Abstract
These guidelines provide an up-date of previous IFCN report on “Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application” (Rossini et al., 1994). A new Committee, composed of international experts, some of whom were in the panel of the 1994 “Report”, was selected to produce a current state-of-the-art review of non-invasive stimulation both for clinical application and research in neuroscience.
Since 1994, the international scientific community has seen a rapid increase in non-invasive brain stimulation in studying cognition, brain–behavior relationship and pathophysiology of various neurologic and psychiatric disorders. New paradigms of stimulation and new techniques have been developed. Furthermore, a large number of studies and clinical trials have demonstrated potential therapeutic applications of non-invasive brain stimulation, especially for TMS. Recent guidelines can be found in the literature covering specific aspects of non-invasive brain stimulation, such as safety (Rossi et al., 2009), methodology (Groppa et al., 2012) and therapeutic applications (Lefaucheur et al., 2014).
This up-dated review covers theoretical, physiological and practical aspects of non-invasive stimulation of brain, spinal cord, nerve roots and peripheral nerves in the light of more updated knowledge, and include some recent extensions and developments.
Keywords: Non-invasive stimulation, Transcranial magnetic stimulation, Human cortex, Clinical neurophysiology, TMS measures, Excitability threshold
1. Introduction
It has been 20 years since the publication of the first IFCN-endorsed report on “Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application” (Rossini et al., 1994). This report has been useful and has been cited 1805 times to date (Google Scholar, 7th December 2014). Over 20 years there have been many developments, some foreshadowed in 1994, some unforeseen. Accordingly, a new Committee has been tasked with updating the report. In 20 years much has been learnt about plasticity of the nervous system in healthy subjects and patients with neurological and neuropsychiatric dysfunction. The development of new techniques, new coils, new stimulus paradigms and the introduction of neuronavigation have rendered research and clinical studies more accurate, more insightful and of greater clinical value. These developments have allowed non-motor areas of the brain to be probed and for non-invasive brain stimulation to be trialled as a therapeutic measure. Comprehensive coverage of the entire field would constitute a sizable monograph and of necessity this Report focuses on those areas of greatest interest to practicing clinical neurophysiologists. Recently published guidelines cover the safety of non-invasive brain stimulation (Rossi et al., 2009), its methodology (Groppa et al., 2012) and therapeutic applications (Lefaucheur et al., 2014).
2. Physiology
Stimulation of the human brain, like the peripheral nerve, involves depolarizing neuronal membranes in order to initiate action potentials. Experience from invasive stimulation during neurosurgery or epilepsy monitoring shows that stimulation parameters for the central nervous system are relatively similar to those needed for peripheral nerve: short pulses with a duration of less than 1 ms and with an amplitude of few milliamperes. Transcranial methods for brain stimulation face the problem of delivering such a stimulus across the high resistance barrier of the periencephalic ‘layers’, including scalp, skull, meninges and cerebrospinal fluid.
Early approaches involved applying high-voltage electric stimuli through electrodes on the scalp. Although a large proportion of the current travels along the scalp between the electrodes, a small portion of the current penetrates the brain and activates neurons. This method, pioneered by Merton and Morton (1980), is known as transcranial electrical stimulation (TES; a term which should be differentiated from the generic term used for any transcranial electrical stimulation method, usually abbreviated as TES, and including those employing weak electric currents, see below). TES did have the huge merit of introducing a neurophysiological technique for studying for the first time excitability and propagation properties along central nervous system fibers in intact and cooperative human beings. However, the fields of application declined rapidly with the introduction of transcranial magnetic stimulation (TMS) in 1985 by Barker et al. (1985) because high-voltage TES is uncomfortable.
Another important electrical stimulation approach, which is not covered in this review, is low-intensity transcranial electrical stimulation using low-intensity currents applied through scalp electrodes (Nitsche and Paulus, 2000). The most common protocol is transcranial direct current stimulation in which a constant current of 1–2 mA is applied continuously for 10–20 min. Currents of this magnitude cannot directly initiate action potentials in a resting cell or its axon; instead they cause small changes in the membrane potential of cell bodies or the axonal terminations of neurons and have been proven to modulate spontaneous firing rates (Nitsche and Paulus, 2011; Paulus et al., 2013; Filmer et al., 2014). This is thought to bias excitability of populations of neurons and influence information transmission in neural networks. Finally, many other techniques of TES have been proposed for more than a century (Guleyupoglu et al., 2013), using, for example, non-polarizing high-frequency pulsed biphasic balanced current (Limoge et al., 1999) or weak brain oscillation-locked alternating current (Reato et al., 2013).
2.1. Transcranial electrical stimulation (TES) using short-duration high-intensity pulses
High-voltage TES delivers a capacitively coupled pulse that has a time constant of 50–100 μs duration and an electrical potential difference of several hundred Volts using a bipolar electrode arrangement. It produces a brisk sensation because it activates local pain receptors in the skin and causes local contraction of scalp muscles. In the motor cortex, neural stimulation occurs preferentially in the cortex underlying the anode and elicits motor evoked potentials (MEPs) on the opposite side of the body. A single pulse of anodal stimulation delivered on the scalp elicits brain current that enters superficial dendrites of pyramidal neurons of layer 5 and exits in deeper layers where it depolarizes the cell membrane and initiates an action potential. Experiments on primates using surface stimulation of the exposed cortex show that activation occurs in the subcortical white matter, a few nodes distant to the axon hillock region (Ranck, 1975; Amassian et al., 1987). The same is thought to be true in humans for TES (see below). As the action potentials descend to the spinal cord via the pyramidal tract, they can be recorded as a D-wave (D = direct, which reflects direct activation of axons). At higher intensities, the stimulus recruits synaptic inputs to the same corticospinal neurones, causing them to discharge at later intervals. This produces I-waves (I = indirect [synaptic] activation of corticospinal neurons), in recordings of the descending volleys from the spinal cord.
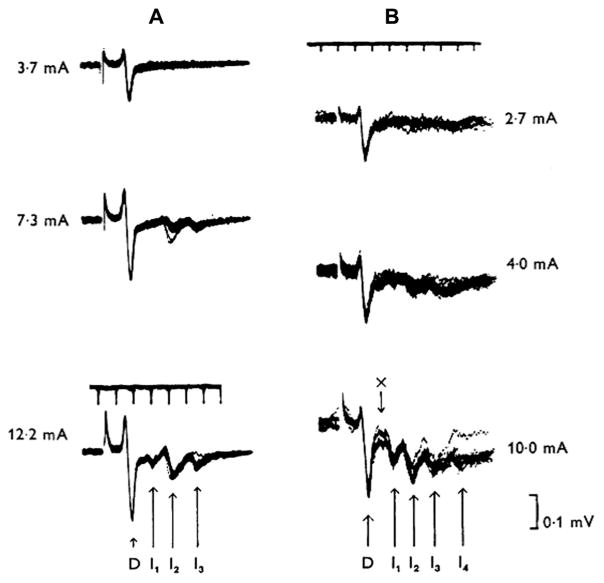
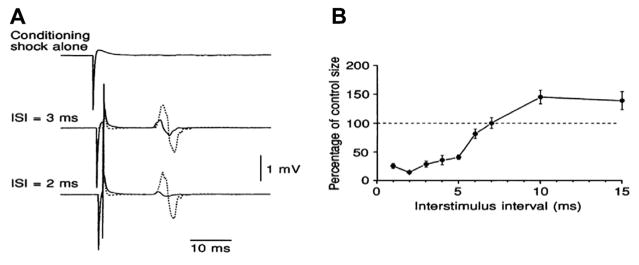
Initial invasive experiments characterised D- and I-waves in monkeys and cats (Adrian and Moruzzi, 1939; Patton and Amassian, 1954; Kernell and Chien-Ping, 1967; Amassian et al., 1987; Edgley et al., 1997) (Fig. 1), and similar data have been obtained in humans by recording the descending activity produced by TES of motor cortex from electrodes inserted into the epidural space of the cervical spinal cord in patients during spinal surgery (Boyd et al., 1986; Berardelli et al., 1990; Burke et al., 1990, 1993; Thompson et al., 1991) and, more recently, in conscious patients implanted for pain control (Di Lazzaro et al., 2004a). The mechanisms that generate I-waves wave are still unclear (Di Lazzaro et al., 2004a): why should corticospinal neurones tend to discharge synchronously at a frequency of around 600 Hz? One possibility is that the pyramidal neurons or a class of excitatory input neurons have an intrinsic capacity to discharge repetitively in response to a strong depolarizing input. Such fast spiking neurons have been described in somatosensory cortex (Ozaki and Hashimoto, 2011) in response to electrical stimulation of afferents in peripheral nerves (Baker et al., 2003). Other possibilities are reverberating chains of oscillating interneurons or even separate monosynaptic, di- and tri-synaptic inputs. Detailed neural models that have been developed incorporate both the intrinsic electrical properties of pyramidal cells as well as reasonable estimates of their inputs and can produce high frequency repetitive activity using known cortical circuitry (Esser et al., 2005; Rusu et al., 2014), but the technology to test the models using direct recordings of cortical neurons during stimulation is still being developed (Mueller et al., 2014).
Fig. 1.
The pyramidal tract waves (from Kernell and Chien-Ping, 1967 – with permission). The records of Fig. 1 are from two different baboons (A and B, respectively), and they were obtained with an electrode resting on the dorsolateral surface of the cervical spinal cord. Weak stimuli are seen to elicit only a brief single ‘wave’ which has an initial positive and a more prominent negative phase (D-wave). At higher stimulus strengths the D wave attains a greater amplitude, and it is then succeeded by a series of rapidly recurring, smaller, and predominantly negative-going waves (I-waves). The interval between the various waves is of the order of 1–2 ms. The various I waves were numbered according to their latency at strong cortical stimuli, and they are referred to as the I1 (arrow marked “x”), I2, I3 and 14 waves, respectively.
TES at just supra-threshold intensity evokes a very short latency response (around 20 ms) in contralateral hand muscles when they are pre-activated (the motor evoked potential, MEP). Very often no response is seen to the same stimulus in volunteers at rest; only when the intensity is increased do responses regularly occur in relaxed individuals, although these are often 2–3 ms or more later than the earliest onset in active muscle (Rossini et al., 1987a; Day et al., 1989). The reason for this “latency jump” between relaxed and contracted MEPs is that just supra-threshold pulses recruit only a single D-wave, which generates a monosynaptic EPSP onto spinal motoneurons. In resting motoneurons, this EPSP will be insufficient to bring the neuron to threshold. However, if motoneu-rons are near their discharge threshold, as most of them are during a mild voluntary contraction, they will fire an action potential and produce a short-latency EMG response in the hand. This hypothesis is partly confirmed by recordings in awake monkeys (Lemon et al., 1998). Recruitment of later I-waves at higher intensities produces a sequence of EPSPs that temporally summate and eventually discharge motoneurons, albeit at a longer latency than the initial D-wave (Day et al., 1989; Rossini et al., 1995). Another – non mutually exclusive – explanation is that the relaxed MEPs mainly reflect the activation of low-threshold, small and slowly propagating pyramidal tract neurons at the cortical level, while the contracted MEPs mainly reflect activation of higher-threshold central and peripheral pyramidal neurons having faster propagations and producing larger action potentials which finally govern large motor units in the target muscle (Liddel and Phillips, 1952; Henneman et al., 1974; Rossini et al., 1995). This issue is still a matter of debate. One should be aware that the volleys recorded from the spinal cord surface in humans do not necessarily reflect those to the target muscle from which the MEPs are recorded.
