Abstract
A nine-year-old domestic cat from Melbourne, Australia, presented with a non-ulcerated nodule on its nasal bridge. A fungal infection of the subcutis was diagnosed based on histopathology and culture of a white mould, which was identified as Sporothrix pallida complex by ITS1–5.8S-ITS2 and β-tubulin gene sequencing. The cat was treated by cytoreduction, itraconazole and subsequently posaconazole, which eventually resulted in regression of residual infected tissues and clinical resolution.
Keywords: Sporotrichosis, Sporothrix pallida, Itraconazole, Posaconazole
1. Introduction
Sporotrichosis is an infection caused by thermally dimorphic fungi of the genus Sporothrix. Numerous cases in humans and animals have been recorded, most commonly caused by species within the Sporothrix schenckii complex [1], [2]. This taxonomic group includes (i) S. schenckii sensu stricto, the first species described as infecting humans, (ii) S. brasiliensis, a highly virulent species infecting cats (primarily), dogs and humans in Brazil, (iii) S. globosa, responsible for ‘sapronoses’ in China, India and Australia, and (iv) the less frequently reported S. luriei [1], [2], [3], [4]. The closely related Sporothrix pallida complex includes S. pallida s. str., S. mexicana, S. chilensis, S. stylites and S. humicola, all largely considered non-pathogenic [1], [5]. This case report documents the first case of sporotrichosis in a cat caused by an isolate within the Sporothrix pallida complex.
Mammalian sporotrichosis is usually the result of traumatic inoculation of the skin or subcutis, either from contaminated plant material or hay, or from the bite or scratch of an infected cat [2]. Outbreaks have been well documented [2], [6], [7]. The warm and nutrient-rich environment of the subcutis, together with macrophage engulfment, induces development of yeast-like cells, which are better able to evade host immune defences [2], [8]. Infection usually presents as a chronic, localised cutaneous infection, forming nodules which may ulcerate and suppurate. It may invade the lymphatic system, creating linear arrangements of lesions, so called sporotrichoid spread. In some instances, it may disseminate widely to involve the lungs, joints, sinuses or central nervous system [2]. Signs in cats are variable, but as a species, they are susceptible to development of severe local disease and systemic spread [2], [9].
Animal infections caused by ‘environmental’ Sporothrix species are rare, with laboratory studies showing little pathogenicity and often self-clearing of S. pallida and S. mexicana infections in experimental murine models [10]. Similar studies using immunocompetent mice show S. chilensis has limited pathogenicity, with an ability to colonise the lungs and cause skin lesions on the forelimbs, around the eyes and on the abdomen, but without fatalities [5]. These experimental infections are also typically cleared spontaneously [5].
Inoculation leading to sporotrichosis is common on the hands and limbs of humans [2], with the bridge of the nose being a common predilection site in cats, resulting from contaminated cat scratch injuries [11]. Cats have been known to spread the pathogen through direct contact with undamaged skin, therefore gloves should always be worn when handling suspect cases [12]. Although less commonly than for other dimorphic fungi, Sporothrix may be spread by inhalation of conidia, and so extra care must be taking when culturing the organism in the laboratory [2]. It is therefore recommended for occupational safety that fresh tissue samples be submitted to a mycology reference laboratory for culture.
