putamen and globus pallidum bilaterally (see Fig. 2, Fig. 3 and Table 1b). These smaller grey matter volumes were reflected in corresponding increases in CSF volumes (Fig. 4). Notably the mean thalamic volumes were not statistically different between the HD group and the normal control group (p < .01) (see Table 2).
Fig. 2.
HD, HDL2 and control groups grey matter and white matter volumes. The estimated least-squares (LS) means from the regression are depicted in a three-way comparison of grey and white matter volume. Error bars denote the 95% confidence intervals for the mean. Significance was set at p < .008. Symbols denote significant differences: **: HD, HDL2 < Control; *: HDL2 < Control; $: HD, Control< HDL2. GM = grey matter, WM = white matter.
Fig. 3.
HD, HDL2 and control groups basal ganglia, thalamic and hippocampal volumes. The estimated least-squares (LS) means from the regression are depicted in the figure in a three-way comparison among HD, HDL2 and control groups. Statistical significance is denoted by a symbol. Error bars denote the 95% confidence interval for the mean. Significance was set at p < .008. Symbols: **: HD, HDL2 < Control, ^: HDL2 < HD < Control, ^^: HDL2 < Control, HD where significance was reached. LS = Least-squares means, Nucleus acc = Nucleus accumbens.
Fig. 4.
HD, HDL2 and control cerebrospinal fluid and total intracranial volumes. The estimated least-squares (LS) means from the regression are depicted in the graph in a three-way comparison among the HD, HDL2 and control groups showing cerebrospinal fluid volumes and total intracranial volumes. Note that total intracranial volume was divided by 20 to fit to the graph scale. Statistical significance is shown with a symbol. Error bars denote the 95% confidence interval for the mean and significance was set at p < .008. Symbols denote #: Control < HD < HDL2. ##: HD, HDL2 < Control where significance was reached. LS = least squares mean, CSF=Cerebrospinal fluid.
Table 2.
Table comparing the Huntington's Disease Like 2, Huntington's Disease and the Normal control groups basal ganglia mean volume significant differences across multiple covariates (controlling for age at MRI, controlling for duration of disease, controlling for abnormal repeat length and controlling for age at onset) Two-tailed significance: P < .008. G. pallidus = Globus Pallidus, HDL2 = Huntington's Disease Like 2, HD = Huntington's Disease, N. Accumbens = Nucleus accumbens.
| Region | Comparison between HDL2, HD and control groups controlling for age at MRI |
Comparison between HDL2 and HD groups controlling for duration of disease |
Comparison between HDL2 and HD groups controlling for abnormal repeat length |
Comparison between HDL2 and HD groups controlling for age at onset |
||||
|---|---|---|---|---|---|---|---|---|
| Significant differences | P value | Significant differences | P value | Significant differences | P value | Significant differences | P value | |
| Left Thalamus | HDL2 < HD, Control | 0,0007 | HDL2 < HD | 0,0015 | HDL2 < HD | 0,0049 | HDL2 < HD | 0,0012 |
| Right Thalamus | HDL2 < HD, Control | 0,0005 | HDL2 < HD | 0,0008 | HDL2 < HD | 0,0032 | HDL2 < HD | 0,0006 |
| Left Putamen | HDL2, HD < Control | <0.0001 | HDL2 < HD | 0,0083 | HDL2 < HD | 0,0063 | Not Significant | 0,011 |
| Right Putamen | HDL2 < HD < Control | <0.0001 | HDL2 < HD | 0,0064 | HDL2 < HD | 0,0014 | HDL2 < HD | 0,0081 |
| Left Caudate | HDL2, HD < Control | <0.0001 | Not Significant | 0,023 | Not Significant | 0,027 | Not Significant | 0,034 |
| Right Caudate | HDL2, HD < Control | <0.0001 | Not Significant | 0,010 | Not Significant | 0,018 | Not Significant | 0,019 |
| Left G. pallidus | HDL2, HD < Control | <0.0001 | Not Significant | 0,45 | Not Significant | 0,41 | Not Significant | 0,42 |
| Right G. Pallidus | HDL2, HD < Control | <0.0001 | Not Significant | 0,81 | Not Significant | 0,80 | Not Significant | 0,97 |
