Abstract
Many older adults receive routine cancer screening even when it is no longer recommended. We sought to identify demographic, health-related, and attitudinal factors that are most predictive of continued breast, colorectal, and prostate cancer screening decisions in older adults under various scenarios. A sample of adults age 65+ (n = 1272) were recruited from a nationally representative panel in November 2016, of which 881 (69.3%) completed our survey. Participants were presented vignettes in which we experimentally varied a hypothetical patient's life expectancy, age, quality of life, and physician screening recommendation. The dependent variable was the choice to continue cancer screening in the vignette. Classification and regression tree (CART) analysis was used to identify characteristics most predictive of screening decisions; both the participants' characteristics and the hypothetical patient's characteristics in the vignettes were included in the analysis. CART analysis uses recursive partitioning to create a classification tree in which variables predictive of the outcome are included as hierarchical tree nodes. We used automated ten-fold cross-validation to select the tree with lowest misclassification and highest predictive accuracy. Participants' attitude towards cancer screening was most predictive of choosing screening. Among those who agreed with the statement “I plan to get screened for cancer for as long as I live” (n = 300, 31.9%), 73.2% chose screening and 57.2% would still choose screening if hypothetical patient had 1-year life expectancy. For this subset of older adults with enthusiasm towards screening even when presented with scenario involving limited life expectancy, efforts are needed to improve informed decision-making about screening.
Keywords: Cancer screening, Life expectancy, Decision-making, CART analysis
Highlights
-
•
Patient attitude about cancer screening is most predictive of screening decisions.
-
•
One-third of older adult participants reported enthusiasm about cancer screening.
-
•
Those enthusiastic about screening chose screening even with limited life expectancies.
1. Introduction
Cancer screening often reduces cancer-related mortality and morbidity through earlier detection (Eckstrom et al., 2013). However, it also may cause harms, including false positive results and over-diagnosis of clinically unimportant cancers (Eckstrom et al., 2013). Clinical practice guidelines recommend against routine cancer screening when the harms outweigh the benefits, often defined using age or life expectancy thresholds (Harris et al., 2015; Kotwal and Schonberg, 2017). Many older adults for whom routine cancer screening is no longer recommended still receive screening for breast, colorectal, and prostate cancers (Royce et al., 2014). It is important to understand what drives older adults' cancer screening decisions.
A myriad of factors have been found to be relevant to patients' cancer screening decisions; these include demographic factors such as age, sex, education, finances; health factors such as smoking history, family history, health status and life expectancy; experiential factors such as previous screening experiences and physician recommendation; and attitudinal factors such as worry about cancer and enthusiasm towards cancer screening (Lewis et al., 2006; Tarasenko et al., 2011; Beydoun and Beydoun, 2008; Schonberg et al., 2007; James et al., 2017; Schwartz et al., 2004). The relative importance of these factors in patients' screening decisions remains unclear. This is a critical knowledge gap that needs to be addressed in order to design targeted, effective interventions to improve cancer screening in older adults that maximize benefit and minimize harms.
In our prior work, we explored the relative influence of four factors – life expectancy, age, quality of life, and physician recommendation – on older adults' cancer screening decisions in a vignette-based national survey and found that, among them, age was the most influential on breast, colorectal, and prostate cancer screening decisions (Janssen et al., 2019). The analytic method, however, did not allow examination of other participant characteristics. We sought to extend this prior work by leveraging data from the same national survey to analyze a much broader set of participant factors in order to identify the factors that are most predictive of decisions for breast, colorectal, and prostate cancer screenings in older adults. Informed by the Health Belief Model (Janz and Becker, 1984), we examined demographic, health-related, and attitudinal factors.
2. Methods
2.1. Study design and sample
We draw our data from a cross-sectional national survey that used vignettes to explore the effect of life expectancy, age, quality of life, and physician recommendation on older adults' cancer screening decisions; the results focused on the relative effect of these four factors are presented elsewhere (Janssen et al., 2019). We leverage the rich data collected about the participants and use a novel method – the Classification And Regression Tree (CART) analysis - to identify what participant or vignette characteristics are most predictive of screening decisions.
