Abstract
Blood flow restriction walking (BFR-W) is becoming more frequently used in aerobic and strength training and it has been proposed that BFR-W can be used in clinical populations. BFR-W may change gait stability yet few studies have assessed gait changes during or following BFR-W. The aim of this study was to assess if spatial-temporal gait parameters change during and following BFR-W. Twenty-four participants completed two walking sessions (>48-hours apart); 1) Unilateral BFR-W applied at the dominant thigh, 2) walking without BFR. In each session participants performed a 5-min warmup, 15-min walking intervention and 10-min active recovery. The warmup and active recovery were performed without BFR on both days. Measurements were attained at baseline, during the intervention and post-intervention using the GAITRite®. Linear mixed models were applied to each measured variable. Fixed factors were timepoint (warmup, intervention, and active recovery), condition (BFR-W and control walking) and condition × timepoint. Random factors were subject and subject × condition. Participants took shorter (3.2-cm (mean difference), CI95%: 0.8–5.6-cm) and wider strides (1.4-cm, CI95%: 0.9–1.9-cm) during BFR-W. For single leg measures, participants took shorter steps (2.8-cm, CI95%: 1.7–4.0-cm) with a faster single support time (7.5-ms, CI95%: 2.9–12.0-ms) on the non-dominant (unoccluded) leg during BFR-W compared to the non-dominant leg during control walking. There were no differences in step length and single support time between the dominant (occluded) leg during BFR-W compared to the dominant leg during control walking. There were no significant changes in velocity, cadence or double support time between BFR-W and control walking (P > 0.05). BFR-W caused small transient changes to several gait parameters. These changes should be considered when using BFR-W in clinical populations.
Keywords: Neuroscience, Physiology, Rehabilitation
1. Introduction
Blood flow restriction (BFR) is a training modality which involves the restriction of blood flow at the proximal portion of the limb while training the more distal limb (Loenneke et al., 2012). Following training regimes ranging from 6 days to 8 weeks, BFR-training increases strength, endurance and muscle size in the trained muscles distal to the blood pressure cuff (Shinohara et al., 1998; Takarada et al., 2002; Ishii et al., 2005; Yasuda et al., 2005, 2014, Patterson and Ferguson, 2010, 2011). Numerous mechanisms for the efficacy of BFR have been proposed including increased muscle protein synthesis, increased neural drive, increased glycogen usage and changes in the expression of certain genes (for overview of mechanisms see Scott et al., 2014). The advantage of BFR is that lower exercise intensities (20%–30% of 1RM) can elicit physiological adaptations similar to those achieved when training at higher intensities (>70% of 1RM) without BFR (Loenneke et al., 2012). BFR-training also increases muscle strength, hypertrophy and local muscular endurance in patient populations compared to conventional training (Abe et al., 2006; Loenneke et al., 2012).
BFR-walking is similar to BFR-training however participants walk instead of performing resistance exercise. For BFR-walking, the cuff is inflated around the thigh (unilaterally or bilaterally) and participants walk with the cuff(s) inflated. Three weeks of daily BFR-walking increases hypertrophy, strength and endurance in muscles distal to the BP cuff (Beekley et al., 2005; Abe et al., 2006, 2009). In addition, a recent systematic review showed that BFR-walking can be an effective training modality across several musculoskeletal patient groups including elderly patients at risk of sarcopenia (Hughes et al., 2017). Given this, BFR-walking has the potential to provide an alternative training modality in a neurorehabilitation setting for patients with brain injury. BFR-walking in these populations could increase strength and endurance more than conventional walk training. Patient populations during walking often experience unilateral weakness to the ankle dorsiflexors resulting in drop-foot (Olney and Richards, 1996). For these populations unilateral BFR-walking has the potential to more effectively increase dorsiflexion strength and endurance than normal walking.
Despite its potential benefits, BFR-walking could alter gait parameters and increase the risk of falls. As BFR-walking could be used clinically, research is required to assess the extent of spatial-temporal changes that occur during training. If these change significantly, BFR-walking could increase the risk or fear of falls (Maki, 1997; Wei et al., 2017). The aim of this study was to investigate the effects of unilateral BFR-walking on spatial-temporal gait parameters including velocity, cadence, stride length, stride width, double support time, step length and single support time when compared to normal walking in a healthy adult population. Some of these parameters have been linked to falls (Wei et al., 2017) and fear of falls (Maki, 1997) in elderly and clinical populations. If these gait parameters change, it is possible that BFR-walking could increase the risk and/or fear of falling.