| Left Amygdala | Not Significant | 0,11 | Not Significant | 0,35 | Not Significant | 0,27 | Not Significant | 0,35 |
| Right Amygdala | Not Significant | 0,27 | Not Significant | 0,22 | Not Significant | 0,16 | Not Significant | 0,25 |
| Left N. Accumbens | HDL2, HD < Control | 0,0016 | Not Significant | 0,38 | Not Significant | 0,16 | Not Significant | 0,54 |
| Right N. Accumbens | Not Significant | 0,011 | Not Significant | 0,018 | Not Significant | 0,10 | Not Significant | 0,10 |
3.2. Comparison between HDL2 and normal controls
Grey matter volume tended to be less in the HDL2 group compared to control groups when controlling for age at the time of the MRI (see Fig. 2). The HDL2 group showed significantly smaller grey matter volumes for both the parietal and temporal lobes and left occipital lobe and significantly smaller white matter volumes for both the frontal and occipital lobes and left parietal lobe compared to the normal control participants (p < .008). Almost all of the CSF spaces were significantly larger in the HDL2 group compared to the control group (see Fig. 4). Most of the basal ganglia including the thalamus were significantly smaller in the HDL2 group compared to the control group (p < .008)(see Table 1b and Fig. 3).
3.3. Comparison between HDL2 and HD
When comparing the HDL2 and HD groups, a similar pattern of grey and white matter volume was noted (see Fig. 2). The mean volumes of the caudate nucleus, globus pallidum, amygdala and the nucleus accumbens tended to be smaller in the HDL2 group compared to the HD group but this did not reach statistical significance when compared across all covariates (Table 1b and Fig. 3). When controlling for age at the time of the MRI, duration of disease, abnormal repeat length and age of onset, the HDL2 groups mean thalamic volumes were significantly smaller compared to the HD participants (Mean [STD] left and right thalamic volumes; HDL2 = left 5.156 cm3 [0.671], right = 4.885 cm3 [0.805], HD left = 6.453 cm3 [0.86], right = 6.232 cm3 [745]).
The HDL2 volumes were smaller when evaluating differences in the unadjusted putamen volumes in HDL2 and HD cases (Mean [STD] left and right putamen volumes; HDL2 = left 2.147 cm3 [0.452], right = 2.249 cm3 [0.389], HD left = 2.615 cm3 [0.233], right = 2.698 cm3 [0.263]). However, when applying the Benjamini-Hochberg procedure and applying a p < .0084, significance was not seen bilaterally for the putamen as was with the thalamus when controlling for age of onset. Furthermore, the thalamus (Left: p < .0012, Right: p < .0006) in comparison to the putamen (Left: p < .011, Right: p < .0081) had greater significant volume difference between the two diseases when controlling for age of disease onset (Table 2).
The unadjusted left and right caudate volumes suggest similar trends as seen in the thalamus and putamen when the unadjusted data are observed with the HDL2 volumes being smaller compared to the HD group caudate volumes (Mean [STD] left and right putamen volumes; HDL2 = left 1.010 cm3 [0.455], right = 1.080 cm3 [0.475], HD left = 1.528 cm3 [0.485], right = 1.667 cm3 [0.488]) (Table 1b). However, the LS mean caudate volumes are small: 3.2 for HD and 2.2 for the HDL2 groups and when applying the same procedure the difference between the HDL2 and HD caudate volumes did not reach significance when controlling for any of the covariates as noted in Table 2.
The relationship between duration of disease, abnormal repeat length, age at onset was separately determined for each volume measurement in both the HDL2 and HD groups. The trend was an inverse correlation for most measurements, as expected, but significance was not reached. In both diseases smaller volumes of the grey matter structures was seen with increasing duration of disease and larger repeat lengths.
Lastly, on blinded clinical review T2 images demonstrated bilateral putaminal rim hyperintensities in HD and HDL2 patients, but not in the control group.