The repetitive discharge evoked by TES combined with the synaptic relays in the pathway from cortex to muscle makes the electromyographic (EMG) responses to TES (MEPs) more complex than the compound muscle action potential (CMAP) recorded from electrical stimulation of a peripheral nerve (Day et al., 1989). Most of our knowledge has come from examination of TES-induced MEPs in small hand muscles to stimulation of the hand area of primary motor cortex (M1). These MEPs differ from CMAPs in three important respects. First, the threshold for evoking a MEP is lower in actively contracting muscle than it is in muscles at rest. Second, the latency of a MEP evoked at rest is often 1–3 ms longer than a response of the same size evoked in active muscle. Third, the MEP amplitude and shape differ from the peripherally evoked CMAPs. The shape of the MEP becomes polyphasic and the MEP has a longer duration than the peripherally evoked CMAP, particularly at high intensities of stimulation. The maximal MEP amplitude evoked by a single stimulus is always substantially lower than the maximal CMAP amplitude evoked by supramaximal peripheral electrical stimulation (Rossini et al., 1987a; Day et al., 1989). In contrast to peripherally evoked CMAPs, cortically evoked MEPs show substantial trial-to-trial variability even when extrinsic stimulation settings, such as stimulus intensity and position are kept constant. This variability can be attributed to intrinsic fluctuations of corticomotor excitability, both cortical and spinal.
The reason why the threshold is lower during contraction than at rest is that, in a contracting muscle, spinal motoneurons are firing randomly and the effect of a liminal excitatory input from the corticospinal tract (CST) will be to advance and synchronize the activity of motoneurons that were just about to discharge (see Day et al., 1989). A single TES evoked descending volley can therefore discharge the spinal motoneurons during a tonic contraction while the same volley cannot do so at rest. Resting motoneurons will require a larger descending input to evoke an MEP. The lowest threshold volley evoked by TES is the D-wave initiated in the cortex and its threshold is unaffected by volitional contraction (Di Lazzaro et al., 1998). H-reflex measurements provide further support for a spinal mechanism mediating at least in part the reduction in corticomotor threshold during voluntary contraction of the target muscle (Day et al., 1987).
Regarding factors that contribute to the shorter latency of MEPs under active conditions, one should note that the latency of descending corticospinal volleys recorded from the spinal cord is similar in active and relaxed muscles (Di Lazzaro et al., 1998, 1999b). As noted above, the D-wave evoked during active contraction produces an excitatory input to the spinal motor pool that is capable of recruiting already-firing motor units and produce a MEP. At rest, TES brings spinal motoneurons to threshold at a later time and this accounts for the later onset of MEP in relaxed muscle (Day et al., 1987). If the stimulus intensity is increased, later I-waves are recruited and their excitatory postsynaptic potentials (EPSPs) summate at motoneurons with the EPSP from the initial D-wave, leading to larger MEPs. It is worth noting that with TES, large MEPs tend to have a shorter latency than small MEPs (particularly in relaxed muscle) (Rothwell et al., 1987; Day et al., 1987). This would be in accordance with the size principle of motor unit recruitment, discussed later, such that larger motoneurons with faster conducting peripheral motor axons are recruited in the larger MEP and therefore the latency is correspondingly shorter (Henneman, 1985). However a larger D wave produced by suprathreshold stimuli would also contribute to this.
The final difference between MEPs and peripheral CMAPs is that MEPs are more polyphasic especially at high stimulus intensities. As the intensity of the pulse increases, the number of descending volleys increases, each of which may produce EPSPs in spinal motoneurons. The net effect is that the motoneurons may discharge on receipt of anyone of these, leading to temporally dispersed activation of motor units and a polyphasic MEP. In fact, the multiple descending inputs can even cause some single units to discharge twice within the same MEP (Day et al., 1989). This can be confirmed by collision methods. If correctly timed, a supramaximal CMAP can collide with the orthodromic activity in peripheral motor axons set up by a high intensity transcranial pulse. If spinal motoneurons discharge only once in response to the transcranial input, then there should be total collision with the antidromic activity set up by the CMAP. In the EMG, all that will be visible is the CMAP. However, if transcranial or proximal peripheral stimulation made motoneurons fire twice the second discharge of the units will be visible following the CMAP, unless the collision is incomplete due to motoneuron loss (Day et al., 1987; see also Magistris et al., 1999, for application to TMS evoked MEPs). Finally, it is important to remember that although the initial response of a muscle to TES is caused by activity in rapidly conducting monosynaptic corticospinal projections (the corticomotoneuronal connection), the same stimulus can also activate other slower conducting and probably multi-synaptic inputs. These are often not visible in the EMG because they sit in the “shadow” of the large corticomotoneuronal MEP. However, they can be revealed by using H-reflexes to test motoneuron excitability in response to sub-MEP threshold stimulation in relaxed muscle (Cowan et al., 1986). Following the initial period of excitation, there is often a short period of inhibition, which is probably due to activation of the Ia reciprocal inhibitory interneuron in the spinal cord. This is followed by a longer phase of excitation, some of which might result from the activation of propriospinal interneurons by the initial descending volley (Mazevet et al., 1996; Pierrot-Deseilligny and Burke, 2012).
As mentioned previously both for TES and TMS, there is a clear “latency jump” of about 3 ms when MEPs are recorded in relaxed and contracting MEPs (Merton and Morton, 1980; Merton et al., 1982). The “latency jump” and amplitude facilitation observed in MEPs during contraction vs. relaxation involves a number of mainly spinal mechanisms, presumably including the size principle of motoneuron recruitment (Henneman et al., 1965; Desmedt, 1983; Rossini et al., 1985, 1987a,b).
2.2. Transcranial magnetic stimulation (TMS)
TMS uses electromagnetic induction as a highly effective painless way to generate suprathreshold current in the brain, much as does TES. A simple TMS device consists of a few circular turns of copper wire connected to the terminals of a large electrical capacitance via a switch. The capacitance is discharged by closing the switch so that a large current of several thousands Amps flows transiently through the wire coil for a period of less than 1 ms. This large current can have a monophasic or biphasic pulse configuration. The monophasic current pulse consists of a strong initial current which is balanced by a dampened return current. Only the first phase of the stimulus produces current flow in the stimulated brain: the dampened reverse current produces no neuronal stimulation. In biphasic pulse configuration an initial current rise is followed by a reversed current and by a subsequent increase of current: therefore, the current direction is reversed twice during a biphasic pulse. Both phases of the biphasic pulse induce physiologically significant current fluxes in brain tissue, and these flow in the same or opposite direction. Because the reversal phase is longer and wider than the initial rising phase and the second phase is more effective for biphasic TMS (Kammer et al., 2001; Groppa et al., 2012). The monophasic or biphasic current pulse produces a rapidly changing and brief magnetic field at the orthogonal angles to the coil plane. The peak magnetic field strength is similar to that of the static field in a magnetic resonance imaging (MRI) scanner (1–2 T). Magnetic fields readily penetrate into the brain without attenuation by the scalp or skull and generate a current according to the Faraday’s law of electromagnetic induction (Fig. 2). Several comprehensive reviews on the technical aspects of magnetic stimulators are available in the literature and are beyond the scope of the present document (Kammer et al., 2001; Sauve and Crowther, 2014).
Fig. 2.

Illustration of direction of current flows in a magnetic coil and the induced current in the brain (from Hallett, 2007 – with permission). In magnetic stimulation, a brief, high-current pulse is produced in a coil of wire, called the magnetic coil. A magnetic field is produced with lines of flux perpendicular to the plane of the coil, which ordinarily is placed tangential to the scalp. The magnetic field can be up to about 2 T and typically lasts for about 100 μs. An electric field is induced perpendicularly to the magnetic field. The voltage of the field itself may excite neurons, but the induced currents are likely to be more important. In a homogeneous medium, spatial change of the electric field will cause current to flow in loops parallel to the plane of the coil, which will be predominantly tangential in the brain. The loops with the strongest current will be near the circumference of the coil itself. The current loops become weak near the center of the coil, and there is no current at the center itself. Neural elements are activated by the induced electric field by two mechanisms. If the field is parallel to the neural element, then the field will be most effective where the intensity changes as a function of distance. If the field is not completely parallel, activation will occur at bends in the neural element.
2.2.1. TMS coil design (Fig. 3)
Fig. 3.
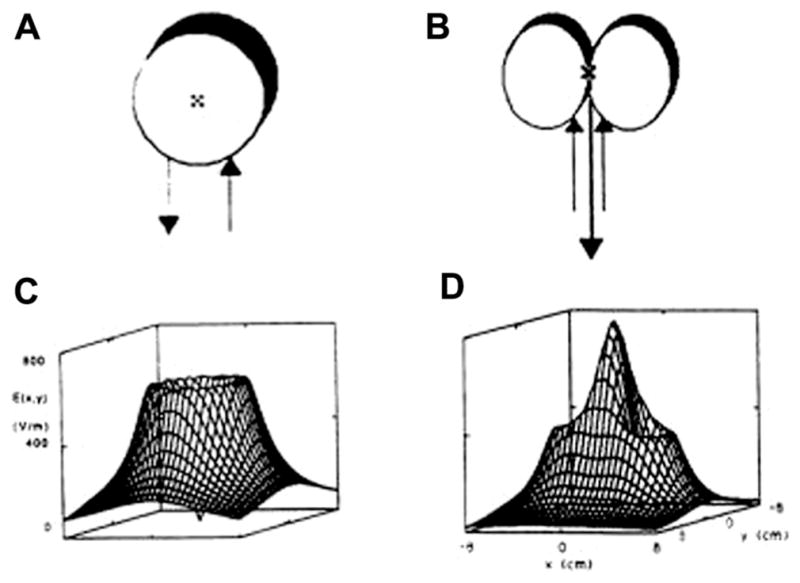
Magnetic coil shape determines the pattern of the electric field (from Hallett, 2007 – with permission). Two magnetic coils with different shapes (A and B) and their resultant electric fields (C and D).