The survey recruited from the KnowledgePanel, a probability-based online survey panel designed to be representative of U.S. adults. Panel members are recruited by random digit dialing and address-based sampling, and are provided computers and Internet access if needed (GfK, n.d.). Panel members who were 65+ years old and English-speaking were invited to participate, with over-sampling of African Americans. This project was approved by a Johns Hopkins School of Medicine institutional review board and complied with code of ethics outlined in the Declaration of Helsinki.
2.2. Survey instrument
The survey examined decision making in a hypothetical scenario about cancer screening. Participants were randomized to questions about colorectal cancer screening or prostate (males) or breast (females) cancer screening. We briefly reviewed the benefits and harms of the specific type of cancer screening. We presented 9 vignettes in which we experimentally varied a hypothetical patient's life expectancy (10 years, 5 years, or 1 year), age (65, 75, or 85 years old), quality of life (good, medium, or poor), and physician recommendation (recommends screening, neutral, or recommends against screening). These values were chosen based on literature review and qualitative interviews with older adults (Schoenborn et al., 2017). We asked the participants whether they would get a screening test if they were the hypothetical patient. Sample vignettes are included in Supplemental Files.
The participants' age, sex, race/ethnicity, education, household income, marital status, geographic region, and employment status were provided by the KnowledgePanel. In addition, we collected the following information, as informed by the Health Belief Model (Janz and Becker, 1984):
-
-
Factors related to the model domain on perceived susceptibility to the disease: cancer history, cancer screening history, history of abnormal screening;
-
-
Factors related to the model domain on perceived benefits/harms of the preventive action: attitude towards cancer screening as measured by agreement with the statement “I plan to be screened for (breast/colorectal/prostate) cancer for as long as I live” (Lewis et al., 2006), health literacy- measured by three validated questions (area under the receiver operating characteristic curve [AUC] 0.87, 0.80, 0.76, respectively, as reported in the validation study) (Chew et al., 2004), numeracy – measured by a three-question scale (SNS-3) that is was highly correlated with the full SNS (correlation coefficient 0.91) which has an AUC of 0.77 as reported in the validation study (McNaughton et al., 2015);
-
-
Factors related to the model domain on perceived severity and threat from the disease – self-reported health and functional status, smoking status, history of life threatening illness, predicted life expectancy using a validated index (c-statistic 0.834 in validation cohort) (Cruz et al., 2013), belief regarding whether doctors can predict life expectancy, preferences around discussing life expectancy, self-perceived life expectancy;
-
-
Factors related to the model domain on cues to action: whether doctors had recommended stopping cancer screening, preferred decision-making roles, trust in doctors.
We pilot tested the survey instrument with ten older adults who were not included in the study and iteratively revised the instrument based on feedback.
2.3. Data collection and analysis
Eligible KnowledgePanel members (N = 1272) were invited to participate in the online survey via email in November 2016. Survey weights were applied to adjust for nonresponse and for oversampling of African Americans to produce nationally-representative estimates.
We used the classification and regression tree (CART) analysis to identify what participant or vignette characteristics are most predictive of screening decisions. CART analysis is a non-parametric statistical method that uses recursive partitioning to create a classification tree in which the variables predictive of an outcome are included as nodes in the tree in a hierarchical fashion – with the most predictive variable at the top of the tree (Breiman et al., 1984). CART analysis offers the advantage of being able to accommodate large number of possible predictors. In this analysis, we included participant characteristics and the four characteristics of the hypothetical patient tested in the vignettes. The variables are described in full in the Supplemental Files. We used an automated ten-fold cross-validation to select the optimal tree with the lowest misclassification and the highest accuracy for prediction (by the AUC). The ten-fold cross-validation procedure split the sample into 10 subsets and uses 9 subsets as learning samples to develop the model and the 10th subset as the testing sample. This procedure is then repeated multiple times and statistics are averaged. CART analysis is often used to identify the optimal prediction algorithm for an outcome, but our analysis focused on identifying the top predictors of the screening decision. Therefore, after an optimal tree was identified, we used successive pruning to reduce the tree to a maximum of 10 nodes (Breiman et al., 1984). All statistical analyses except for CART modeling were performed using STATA version 13. CART was performed using Salford Predictive Modeler version 8.2.