2. Methods
2.1. Participants
Twenty-four participants were included in the study (16 males, 8 females; age: 27 ± 4 years (mean ± SD); height: 1.77 ± .10 m; weight: 76 ± 13 kg; systolic blood pressure: 128 ± 8 mmHg; diastolic blood pressure: 78 ± 9 mmHg). Twenty-one participants were right leg dominant. Participants were over 18 years, with no recent history of musculoskeletal or neurological issues. The study was approved by the Macquarie University Human Research Ethics Committee (approval number: 5201600533) and conformed to the Declaration of Helsinki. All participants provided written informed consent.
2.2. Measures
Outcome measures were velocity, cadence, stride length, stride width, double support time, step length and single support time. Outcome measures were automatically generated by the GAITRite® Electronic Walkway software (see GAITRite® Electronic Walkway Technical Reference, 2013).
2.3. Design and procedures
Participants trained on two sessions spaced 4 ± 2 days apart with at least 48 hours between sessions. The order of intervention was randomised to control for session order effect and participants performed either walking with BFR (BFR-condition) or walking without BFR (CON-condition) on their first session. The other condition was performed on the second session. Participants wore comfortable shoes for all walking and wore the same shoes for each session. BFR was performed on the dominant leg.
Prior to the experiment, brachial blood pressure of the right arm was measured in supine-lying using a sphygmomanometer (81-OB, Prestige Medical, Northridge CA) in accordance with the clinical guidelines recommended by the Japanese Society of Hypertension (2014).
Measurement of spatial-temporal gait parameters were performed on a GAITRite® portable walkway system (overall length: 9 m, active area: 8 m, CIR Systems Inc. Franklin, NJ). The GAITRite® walkway uses pressure sensitive sensors to record the location and timing of footfalls during walking. It is a valid (Webster et al., 2005) and reliable measure for numerous spatial-temporal parameters of gait in younger adults (Menz et al., 2004; Van Uden and Besser, 2004), older (Menz et al., 2004) and clinical populations (Kuys et al., 2011; Lewek and Randall, 2011).
2.4. Walking protocol
Fig. 1 is a schematic of the walking protocol for the BFR-condition and CON-condition. For both conditions, participants completed three bouts of treadmill walking (BodyWorx® treadmill, Model: JSPORT3000) prior to using the GAITRite walkway; warmup (5 min), BFR-walking/CON-walking (intervention) (15 min) and active recovery (post-intervention) (10 min). For BFR-walking, warmup and active recovery treadmill walking (and GAITRite® measurements following these) were performed without the blood pressure cuff. On the first session, during the first minute of the warmup, participants were asked to choose a self-selected walking speed (1.23 ± 0.16 m/s) which they would feel comfortable maintaining for 30 min. This speed was used for all subsequent treadmill walking on both session 1 and session 2. Following the warmup, intervention and active recovery, participants were asked to remain on the treadmill for 30 s once the treadmill had become stationary. Participants stepped off the treadmill, waited 10 seconds, and walked over the GAITRite® walkway (9 meters) three times at a comfortable speed. An additional 2 meters was marked at the start and end of the mat to allow for a natural stride through the active section of the mat. Participants were asked to focus on a target placed at eye level in front of the mat and asked not to alter their stride when approaching the walkway. Prior to the experiment, participants practised walking on the mat and feedback was given when required.
Fig. 1.
Schematic representation of the experimental protocol. Experimental protocol for blood flow restriction walking condition (BFR-condition) and walking without BFR (CON-condition). Note that the figure is not to scale. The BFR-condition and CON-condition were conducted on two separate days. On the first day participants chose their self-selected walking speed during the warm-up period which was used for treadmill walking for the remainder of the experiment on both days. For the-BFR condition and CON-condition, warmup and active recovery were performed without BFR. During the intervention, for the BFR-session, participants walked with a blood pressure cuff inflated to systolic blood pressure around the proximal thigh of the dominant leg. The blood pressure cuff was inflated and deflated over 1 minute. During this time on the CON-session, participants stood quietly. GAITRite® measures were performed overground (OG) on three occasions at a comfortable walking speed during each of the three timepoints, pre-intervention (PRE-measures), intervention (IN-measures) and post-intervention (POST-measures). On the BFR-session, participants performed the IN-measures with the blood pressure cuff inflated.
2.5. Blood flow restriction walking
Prior to the BFR-intervention, participants were fitted with a blood pressure cuff (width: 7 cm; The Occlusion Cuff®, SKU: PB216, Perform Better Limited, Warwickshire, UK). The cuff was placed around the dominant thigh at 25% of the distance from the greater trochanter to the lateral femoral condyle. Once secured, the participants unloaded the leg and the cuff was inflated over 1 min to the resting systolic blood pressure, determined on the first session. Participants completed the BFR-intervention and subsequent GAITRite® walkway testing with the cuff affixed and inflated. The cuff pressure was monitored throughout the BFR-intervention and adjusted as required. Once the BFR-intervention and subsequent GAITRite® measures were completed, the cuff was deflated over 60 seconds and removed before active recovery.