TMS was introduced using large circular coils of wire with a diameter of around 10 cm. When a circular coil configuration is used for TMS, stimulation is most effective circularly under the coil with minimal stimulation in the center of the ring. When the circular coil is placed tangentially on the scalp, the site of stimulation covers a large area of brain but the depth of penetration into the brain is small: the intensity of the induced current falls as a matter of distance following a mathematical function; for instance, at a given strength of stimulation, the resulting current intensity induced 5 cm from the coil surface is only about 1/3 of the peak value (Mills et al., 1987). Thus, the stimulation of deep structures always comes at the cost of stimulation at higher intensity of the more shallow ones using “classical” coils. This problem has not been solved even by specific coils which have been designed to stimulate deep structures, such as the “H-coil” (Zangen et al., 2005). Shallower stimulation will result in a concurrent and more intense focal neural stimulation, achieved by overlapping two small round coils with oppositely directed currents into a figure-of-eight shape (Ueno and Matsuda, 1992). The figure-of-eight coil is classically used to stimulate a given brain region more selectively, for example in the context of therapeutic applications of TMS or for brain mapping. The smaller the diameter the more focal the TMS and the more rapidly the coil heats up during repetitive stimulation. Recent reviews have addressed this issue, comparing the various types of coil used in TMS practice (see Peterchev et al., 2013; Deng et al., 2014; Mueller et al., 2014).
2.2.2. TMS recruits I-waves at lower thresholds than D-waves
Most of the basic principles discussed earlier for TES are also valid for TMS. However, there is one important difference between TMS and TES. TMS of the motor cortex tends to activate I-waves at lower intensities than the D-wave, although this depends on coil orientation (Di Lazzaro et al., 2004a).
Most of the reported data comes from motor cortex stimulation, and the most direct evidence comes from epidural recordings from the spinal cord in conscious patients. They show that the earliest wave recruited by TES, the presumed D-wave, is not recruited at threshold by a TMS pulse. Dependent on coil orientation, TMS of the motor cortex evokes a wave corresponding to the I1 wave, and the D-wave is only recruited at intensities much greater than threshold. The resulting effect of this can be observed in MEP recordings. When MEPs are recorded in voluntarily activated muscle, the minimal latencies can be measured, and MEP latency at just-suprathreshold intensities is usually approximately 1.5 ms longer than the latency of similarsized MEPs evoked by TES. At higher intensities, the latency to TMS shortens and becomes equal to TES, because the stronger TMS evokes D-waves (Day et al., 1989; Di Lazzaro et al., 2004b). Whether this concept generalizes to all areas of cortex is unknown, and more research on non-motor areas should be carried out. Even in the motor cortex there is some debate over the extent to which this is true for TMS of the leg area as compared with the arm area. Early studies that used the same single motor unit and surface EMG approaches in the tibialis anterior muscle suggested that, in contrast to stimulation of the hand area, TES and TMS of the leg area both evoke D-waves (Priori et al., 1993; Terao et al., 1994; Nielsen et al., 1995). However, recordings from the thoracic cord suggested that TMS tended to evoke I-waves rather than D-waves (Di Lazzaro et al., 2001b; Terao et al., 2000).
2.2.3. What and where does TMS stimulate?
Both TES and TMS stimulate axons rather than cell bodies of neurons since the latter have a much longer electrical time constant and higher threshold. This has been confirmed experimentally by comparing the strength–duration (S–D) time constants of peripheral nerve and cortex. Measurements made with varying durations of electrical pulses yield similar S–D time constants for both nerve and cortex suggesting that their targets are large diameter myelinated axons (Burke et al., 2000). Two key features need to be considered. The first is that the currents induced in the brain by TMS (and TES) have an important directional component. In the case of TMS, early modeling studies of the induced electric field showed that charge build-up at surface boundaries makes the majority of induced current flow parallel to the surface of the brain, rather than perpendicular to the grey matter (Tofts and Branston, 1991). The second concept is that the threshold for stimulation of neurons depends strongly on the relative current direction: axons are best stimulated by that component of current that flows nearly in parallel with their main orientation. This explains why electrical stimulation occurs best with the anode and cathode placed along the length of a peripheral nerve rather perpendicular to it. In fact, the physics of nerve stimulation state that if we take an axon, then it will be best activated at the point where the second spatial derivative of the electric field along its length is maximal. With respect to TMS, this means that stimulation often occurs at the point where the axon bends out of the field and the change in electric field is greatest (Maccabee et al., 1993). Thus EMG responses to TMS at just supra-threshold intensities are usually 1.5 ms or so later than after TES. At higher intensities, the latency to TMS shortens and approaches that of TES when TMS finally evokes D-waves (Di Lazzaro et al., 2004b). Detailed modeling studies of the electric field distributions induced by TMS in realistic models of the brain are still being undertaken (Thielscher et al., 2011; Laakso et al., 2014a, 2014b).
Day et al. (1989) initially argued that with TMS stimulation is most likely to occur in the part of the motor cortex nearest to the scalp surface, which would correspond to the crown of the anterior bank of the central sulcus. If TMS induced horizontal current flow through this region, then it would be unlikely to activate pyramidal neurons directly (and evoke D-waves). Instead, it was postulated that TMS might preferentially activate horizontally oriented axons of cortical interneurons that activated pyramidal neurons trans-synaptically (I1-waves). Later, imaging studies have suggested that this assumption was erroneous, because motor cortex TMS evoked activation deep in the central sulcus using PET assessment (Fox et al., 2004) or distant from the site of stimulation using fMRI assessment (Bestmann et al., 2004). However, these results do not allow identification of the precise location of TMS-induced axonal activation, because this site may be different from the neural structures identified by functional brain imaging. The biological effect depends on the neuronal circuits that are finally recruited and can be anatomically different from the site where axons are activated by the TMS-induced electrical field. An example is seen with the analgesic effects resulting from motor cortex stimulation (Lefaucheur, 2013). Therefore, modeling studies may be more relevant than imaging studies to determine where TMS activation occurs in the brain. Several detailed modeling studies, mostly of motor cortex stimulation, have taken into account tissue inhomogeneities in the cortex as well as boundaries between cerebrospinal fluid (CSF/grey and grey/white matter), and have shown that induced electric fields are strongest in the crown of the gyrus, although there can also be hot spots within the subcortical white matter (Opitz et al., 2013). When this type of calculation is combined with models of typical varieties of cortical and subcortical neurons, it seems likely that TMS of the motor cortex will activate cortical interneurons in the gyral crown or lip of the sulcus, as well as pyramidal neurons in the lip of the sulcus or slightly deeper (Salvador et al., 2011; Opitz et al., 2013). Excitation threshold depends on the orientation and membrane properties of the axons impacted by the TMS-induced electrical field. The influence of subcortical white matter activation remains to be studied in detail, but could well be important since the subcortical white matter is primarily composed of the axons of cortico-cortical loop fibers which may well have strong connections to the corticospinal output neurons. Indeed, another modeling study has proposed that the activation site in TMS involves the crown, anterior bank and white matter (Laakso et al., 2014a). The results of imaging studies employing neurovascular coupling reflect the cumulative effects of TMS over several seconds, not merely the one triggered by the initial part of the stimulus as reflected in the MEP, and prudence should be employed in considering the conclusions of such studies.
2.2.4. Stimulation with TMS is directionally specific
When a figure-of-eight TMS coil is placed over the hand area of motor cortex, thresholds from a monophasic stimulator are lowest when the coil is oriented to produce an approximately posterior–anterior (PA) current flow perpendicular to the central sulcus (Werhahn et al., 1994; Sakai et al., 1997). Recordings of descending corticospinal activity from the spinal epidural space suggest preferential recruitment of the first I-wave (I1-wave) (Di Lazzaro et al., 2001c). If the coil orientation on the scalp is reversed to induce an anterior–posterior (AP) current then the first recruited I-waves often occur 1–3 ms later (Di Lazzaro et al., 2001c). Similar differences in latency are evident in the surface EMG recorded MEPs (Werhahn et al., 1994; Sakai et al., 1997). It is not clear whether the late I-waves from AP stimulation are the same late I-waves as recruited by higher intensity PA current (Di Lazzaro and Ziemann, 2013). Nevertheless, changing the current direction seems to recruit different inputs to the corticospinal output neurons. Orienting the coil to induce a latero-medial current reduces the threshold for D-wave activation (Di Lazzaro et al., 1998). All these differences are less evident at higher intensities of stimulation. Interestingly, the extent to which they are present differs between individuals, possibly meaning that they depend on details of individual neuroanatomy and tissue anisotropy, such as the orientation and location of neurons relative to the TMS coil; needless to say, this aspect is valid for any type of cortical area and not only for the motor cortex. Models of electric fields induced by TMS can account for some of this selectivity and have been recently implemented (Rotem et al., 2014). They show that larger fields are induced in the crown of the precentral gyrus by perpendicular than parallel stimulation. However, they do not readily explain why there is a difference between PA and AP stimulation, particularly if TMS primarily activates horizontal interneurons at the gyral surface (Salvador et al., 2011; Day et al., 1989). This is because these neurons are distributed isotropically and therefore should be equally well activated by both AP and PA directionality of TMS. Since this cannot be the case, it suggests that some other (at present unknown) elements are stimulated preferentially by PA stimulation.
These observations relate to monophasic magnetic pulses, which are commonly used for single-pulse experiments, while repetitive TMS (rTMS) is usually performed with biphasic stimuli because of a lower energy requirement (Sommer et al., 2006). Biphasic stimulation is thought to be more powerful than monophasic stimulation, in particular in producing motor evoked potentials (MEPs) (Kammer et al., 2001). However, rTMS using monophasic pulses activates a relatively uniform population of neurons and could therefore be more effective in producing sustained after-effects than biphasic pulses which generate a more complex pattern of neural activation. For example, MEP size reduction following 1 Hz-rTMS delivered over M1 (Taylor and Loo, 2007) and MEP enhancement following 10 Hz-rTMS (Arai et al., 2007) are more marked and prolonged when monophasic pulses are used. Importantly, the effects of monophasic and biphasic magnetic pulses can be compared only if the second and decisive phase of the biphasic pulse is taken as the equivalent of the initial monophasic pulse (Di Lazzaro et al., 2001a; Sommer et al., 2013). Studies may be confusing when the initial phase of the biphasic pulse is retained for comparison, also given that the direction of the current can be reversed depending on the manufacturer (Kammer et al., 2001).
3. TMS in clinical settings
TMS of the motor cortex has a well-established role in clinical neurophysiology and is used worldwide to assess the conduction of the descending cortico-nuclear and cortico-spinal connections (Table 1). The motor cortex is a favoured target area for neuros-cientific studies because changes in motor activation and excitability can be readily assessed by recording MEPs. Neurophysiological measures, such as corticomotor threshold (MT), MEP amplitude and latency, Cortical Silent Period (CSP) duration, Central Motor Conduction Time (CMCT) or MEP recruitment curves among others, can be used to provide evidence of disease-related changes in motor cortical control or corticospinal output in patients. However, as for any other neurophysiological variables, each laboratory needs to establish its own normative data set for reliable diagnostic testing in clinical practice.
Table 1.
Neurophysiological measurements in various neurological disorders.
| Neurological disorder | MEP amplitude | CMCT | MT | CSP |
|---|---|---|---|---|
| Multiple sclerosis | Reduced | Increased | Increased | Prolonged |
| Stroke | Reduced | Increased | Increased or reduced | Shortened or prolonged* |
| Cervical myelopathy | Reduced | Increased | Increased | Shortened |
| Amyotrophic lateral sclerosis | Reduced | Increased | Increased (late) reduced (early) | Normal or shortened |
| Parkinson’s disease | Facilitated (r) | Normal | Normal | Shortened |
| Dystonia | Normal (r) facilitated (a) | Normal | Normal | Shortened |
| Cerebellar ataxias | Normal or reduced | Increased | Increased | Prolonged |
| Epilepsies | Normal or reduced | Normal | Normal, reduced or increased | Normal, shortened or prolonged |
MEP, motor evoked potential; CMCT, central motor conduction time; MT, motor threshold; CSP, cortical silent period; (r), at rest; (a), during activation.