2. Case
2.1. Investigation
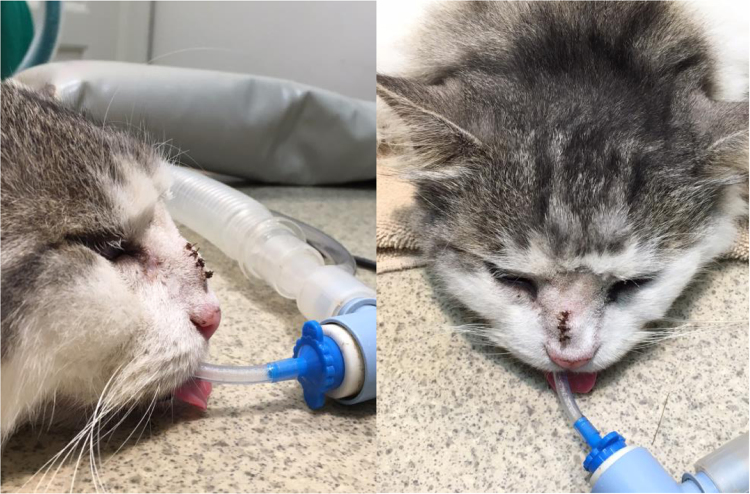
A nine-year-old, castrated domestic short hair cat (5.9 kg) from Melbourne, Australia, presented for veterinary attention in July 2016 (day 0) because of a swelling on the bridge of its nose. The lesion consisted of a soft, tan-coloured, raised, non-ulcerated nodule (1 cm diameter). On day 7, debulking by marginal resection provided material for laboratory studies to assess an infectious or neoplastic cause (Fig. 1). Tissue was submitted to a veterinary pathology laboratory in buffered formalin for histological examination. The remaining specimen was maintained in a sterile pot in the 4 °C refrigerator at the veterinary practice.
Fig. 1.
Side and front view of the cat immediately following debulking surgery. Residual swelling of the bridge of the nose is present.
2.2. Histology
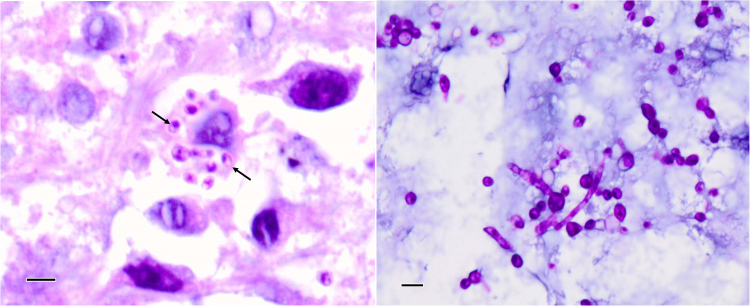
Tissue sections were stained with haematoxylin and eosin (H&E), Grocott's methanimine silver (GMS) and periodic acid-Schiff (PAS). Microscopically, the tissue was a deep pyogranuloma, with no epithelium or other attachments present in the fragments. Sections displayed a diffuse mixture of neutrophils, small lymphocytes, plasma cells and large, activated macrophages within a background of fibrous connective tissue. Numerous fragments of hyphae with yeast-like swellings at the tips were observed (Fig. 2B). Multiple 3–5 µm ovoid bodies resembling fungal elements were also observed within many macrophages, with eccentrically placed and sometimes crescentic nuclear spots.
Fig. 2.
(Left) H&E-stained histology section showing intracellular eukaryotic organisms (arrows) as well as some scattered extracellular organisms. (Right) PAS-stained section revealing hyphae and conidia and/or yeast forms. The measure bar in each photo is 10 µm.