2.6. Control walking
For the CON-condition, CON-walking was performed as above without BFR. The timings were the same as the BFR-condition. During the times when the blood pressure cuff was inflated and deflated during the BFR-condition, participants were standing.
2.7. Statistical analysis
Partial footsteps on the GAITRite® were discarded as only complete footsteps could be used for analysis. For the BFR-condition, footsteps were labelled BFR-dominant and BFR-non-dominant. For the CON-condition footsteps were labelled CON-dominant and CON-non-dominant. The variable, condition, consisted of the BFR-condition and CON-condition and the variable, timepoint, consisted of baseline (warmup), intervention, and post-intervention (active recovery). For all outcome variables, linear mixed models were performed with subject and subject*condition as random factors and timepoint, condition and condition*timepoint as fixed factors. One linear mixed model was performed for the variables velocity, cadence, stride length, stride width and double support time as these were combined (composite) measures from both dominant and non-dominant legs. As the variables step length and single support time were single leg measures, separate mixed models were performed for dominant and non-dominant legs. If the linear mixed models were significant for an interaction, post-hoc tests were performed to determine the effect sizes and location of the differences and no assessment of the main effects was performed. If the condition*timepoint interaction was non-significant, the effect sizes of the main effects of time and condition were reported. Statistical analyses were performed using SPSS (version 21). Significance was set to P < 0.05.
3. Results
A summary of the results is provided in Table 1.
Table 1.
Mean (95% confidence intervals) for the modelled spatial-temporal gait parameters.
| BFR-condition |
CON-condition |
|||||
|---|---|---|---|---|---|---|
| Baseline | Intervention | Post-intervention | Baseline | Intervention | Post-intervention | |
| Velocity (m/s) | 1.47 (1.40–1.55) | 1.47 (1.40–1.54) | 1.50 (1.42–1.56) | 1.46 (1.39–1.53) | 1.48 (1.41–1.56) | 1.48 (1.41–1.56) |
| Cadence (steps/min) | 114 (111–116) | 115 (112–118) | 115 (112–118) | 113 (111–116) | 114 (111–117) | 114 (111–117) |
| Stride length (cm)† | 155 (149–162) | 153 (147–159) | 156 (150–162) | 154 (149–161) | 156 (150–162) | 156 (150–162) |
| Stride width (cm)‡ | 10 (10–11) | 12 (11–13) | 10 (9–11) | 10 (10–11) | 10 (10–11) | 10 (9–11) |
| Double support time (ms) | 251 (237–265) | 246 (232–260) | 245 (231–259) | 251 (237–265) | 246 (232–260) | 245 (232–261) |
| BFR-dominant and CON-dominant | ||||||
| Step length (cm) | 78 (75–81) | 78 (75–81) | 78 (75–81) | 77 (74–80) | 78 (75–81) | 78 (75–81) |
| Single support time (ms) | 405 (395–414) | 402 (392–411) | 401 (392–411) | 405 (396–415) | 404 (395–414) | 405 (396–415) |
| BFR-non-dominant and CON-non-dominant | ||||||
| Step length (cm)‡ | 78 (75–81) | 75 (72–78) | 78 (75–81) | 77 (74–80) | 78 (75–81) | 78 (75–81) |
| Single support time (ms)* | 406 (396–415) | 399 (389–408) | 402 (392–411) | 407 (398–415) | 406 (397–416) | 407 (397–416) |
BFR-dominant = The dominant (occluded) leg during the BFR-condition, CON-dominant = The dominant leg during the CON-condition, BFR-non-dominant = The non-dominant (unoccluded) leg for the BFR-condition day, CON-non-dominant = The non-dominant leg for the CON-condition. ‘*’, †, ‘‡’ represent variables with a significant condition*timepoint interaction to P < 0.05, P < 0.01 and P < 0.001, respectively.