4. Discussion
This is the first quantitative volumetric MRI study in patients with HDL2 and the first comparison to a HD group and an unaffected control group and done blind to the patients diagnosis. Consistent with the previously reported qualitative review of individual cases (Anderson et al., 2017b; Margolis et al., 2001). The analysis indicated similar atrophy of the grey and white matter structures including the basal ganglia and enlarged CSF spaces in the two disease groups compared to the normal control group. However, smaller thalamic volumes were noted in the HDL2 group compared to the HD group. There was no evidence of relative caudate sparing, as observed in one previous HDL2 case (Bardien et al., 2007).
The HD group showed white and grey matter loss, enlarged CSF spaces, and basal ganglia changes that replicated the findings of previous studies using similar analytic methods but much larger sample sizes and provides support for the methods and patient population used in this study (Paulsen et al., 2008; Tabrizi et al., 2009; van den Bogaard et al., 2011). The key finding of this analysis is the similarity between the MRIs of HD and HDL2 patients, with the notable exception that the average thalamic volume was 21% smaller in the HDL2 group compared to the HD group. In contrast to this difference, the mean thalamic volume in the HD group was similar to those recorded in the normal control participants.
Initial neuroimaging studies found no difference in thalamic volumes between HD patients and controls (Rosas et al., 2003). Later studies have demonstrated thalamic volume loss in HD but have suggested that this reflects disease progression. Studies comparing MRI brain volumes in HD with normal controls often used different volumetric methods and showed HD thalamic volumes being between 2 and 8% less than the unaffected control groups (Coppen et al., 2018; Kassubek et al., 2004). Thalamic atrophy in HD appears to reflects whole brain atrophy and is proportional to overall degeneration (van den Bogaard et al., 2011). Therefore in keeping with previous reports of HD thalamic volumes, the mean thalamic volumes of the HD group in this study was 2% less than the control group. However, in the HD and HDL2 groups, regions like the putamen and caudate showed typically smaller volumes compared to the normal control group as has been described previously in HD (Tabrizi et al., 2009; van den Bogaard et al., 2011). The striking finding from this study was that HDL2 thalamic volume was significantly smaller than in the HD group when controlling for age at the time of the MRI, duration of disease, abnormal repeat length and age of onset of disease.
Attempts to find features differentiating HDL2 from HD are available, but findings have been anecdotal, inconsistent or disproved. For example, the hypothesis that HDL2 is a neuroacanthocytosis syndrome was recently refuted by a blinded controlled study (Anderson et al., 2017a). Descriptions of the HDL2's phenotype have introduced the possibility that HDL2 may disproportionately present with bradykinetic/parkinsonian features which likely reflects the unique phenotype and relatively long expanded repeat length, but this remains unverified (Margolis et al., 2001)(Schneider et al., 2012). The characterization of the clinical phenotype of HDL2 will remain incomplete while based only on retrospective case series (Anderson et al., 2017b). Thus, future research is needed, and our findings regarding the differences in thalamic volumes between HDL2 and HD may provide a new and exciting guide to explore into differentiating between these two mutations.
The possible clinical implications and pathogenic mechanisms causing greater thalamic atrophy in HDL2 would need to be accounted for. The pathogenesis of HDL2 is complex, with likely contributions from toxic properties of JPH3 RNA with the repeat expansion, loss of expression of JPH3, and perhaps expanded tracts of polyglutamine expressed from a cryptic gene on the antisense strand at the HDL2 locus (Rudnicki et al., 2007; Seixas et al., 2012; Wilburn et al., 2011). The finding of smaller putamen and thalamic volumes in HDL2 compared to HD suggests differential neuronal and/or glial vulnerability in these areas to the HDL2 mutation compared to the HD mutation. This may have implications first in terms of phenotype. It has been suggested that there are clinical differences such as more myoclonus and parkinsonism although this needs further validation (Anderson et al., 2017b; Margolis et al., 2001). Secondly although little difference has been reported in the gross or histopathological findings between five patients with HDL2 compared to matched cases with HD (Rudnicki et al., 2008), it is possible that the thalamic differences may be the consequence of histopathological discrepancies between these mutations that require larger samples of HDL2 specimens (Rudnicki et al., 2008).