2.3. Mycology
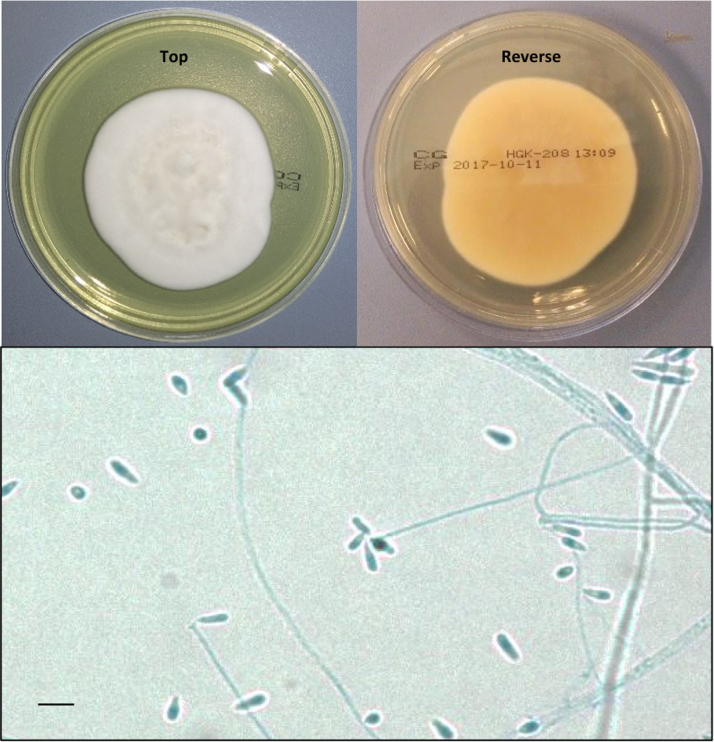
Given the histological observation of fungal elements, tissue was submitted to the Veterinary Diagnostic Laboratory, The University of Adelaide, for bacterial and fungal microscopy and culture. No bacteria were isolated. A pure heavy growth of a white mould grew within 48 h on Sabouraud's dextrose agar at 26 °C and was referred to the National Mycology Reference Centre, SA Pathology, Adelaide for identification. Colonies were snow white with velvety texture and light reverse, developing a duller cream to beige and glabrous centre after approximately 5 days incubation. Microscopic examination of the culture revealed long, delicate conidiophores with sympodial denticles bearing small ovoid to cylindrical smooth-walled conidia with pointed bases (Fig. 3). No melanin pigmentation was observed. Cultures grew faster at lower temperature (25 °C) and on richer media (brain heart infusion, horse blood agar, chocolate agar) and grew poorly at 37 °C. Macroscopic and microscopic morphology was similar on all media and at all temperatures. No yeast form was observed in vitro despite concerted attempts at culture on a variety of media, at a range of temperatures (25 °C, 35 °C, 37 °C), in both ambient conditions and 5% CO2.
Fig. 3.
(Top) Colony morphology on Sabouraud's dextrose agar after 14 days at 26 °C, and (bottom) lactophenol cotton blue wet preparation showing clavate conidia typical of Sporothrix spp. The measure bar is 5 µm.
Specific identification was attempted by DNA sequencing of the ribosomal Internal Transcribed Spacer regions (ITS1–5.8S-ITS2) and the β-tubulin gene, as described previously [13], [14]. The resulting sequences were aligned to both the NCBI Genbank (http://www.ncbi.nlm.nih.gov/genbank) and the Westerdijk Fungal Biodiversity Institute databases (http://www.westerdijkinstitute.nl). Neither the ITS1–5.8S-ITS2 rDNA region nor the β-tubulin sequences yielded a 100% match to any species in either sequence database. Sequence identities of 99% (ITS1–5.8S-ITS2) and 92% (β-tubulin) to S. mexicana, S. stylites and S. chilensis were observed, all being members of the S. pallida complex. S. mexicana was excluded as the causative organism due to the lack of melanin pigmentation on the culture. Comparison of concatenated β-tubulin and ITS1–5.8S-ITS2 sequence (982 nucleotides) to NCBI Genbank provided a best match of a 95.5% sequence identity to S. chilensis, although this is insufficient to conclude that the isolate is S. chilensis. Both sequences were submitted to Genbank (ITS1–5.8S-ITS2 accession MK367810; β-tubulin accession MK380725).
Antifungal susceptibility testing was conducted using the Sensititre YeastOne YO10 kit (Thermo Scientific). Inoculum preparation and endpoint reading was in accordance with the Clinical and Laboratory Standards Institute CLSI M38-A3 guidelines, at an incubation time of 72 h [15]. Minimum inhibitory concentrations (mg/L) were as follows: anidulafungin > 8, micafungin > 8, caspofungin > 8, 5-fluorocytosine 64, itraconazole 1, posaconazole 1, voriconazole 2, fluconazole 64, amphotericin B 4. No clinical breakpoints are currently available for Sporothrix spp.