3. Results
A total of 881 (69.3%) adults completed the survey. Majority of the participants were women (n = 464, 55.2%) and were white (n = 576, 77.2%), with average age of 73.4 years (Table 1). Compared to the non-responders, the responders were similar in age (p = 0.77) and education (p = 0.19) but were less likely to be female (52.7% versus 59.3%, p = 0.03) and more likely to be non-Hispanic white (65.4% versus 49.6%, p < 0.001).
Table 1.
| Characteristics | Aggregate (n = 881) | Screening attitude: I plan to get screened for breast/prostate/colorectal cancer for as long as I livec |
p-Value | ||
|---|---|---|---|---|---|
| Strongly agree/agree (n = 300) | Neither agree nor disagree (n = 260) | Strongly disagree/disagree (n = 266) | |||
| Age, year - mean (SD) | 73.4 (6.1) | 72.2 (5.3) | 72.0 (5.5) | 75.7 (6.9) | <0.001 |
| Female sex | 464 (55.2%) | 165 (60.6%) | 128 (50.3%) | 147 (55.3%) | 0.16 |
| Race | 0.002 | ||||
| White, non-Hispanic | 576 (77.2%) | 180 (75.0%) | 162 (70.2%) | 201 (84.7%) | |
| Black, non-Hispanic | 216 (8.8%) | 91 (11.6%) | 63 (7.6%) | 44 (6.5%) | |
| Hispanic | 47 (8.2%) | 20 (11.0%) | 17 (10.3%) | 8 (4.2%) | |
| Other | 42 (5.8%) | 9 (2.5%) | 18 (11.9%) | 13 (4.7%) | |
| Cancer screening type in the survey | 0.003 | ||||
| Prostate | 208 (22.5%) | 58 (15.8%) | 66 (26.6%) | 58 (21.6%) | |
| Colorectal | 441 (49.8%) | 146 (47.5%) | 139 (51.2%) | 148 (57.6%) | |
| Breast | 232 (27.7%) | 96 (36.6%) | 55 (22.2%) | 60 (20.8%) | |
| Has ever had a mammogram/prostate-specific antigen (PSA) test/colonoscopyd | 744 (81.2%) | 275 (92.1%) | 218 (75.7%) | 196 (72.4%) | <0.001 |
| Has had an up-to-date mammogram/PSA test/colonoscopye | 631 (66.0%) | 260 (85.2%) | 174 (55.4%) | 148 (53.7%) | <0.001 |
| Physician have recommended stopping mammogram/PSA test/colonoscopy | 76 (9.7%) | 6 (2.0%) | 19 (6.8%) | 46 (20.1%) | <0.001 |
| <10 year predicted life expectancyf | 197 (31.1%) | 51 (22.7%) | 47 (26.9%) | 70 (36.5%) | 0.03 |
| <10 year self-perceived life expectancy | 110 (16.6%) | 25 (11.7%) | 27 (14.1%) | 54 (24.9%) | 0.008 |
| Education | 0.23 | ||||
| <High school | 61 (14.4%) | 22 (16.4%) | 20 (17.8%) | 17 (9.9%) | |
| High school | 271 (33.3%) | 81 (29.7%) | 91 (34.3%) | 82 (35.3%) | |
| <4 year college | 243 (24.1%) | 93 (26.6%) | 69 (24.8%) | 69 (21.8%) | |
| College or higher | 306 (28.2%) | 104 (27.4%) | 80 (23.2%) | 98 (33.0%) | |
| Health literacy (Chew et al., 2004) (3–15) – mean (SD) | 13.1 (2.1) | 13.0 (2.3) | 12.8 (2.3) | 13.4 (1.8) | 0.002 |
| Numeracy (McNaughton et al., 2015) (3–18) - mean (SD) | 13.8 (3.5) | 13.9 (3.6) | 13.4 (3.7) | 14.0 (3.4) | 0.56 |
| Decision making preferences | 0.18 | ||||
| Make own decisions | 533 (62.5%) | 163 (55.2%) | 163 (66.8%) | 174 (67.3%) | |
| Make decisions together | 331 (36.1%) | 130 (43.7%) | 92 (31.0%) | 88 (31.6%) | |
| Leave decision to doctor | 7 (1.4%) | 1 (1.2%) | 3 (2.2%) | 3 (1.1%) | |
Means and percentages are weighted.