CSP duration is variable in stroke, depending on the site of the stroke. The CSP is typically shortened if M1 is affected, but often grossly prolonged if areas outside M1 (e.g. S1 or parietal cortex) are affected.
4. Motor threshold
In clinical practice and in scientific studies, the intensity of TMS is individually adjusted to the “cortical” Motor Threshold (MT) defined as the minimal intensity of motor cortex stimulation required to elicit a reliable MEP of minimal amplitude in the target muscle. Lowest thresholds are found for hand and forearm muscles, followed by progressively higher thresholds for truncal, lower limb and pelvic musculature (Tables 2 and 3). Face musculature can also be examined, but this is more difficult because the facial nerve and muscles can be directly stimulated in the TMS-induced electrical field, resulting in contamination of “cortical” MEPs by “direct” peripheral neuromuscular responses and facial muscle reflexes (i.e. blink reflex).
Table 2.
Normative values of motor threshold (MT) obtained for a group of 50 healthy subjects (modified from Rossini et al. (1994)).
| Muscle | MT (%) |
|---|---|
| Deltoid | 50–60 |
| Biceps | 50–60 |
| Extensor digitorum brevis | 38–45 |
| Thenar* | 39–46 |
| Recti abdomini | 55–65 |
| Quadriceps | 60–80 |
| Tibialis anterior | 60–80 |
| Soleus | 70–90 |
| Abductor hallucis** | 55–75 |
| Anal sphincter | 75–100 |
| Bulbo-cavernous | 75–100 |
Threshold is expressed as a percentage of the maximal stimulator output (%) connected to a circular coil of 7 cm diameter.
In young subjects (18–44 years) = 39.4 ± 3.5% S.D.; in elderly subjects (45–80 years) = 44.2 ± 6.1% S.D.
In young subjects (18–44 years) = 56.3 ± 6.7% S.D.; in elderly subjects (45–80 years) = 66.2 ± 10.1% S.D.
Table 3.
Summary of different MT normative measurements (modified from Mills and Nithi (1997)).
| Muscle | Age | MT% (M ± SD) | References |
|---|---|---|---|
| FDI | 39.6 ± 13.8 | 48 ± 6 | Maertens de Noordhout et al. (1992) |
| ADM | – | 56 ± 7.2 | Reutens and Berkovic (1992) |
| ADM | 12–49 | 55.8 ± 12.9 | Reutens et al. (1993) |
| APB | – | 61.3 ± 9.6 | Valls-Solé et al. (1994) |
| R APB | – | 38.5 ± 6 | Triggs et al. (1994) |
| L APB | – | 41.6 ± 7 | Triggs et al. (1994) |
| ADM | – | 50.4 ± 8.3 (range 33–67) | Di Lazzaro et al. (1994) |
| FDI | – | − (range 41–83) | Ridding et al. (1995a) |
| APB | 16–35 | 39.4 ± 3.5 | Rossini et al. (1992) |
| APB | 51–86 | 43.9 ± 6.4 | Rossini et al. (1992) |
| ADM | 28–47 | 49 ± 8 | Macdonell and Donnan (1995) |
MT%, motor threshold expressed as a percentage of maximal stimulator output; M, mean; SD, standard deviation; FDI, first dorsal interosseous; ADM, abductor digiti minimi; APB, abductor pollicis brevis; R, right; L, left.
As previously stated the MT can be defined as the lowest transcranial stimulus intensity at which TMS of motor cortex produces an EMG response in the ‘target’ muscle or a visible muscle twitch. However, even if a twitch-based MT estimation is easier to perform, MT determination based on this is discouraged because it is associated with high intra- and inter-rater variability. In addition, visually estimated twitch-based MTs are approximately 10% (0–30%) higher than MTs determined on EMG recordings (Westin et al., 2014).
Intrinsic fluctuations of the excitability of cortical and spinal neurons cause trial-to-trial variability in MEP amplitude. This “physiological noise” introduces some uncertainty when estimating the MT (Adrian and Moruzzi, 1939). While physiological noise cannot be eliminated, other technical and physiological variables can and should be kept constant during MT measurements, such as coil position and orientation, the motor state (e.g. background activity of the target muscle), the individual arousal level, and environmental noise. Before determining MT, the optimal position and orientation of the coil for stimulation of the target muscle has to be identified (cf. sections on mapping and basic physiology).
4.1. Determining the “cortical” motor threshold
Resting MT (RMT) is determined while the target test muscle is at rest. Complete relaxation can be controlled by checking the absence of EMG at high-gain amplification either visually or by acoustic feedback or both. Active MT (AMT) is usually determined during a slight tonic contraction of the target muscle at approximately 20% of the maximal muscle strength. The active MT corresponds closely to the threshold for inducing descending volleys in the fast-conducting neurons of the corticospinal tract. This is because the first recruited descending volleys are able to effectively discharge those spinal motoneurons that are close to firing threshold in an active condition.
MEP responses to individual, successive stimuli when elicited in active muscles using threshold intensities may fluctuate in amplitude from 0 to about 1 mV, with a median value around 0.2 mV. If relaxed, variability is less, around 0–0.5 mV.
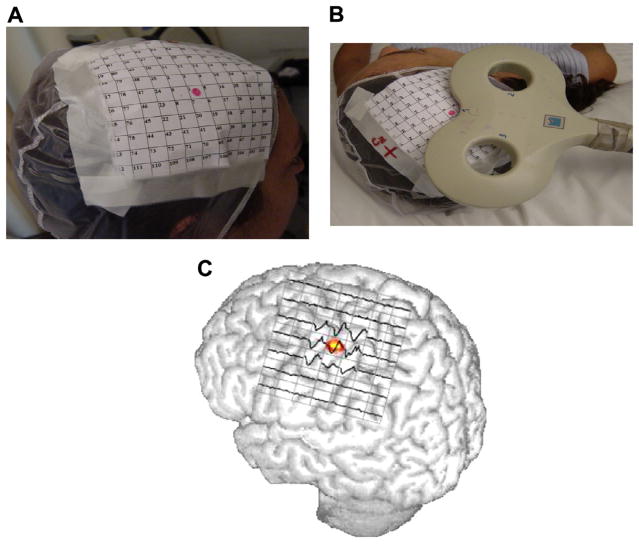
The following procedure is recommended to define the MT precisely using a figure-of-eight coil (see also Groppa et al., 2012) (Figs. 4 and 5). Localize the “hot spot” with the coil on M1 contralateral to the examined limb (cf. section on mapping). The lowest threshold for the hand area can usually be determined by orienting the coil 45° towards the contralateral forehead in order to guarantee current flow approximately perpendicular to the central sulcus. Orientation is at least as critical as position (Sakai et al., 1997; Opitz et al., 2013). For stimulation of the foot area (abductor hallucis muscle) a lateral orientation of the handle of the butterfly or figure-of-8 perpendicular to the interhemispheric cleft (i.e. lateral) produces the highest MEP amplitudes and shortest latencies. However Richter et al. (2013) report that rotating the coil ~30° anteriorly results in a MT that is lower by 8.0 ± 5.9% of maximal stimulator output compared with the standard lateral orientation.
Fig. 4.
Coil placement for MT determination of an intrinsic hand muscle (from Groppa et al., 2012 – with permission). In this and in the following figure the current flowing in the brain has an opposite direction to that flowing in the coil. Optimal coil orientation refers to monophasic pulse or the second phase of a biphasic pulse. Current direction in the coil can differ across commercially available stimulators.
Fig. 5.
Coil placement for MT determination of a leg muscle (from Groppa et al., 2012 – with permission).
The following methods have been used for MT determination.
4.1.1. Relative frequency methods
The relative frequency method has been described in the 1994 “Report” (Rossini et al., 1994) and recently modified slightly (Groppa et al., 2012): TMS should start with a subthreshold intensity. One may start with a stimulus intensity of 35% of the maximal stimulator output (MSO) with the coil placed over the optimal site for stimulation. To determine RMT, stimulus intensity is gradually increased in steps of 5% MSO until TMS consistently evokes MEPs with peak-to-peak amplitudes of >50 μV in each trial. Thereafter, stimulus intensity is gradually lowered in steps of 1% MSO until there are less than 5 positive responses out of 10 trials. This stimulus intensity plus 1 is then defined as RMT. In active muscles with ongoing activity, MEPs greater than 0.1 mV (100 μV) are judged to be positive. Of note, an analogous procedure for MT determination has been used in classic physiology with direct cortical stimulation in experimental preparations (Patton and Amassian, 1954). The procedure is more complicated for AMT than RMT, since TMS interferes with the ability to maintain a steady muscle contraction with stable background EMG activity of 10–20% of maximal contraction (Rossini et al., 1995). As with any other method, accuracy increases with the number of stimuli per intensity level.
However, when using at least 5 positive MEPs out of 10 trials (Groppa et al., 2012) it has been calculated that measurement accuracy, defined as “probability to obtain a diagnostically acceptable estimator for this subject exceeds 0.95” (Awiszus, 2012), is only 47.6%. With 10 MEPs out of 20 trials (Rossini et al., 1994) accuracy is 96.2% (Awiszus, 2012). On this basis it can be said that for both for clinical and research purposes 10 out of 20 trials are required to produce reproducible results.
4.1.2. Adaptive methods
Adaptive methods use an S-shaped metric function to model the probabilistic nature of MT and the relationship between TMS intensity and the probability of eliciting a MEP. At each trial, the model recalculates by an adaptive stair-case procedure a TMS intensity that yields a 50% probability of evoking a MEP which is then selected as the intensity for the next TMS pulse. Examples of adaptive methods to determine the MT are Parameter Estimation by Sequential Testing (PEST) (Awiszus, 2003) and Maximum Likelihood Regression. A computer program is necessary to run the maximum-likelihood threshold tracking algorithm, and was made freely available by Awiszus and Borckardt (“Adaptive PEST” http://www.clinicalresearcher.org/software.htm (Mishory et al., 2004)).
As summarized recently (Groppa et al., 2012), a typical program starts with defining an upper and lower boundary. A conservative approach uses 0% of MSO as lower boundary and 100% as upper boundary. More effectively, one might select the boundaries based on the known threshold distribution in the target population (e.g. ±20%). 14 up to 17 stimuli without specific a priori assumptions have been calculated to be necessary for reliable MT estimation (Awiszus, 2011). In the only study carrying out a comparative analysis for adaptive methods (Silbert et al., 2013) it was concluded that the relative frequency method requires significantly more trials than the PEST method (see below). The median difference between PEST and relative frequency estimates of MT was 2.3% of MSO with a maximal individual difference of up to 5%: higher MTs were found for PEST (Silbert et al., 2013).