2.4. Clinical course
The wound healed unremarkably with minimal scarring. As the interim histological assessment suggested a primary fungal aetiology, itraconazole (10 mg/kg (50 mg) orally once daily) was commenced on day 7, taken on an empty stomach. The cat became lethargic by day 11, so the dose was reduced to 5 mg/kg once daily, before incrementally increasing on days 18 and 25 back to the original dose. At day 49 the itraconazole was given immediately before feeding rather than on an empty stomach, which appeared to improve the lethargy.
Upon review at days 67 and 73, an increase in vomiting was noted, and a serum biochemical panel showed elevated alanine aminotransferase (ALT; 550 U/L [5–80]) and aspartate aminotransferase (AST; 187 U/L [10–60]) activities, consistent with itraconazole-induced hepatic injury. Therapy was therefore changed to posaconazole (day 81; 7.5 mg/kg (40 mg) orally with food once daily). This resulted in resolution of vomiting and a significant decrease but not normalization in liver enzyme activities by day 137 (ALT 296, AST 106), and the cat continued to lose weight. At this time the owner elected to discontinue treatment. The lesion began to regrow over a period of months, estimated at 20% bigger at day 239, before eventually rupturing (approx. day 315), regressing and healing. The cat was variably unwell during this period, however, at day 389 the owner reported that the cat was back to normal. The cat was euthanased approximately one year later (day 745) for unrelated reasons.
3. Discussion
This report describes focal subcutaneous disease caused by a species of the S. pallida complex in an apparently immunocompetent cat. The anatomical location of the subcutaneous lesion in this cat is most consistent with an introduced saprophyte, most likely via a penetrating cat scratch injury; cat claws are frequently contaminated with saprophytic bacteria and fungi, particularly in those with access to the outdoors, where they dig and bury faecal waste in soil. This Sporothrix isolate had high MICs to most antifungal drugs available for testing, and despite reversible itraconazole-induced hepatic injury and variable compliance, the infection eventually responded to therapy consisting of marginal excision, followed by itraconazole and subsequently 100 days of posaconazole. The difficulty in making an exact identification of the isolate, despite the use of two well accepted fungal barcodes, is in part a reflection of the very limited number of sequences available in publicly accessible sequence databases. The favourable response to therapy despite high in vitro MICs suggests that the normal adaptive immune response of the host and initial cytoreductive surgery played important roles in resolving the infection. Case studies such as this, which combine clinical notes with molecular characterisation of the isolate, are important for properly understanding the aetiopathogenesis of such rare infections and to optimise treatment regimens for such uncommon Sporothrix species.
To date, only two S. pallida complex infections have been reported, both in humans: an infected corneal transplant [16] and a proven non-dermatophyte onychomycosis [5], [17]. Both cases were acquired from environmental sources and remained localised to cool superficial anatomic locations, with no lymphatic spread. The causative organisms were identified on the basis of β-tubulin sequencing as S. pallida s. str. and S. chilensis, respectively, with ITS1–5.8S-ITS2 and large subunit rDNA sequencing being insufficient for definitive identification in both cases [5], [16]. It should be noted, that S. schenckii complex case reports based upon phenotypic identification alone would be insufficient to differentiate the S. pallida complex; as a result, these infections may be under-reported in the literature [3], [5].