Proportion of missing values were 1.0% or less for all variables except for health literacy (composite of 3 questions) which had a cumulative missing value of 1.2%, numeracy (composite of 3 questions) which had a cumulative missing value of 2.5%, and predicted life expectancy (composite of 12 variables) which had a cumulative missing value of 7.2%.
Participants with history of relevant cancer (n = 52) were excluded from this question because they would be under surveillance for recurrence of cancer, rather than screening. Three additional participants declined to answer this question.
Participants randomized to breast cancer screening questions were asked about receipt of mammogram; participants randomized to prostate cancer screening questions were asked about receipt of prostate-specific antigen test; participants randomized to colorectal cancer screening questions were asked about receipt of colonoscopy.
Up to date mammogram and PSA test were defined to be within 2 years; up to date colonoscopy was defined to be within 10 years.
Using the mortality risk index developed by Cruz et al. (2013).
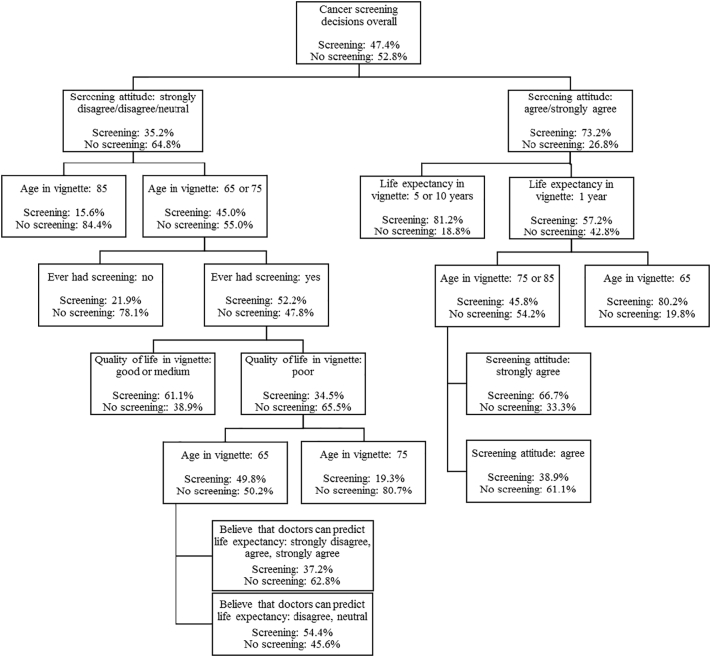
We found an optimal tree with 57 nodes based on 10-fold cross-validation. We reduced the tree to 10 nodes (Fig. 1) via successive pruning since the goal of our analysis was to identify top predictors of the screening decision. The AUC for the optimal tree averaged 0.847 (0.792 for the reduced tree) for the learning samples, 0.806 (0.786 for the reduced tree) for the testing samples. The overall misclassification rate was 0.197 (0.258 for the reduced tree) and 0.246 (0.265 for the reduced tree) for the learning and testing samples, respectively.
Fig. 1.
Ten-node tree from CART analysis showing the top predictors of hypothetical breast, colorectal, and prostate cancer screening decisions among 881 older adults who completed a national online survey in November 2016.