As a conclusion, each of the methods described above can be used in research or clinical settings to provide a sufficiently accurate MT estimation. However, adaptive methods based on threshold-tracking algorithms provide a more accurate and usually faster MT estimation because it may require a smaller number of stimuli (Awiszus, 2011; Qi et al., 2011).
5. MEP amplitude
The MEP is commonly recorded over the target muscle using surface electrodes in a bipolar belly-tendon arrangement. The placement of the electrodes and the filter and amplification settings are identical to the standards used for recording the CMAP evoked by peripheral nerve stimulation (PNS). However, the peripherally evoked and transcranially evoked motor responses have different neurobiological properties, and these need to be considered when using the MEP amplitude as a neurophysiological measure of corticomotor excitability. These differences were discussed earlier and explain why the TMS-evoked and PNS-evoked motor responses only resemble each other: there are differences in amplitude, duration, and shape.
5.1. Temporal dispersion of corticomotor excitation
As described in detail in the previous sections a single TMS pulse applied to M1 gives rise to a series of temporally dispersed descending corticospinal volleys (Di Lazzaro et al., 2004a; Groppa et al., 2012; but see Rusu et al., 2014 on the plausibility of an alternative mechanism behind D- and I-wave generation through computational modeling). At spinal level the dispersed corticospinal volleys result in activation of motoneurons at slightly different latencies, dependent on their thresholds, and this asynchrony is accentuated by conduction in the peripheral nerve. This produces phase cancellation of motor unit potentials (MUAPs) and MEPs that are significantly less synchronized, more prolonged, and of lower amplitude than CMAPs (Rossini et al., 1995; Magistris et al., 1998). This observation cannot be overcome during brain stimulation by increasing the TMS intensity. In fact, phase cancellation of the motor unit potentials contributing to the whole MEP is prominent at high stimulation intensities and causes a discrepancy between the size of the mechanical and electrical muscle response to TMS. At TMS intensities that cause maximal stimulation of the fast-conducting corticomotor pathway, TMS elicits a muscle twitch in the target muscle that can exceed the force of muscle twitches evoked by supramaximal peripheral nerve stimulation. This is because some motoneurons may discharge more than once to the intense corticospinal volley.
Phase cancellation of MUAPs can be reduced to a level that is comparable to PNS with a triple stimulation technique (TST). The TST is a collision method that has been introduced by Magistris et al. (1998) and “resynchronizes” corticomotor excitation at the level of the peripheral motor axon. MEP amplitudes recorded with TST closely match that of the maximal electrically evoked CMAP so that the MEP/CMAP ratio in normal subjects is close to 1. TST can be implemented as a clinical routine procedure to assess corticomotor conduction to distal limb muscles in patients, but has been rarely employed in neuroscientific studies on healthy individuals.
5.2. The effect of stimulus intensity
Both, extrinsic factors (e.g. ‘conditioning’ stimuli preceding a ‘test’ TMS stimulus) and intrinsic factors (e.g. the mental activity) can change corticomotor excitability and change MEP amplitude. As a general rule, increasing the stimulus intensity will induce a stronger descending excitatory drive resulting in faster temporospatial summation at the cortico-motoneuronal synapses, and MEP amplitude increases gradually with increasing stimulus intensity. The relationship between stimulus intensity and MEP amplitude can be formally modeled by a cumulative Gaussian and described by a sigmoid curve (Devanne et al., 1997; Pitcher et al., 2003; Möller et al., 2009). The sigmoid curve indicating the relationship between stimulus intensity and MEP amplitude is called the “stimulus–response curve”, “recruitment curve”, or “input–output curve”. The initial segment of the sigmoid curve is relatively flat and deviates from zero at the stimulus intensity that corresponds to the MT. The second part of the sigmoid curve is an ascending line caused by an approximately linear increase in MEP amplitude with increasing stimulus intensity. This part of the recruitment curve corresponds to TMS intensities between 120% and 140% of resting MT (Davey et al., 1999; Han et al., 2001). The stimulus–response relationship can then be assessed by calculation of the amplitude ratio of the MEP obtained at 140% of resting MT to that obtained at 120% (Lefaucheur et al., 2006a) or of the slope of the IO-curve within this range of stimulation intensities (Lefaucheur et al., 2012). This slope reflects the gain in MEP amplitude with increasing stimulus intensity. Proton magnetic resonance spectroscopy has revealed a positive correlation between the slope of the MEP input–output curve (IO-curve) and cortical glutamate levels in the motor cortex, suggesting a link between glutamatergic neurotransmission and corticospinal excitability (Stagg et al., 2011). At higher stimulus intensities, the stimulus–response curve plateaus with no further increase in MEP amplitude despite of an increase in stimulus intensity. The plateau at high stimulus intensities is due in part to the increasing phase cancellation of the motor unit action potentials (MUAPs) that constitute the MEP (Magistris et al., 1998). In summary, the stimulus–response curve is determined by the progressively higher number of recruited corticospinal fibers and the temporal dispersion of the spikes propagating along the corticomotor pathways. Therefore, clinical use of the MEP IO-curve has been promoted in acute (e.g. stroke) and slowly progressive (e.g. amyotrophic lateral sclerosis) diseases affecting the number of pyramidal tract fibers.
An increase in neural excitability at the cortical or spinal level, due to, e.g., voluntary contraction of the target muscle, will facilitate corticomotor excitability and result in larger MEP amplitudes without a change in stimulus intensity. This implies that the sigmoid stimulus–response function reflecting the relation between stimulus intensity and MEP amplitude is not invariant, but is subject to dynamic changes which reflect the present physiological state of the motor system. For instance, different IO-curves will be obtained in the same muscle depending on whether the target muscle is relaxed or active, in a movement preparation period, or in a condition of mental motor imagery without real contraction (Starr et al., 1988; Rossini et al., 1988; Tomberg and Caramia, 1991). The flexible tuning of the stimulus–response function by physiological variables explains why measurements of MEP amplitude are the most popular electrophysiological “read out” to assess after-effects of repetitive TMS and other non-invasive brain stimulation protocols applied over M1 on corticospinal excitability (Siebner and Rothwell, 2003; Ziemann et al., 2008).
5.3. Inter-trial variability of MEP amplitude
Intrinsic fluctuations in neural excitability at the cortical and spinal levels render the MEP amplitude highly variable even in an apparently resting state with complete relaxation of the ‘target’ muscle. This intrinsic trial-to-trial variability has to be taken into account when measuring threshold under resting conditions (Rossini et al., 1994) and using the mean MEP amplitude as a state marker of corticomotor excitability (Wassermann, 2002). Recent TMS–EEG studies have shown that TMS-induced MEP amplitudes depend on the state of ongoing EEG phase and power fluctuations which may account at least partially for the inter-trial variability of MEP amplitudes (Bergmann et al., 2012; Ferreri et al., 2014b; Keil et al., 2014). For instance, MEPs are consistently larger when evoked during the up-states than during down-states of slow oscillations in non-REM sleep (Bergmann et al., 2012). During wakefulness, the MEP amplitude correlate with the power and phase of EEG and EMG activity in a frequency band around 18 Hz, and high beta-band cortico-muscular coherence shows a positive linear relationship with the MEP amplitude (Keil et al., 2014). Together, these results show that the inter-trial variability of MEP amplitudes may contain important information about the state-dependency of corticomotor excitability (Ferreri et al., 2014b).
5.4. Measuring MEP size
The size of a single MEP is usually expressed as peak-to-peak amplitude, but the “area under the curve” of the rectified MEP or the amplitude from pre-MEP baseline can also be used. As mentioned above, intrinsic fluctuations of neural excitability at the cortical and spinal levels introduce a substantial variability of the MEP amplitude from trial to trial. Therefore, several MEPs need to be consecutively recorded to obtain a reliable estimate of the MEP size. This is particularly relevant in TMS studies during which MEP amplitude measurements need to be repeated several times during the same experiment. In this context neuronavigated TMS is advantageous to monitor coil position relative to the cortical target site and to correct any shift in coil position or angulation during serial measurements.
MEP amplitude measured at a single TMS intensity provides no information about the stimulus–response characteristics of corticospinal excitability. TMS intensities are usually expressed as a percentage of either individual MT or MSO. MEP measurements can be performed during relaxation or during tonic contraction of the target muscle. The stimulus–response relationship between TMS intensity and MEP amplitude can then be determined by averaging the MEP amplitudes for each intensity level. Alternatively, the MEP amplitudes for each trial are plotted against the corresponding TMS intensity and the stimulus–response function is derived by curve fitting, e.g., by fitting a Boltzmann function. A change in corticospinal excitability may result in a right- or leftward shift of the entire stimulus–response curve and/or a change in its slope depending on whether the excitability change is similarly expressed across the entire intensity range or not. Hysteresis effects have been described for TMS at a relatively short inter-trial interval of 5 s (but not 20 s) in the relaxed (but not active) target muscle and may introduce systematic biases in clinical and research studies (Möller et al., 2009). Stimulus–response curves are not static but undergo rapid modifications under physiological conditions. A simple example is a change in motor state of the target muscle from rest to tonic contraction, which will result in a leftward shift of the curve, a steeper slope and a higher maximal amplitude. Motor skill training can also change the recruitment curve. After motor skill training of rapid wrist extension, the slope of the recruitment curve of the agonist muscle increased, while the slope decreased in the antagonist muscle (Suzuki et al., 2012). Further, stimulus–response curves can be modified by CNS-active drugs. For example, lorazepam and lamotrigine suppress the stimulus–response curve in healthy individuals (Boroojerdi et al., 2001) (for recent review, see Ziemann et al., 2014).
The stimulus–response curve is altered by neurological diseases that impair corticomotor conduction. Within the central nervous system, a disease-related loss of corticospinal axons, conduction block or demyelination may result in an attenuated slope of the stimulus–response curve and reduced maximal amplitude. Accordingly, abnormal stimulus–response curves have been reported in patients with motor stroke (Ward et al., 2006) and amyotrophic lateral sclerosis [ALS] (Vucic and Kiernan, 2007). In patients with chronic motor stroke, the slope of the stimulus–response curve predicted the magnitude of task-related brain activation (Ward et al., 2006). The flatter the slope of the stimulus–response curve, the more patients recruited secondary motor networks in both hemispheres “in an attempt to generate motor output to spinal cord motoneurons” (Ward et al., 2006). In ALS, the stimulus–response curve often shows hyperexcitability of the corticospinal neurons, especially in the early stages of the disease (Vucic et al., 2013). It is worth pointing out that the stimulus–response curve is also sensitive to disease-related alterations in corticospinal excitability in conditions which do not cause structural damage to the CST, e.g. Parkinson’s disease (Valls-Solé et al., 1994) and dystonia (Siebner et al., 1999) (cf. Table 1).