The treatment and outcome of sporotrichosis is dependent on several factors, most notably the species identified and the extent of initial infection [7], [8], [9], [10]. Standard treatment (among humans) for ‘classical’ cutaneous sporotrichosis is itraconazole, a common and well-tested front-line drug. In cats itraconazole is also considered the first-line treatment, although liver toxicity is a not uncommon side effect particularly with doses exceeding 10 mg/kg/day that are often required for effective therapy [18]. Studies indicate varying itraconazole susceptibilities among the genus in vitro, but broadly correlating with species [18], [19]. Susceptibility testing of this isolate indicated itraconazole and posaconazole MICs of 1 mg/L; while interpretive criteria are lacking for any species of the S. pallida complex, these are below the epidemiological cut-off values of 2 mg/L proposed for both antifungals against S. schenckii and S. brasiliensis [20]. However, the relevance of these data in S. pallida complex is unknown. Posaconazole has shown equivalence with itraconazole in both in vivo and in vitro trials [21], with readily achievable effective blood concentrations, fewer side effects, and with easier administration in cats due to a palatable liquid formulation [22]. Terbinafine is also very effective against Sporothrix spp., with low MICs and less impact on the liver, although S. pallida (reported by its former name, S. albicans) appears to have higher MICs than other Sporothrix species [19]. Although not used in the present case, intralesional amphotericin B can be a very useful adjunct to therapy in localised feline sporotrichosis [23].
Treatment of feline sporotrichosis poses challenges, particularly for S. brasiliensis infections which are known to be intrinsically more virulent and contagious in cats, and where nasal mucosal involvement, respiratory signs and high fungal loads in skin lesions are predictors of treatment failure despite overall low MICs to several antifungals [11], [19]. In contrast, considering that the predicted maximum concentration (CMAX) for posaconazole in cats given an oral loading dose of 15 mg/kg is 1.19 mg/L ± 0.52 mg/L [22], peak serum concentrations would, at best, be comparable to the MIC for this isolate (1 mg/L). Taken together with the delayed clinical response to therapy, we consider that posaconazole therapy, debulking surgery and the cat's adaptive immunological response to therapy all contributed to the eventual successful clinical outcome.
Australia has sporadic human cases of sporotrichosis (due to either S. schenckii s. str., S. globosa or S. mexicana) [3], [4], although none of the isolates from reported feline cases had been characterised by molecular methods to the best of our knowledge. The lack of melanisation of the mature fungal colony in this instance immediately suggested a species other than S. schenckii and S. mexicana, as both these species typically produce melanised conidia, turning the colonies brown to black in appearance [2], [5]. ITS1–5.8S-ITS2 sequence analysis is the recognised standard ‘barcode’ for differentiating most fungi, while the β-tubulin gene is commonly used for further differentiation between Sporothrix species [1], [5], [13]. Sequence analysis indicated that the isolate was most closely related to S. chilensis, with the possibility that this is an uncommon strain of S. chilensis, or a closely related species not yet represented by a sequence in publicly available databases. Additional sequencing of Calmodulin and Translation Elongation Factor-1α (TEF-1α) genes or indeed of the whole genome, may have aided a definitive identification, but were beyond the scope of this clinical investigation [5].
The rarity of Sporothrix cases in Australian mammals makes this case of great interest. The key finding was the unequivocal pathogenicity of a member of S. pallida complex, typically considered a non-pathogenic fungal saprophyte [8]. Appropriate specimen collection and testing, accurate fungal identification, and prompt treatment of sporotrichosis is critical to a good outcome and appropriate therapy. A retrospective case review in New South Wales, Australia, found that approximately one-third of 19 human cases for which treatment data were available, were treated with multiple or prolonged courses of antibiotics before a fungal cause was identified [24]. Even more serious, a recent report highlighted five cases where misdiagnosis as pyoderma gangrenosum led to treatment with immunosuppressive drugs, causing a severe decline in condition and death in two cases [25]. Such errors may be common in the veterinary setting where costs often restrict appropriate diagnostic investigations.
Acknowledgements
The authors would like to acknowledge the contributions of Ken Lee (Veterinary Diagnostic Laboratory, University of Adelaide, SA), Hugh Wackett (Glenhuntly Road Veterinary Clinic, Glenhuntly, Vic.), and Helen Alexiou and Ian Ross (National Mycology Reference Centre, SA Pathology, Adelaide).