This study is based on the uniquely high prevalence of HDL2 in South Africa, and would be difficult to accomplish in any other location. Even in this region HDL2 is a rare disorder and therefore the sample size is small. The results do however establish the general neuroradiological similarity of HD and HDL2 and are consistent with their reportedly analogous clinical phenotypes. The selectively smaller thalamic volumes in HDL2 is an interesting finding, but the sample size imposes limits on data interpretation. In both HD and HDL2, the relationship between intracerebral volumes and duration of disease and abnormal repeat length do not reach significance, though most measurements trend to show smaller volumes with increasing duration of disease and larger abnormal repeat lengths. These are well established negative correlations for HD demonstrated in larger studies (Tabrizi et al., 2009). We would predict that in HDL2, like HD, these trends would be confirmed with larger cross-sectional cohorts of patients or with longitudinal analyses of smaller cohorts. In support of this, sequential MRIs five years apart have been reported for a single HDL2 case which qualitatively demonstrated progressive brain atrophy in a similar pattern to HD (Rodrigues et al., 2008).
Lastly, we found putaminal rim hyperintensities in all of the HD and HDL2 cases. This sign has been described before in HDL2 (Schneider et al., 2012). Other neurodegenerative disorders and normal controls have been shown to have putaminal rim hyperintensities and this is therefore unlikely to help distinguish HDL2 from other conditions (Lee et al., 2005; Tha et al., 2012). To our knowledge, the putaminal rim sign has not been commented on in HD previously.
5. Conclusion
Huntington's disease like 2 and HD are remarkably similar clinically and radiologically. When HDL2 is objectively compared to a HD group that is matched for age, abnormal repeat length and duration of disease there is a similar pattern emerging of smaller volumes in the grey and white matter as well as in the basal ganglia. The results presented here, now demonstrate the similarities between HDL2 and HD extend to spatial patterns of neurodegeneration, with the interesting exception of the thalamus, where selective thalamic volume loss tends to be greater in HDL2.
These findings raise intriguing possibilities about both the convergent and the divergent pathogenic pathways of the two diseases and opens future paths of research into how this relates to their phenotypes.
Disclosures and conflict of interest
Ronald Pierson is a shareholder in NeuroAnalytica and received fees from the University of the Witwatersrand for analysis of the MRIs.
Study funding
Funding was received from The Medical Research Council of South Africa for this study. RLM received support from the ABCD Charitable Trust.
Data sharing and data accessibility
The authors encourage others to make use of this data and will make this large, anonymised data set available to any researchers or groups of good standing.
Acknowledgements
We wish to thank the Department of Radiology at the University of the Witwatersrand Donald Gordon Medical Centre, Dr. TJ Nel and Partners for generously donating all the MRI scans for this study and all the staff at the Department for their helpfulness and participation. We would also like to acknowledge and thank Marianne Gomes for co-ordinating and counselling the patients and families in this study and Dr. Petra Gaylard for her assistance with the statistical analysis of the data. Most importantly, we would like to thank the patients and their families for their time and kind participation in this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2019.101666.
Appendix A. Author contribution table
| Name | Location | Role | Contribution |
|---|---|---|---|
| David G. Anderson | The University of the Witwatersrand Donald Gordon Medical Centre | Author | : Study concept and design, organization, acquisition of data, analysis and interpretation, writing of manuscript, critical revision of the manuscript for important intellectual content. |
| Mark Haagensen | The University of the Witwatersrand Donald Gordon Medical Centre | Author | : Acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content |
| Aline Ferreira-Correia | Department of Psychology, School of Human and Community Development, University of the Witwatersrand | Author | : Acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content. |
| Ronald Pierson | Brain Image Analysis, LLC, Simi Valley CA USA | Author | :Analysis and interpretation, critical revision of the manuscript for important intellectual content. |
| Jonathan Carr | Division of Neurology, Department of Medicine, University of Stellenbosch, Cape Town | Author | Study supervision, critical revision of the manuscript for important intellectual content. |
| Amanda Krause | Division of Human Genetics, National Health Laboratory Service and School of Pathology, Faculty of Health Sciences, The University of the Witwatersrand | Author | : Study supervision, Analysis and interpretation, critical revision of the manuscript for important intellectual content, senior author. |
| Russell L. Margolis | Departments of Psychiatry and Neurology, Program in Cellular and Molecular Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, USA. | Author | Study supervision, Analysis and interpretation, critical revision of the manuscript for important intellectual content, senior author. |
Co author email addresses
Mark Haagensen: markhaagensen@mac.com
Aline Ferreira Correia: Aline.FerreiraCorreia@wits.ac.za
Ronald Pierson: ronald@brainimageanalysis.com
Jonathan Carr: jcarr@sun.ac.za
Russell L. Margolis: rmargoli@jhmi.edu
Amanda Krause: amanda.krause@nhls.ac.za
Appendix B. Supplementary data
Supplementary Information describing the MRI sequences and Brain Research: Analysis of Images, Networks, and Systems (BRAINS) protocol used to analyse the images for this study.