5.5. Practical considerations
The estimation of the probabilistic distribution of MEP amplitudes is relevant in scientific studies of corticospinal excitability and requires a large number of MEPs to be recorded for each muscle. In many studies, however, experimental constraints allow only the assessment of the MEP size at a single intensity rather than assessing the entire stimulus–response curve. In these situations, TMS intensity is usually set to 115–125% of the individual’s RMT to ensure that the experiment probes the MEP size on the rising phase of the stimulus–response curve where there is a roughly linear increase with TMS intensity. Many plasticity studies target an intensity that generates a MEP size of about 1 mV, or MEP size of half maximum.
In contrast to scientific studies, the primary goal of diagnostic TMS is to elicit a maximal corticomotor response. Hence, TMS intensity should be sufficiently high to excite all high-threshold, fast-conducting corticospinal neurons and spinal motoneurons (Groppa et al., 2012). An optimal intensity for diagnostic TMS is an intensity that marks the transition from the rising slope to the flat portion (plateau) of the sigmoid stimulus–response curve: 140% of RMT, corresponding to approximately 170% of AMT (Groppa et al., 2012). In addition, the efficacy of TMS in exciting the corticomotor output may be increased by asking the patient/subject to preactivate the target muscle at 10–20% of maximum strength. For each muscle, 5–6 consecutive MEPs should be recorded during tonic contraction and only the MEP with the largest amplitude should be considered. In patients with an inability to contract the target muscle (for instance due to severe paresis), CST excitation may be facilitated by voluntary activation of the homologous muscle of the other side, motor imagery of target muscle contraction, or tonic vibration of the target muscle, though to a lesser extent than voluntary contraction of the target muscle.
There is a further practical consideration here. In cognitive studies, where repetitive TMS around threshold intensity is used, one cannot assume a stable state of the cortex being stimulated for many hundreds of trials; nor can we directly measure the cognitive outputs as we can do with the motor cortex. This is a complex issue, but not one that can be ignored (see Silvanto et al., 2008)
6. Cortical Silent Period
As initially mentioned by Merton and Morton (1980), a period of electrical silence in the surface EMG activity occurs immediately after the MEP when TES is delivered to M1 during a tonic muscle contraction. Using focal TMS of the motor cortex hand representation using suprathreshold intensities, a silent period (cortical silent period, CSP) can be evoked in a contralateral hand muscle, lasting up to 100–300 ms following the MEP. The duration of the CSP gradually increases with the intensity of TMS (Fig. 6), while the level of contraction plays an insignificant role (Haug et al., 1992; Inghilleri et al., 1993; Roick et al., 1993; Rossini et al., 1995; Orth and Rothwell, 2004; Kimiskidis et al., 2005). The physiology of this inhibitory phenomenon is particularly complex and different spinal and supraspinal sources contribute to its genesis. It is now generally agreed that several spinal inhibitory mechanisms contribute to the CSP (Pierrot-Deseilligny et al., 1976; Person and Kozhina, 1978), including recurrent inhibition due to activation of Renshaw cells, the refractoriness of spinal motor neurons after excitation, postsynaptic inhibition by activation of Ia inhibitory interneurons. However, because of their short duration, these spinal mechanisms are limited to the early party of CSP, i.e. the initial ~50 ms (5–10 ms, except recurrent inhibition ~35 ms; Fuhr et al., 1991; see Pierrot-Deseilligny and Burke, 2012) while the later part of CSP is generated by inhibitory mechanisms within M1. Hence, the total duration of the CSP is usually altered only by cortical mechanisms. TMS applied even at moderate stimulation intensities produces a CSP significantly longer than the one induced by TES (Inghilleri et al., 1993; Brasil-Neto et al., 1995); as TMS preferentially activates axons of excitatory intracortical interneurons which in turn stimulate pyramidal neurons (Day et al., 1989; Di Lazzaro et al., 2004a) while TES directly depolarizes subcortical axons of pyramidal neurons (Edgley et al., 1990). This observation confirms the above finding and suggests a predominant role of intra-cortical inhibitory phenomena in the genesis of CSP. The recording from epidural electrodes by Chen et al. (1999b) demonstrated that I-waves of descending corticospinal volley evoked by TMS were suppressed if a second TMS pulse was given 100–200 ms after a conditioning first TMS pulse, i.e., during the CSP.
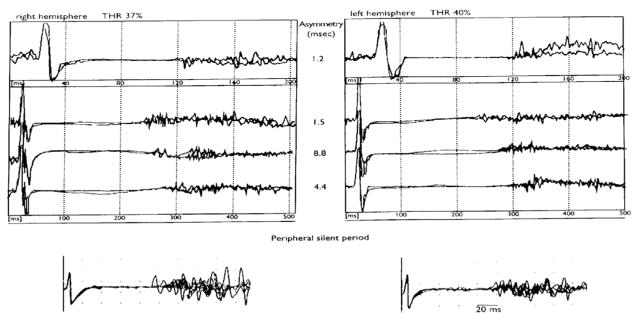
Fig. 6.
A representative case of a linear pattern of increase of the CSP duration (right and left hemisphere, recordings from left and right FDI, respectively) as a function of transcranial stimulation intensity which was increased by about 20% from motor threshold (from Cicinelli et al., 2000 – with permission). The values of the CSP duration interhemispheric asymmetry (ms) are shown. The bottom part of the figure shows the peripheral SP recorded from FDI muscles following supramaximal electrical stimulation of the ulnar nerve at the wrist.
The inter-hemispheric difference in CSP duration is very small, typically less than 10 ms, and interindividual differences and the inter-session variability of the CSP duration are larger (Haug et al., 1992; Cicinelli et al., 1997; Orth and Rothwell, 2004), ranging from 20% to 35% (Orth and Rothwell, 2004).
Since the duration of the CSP mostly reflects cortical mechanisms and it can be readily probed with single-pulse TMS, the CSP is a useful measure of intracortical inhibition in human M1 and can be used to detect intracortical excitability changes in brain diseases (i.e. in epilepsy, see Cicinelli et al., 2000) during clinical studies.
The following two methods can be used to estimate the CSP duration, as recommended by Groppa et al., 2012 (based on 5/6 trials per muscle):
The first method calculates the mean CSP duration (or median) based on trial-by-trial measurements of the CSP duration. In a single trial, the CSP is measured as the time elapsing from the onset of the MEP until the recurrence of voluntary tonic EMG activity.
The second method averages 5–6 MEP/CSP rectified traces. The rectified and averaged trace provides a good visualization of the voluntary EMG activity level at baseline (i.e., prior to the TMS pulse). Therefore, the end of the CSP can be defined more precisely by the reappearance of voluntary EMG activity relative to the tonic baseline EMG level.
Pharmacological studies suggested that the CSP reflects particularly long-lasting cortical inhibition mediated through gamma-aminobutyric type B receptors (GABABR). Stetkarova and Kofler (2013) demonstrated that CSP duration increased progressively after the administration of intrathecal baclofen (a specific GABAB receptor agonist). These findings contrast with previous observations: no effect after single oral (McDonnell et al., 2006) or intravenous (Inghilleri et al., 1996) doses of baclofen, but they concur with the significant CSP prolongation observed during the continuous administration of high-dose intrathecal baclofen in a patient with generalized dystonia (Siebner et al., 1998). These discrepancies can be explained by different routes of administration, dose, effective drug concentration and observation periods. Other studies (Werhahn et al., 1999; Pierantozzi et al., 2004) demonstrated that the administration of tiagabine (a GABA re-uptake inhibitor) or vigabatrin (an inhibitor of the GABA-degrading enzyme GABA transaminase) lengthen the CSP, suggesting the involvement of both GABAAR and GABABR. In relation to this, it has been shown that lorazepam (a GABAAR positive allosteric modulator) prolonged CSP duration if assessed at low stimulation intensity but shortened it if assessed using a high stimulation intensity (Kimiskidis et al., 2006). These data support the idea that at the lower range of stimulus intensities (i.e. short CSPs are elicited) CSP duration reflects the activation of GABAARs, while at the higher range of stimulus intensities (i.e., when long CSPs > 100 ms are elicited) it reflects the activation of GABABRs.
7. Central motor conduction measurements
After the introduction of TMS by Barker et al. (1985), the first clinical use of this new technique was to estimate the CMCT in humans by recording MEPs to stimulation of the motor cortex and spinal roots (Table 4). We here summarize the most frequently employed techniques.
Table 4.
Normative data for the upper and lower limbs (modified from Wassermann et al. (2008)).
| Muscle | CML (ms) | R/L diff. (ms) | PML (ms) | CMTCm (ms) | R/L diff. (ms) | CMCTf (ms) | R/L diff. (ms) | References |
|---|---|---|---|---|---|---|---|---|
| Biceps brachii | 9.4 ± 1.7 | 6.0 ± 1.2 | Eisen and Shtybel (1990) | |||||
| 12.5 ± 1.2 | 0.65 ± 0.6 | 7.1 ± 1.1 | 0.60 ± 0.51 | Furby et al. (1992) (21–54 y.) | ||||
| 10.2 ± 0.5 | 5.1 ± 0.3 | Abbruzzese et al. (1993) | ||||||
| 7.6 (3SD) | Di Lazzaro et al. (1999a) | |||||||
| Abductor digiti minimi | 20.5 ± 1.7 | 7.4 ± 1.2 | Barker et al. (1987) | |||||
| 18.8 ± 1.2 (f) | 11.8 ± 1.0 | 7.0 ± 0.8 | Chu (1989) | |||||
| 19.7 ± 1.0 (m) | 12.7 ± 1.1 | 7.1 ± 1.1 | Chu (1989) | |||||
| 19.7 ± 1.0 | 14.0 ± 1.5 | 6.0 ± 0.9 | 2.4 | 5.8 ± 0.8 | 1.8 | Claus (1990) | ||
| 7.0 ± 0.9 | 6.1 ± 1.0 | Furby et al. (1992)(21–54 y.) | ||||||
| Abductor pollicis brevis | 21.1 ± 1.5 | 8.0 ± 1.2 | Barker et al. (1987) | |||||
| 21.8 ± 1.8 | 14.4 ± 1.4 | 7.2 ± 1.8 | Tabaraud et al. (1989) | |||||
| 21.4 ± 1.5 | 14.8 ± 1.2 | 6.6 ± 1.4 | Ludolph et al. (1989) | |||||
| 20.2 ± 1.6 | 6.5 ± 2.0 | 7.9 ± 2.1 | 6.5 ± 2.0 | Eisen and Shtybel (1990) | ||||
| 5.66 + 0.84 | Rossini et al. (1992) (16–35 y.) | |||||||
| 5.45 + 0.72 | Rossini et al. (1992) (51–86 y.) | |||||||
| Rectus femoris | 21.5 ± 1.7 | 0.88 ± 0.85 | 14.2 ± 1.5 | 0.93 ± 0.90 | Furby et al. (1992)(21–54 y.) | |||
| 16.6 (3SD) | 1.8 | Di Lazzaro et al. (1999a) | ||||||
| Tibialis anterior | 29.1 ± 1.4 | 14.4 ± 0.9 | Abbruzzese et al. (1993) | |||||
| 27.4 ± 2.6 | 14.2 ± 1.7 | 10.7 ± 1.77 | Garassus et al. (1993) | |||||
| 16.1(3SD) | 17.1 (3SD) | 2.0 | 14.7 (3 SD) | 2.1 | Di Lazzaro et al. (1999a) | |||
| Abductor hallucis | 41.2 ± 3.4 | 16.7 ± 2.4 | Barker et al. (1987) | |||||
| 17.1(3SD) | 18.2 (3SD) | 15.9 (3SD) | Di Lazzaro et al. (1999a) | |||||
| 39.1 ± 2.5 | 1.5 ± 1.13 | 12.5 ± 2.2 | 1.6 ± 1.02 | Osei-Lah and Mills (2004) | ||||
| 39.4 ± 2.7 | 0.8 ± 0.6 | 16.9 ± 0.9 | 0.5 ± 0.4 | 12.4 ± 1.2 | 0.9 ± 0.4 | Di Lazzaro et al. (2004c) |
CML, cortical motor latency (after cortical magnetic stimulation); R/L diff., side-to-side difference; PML, peripheral motor latency (after paravertebral magnetic stimulation); CMCTm, central motor conduction time calculated using magnetic nerve root stimulation; CMCTf, central motor conduction time calculated using the F-wave method; SD, standard deviation; (f), females; (m), males.