Acknowledgments
Conflict of interest
There are none.
Ethical form
Please note that this journal requires full disclosure of all sources of funding and potential conflicts of interest. The journal also requires a declaration that the author(s) have obtained written and signed consent to publish the case report from the patient or legal guardian(s).
The statements on funding, conflict of interest and consent need to be submitted via our Ethical Form that can be downloaded from the submission site www.ees.elsevier.com/mmcr. Please note that your manuscript will not be considered for publication until the signed Ethical Form has been received.
References
- 1.de Meyer E.M., de Beer Z.W., Summerbell R.C. Taxonomy and phylogeny of new wood- and soil-inhabiting Sporothrix species in the Ophiostoma stenoceras-Sporothrix schenckii complex. Mycologia. 2008;100:647–661. doi: 10.3852/07-157r. [DOI] [PubMed] [Google Scholar]
- 2.Barros M.B.L., Paes R.A., Schubach A.O. Sporothrix schenckii and sporotrichosis. Clin. Microbiol. Rev. 2011;24:633–654. doi: 10.1128/CMR.00007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y., Hagen F., Stielow B. Phylogeography and evolutionary patterns in Sporothrix spanning more than 14,000 human and animal case reports. Persoonia. 2015;35:1–20. doi: 10.3767/003158515X687416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.New D., Beukers A.G., Kidd S.E., Merritt A.J., Weeks K., van Hal S.J., Arthur I. Identification of multiple species and subpopulations among Australian clinical Sporothrix isolates using whole genome sequencing. Med. Mycol. 2018 doi: 10.1093/mmy/myy126. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Rodrigues A.M., Choappa R.M.C., Fernandes G.F., de Hoog S., de Camargo Z.P. Sporothrix chilensis sp. nov (Ascomycota: ophiostomatales), a soil-borne agent of human sporotrichosis with mild-pathogenic potential to mammals. Fungal Biol. 2016;120:46–64. doi: 10.1016/j.funbio.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Feeney K.T., Arthur I.H., Whittle A.J., Altman S.A., Speers D.J. Outbreaks of sporotrichosis, Western Australia. Emerg. Infect. Dis. 2007;13:1228–1231. doi: 10.3201/eid1308.061462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGuinness S.L., Boyd R., Kidd S., McLeod C., Krause V.L., Ralph A.P. Epidemiological investigation of an outbreak of cutaneous sporotrichosis, Northern Territory, Australia. BMC Infect. Dis. 2016;16:16. doi: 10.1186/s12879-016-1338-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Téllez M.D., Batista-Duharte A., Portuondo D. Sporothrix schenckii complex biology: environment and fungal pathogenicity. Microbiology. 2014;160:2352–2365. doi: 10.1099/mic.0.081794-0. [DOI] [PubMed] [Google Scholar]
- 9.Yegneswaran P.P., Sripathi H., Bairy I. Zoonotic sporotrichosis of lymphocutaneous type in a man acquired from a domesticated feline source: report of a first in southern Karnataka, India. Int. J. Dermatol. 2009;48:1198–1200. doi: 10.1111/j.1365-4632.2008.04049.x. [DOI] [PubMed] [Google Scholar]
- 10.Arrillaga-Moncrieff I., Capilla J., Mayayo E., Marimon R., Mariné M., Gené J., Cano J., Guarro J. Different virulence levels of the species of Sporothrix in a murine model. Clin. Microbiol. Infect. 2009;15(7):651–655. doi: 10.1111/j.1469-0691.2009.02824.x. [DOI] [PubMed] [Google Scholar]