Participants' attitude towards cancer screening was the most predictive of whether a participant chose screening in the vignettes. About one-third of the participants (n = 300, 31.9%) agreed or strongly agreed with the statement “I plan to get screened for breast/colorectal/prostate cancer for as long as I live”; 260 (29.2%) neither agreed nor disagreed, and 266 (33.2%) disagreed or strongly disagreed with this statement (Table 1). Compared to those who disagreed with the statement, those who agreed with the statement were younger, more likely to be non-white, more likely to be answering questions about breast cancer screening, less likely to have <10 year life expectancy by prediction or self-perception, and much less likely to have been recommended to stop screening by their doctors. There were no differences in sex, education, numeracy, or decision-making preferences.
Among those who agreed or strongly agreed with the statement “I plan to get screened for breast/colorectal/prostate cancer for as long as I live”, the rate of choosing screening in the vignettes was 73.2%. For these participants, life expectancy of the hypothetical patient in the vignette was the second most predictive of screening decisions, followed by the age of the hypothetical patient (Fig. 1). However, even when the hypothetical patient only had a life expectancy of one year, over half (57.2%) of the participants still chose screening; 45.8% of the participants chose screening even if the hypothetical patient had a life expectancy of one year and was 75 years or older.
Among those who disagreed or felt neutral about the statement, the rate of choosing screening in the vignettes was 35.2% and the age of the hypothetical patient in the vignette was the second most predictive of screening decisions, followed by history of ever having screening and quality of life of the hypothetical patient. Participants were more likely to choose screening when the hypothetical patient was younger, if the participants have had prior screening, and if the hypothetical patient had a good or medium, as opposed to poor, quality of life (Fig. 1). In a sensitivity analysis where we conducted separate analyses by cancer screening type, all three types of cancer screening (breast, colorectal, prostate), screening attitude remained the most predictive of the screening decisions.
4. Discussion
This is the first study, to our knowledge, to examine the relative effects of a rich set patient demographic, health, and attitudinal factors on hypothetical cancer screening decisions in a national sample. It is also the first study using CART analysis to examine predictors of cancer screening. We found that attitude towards screening was the strongest predictor of participants' choice to pursue cancer screening in the vignettes. The importance of enthusiastic attitude towards screening has been shown in previous studies (Lewis et al., 2006; Schwartz et al., 2004). For example, in a survey of 116 older adults, most participants wanted cancer screening throughout their lives and believed that those living in nursing homes, with Alzheimer's disease, or were totally dependent on others should still get screened for cancer (Lewis et al., 2006).
Our finding that those who agreed with the statement “I plan to be screened for cancer for as long as I live” would then choose to get screened in the survey vignettes is unsurprising in itself; what is interesting is that this enthusiasm towards screening is more important in predicting screening decisions than other demographic and health factors, including both age and life expectancy. In this national sample, participants with favorable attitude towards screening represented about one-third of the total sample. This has important implications since a sizable subset of older adults may have persistent beliefs and attitudes that lead to screening despite older age or short life expectancy, which are thresholds outlined in guidelines for stopping routine screening (Harris et al., 2015; Kotwal and Schonberg, 2017).
Although one interview study found that older adults may be amenable to stopping cancer screening in the context of trusting relationships with their doctors (Schoenborn et al., 2017), we did not find physician recommendation to be a top predictor of screening decisions in this study. How the subset of older adults who hold favorable attitudes towards screening would react to physician counseling to stop routine screening is unknown and needs to be explored in future studies. Even if these older adults were amenable to being persuaded by their physicians, physicians may be hesitant to try. Physicians have reported that patient request is a significant barrier to stopping routine cancer screening and are often reluctant to counter patients' views for fear of how patients would react (Schoenborn et al., 2016; Pollack et al., 2012). Messaging approaches to reshape patient attitudes, for example, through social network or public health messages, have not been explored and offer opportunities to improve informed decision making about cancer screening among older adults.