1. Central motor conduction time (CMCT)
CMCT is a neurophysiological measure that reflects conduction between the primary motor cortex and spinal cord. With electrical stimulation, it includes the times for impulse propagation via the fast-conducting neurons in the corticospinal tract and excitation of the spinal motoneurons sufficient to exceed their firing threshold. With TMS, it also includes the times for trans-synaptic excitation of the cortical motoneurons in the M1 via cortical interneurons. CMCT can be estimated by subtracting the conduction time from the spinal roots/nerves to the muscle, referred to as peripheral motor conduction time (PMCT) from the latency of MEPs evoked electrically or magnetically by transcranial cortical stimulation (Fig. 7).
Fig. 7.
Schematic representation of the calculation of central motor conduction time (CMCT) (from Kobayashi and Pascual-Leone, 2003 – with permission). Motor evoked potential induced by TMS. (b) MEP after cervical spinal root stimulation. (c) F-waves after ulnar nerve electric stimulation. CMCT is estimated by onset latency of T1 minus onset latency of T2. By use of F-wave latency CMCT can be estimated more precisely as T1–(F + M − 1)2. T1 = onset latency of MEP elicited by TMS; T2 = onset latency of MEP elicited by the coil placed on the back of cervical spine. M = onset latency of M-wave by electrical ulnar nerve stimulation. F = onset latency of F-wave by electrical ulnar nerve stimulation.
Two methods are employed to measure PMCT: motor root stimulation and the F wave technique (Merton et al., 1982; Rossini et al., 1985, 1986, 1987a,b). The first approach activates motor roots (spinal nerves) at their exit foramina using electrical or magnetic stimulation over the spinal enlargements (Mills and Murray, 1986; Ugawa et al., 1989b; Matsumoto et al., 2013b). In this method, CMCT includes the time taken for at least one synaptic delay and the time in proximal motor root in the spinal canal, in addition to the true CMCT (time needed for conduction in the CST) (Cowan et al., 1984; Mills and Murray, 1985; Hess et al., 1987; Rossini et al., 1987a; Ugawa et al., 1988a,b, 1989a, 1990). The second approach is the use of F-waves (Rossini et al., 1987a; Chu, 1989; Eisen and Shtybel, 1990; Claus, 1990). The F wave latency measures antidromic conduction in motor axons to the spinal motoneuronal pool, the “turn-around” time at the motoneuron pool (generally considered to be 1 ms) and then orthodromic conduction from the motoneuron pool to the muscle. Accordingly, the conduction time from the motoneuron pool can be estimated by taking half of the result of adding the F wave latency and the M wave latency to nerve stimulation (cathode proximal) and subtracting 1 ms, i.e. (F + M − 1)/2 (Rossini et al., 1987a). The peripheral conduction time measured using F waves is slightly longer (1–1.5 ms) than the latency of the CMAP produced by “spinal stimulation”. This is because the site of stimulation with the latter is not at the motoneuron pool, as discussed below.
Both techniques have disadvantages:
- F waves
The major disadvantage of this method is that F waves can be measured routinely only for distal muscles, unless complex collision techniques are used. In addition, the latency of the fastest F waves provides a measure of conduction in the fastest motor axons, but their motoneurons are not those recruited first by corticospinal volleys particularly with near-threshold stimuli and a relaxed target muscle. The IO-curve for motor axons is normally quite steep, so that the resulting discrepancy will be small if the MEP is relatively large and well-synchronized. However, in presence of damage to the descending motor pathways or the spinal motoneurons the MEP may be small, reflecting only the recruitment of the lowest-threshold motoneurons. On the other hand, peripheral nerve damage may disperse the MEP, and this could create difficulties when trying to exclude central abnormalities in patients with peripheral nerve damage.
If F wave persistence is low, whether this is normal for that particular muscle (e.g., tibialis anterior) or due to disease, the recorded F wave sequence may not sample the fastest axons. This will produce a spuriously short CMCT.
- Stimulation over the spinal segment
Here the latency of the CMAP produced by stimulation over the spinal segment is subtracted from the latency of the MEP. Stimulation may be electrical or electromagnetic. Electrical stimuli are commonly delivered using the high-voltage stimulators developed for TES. As in conventional nerve conduction studies, the effective stimulus is cathodal (unlike the optimal polarity for transcranial stimulation of the motor cortex; Rothwell et al., 1987; Burke et al., 1990). The cathode is placed over T1 for the upper limb and the relevant root exit zone for the lower limb, with the anode over the spine, some centimeters more rostrally, e.g., over C5 for the upper limb. Using TMS, the coil is centered over the cervical or lumbar root exit zone.
When recordings are carried out from muscles of small volume surrounded by other muscles the final MEP will always be somewhat “contaminated” by cross-talk: volumetric spread of MEPs from adjacent muscles, often innervated by different spinal roots and/or nerve trunks. Only with needle electrodes can one be certain that the MEP arises from a specific muscle, and this is particularly so when recording from atrophic muscles. On the other hand, MEPs can be recorded from muscles which are anatomically relatively isolated (e.g. ADM for the hand).
With both high-voltage surface stimulation and electromagnetic stimulation, current is sprayed over a large area, even when focal coils (e.g., figure-of-8) are used. Motor axons may then be activated at some distance from the cathode. However, the bend in axons and the tissue inhomogeneities alter the electrical and electromagnetic fluxes such that motor axons are preferentially activated at the vertebral foramina for both the upper and lower limb outflows (e.g. Mills and Murray, 1986; Ugawa et al., 1989b; Alfonsi et al., 2003). As mentioned above, this implies that the measure of CMCT derived using peripheral stimulation is contaminated by the inclusion of conduction across the motoneuron and its axon. This will be greater with lower limb muscles because of the longer conduction path through the cauda equina. Whatever the method of stimulation employed it is important to monitor the CMAP carefully because, as stimulus intensity is increased to supramaximal, the site of activation of some axons will shift distally, even as far as the brachial plexus with stimulation over the cervical spinal cord (Plassman and Gandevia, 1989). Stimulation of nerve roots or the plexus will activate axons projecting to muscles other than the target one, regardless of whether high voltage or electromagnetic stimuli are employed. Contamination of the EMG recording over the target muscle by activity of neighboring muscles (cross-talk) is inevitable, and may be hard to detect when there is peripheral nerve pathology. In addition the electrical and electromagnetic stimuli inevitably activate afferent axons, and reflex discharges could contribute to late components of a dispersed CMAP.
There is no perfect technique that will be optimal for all occasions: for measuring CMCT the F wave technique is more accurate, while for peripheral nerve/root pathology, spinal stimulation may be preferred. In diagnostic studies, routine usage of the TST as described by Magistris et al. (1999) is more complicated (and uncomfortable), but its use may be necessary to clarify the findings in individual patients as well as in specific conditions in research studies.
For CMCT measurements TMS is usually delivered during voluntary contraction of the target muscle, thereby providing the shortest MEP latency. In this situation, the spinal motoneuronal pools will be close to firing threshold and a discharge could be generated by the earliest descending volley. In some disorders, however (i.e. in multiple sclerosis), a prolonged CMCT may be due to impaired temporal and spatial summation of descending volleys at the spinal motoneurons. For CMCT measurement, after superimposing the responses, the reproducible onset latency should be measured. If conduction block in proximal peripheral nerves is suspected, supramaximal electrical or magnetic root stimulation should be tried to identify the conduction block (Mills and Murray, 1986; Matsumoto et al., 2009a, 2010e, 2013b,c,d).
CMCT and MEP latency mature in parallel with the development of the central nervous system, specifically with maturation of the corticospinal tracts, and in neonates they are markedly longer in latency than in adults, particularly given size (Duron and Khater-Boidin, 1988; see Fig. 8). While CMCT reaches adult values around 3 years of age, the threshold of transcranial stimulation is too high for reliable responses until about 10 years of age (Koh and Eyre, 1988; Müller et al., 1991; Caramia et al., 1993; Fietzek et al., 2000). In adults, CMCT has no correlation or only a weak correlation with age (Ugawa et al., 1989a; Claus, 1990; Eisen and Shtybel, 1990; Mano et al., 1992; Rossini et al., 1992; Mills and Nithi, 1997; Matsumoto et al., 2012). CMCT for upper limbs has no correlation or only a weak correlation with body height, whereas CMCT for lower limbs is strongly correlated with height (Rossini et al., 1987b; Chu, 1989; Ugawa et al., 1989a; Claus, 1990; Ghezzi et al., 1991; Ravnborg et al., 1991; Toleikis et al., 1991; Furby et al., 1992; Wochnik-Dyjas et al., 1997; Matsumoto et al., 2010a). Most studies show no gender differences in CMCT once data are corrected for height (Ugawa et al., 1989a; Claus, 1990; Toleikis et al., 1991; Furby et al., 1992; Mills and Nithi, 1997; Tobimatsu et al., 1998). Most studies have reported no significant side differences in CMCT in the healthy subjects (Ugawa et al., 1989a; Eisen and Shtybel, 1990; Mills and Nithi, 1997); the side-to-side difference in CMCT is therefore a clinically useful measure.
Fig. 8.
Age effects on absolute latencies and ‘latency jump’ between “relaxed” and “contracted” motor evoked potentials (from Caramia et al., 1993 – with permission). Magnetic brain stimulation was carried out in children from 2 to 12 years. The latency of MEPs recorded during voluntary contraction increased in a linear fashion with age and body size. The latency of MEPs recorded when relaxed had a much slower “maturation,” and gained the adult value at about 10–12 years of age. The same effect is observed with TES.