3.1. Velocity, cadence and stride measurements
There was a significant condition*timepoint interaction for stride length (P = 0.001) and stride width (P < 0.001). For the BFR-condition, participants took significantly shorter strides during the intervention compared to the CON-condition (P = 0.01) and significantly shorter strides compared to baseline (P = 0.001) (Fig. 2A). For the CON-condition, there was a small but significantly longer stride length, compared to baseline, during the intervention (P = 0.034) and post-intervention (P = 0.033) (Fig. 2A). For the BFR-condition, participants took significantly wider strides during the intervention compared to CON-condition (P < 0.001) and significantly wider strides compared to baseline (P < 0.001) (Fig. 2B). There was no significant condition*timepoint interaction for velocity (P = 0.087), cadence (P = 0.499) or double support time (P = 0.890). There was a main effect of timepoint for velocity (P = 0.009), cadence (P = 0.006) and double support time (P = 0.031). For both BFR-walking and CON-walking, participants walked faster post-intervention compared to baseline (Fig. 2C), participants took more steps during the intervention and post-intervention compared to baseline (Fig. 2D) and participants had reduced double support time during the intervention and post-intervention (Fig. 2E). There was no main effect of condition for velocity (P = 0.866, Fig. 2C), cadence (P = 0.205, Fig. 2D) and double support time (P = 0.877, Fig. 2E).
Fig. 2.
Combined leg measures. A and B. Modelled post-hoc contrasts for the condition*time interaction for stride length (A) and stride width (B). Black and grey filled circles represent mean differences and 95% confidence intervals of the differences for intervention values (IN) and post-IN values minus baseline values for walking with blood flow restriction (BFR-condition) and walking without BFR (CON-condition), respectively. Unfilled circles represent mean differences and 95% confidence intervals for BFR-condition values minus CON-condition values for baseline, IN and post-IN values. C–E. Modelled post-hoc contrasts of the main effects for velocity (C), cadence (D) and double support time (E). Main effects were compared as there was no significant condition*time interaction effect for these variables. Black filled squares represent the main effect of timepoint whereby mean differences and 95% confidence intervals of the differences for IN values and post-IN values minus baseline are shown. Unfilled squares represent the main effect of intervention whereby mean differences and 95% confidence intervals of the difference for BFR-values minus CON-values. A–E. The horizontal dotted line represents no difference between contrasts. ‘*’, ‘†’ and ‘‡’ represent significant differences for the given contrasts to P < 0.05, P < 0.01 and P < 0.001, respectively.
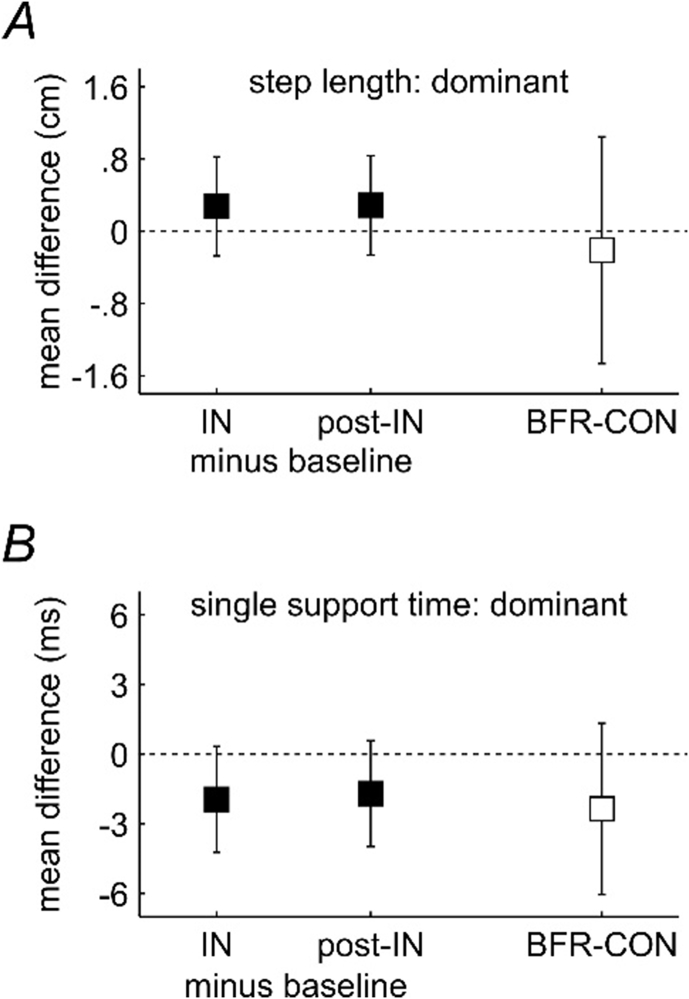
3.2. BFR-dominant (occluded) and CON-dominant step measurements
For step length and single support time, there was no significant condition*timepoint interaction (P = 0.111 and P = 0.343, respectively), no main effect of timepoint (P = 0.502 and P = 0.191, respectively) and no main effect of condition (P = 0.732 and P = 0.199, respectively) (Fig. 3). Therefore, for the BFR-condition, there were no differences between baseline, intervention and post-intervention step length and single support time and no differences when comparing the BFR-condition (dominant occluded leg) and CON-condition (dominant leg) for step length and single support time.