References
- Anderson D.G., Carmona S., Naidoo K., Coetzer T.L., Carr J., Rudnicki D.D., Walker R.H., Margolis R.L., Krause A. Absence of acanthocytosis in Huntington's disease-like 2: a prospective comparison with Huntington's disease. Tremor Other Hyperkinet Mov (N Y) 2017;7:512. doi: 10.7916/D81J9PDX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D.G., Walker R.H., Connor M., Carr J., Margolis R.L., Krause A. a systematic review of the Huntington disease-like 2 phenotype. J. Huntingtons Dis. 2017;6:37–46. doi: 10.3233/JHD-160232. [DOI] [PubMed] [Google Scholar]
- Baine F.K., Krause A., Greenberg L.J. The frequency of Huntington disease and Huntington disease-like 2 in the South African population. Neuroepidemiology. 2016;46:198–202. doi: 10.1159/000444020. [DOI] [PubMed] [Google Scholar]
- Bardien S., Abrahams F., Soodyall H., van der Merwe L., Greenberg J., Brink T., Carr J. A South African mixed ancestry family with Huntington disease-like 2: clinical and genetic features. Mov. Disord. 2007;22:2083–2089. doi: 10.1002/mds.21672. [DOI] [PubMed] [Google Scholar]
- Coppen E.M., Jacobs M., van den Berg-Huysmans A.A., van der Grond J., Roos R.A.C. Grey matter volume loss is associated with specific clinical motor signs in Huntington's disease. Parkinsonism Relat. Disord. 2018;46:56–61. doi: 10.1016/j.parkreldis.2017.11.001. [DOI] [PubMed] [Google Scholar]
- van den Bogaard S.J.A., Dumas E.M., Acharya T.P., Johnson H., Langbehn D.R., Scahill R.I., Tabrizi S.J., van Buchem M.A., van der Grond J., Roos R.A.C., TRACK-HD Investigator Group Early atrophy of pallidum and accumbens nucleus in Huntington's disease. J. Neurol. 2011;258:412–420. doi: 10.1007/s00415-010-5768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris G., Andreasen N.C., Cizadlo T., Bailey J.M., Bockholt H.J., Magnotta V.A., Arndt S. Improving tissue classification in MRI: a three-dimensional multispectral discriminant analysis method with automated training class selection. J. Comput. Assist. Tomogr. 1999;23:144–154. doi: 10.1097/00004728-199901000-00030. [DOI] [PubMed] [Google Scholar]
- Holmes S.E., O'Hearn E., Rosenblatt A., Callahan C., Hwang H.S., Ingersoll-Ashworth R.G., Fleisher A., Stevanin G., Brice A., Potter N.T., Ross C.A., Margolis R.L. A repeat expansion in the gene encoding junctophilin-3 is associated with Huntington disease-like 2. Nat. Genet. 2001;29:377–378. doi: 10.1038/ng760. [DOI] [PubMed] [Google Scholar]
- Kassubek J., Juengling F.D., Ecker D., Landwehrmeyer G.B. Thalamic atrophy in Huntington's disease co-varies with cognitive performance: a morphometric MRI analysis. Cereb. Cortex. 2004;15:846–853. doi: 10.1093/cercor/bhh185. [DOI] [PubMed] [Google Scholar]
- Krause A., Mitchell C., Essop F., Tager S., Temlett J., Stevanin G., Ross C., Rudnicki D., Margolis R. Junctophilin 3 (JPH3) expansion mutations causing Huntington disease like 2 (HDL2) are common in South African patients with African ancestry and a Huntington disease phenotype. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2015;168:573–585. doi: 10.1002/ajmg.b.32332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W.-H., Lee C.-C., Shyu W.-C., Chong P.-N., Lin S.-Z. Hyperintense putaminal rim sign is not a hallmark of multiple system atrophy at 3T. AJNR Am. J. Neuroradiol. 2005;26:2238–2242. [PMC free article] [PubMed] [Google Scholar]