Strengths of this study include the use of large national sample of older adults and the novel use of the CART analysis to examine predictors of cancer screening. Compared to more traditional methods such as logistic regression, CART analysis offers several advantages. It is able to better handle non-linear relationships between outcome and predictors. Second, CART is able to handle a large number of predictors and has sophisticated methods for dealing with missing values (Breiman et al., 1984). Limitations of the study include the use of hypothetical scenarios that may not fully reflect actual behavior. However, the results showed that the participants' attitudes towards screening were the most influential of their decisions in the hypothetical scenario and were also highly correlated with their past screening behavior, suggesting that there may be sufficient correlation between the participants' responses in the survey and decisions in real life. Although our analysis included a number of participant characteristics, there may be other factors that impact cancer screening decision making that we did not examine, including psychosocial factors such as self-efficacy, attitudes towards preventive care, attitudes towards risk/uncertainty, etc. Our findings could also be susceptible to non-response bias. Responders were less likely to be female and more likely to be non-Hispanic white which we adjusted for using post-stratification weights. Lastly, the study included a national sample of older adults in the United States and may not be representative of other countries.
5. Conclusion
We found older adults' attitude towards cancer screening to be the most predictive factor of their screening decisions. A subset of older adults who are enthusiastic about screening may choose screening even when presented with a scenario involving limited life expectancy. Interventions are needed to improve balanced perceptions and informed decision-making based on the benefits and harms of screening among older adults.
Acknowledgments
Acknowledgements
Conflict of interest
No authors had any conflict of interest. Dr. Pollack has stock ownership in Gilead Sciences, Inc. However, we do not believe this has resulted in any conflict with the design, methodology, or results presented in this manuscript.
Funding/support
This work was supported by the National Institute on Aging of the National Institutes of Health under Award Number R03AG050912. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
In addition, Dr. Schoenborn was supported by a T. Franklin Williams Scholarship Award with funding provided by: Atlantic Philanthropies, Inc., the John A. Hartford Foundation, the Alliance for Academic Internal Medicine-Association of Specialty Professors, and the American Geriatrics Society; the Johns Hopkins KL2 Clinical Scholars program funded by KL2TR001077 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research; a Cancer Control Career Development Award from the American Cancer Society (CCCDA-16-002-01), and K76AG059984 from the National Institute on Aging. Dr. Boyd was supported by 1K24AG056578 from the National Institute on Aging. Dr. Xue was supported by P30AG021334 from the National Institute on Aging.
None of the funding sources had any involvement in study design, collection, analysis and interpretation of data, or writing of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pmedr.2019.01.007.
Contributor Information
Nancy L. Schoenborn, Email: nancyli@jhmi.edu.
Qian-Li Xue, Email: qxue1@jhu.edu.
Craig E. Pollack, Email: cpollac2@jhmi.edu.
Ellen M. Janssen, Email: ellen.janssen@iconplc.com.
John F.P. Bridges, Email: john.bridges@osumc.edu.
Antonio C. Wolff, Email: awolff@jhmi.edu.
Cynthia M. Boyd, Email: cyboyd@jhmi.edu.