- 11.de Souza E.W., Borba C.M., Pereira S.A., Gremião I.D.F., Langohr I.M., Oliveira M.M.E., de Oliveira R.V.C., da Cunha C.R., Zancopé-Oliveira R.M., de Miranda L.H.M., Menezes R.C. Clinical features, fungal load, coinfections, histological skin changes, and itraconazole treatment response of cats with sporotrichosis caused by Sporothrix brasiliensis. Sci. Rep. 2018;8(1):9074. doi: 10.1038/s41598-018-27447-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barros M.B.L., Schubach A.O., Valle A.C.F. Cat-transmitted sporotrichosis epidemic in Rio de Janeiro, Brazil: description of a series of cases. Clin. Infect. Dis. 2004;38:529–535. doi: 10.1086/381200. [DOI] [PubMed] [Google Scholar]
- 13.White T.J., Bruns T., Lee S., Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M., Gelfand D., Shinsky J., White T., editors. Vol. 3. Academic Press; San Diego: 1990. pp. 315–322. (PCR Protocols: A Guide to Methods and Applications). [Google Scholar]
- 14.Marimon R., Gené J., Cano J. Molecular phylogeny of Sporothrix schenckii. J. Clin. Microbiol. 2006;44:3251–3256. doi: 10.1128/JCM.00081-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CLSI . Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi. 3rd edition. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2017. (M38-A3) [Google Scholar]
- 16.Morrison A.S., Lockhart S.R., Bromley J.G., Kim J.Y., Burd E.M. An environmental Sporothrix as a cause of corneal ulcer. Med. Mycol. Case Rep. 2013;2:88–90. doi: 10.1016/j.mmcr.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choappa R.M.C., Oyarzo P.I.V., Silva L.C.C. Isolation of Sporothrix pallida complex in clinical and environmental samples from Chile. Rev. Argent. Microbiol. 2014;46:311–314. doi: 10.1016/S0325-7541(14)70088-4. [DOI] [PubMed] [Google Scholar]
- 18.Mahajan V.K. Sporotrichosis: an overview and therapeutic options. Dermatol. Res. Prac. 2014;2014:272376. doi: 10.1155/2014/272376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marimon R., Serena C., Gené J., Cano J., Guarro J. In vitro antifungal susceptibilities of five species of Sporothrix. Antimicrob. Agents Chemother. 2008;52:732–734. doi: 10.1128/AAC.01012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Espinol-Ingroff A., Abreu D.P.B., Almeida-Paes R. Multicenter, international study of MIC/MEC distributions for definition of epidemiological cut-off values for Sporothrix species identified by molecular methods. Antimicrob. Agents Chemother. 2017;61:e01057–17. doi: 10.1128/AAC.01057-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernández-Silva F., Capilla J., Mayayo E., Guarro J. Efficacy of posaconazole in murine experimental sporotrichosis. Antimicrob. Agents Chemother. 2012;56:2273–2277. doi: 10.1128/AAC.05376-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mawby D.I., Whittemore J.C., Fowler L.E., Papich M.G. Posaconazole pharmacokinetics in healthy cats after oral and intravenous administration. J. Vet. Intern. Med. 2016;30:1703–1707. doi: 10.1111/jvim.14523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gremião I., Schubach T., Pereira S., Rodrigues A., Honse C., Barros M. Treatment of refractory feline sporotrichosis with a combination of intralesional amphotericin B and oral itraconazole. Aust. Vet. J. 2011;89:346–351. doi: 10.1111/j.1751-0813.2011.00804.x. [DOI] [PubMed] [Google Scholar]
- 24.Sivaganam S., Bannan A.M., Chen S.C.-A., Ralph A.P. Sporotrichosis (Sporothrix schenckii infection) in the new South Wales mid-north coast, 2000–2010. Med. J. Aust. 2012;196:588–590. doi: 10.5694/mja11.10755. [DOI] [PubMed] [Google Scholar]
- 25.Lima R.B., Jeunon-Sousa M.A.J., Jeunon T. Sporotrichosis masquerading as pyoderma gangrenosum. J. Eur. Acad. Dermatol. 2017;31:e539–e541. doi: 10.1111/jdv.14421. [DOI] [PubMed] [Google Scholar]