- Margolis R.L., O'Hearn E., Rosenblatt A., Willour V., Holmes S.E., Franz M.L., Callahan C., Hwang H.S., Troncoso J.C., Ross C.A. A disorder similar to Huntington's disease is associated with a novel CAG repeat expansion. Ann. Neurol. 2001;50:373–380. doi: 10.1002/ana.1312. [DOI] [PubMed] [Google Scholar]
- Margolis R.L., Holmes S.E., Rosenblatt A., Gourley L., O'Hearn E., Ross C.A., Seltzer W.K., Walker R.H., Ashizawa T., Rasmussen A., Hayden M., Almqvist E.W., Harris J., Fahn S., MacDonald M.E., Mysore J., Shimohata T., Tsuji S., Potter N., Nakaso K., Adachi Y., Nakashima K., Bird T., Krause A., Greenstein P. Huntington's disease-like 2 (HDL2) in North America and Japan. Ann. Neurol. 2004;56:670–674. doi: 10.1002/ana.20248. [DOI] [PubMed] [Google Scholar]
- Mariani L.-L., Tesson C., Charles P., Cazeneuve C., Hahn V., Youssov K., Freeman L., Grabli D., Roze E., Noël S., Peuvion J.-N., Bachoud-Levi A.-C., Brice A., Stevanin G., Durr A. Expanding the spectrum of genes involved in Huntington disease using a combined clinical and genetic approach. JAMA Neurol. 2016:1–10. doi: 10.1001/jamaneurol.2016.2215. [DOI] [PubMed] [Google Scholar]
- Paulsen J.S., Langbehn D.R., Stout J.C., Aylward E., Ross C.A., Nance M., Guttman M., Johnson S., MacDonald M., Beglinger L.J., Duff K., Kayson E., Biglan K., Shoulson I., Oakes D., Hayden M., Predict-HD Investigators and Coordinators of the Huntington Study Group Detection of Huntington's disease decades before diagnosis: the Predict-HD study. J. Neurol. Psychiatry. 2008;79:874–880. doi: 10.1136/jnnp.2007.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson R., Johnson H., Harris G., Keefe H., Paulsen J.S., Andreasen N.C., Magnotta V.A. Fully automated analysis using BRAINS: autoworkup. NeuroImage. 2011;54:328–336. doi: 10.1016/j.neuroimage.2010.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell S., Magnotta V., Johnson H.J., Jammalamadaka V.K., Pierson R., Andreasen N. Registration and machine learning-based automated segmentation of subcortical and cerebellar brain structures. NeuroImage. 2008;39:238–247. doi: 10.1016/j.neuroimage.2007.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues G.G.R., Walker R.H., Brice A., Cazeneuve C., Russaouen O., Teive H.A.G., Munhoz R.P., Becker N., Raskin S., Werneck L.C., Junior W.M., Tumas V. Huntington's disease-like 2 in Brazil-report of 4 patients. Mov. Disord. 2008;23:2244–2247. doi: 10.1002/mds.22223. [DOI] [PubMed] [Google Scholar]
- Rosas H.D., Koroshetz W.J., Chen Y.I., Skeuse C., Vangel M., Cudkowicz M.E., Caplan K., Marek K., Seidman L.J., Makris N., Jenkins B.G., Goldstein J.M. Evidence for more widespread cerebral pathology in early HD: an MRI-based morphometric analysis. Neurology. 2003;60:1615–1620. doi: 10.1212/01.wnl.0000065888.88988.6e. [DOI] [PubMed] [Google Scholar]
- Rudnicki D.D., Holmes S.E., Lin M.W., Thornton C.A., Ross C.A. Huntington's disease--like 2 is associated with CUG repeat-containing RNA foci. Ann. Neurol. 2007;61:272–282. doi: 10.1002/ana.21081. [DOI] [PubMed] [Google Scholar]