Fig. 3.
Results of single leg measures: BFR-dominant (occluded) and CON-dominant limb. A and B. Modelled main effects for BFR-dominant (occluded) and CON-dominant limb for step length (A) and single support time (B). Main effects were compared as there was no significant condition*time interaction effect for these variables. Black filled squares represent the main effect of timepoint whereby mean differences and 95% confidence intervals of the differences for IN values and post-IN values minus baseline are shown. Unfilled squares represent the main effect of condition whereby mean differences and 95% confidence intervals of the difference for BFR-dominant (occluded) values minus CON-dominant values. The horizontal dotted line represents no difference between contrasts.
3.3. BFR-non-dominant (unoccluded) and CON-non-dominant step measurements
For step length and single support time, there was a significant condition*timepoint interaction (P < 0.001 and P = 0.042, respectively) (Fig. 4). For step length, compared to baseline, participants took shorter steps during the intervention on the BFR-non-dominant (unoccluded) leg (P < 0.001) which returned to baseline levels post-intervention (Fig. 4). During the intervention, participants took shorter steps on the BFR-non-dominant (unoccluded) leg when compared to CON-non-dominant leg (P < 0.001) (Fig. 4). For single support time, compared to baseline, participants spent less time on the BFR-non-dominant (unoccluded) leg during the intervention (P < 0.001) which almost returned to baseline levels post-intervention (P = 0.026) (Fig. 4). During the intervention, participants spent less time on the non-dominant (unoccluded) leg during BFR-walking when compared to the non-dominant leg (P = 0.042) (Fig. 4).
Fig. 4.
Results of single leg measures: BFR-non-dominant (unoccluded) and CON-non-dominant limb. A and B. Modelled post-hoc contrasts for the condition*time interaction for BFR-non-dominant (unoccluded) and CON-non-dominant for step length (A) and single support time (B). Black and grey filled circles represent mean differences and 95% confidence intervals of the differences for the intervention values (IN) and post-IN values minus baseline values for the unoccluded limb while walking with blood flow restriction (BFR-condition) and the non-dominant limb walking without BFR (CON-condition), respectively. Unfilled circles represent mean differences and 95% confidence intervals for BFR-non-dominant (unoccluded) condition values minus CON-non-dominant walking values for baseline, IN and post-IN values. The horizontal dotted line represents no difference between contrasts. ‘*’, ‘†’ and ‘‡’ represent significant differences for the given contrasts to P < 0.05, P < 0.01 and P < 0.001, respectively.
4. Discussion
To our knowledge, this study is the first to examine changes in spatial-temporal gait parameters during and following an acute bout of unilateral BFR-walking in healthy individuals. There was a reduced stride length and increased stride width during the BFR-intervention compared to during the CON-intervention. There was no difference between BFR and CON conditions for velocity, cadence or double support time although there were timepoint dependent main effects. Unilaterally, a shorter step length and reduced single support time occurred in the non-dominant (unoccluded) leg during the BFR-intervention compared to the non-dominant leg during the CON-intervention, however no differences occurred for the dominant leg during the BFR-intervention (occluded leg) compared to the CON-intervention. Changes to gait parameters were transient, returning to baseline (or near baseline), 10 minutes post BFR-intervention.
4.1. Reasons for altered gait parameters
Decreased step length, shorter stride length (Chamberlin et al., 2005) and increased stride width (Maki, 1997; Chamberlin et al., 2005) are compensatory mechanisms that occur to reduce the risk of falls arising from an increased fear of falling. Biomechanically, increasing stride width and reducing stride length limits the distance the center of mass needs to travel outside the base of support. If the center of mass travels too far beyond the base of support, instability, and subsequently the risk of falls, increases. By decreasing step length, participants can mediate the degree to which the center of mass needs to travel before returning inside the base of support. Therefore, participants feel they have greater control and stability. This would indicate that BFR-walking promotes the perception of instability and hence a more conservative gait pattern is adopted.