Appendix A. Supplementary data
Supplementary material
References
- Beydoun H.A., Beydoun M.A. Predictors of colorectal cancer screening behaviors among average-risk older adults in the United States. Cancer Causes Control. 2008;19(4):339–359. doi: 10.1007/s10552-007-9100-y. [DOI] [PubMed] [Google Scholar]
- Breiman L., Freidman J.H., Olshen R.A., Stone C.J. Chapman & Hall; Boca Raton: 1984. Classification and Regression Trees. [Google Scholar]
- Chew L.D., Bradley K.A., Boyko E.J. Brief questions to identify patients with inadequate health literacy. Fam. Med. 2004;36(8):588–594. [PubMed] [Google Scholar]
- Cruz M., Covinsky K., Widera E.W., Stijacic-Cenzer I., Lee S.J. Predicting 10-year mortality for older adults. JAMA. 2013;309(9):874–876. doi: 10.1001/jama.2013.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstrom E., Feeny D.H., Walter L.C., Perdue L.A., Whitlock E.P. Individualizing cancer screening in older adults: a narrative review and framework for future research. J. Gen. Intern. Med. 2013;28(2):292–298. doi: 10.1007/s11606-012-2227-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GfK KnowledgePanel recruitment and sample survey methodologies. http://www.gfk.com/fileadmin/user_upload/dyna_content/US/documents/KnowledgePanel_Recruitment_Sample_Survey_Methodology.pdf
- Harris R.P., Wilt T.J., Qaseem A., High Value Care Task Force of the American College of Physicians A value framework for cancer screening: advice for high-value care from the American College of Physicians. Ann. Intern. Med. 2015;162(10):712–717. doi: 10.7326/M14-2327. [DOI] [PubMed] [Google Scholar]
- James L.J., Wong G., Craig J.C. Men's perspectives of prostate cancer screening: a systematic review of qualitative studies. PLoS One. 2017;12(11) doi: 10.1371/journal.pone.0188258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen E.M., Pollack C.E., Boyd C.M. What is important to older adults when making cancer screening decisions? – results from a national survey using discrete choice experiment. J. Med. Decis. Making. 2019 doi: 10.1177/0272989X19853516. (under review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janz N.K., Becker M.H. The health belief model: a decade later. Health Educ. Behav. 1984;11(1):1–47. doi: 10.1177/109019818401100101. [DOI] [PubMed] [Google Scholar]
- Kotwal A.A., Schonberg M.A. Cancer screening in the elderly: a review of breast, colorectal, lung, and prostate cancer screening. Cancer J. 2017;23(4):246–253. doi: 10.1097/PPO.0000000000000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C.L., Kistler C.E., Amick H.R. Older adults' attitudes about continuing cancer screening later in life: a pilot study interviewing residents of two continuing care communities. BMC Geriatr. 2006;6:10. doi: 10.1186/1471-2318-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton C.D., Cavanaugh K.L., Kripalani S., Rothman R.L., Wallston K.A. Validation of a short, 3-item version of the subjective numeracy scale. Med. Decis. Mak. 2015;35(8):932–936. doi: 10.1177/0272989X15581800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack C.E., Platz E.A., Bhavsar N.A. Primary care providers' perspectives on discontinuing prostate cancer screening. Cancer. 2012;118(22):5518–5524. doi: 10.1002/cncr.27577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royce T.J., Hendrix L.H., Stokes W.A., Allen I.M., Chen R.C. Cancer screening rates in individuals with different life expectancies. JAMA Intern. Med. 2014;174(10):1558–1565. doi: 10.1001/jamainternmed.2014.3895. (Oct) [DOI] [PubMed] [Google Scholar]
- Schoenborn N.L., Bowman T.L., Cayea D., Boyd C., Feeser S., Pollack C.E. Discussion strategies used by primary care clinicians when stopping cancer screening among older adults. J. Am. Geriatr. Soc. 2016;64(11):e221–e223. doi: 10.1111/jgs.14444. (Nov) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenborn N.L., Lee K., Pollack C.E. Older adults' views and communication preferences around cancer screening cessation. JAMA Intern. Med. 2017;177(8):1121–1128. doi: 10.1001/jamainternmed.2017.1778. (Aug) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonberg M.A., McCarthy E.P., York M., Davis R.B., Marcantonio E.R. Factors influencing elderly women's mammography screening decisions: implications for counseling. BMC Geriatr. 2007;7:26. doi: 10.1186/1471-2318-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz L.M., Woloshin S., Fowler F.J., Jr., Welch H.G. Enthusiasm for cancer screening in the United States. JAMA. 2004;291(1):71–78. doi: 10.1001/jama.291.1.71. [DOI] [PubMed] [Google Scholar]
- Tarasenko Y.N., Wackerbarth S.B., Love M.M., Joyce J.M., Haist S.A. Colorectal cancer screening: patients' and physicians' perspectives on decision-making factors. J. Cancer Educ. 2011;26(2):285–293. doi: 10.1007/s13187-010-0145-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material