- Rudnicki D.D., Pletnikova O., Vonsattel J.-P.G., Ross C.A., Margolis R.L. A comparison of Huntington disease and Huntington disease-like 2 neuropathology. J. Neuropath Neuro. 2008;67:366–374. doi: 10.1097/NEN.0b013e31816b4aee. [DOI] [PubMed] [Google Scholar]
- Schneider S.A., Marshall K.E., Xiao J., LeDoux M.S. JPH3 repeat expansions cause a progressive akinetic-rigid syndrome with severe dementia and putaminal rim in a five-generation African-American family. Neurogenetics. 2012;13:133–140. doi: 10.1007/s10048-012-0318-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seixas A.I., Holmes S.E., Takeshima H., Pavlovich A., Sachs N., Pruitt J.L., Silveira I., Ross C.A., Margolis R.L., Rudnicki D.D. Loss of junctophilin-3 contributes to Huntington disease-like 2 pathogenesis. Ann. Neurol. 2012;71:245–257. doi: 10.1002/ana.22598. [DOI] [PubMed] [Google Scholar]
- Tabrizi S.J., Langbehn D.R., Leavitt B.R., Roos R.A., Durr A., Craufurd D., Kennard C., Hicks S.L., Fox N.C., Scahill R.I., Borowsky B., Tobin A.J., Rosas H.D., Johnson H., Reilmann R., Landwehrmeyer B., Stout J.C. Biological and clinical manifestations of Huntington's disease in the longitudinal TRACK-HD study: cross-sectional analysis of baseline data. Lancet Neurol. 2009;8(9):791–801. doi: 10.1016/S1474-4422(09)70170-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tha K.K., Terae S., Tsukahara A., Soma H., Morita R., Yabe I., Ito Y.M., Sasaki H., Shirato H. Hyperintense putaminal rim at 1.5 T: prevalence in normal subjects and distinguishing features from multiple system atrophy. BMC Neurol. 2012;12:39. doi: 10.1186/1471-2377-12-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R.H., Jankovic J., O'Hearn E., Margolis R.L. Phenotypic features of Huntington's disease-like 2. Mov. Disord. 2003;18:1527–1530. doi: 10.1002/mds.10587. [DOI] [PubMed] [Google Scholar]
- Walker R.H., Gatto E.M., Bustamante M.L., Bernal-Pacheco O., Cardoso F., Castilhos R.M., Chana-Cuevas P., Cornejo-Olivas M., Estrada-Bellmann I., Jardim L.B., López-Castellanos R., López-Contreras R., Maia D.P., Mazzetti P., Miranda M., Rodríguez-Violante M., Teive H., Tumas V. Huntington's disease-like disorders in Latin America and the Caribbean. Parkinsonism Relat. Disord. 2018:1–12. doi: 10.1016/j.parkreldis.2018.05.021. [DOI] [PubMed] [Google Scholar]
- Wilburn B., Rudnicki D.D., Zhao J., Weitz T.M., Cheng Y., Gu X., Greiner E., Park C.S., Wang N., Sopher B.L., La Spada A.R., Osmand A., Margolis R.L., Sun Y.E., Yang X.W. An antisense CAG repeat transcript at JPH3 locus mediates expanded polyglutamine protein toxicity in Huntington's disease-like 2 mice. Neuron. 2011;70:427–440. doi: 10.1016/j.neuron.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information describing the MRI sequences and Brain Research: Analysis of Images, Networks, and Systems (BRAINS) protocol used to analyse the images for this study.
Data Availability Statement
The authors encourage others to make use of this data and will make this large, anonymised data set available to any researchers or groups of good standing.