There was reduced single support time in the BFR-non-dominant leg during the intervention. This measure is synonymous with reduced single leg swing time in the BFR-dominant (occluded) leg. Therefore, participants swung the occluded leg more quickly through the swing phase of gait for that leg. This is an unexpected result as we would expect that the single support time would increase in the BFR-non-dominant (unoccluded) leg to favour the more stable (unoccluded) leg. This could be due to the requirement to more quickly place the occluded leg due to proprioceptive uncertainty. BFR can change proprioception, including afferent feedback mechanisms (Mazzaro et al., 2005) and stretch reflexes (Grey et al., 2001) at higher pressures. Although these adaptations will be reduced at lower pressures they still might be present and have an effect, as observed in the upper limb (Mittal et al., 2008). Another possibility is mechanical restriction. This may cause the participants to reduce swing time. The location of the cuff potentially resulted in restriction to the hamstrings and/or hip flexors and therefore adjustment of gait to avoid chafing/rubbing against the other leg could have occurred. However, if mechanical restriction was the reason for the reduced step time, we may expect that there would also be a reduced step length in the BFR-dominant (occluded) leg, which was not observed. While we cannot provide a definite explanation to the reason for the reduced swing time in the BFR-dominant leg during the intervention, there are a number of adaptations to gait due to the blood pressure cuff, either neurally or mechanically mediated. These adaptations may have implications if used in clinical populations.
4.2. Implications
The current study was performed on young adults and therefore the implications to clinical populations can only be speculative. However, as this type of intervention has been proposed in clinical populations the potential consequences should be discussed. The magnitude of gait parameter changes were small and may not be clinically significant. However, although changes were small, the unilateral changes were transient and transferring this protocol to patients could result in two possibilities; 1) The gait parameter changes due to BFR-walking, although significant, are minor and therefore this type of intervention is warranted in clinical populations or 2) There were small alterations in gait parameters and these alterations could be exacerbated (greater) in clinical populations. Given the uncertainty of the effects of BFR-walking in populations with already altered spatial-temporal parameters, clinicians should be cautious when using BFR walking in clinical populations. More research should be conducted on specific clinical populations in a controlled laboratory setting.
While the reason for the altered gait patterns in the current study could be due to the cuff or altered proprioceptive feedback, the implications, regardless of the reason are important to studies utilising ischemia to influence nerve excitability during walking. Numerous studies have utilised ischemia (BFR) to progressively alter reflex afferent discharge (for example; Sinkjaer et al., 2000; Grey et al., 2001; Mazzaro et al., 2005; Zakutansky et al., 2005; Friemert et al., 2010). During walking and using ischemia, these studies reduce large diameter afferent feedback and assess changes in reflex excitability with this feedback reduced. When applying ischemia, in studies modulating reflex excitability, it is important that temporal and spatial gait parameters remain the same, otherwise alterations in reflex excitability could be a result of the changing spatial and temporal aspects of gait, and not the removal of afferent feedback, itself. The current study indicates that spatial and temporal aspects of gait are altered, even with the application of a blood pressure cuff at low pressure and as such, these studies should consider this in the interpretation of their findings.
4.3. Limitations
A limitation of this study was that the baseline, intervention and post-intervention walking were conducted on a treadmill, however assessment of gait was conducted walking overground. Several reasons necessitated this decision; 1) To ensure occlusion cuff pressures were maintained, monitoring of pressure during BFR-walking was required without interfering with the subject's gait as the use of overground walking may have caused the assessors to compromise the gait pattern of the participants to check cuff pressures; 2) The attempt to minimize potential walking speed variability by ensuring participants maintained a consistent speed for both the BFR-condition and CON-condition. Our attempt to do this was successful as there was no difference between the BFR-condition and CON-condition in overground walking velocity between conditions. Although participants walked over the treadmill during prolonged walking and walked overground for testing, and there can be small but significant differences for some parameters between overground and treadmill walking (Riley et al., 2007), as both CON and BFR-conditions used the same methodology any biases associated with using treadmill training and walking overground were controlled for. Another limitation is that different subjects wore different shoe types during testing. Although, this may result in some inter-subject differences, we felt it was best, pragmatically, for the subjects to walk in their own shoes. Despite this, intra-subject differences were controlled as subjects walked with the same shoes each session. A final limitation, relating to the applicability of our results, involves the cuff-width of the cuff used to perform BFR. Differing cuff widths can alter the amount of restriction and the types of post-BFR adaptations (Loenneke et al., 2012a). Although the cuff-width in the present study was 7 cm and is consistent with most cuff widths in other studies (from 5–7.6 cm), this isn't universal, and cuffs can range from 5–20.5 cm (Loenneke et al., 2012a). As such, the results of the current study may not be transferrable to cuffs of markedly different widths.
5. Conclusion
The current study showed small but significant transient changes during the BFR-intervention compared to CON-intervention and baseline. It is difficult to ascertain if the small changes should be interpreted that the changes to gait parameters are minor and this type of intervention is warranted in elderly and patient populations or if the changes, although minor, would be exacerbated in elderly or patient populations leading to an increased risk of falls. More research is required in a controlled laboratory setting conducted specifically on the populations using BFR-walking for rehabilitation.
Declarations
Author contribution statement
Timothy John Faras, Michael David Laporte, Remi Sandoval, Fadi Najjar: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Peter Stubbs: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Vanessa Ade: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
Peter William Stubbs was supported by Hammel Neurorehabilitation Center and the Health Research Fund of the Central Denmark Region.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to thank Dale Schalkwijk, Fletcher Rowe and Dario Rossi for assistance in data collection.
References
- Abe T., Kearns C., Sato Y. Muscle size and strength are increased following walk training with restricted venous blood flow from the leg muscle, Kaatsu-walk training. J. Appl. Physiol. 2006;100:1460–1466. doi: 10.1152/japplphysiol.01267.2005. [DOI] [PubMed] [Google Scholar]
- Abe T., Kearns C.F., Fujita S., Sakamaki M., Sato Y., Brechue W.F. Skeletal muscle size and strength are increased following walk training with restricted leg muscle blood flow: implications for training duration and frequency. Int. J. KAATSU Train. Res. 2009;5:9–15. [Google Scholar]
- Beekley M.D., Sato Y., Abe T. KAATSU-walk training increases serum bone-specific alkaline phosphatase in young men. Int. J. KAATSU Train. Res. 2005;1:77–81. [Google Scholar]
- Chamberlin M.E., Fulwider B.D., Sanders S.L., Medeiros J.M. Does fear of falling influence spatial and temporal gait parameters in elderly persons beyond changes associated with normal aging? J. Gerontol. 2005;60:1163–1167. doi: 10.1093/gerona/60.9.1163. [DOI] [PubMed] [Google Scholar]
- Friemert B., Franke S., Gollhofer A., Claes L., Faist M. Group I afferent pathway contributes to functional knee stability. J. Neurophysiol. 2010;103:616–622. doi: 10.1152/jn.00172.2009. [DOI] [PubMed] [Google Scholar]
- Grey M., Ladouceur M., Andersen J.B., Nielsen J.B., Sinkjaer T. Group II muscle afferents probably contribute to the medium latency soleus stretch reflex during walking in humans. J. Physiol. 2001;534:925–933. doi: 10.1111/j.1469-7793.2001.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes L., Paton B., Rosenblatt B., Gissane C., Patterson S.D. Blood flow restriction training in clinical musculoskeletal rehabilitation: a systematic review and meta-analysis. Br. J. Sports Med. 2017;51:1003–1011. doi: 10.1136/bjsports-2016-097071. [DOI] [PubMed] [Google Scholar]
- Ishii N., Madarame H., Odagiri K., Naganuma M., Shinoda K. Circuit training without external load induces hypertrophy in lower-limb muscles when combined with moderate venous occlusion. Int. J. KAATSU Train. Res. 2005;1:24–28. [Google Scholar]
- Japanese Society of Hypertension Chapter 2. Measurement and clinical evaluation of blood pressure. Hypertens. Res. 2014;37:266–278. [Google Scholar]
- Kuys S.S., Brauer S.G., Ada L. Test-retest reliability of the GAITRite system in people with stroke undergoing rehabilitation. Disabil. Rehabil. 2011;33:1848–1853. doi: 10.3109/09638288.2010.549895. [DOI] [PubMed] [Google Scholar]
- Lewek M.D., Randall E.P. Reliability of spatiotemporal asymmetry during overground walking for individuals following chronic stroke. J. Neurol. Phys. Ther. 2011;35:116–121. doi: 10.1097/NPT.0b013e318227fe70. [DOI] [PubMed] [Google Scholar]
- Loenneke J., Fahs C., Rossow L., Sherk V., Thiebaud R., Abe T., Bemben D., Bemben M. Effects of cuff width on arterial occlusion: implications for blood flow restricted exercise. Eur. J. Appl. Physiol. 2012;112:2903–2912. doi: 10.1007/s00421-011-2266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loenneke J.P., Wilson J.M., Marín P.J., Zourdos M.C., Bemben M.G. Low intensity blood flow restriction training: a meta-analysis. Eur. J. Appl. Physiol. 2012;112:1849–1859. doi: 10.1007/s00421-011-2167-x. [DOI] [PubMed] [Google Scholar]
- Maki B.E. Gait changes in older adults: predictors of falls or indicators of fear. J. Am. Geriatr. Soc. 1997;45:313–320. doi: 10.1111/j.1532-5415.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- Mazzaro N., Grey M.J., Sinkjaer T. Contribution of afferent feedback to the soleus muscle activity during human locomotion. J. Neurophysiol. 2005;93:167–177. doi: 10.1152/jn.00283.2004. [DOI] [PubMed] [Google Scholar]
- Menz H.B., Latt M.D., Tiedemann A., Kwan M.M.S., Lord S.R. Reliability of the GAITRite® walkway system for the quantification of temporo-spatial parameters of gait in young and older people. Gait Posture. 2004;20:20–25. doi: 10.1016/S0966-6362(03)00068-7. [DOI] [PubMed] [Google Scholar]
- Mittal P., Shenoy S., Sandhu J.S. Effect of different cuff widths on the motor nerve conduction of the median nerve: an experimental study. J. Orthop. Surg. Res. 2008;3:1. doi: 10.1186/1749-799X-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney S.J., Richards C. Hemiparetic gait following stroke. Part I: characteristics. Gait Posture. 1996;4:136–148. [Google Scholar]
- Patterson S., Ferguson R. Increase in calf post-occlusive blood flow and strength following short-term resistance exercise training with blood flow restriction in young women. Eur. J. Appl. Physiol. 2010;108:1025–1033. doi: 10.1007/s00421-009-1309-x. [DOI] [PubMed] [Google Scholar]
- Patterson S., Ferguson R. Enhancing strength and postocclusive calf blood flow in older people with training with blood-flow restriction. J. Aging Phys. Activ. 2011;19:201–213. doi: 10.1123/japa.19.3.201. [DOI] [PubMed] [Google Scholar]
- Riley P.O., Paolini G., Della Croce U., Paylo K.W., Kerrigan D.C. A kinematic and kinetic comparison of overground and treadmill walking in healthy subjects. Gait Posture. 2007;26:17–24. doi: 10.1016/j.gaitpost.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Scott B., Slattery K., Sculley D., Dascombe B. Hypoxia and resistance exercise: a comparison of localized and systemic methods. Sports Med. 2014;44:1037–1054. doi: 10.1007/s40279-014-0177-7. [DOI] [PubMed] [Google Scholar]
- Shinohara M., Kouzaki M., Yoshihisa T., Fukunaga T. Efficacy of tourniquet ischemia for strength training with low resistance. Eur. J. Appl. Physiol. Occup. Physiol. 1998;77:189–191. doi: 10.1007/s004210050319. [DOI] [PubMed] [Google Scholar]
- Sinkjaer T., Andersen J., Landouceur M., Christensen L., Nielsen J. Major role for sensory feedback in soleus EMG activity in the stance phase of walking in man. J. Physiol. 2000;523:817–827. doi: 10.1111/j.1469-7793.2000.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takarada Y., Sato Y., Ishii N. Effects of resistance exercise combined with vascular occlusion on muscle function in athletes. Eur. J. Appl. Physiol. 2002;86:308–314. doi: 10.1007/s00421-001-0561-5. [DOI] [PubMed] [Google Scholar]
- Van Uden C.J.T., Besser M.P. Test-retest reliability of temporal and spatial gait characteristics measured with an instrumented walkway system (GAITRite®) BMC Muscoskelet. Disord. 2004;5:13. doi: 10.1186/1471-2474-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster K.E., Wittwer J.E., Feller J.A. Validity of the GAITRite walkway system for the measurement of averaged and individual step parameters of gait. Gait Posture. 2005;22:317–321. doi: 10.1016/j.gaitpost.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Wei T. Sen, Liu P.T., Chang L.W., Liu S.Y. Gait asymmetry, ankle spasticity, and depression as independent predictors of falls in ambulatory stroke patients. PLoS One. 2017;12:1–14. doi: 10.1371/journal.pone.0177136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda T., Abe T., Sato Y., Midorikawa T., Kearns C.F., Inoue K., Ryushi T., Ishii N. Muscle fiber cross-sectional area is increased after two weeks of twice daily KAATSU-resistance training. Int. J. KAATSU Train. Res. 2005;1:65–70. [Google Scholar]
- Yasuda T., Fukumura K., Fukuda T., Uchida Y., Iida H., Meguro M., Sato Y., Yamasoba T., Nakajima T. Muscle size and arterial stiffness after blood flow-restricted low-intensity resistance training in older adults. Scand. J. Med. Sci. Sports. 2014;24:799–806. doi: 10.1111/sms.12087. [DOI] [PubMed] [Google Scholar]
- Zakutansky D.W., Kitano K., Wallace J.P., Koceja D.M. H-reflex and motor responses to acute ischemia in apparently healthy individuals. J. Clin. Neurophysiol. 2005;22:210–215. [PubMed] [Google Scholar]