2. Cortico-brainstem and brainstem-spinal root conduction times (C-BST and BST-R CTs)
Stimulation at the brainstem or foramen magnum levels (Ugawa, 1999a; Terao and Ugawa, 2002) (Fig. 9) can be achieved by electrical (Ugawa et al., 1991b, 1995a) or magnetic stimulation (Ugawa et al., 1994). For TMS the center of a double cone coil is placed over the inion or the midpoint between the inion and the ipsilateral mastoid process to induce upward current in the brain (Ugawa et al., 1994; Shirota et al., 2011) where it likely activates the CST at the pyramidal decussation due to high induced current concentration at the foramen magnum (Ugawa et al., 1992, 1996). It produces a single descending volley (Ugawa et al., 1994), in contrast to the multiple descending volleys with cortical stimulation. Brainstem stimulation can be employed to calculate the cortical–brainstem conduction time (C–BST CT) and the brainstem–spinal root conduction time (BST–R CT) (Ugawa et al., 1992, 1996). Such measurements enable localization of a CST lesion above or below the pyramidal decussation. Brainstem stimulation may also be used in research to investigate changes in spinal excitability (Ugawa, 2002). Possible drawbacks of this method are that slowly conducting descending tracts may be activated in patients with severe damage to the CST (Ugawa and Kanazawa, 1999b) and the inability to elicit a MEP in patients with severe CST involvement. In such cases, paired-pulse magnetic brainstem stimulation may be able to elicit MEPs by producing artificial temporal summation of EPSPs at the spinal motoneurons (Matsumoto and Ugawa, 2008; Matsumoto et al., 2010b).
Fig. 9.
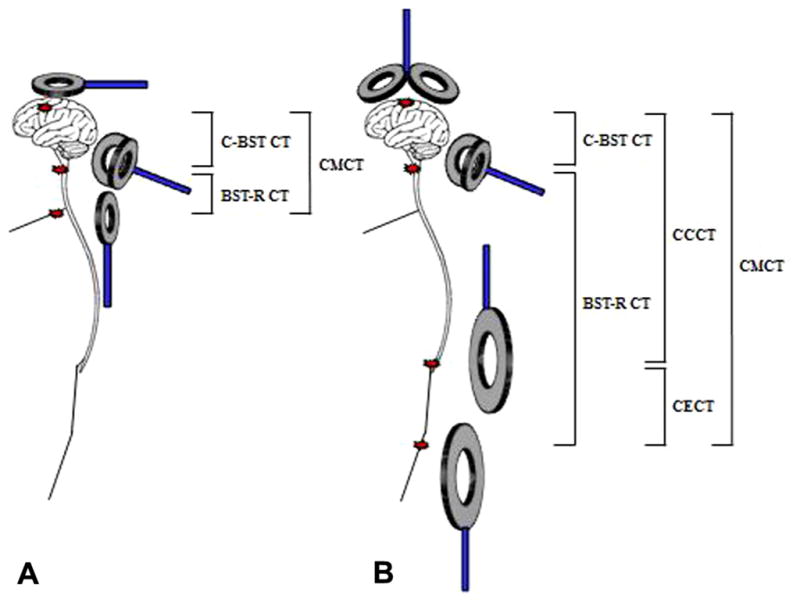
Central motor conduction studies using magnetic stimulation in humans. (A) CMCT for upper limbs, (B) CMCT for lower limbs. For upper limbs, cortical-brainstem conduction time (C–BST CT) and brainstem-cervical root conduction time (BST–R CT) can be measured as well as CMCT. For motor cortex and motor root stimulation, a round coil is usually used, whereas for brainstem stimulation, a double cone coil is required. For lower limbs, cortical-brainstem conduction time (C-BST CT) and brainstem-lumbar root conduction time (BST–R CT) also can be measured. Furthermore, cortico-conus motor conduction time (CCCT) and cauda equina conduction time (CECT) can be measured. For cortical stimulation, a round coil or double cone coil is used, whereas for motor root stimulation, a round coil or MATS coil is used. For conus stimulation, the MATS coil is required.
3. Cortico-conus and cauda equina conduction times (CCCT and CECT)
A newly developed TMS method is able to activate the conus medullaris in the spinal canal (Matsumoto et al., 2009a, 2013b) (Fig. 9). This method is not currently recommended for routine clinical activity. Conus activation can be achieved by electrical (Ugawa et al., 1988a,b, 1989a, 1990, 1995a; Claus, 1990) or magnetic stimulation (Maccabee et al., 1996; Maegaki et al., 1997; Matsumoto et al., 2009b, 2010a,c,d). For magnetic conus stimulation, a 20-cm diameter coil designated as a Magnetic Augmented Translumbosacral Stimulation (MATS) coil has been developed, with which the induced currents are strong enough to elicit MEPs in leg muscles reliably (Matsumoto et al., 2009a, 2009b). With conus stimulation both electrical and magnetic pulses are likely to activate axons at the root exit zone from the conus medullaris, i.e. the most proximal cauda equina, where the electrical conductivity changes abruptly (Ugawa et al., 1995a; Maccabee et al., 1996; Laakso et al., 2014b). These activation sites with the MATS coil have recently been confirmed by the induced current estimation using a spinal cord and canal model (Laakso et al., 2014b). The latency difference between MEPs to cortical stimulation and conus stimulation (cortico-conus motor conduction time (CCCT)) estimates the conduction time within the CST not including any peripherally generated components. In patients with severe peripheral neuropathy, therefore, CCCT was found to be not prolonged while CMCT was, because of peripheral conduction delays within the cauda equina (Matsumoto et al., 2010d). This method also enables calculation of cauda equina conduction time (CECT) which reflects conduction within the cauda equina (Matsumoto et al., 2010a, 2013a). However, supramaximal stimulation of the conus is usually impossible, limiting the ability to detect conduction block in the cauda equina.
8. Cortical mapping of motor representations
TMS can be used to map brain function and explore the excitability of different cortical regions (Rossini et al., 1994; Hallett, 2007; Wagner et al., 2007; Dayan et al., 2013). The only widespread experience in brain mapping with TMS has been of the motor cortex. In theory, any TMS effect can be mapped to its cortical location, but the most commonly employed is the motor homunculus because the MEP constitutes a reliable output measure. For motor mapping, the territory where MEPs can be produced is identified. Stimulation intensity is generally fixed at a percentage of the MSO above resting MT. Stimuli are applied at various scalp sites using a figure-of-eight coil and a coordinate spatial system referenced to the vertex (Thickbroom et al., 1999), and the amplitude of MEPs evoked in contralateral muscles is measured. Then, a map of sites on the scalp from which responses can be obtained in each muscle of interest is defined. The target muscle representation has a maximum value (optimal site), a center of gravity (CoG) (elements of the representation can be used to form a weighted average of their location, in which the weights are given by the normalized value of the element), and a surface area. The CoG provides a spatial average optimal site rather than the one site of largest MEP response, which is the “hot spot”. This approach can allow the distinction of the representations for two or more hand muscles (Figs. 10 and 11). Maps of the M1 representation for APB and ADM muscles were produced by fitting a continuously defined 3D function to findings gathered from stimulation at specific scalp sites and projecting such function onto a 2D surface via a radial projection (Wilson et al., 1993). The maps of APB and ADM overlap, but with a statistically significant separation, the APB map being more lateral than the ADM map. Changing the coil direction may also help refine maps. Maps for ADM, APB and FDI muscles have been studied by systematically rotating the coil to determine the direction of induced current for each scalp site of the map expressing the optimal ones as an angle relative to individual central sulcus directionality (Bashir et al., 2013). There is considerable evidence that CoG-based TMS mapping is spatially accurate, especially when using a navigation system (see next section), and has been recently validated by direct electrical stimulation of cortex in patients with brain tumors (Opitz et al., 2014).
Fig. 10.
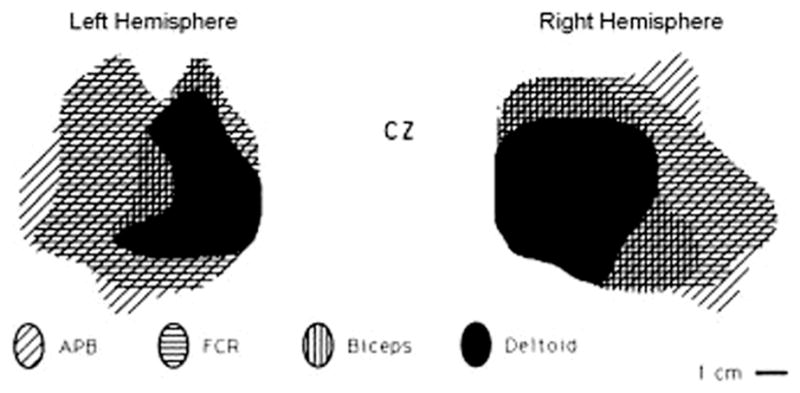
TMS mapping of upper extremity muscles in right and left sides of one normal subject after stimulation of the contralateral M1s (from Wassermann et al., 1992 – with permission). Note that hand muscles are represented more laterally than proximal arm muscles. CZ, vertex.
Fig. 11.
Cortical mapping procedure. (A) Threshold defined on the “hot spot” of the “target muscle”. (B) 4–8 stimuli for each position at intensity of MT +10%. (C) Create MEP amplitude maps and calculate the center of gravity.
Another mapping variable is the map volume, i.e., the sum of the average MEP amplitude at each location stimulated, normalized to the average MEP at the location of the largest response. This measure provides information on the overall excitability of the cortical representation but does not inform about the topography of the representation of a given muscle. The area of the MEP map increases with the intensity of TMS (Najib et al., 2011), and changes in spinal cord excitability may influence the magnitude of motor maps.
The vast majority of reports agree that upper limb/hand muscle maps are quite symmetrical in terms of spatial coordinates on the two hemispheres (Wassermann et al., 1992; Wilson et al., 1993; Cicinelli et al., 1997). This means that intra-individual interhemi-spheric mapping differences are normally small or absent, regardless of the inter-individual differences in the absolute values of such coordinates. Therefore, in case of a lesion or excitability changes affecting the motor cortical system of one hemisphere, the spatial characteristics of the maps from the hemisphere not involved in the lesion/experiment could be used as a reference for a given subject (Traversa et al., 1997, 1998), although plastic changes can impact on the unaffected hemisphere via transcallosal influences. Thus, the right/left or affected/unaffected hemispheres can be reliably compared on follow-up studies, if the mapping procedure is rigorously performed (e.g., with the use of a navigation system) and the method is kept constant (Traversa et al., 1997; Cicinelli et al., 2000).
In some circumstances, both physiological and pathological, it is possible to identify topographic differences in the localization of the CoG of a muscle representation with respect to normal and/or the unaffected hemisphere (Liepert et al., 1999). For example, a shift of the CoG of a hand muscle representation towards the face representation may indicate plastic reorganization (either “functional” or “aberrant”) in the motor cortex. The representations of different body parts, such as arm and leg, are generally separate, but there is overlap for muscle representations in the same body part, much as has been documented in experimental models and in fMRI mapping (Pascual-Leone et al., 1993; Donoghue and Sanes, 1994; Melgari et al., 2008). Motor mapping can also demonstrate the presence of weak and probably polysynaptic ipsilateral corticospinal pathways to upper extremity muscles (Wassermann et al., 1994; Ziemann et al., 1999). Additionally, CSPs can be mapped (Wassermann et al., 1993).
9. Principles of neuronavigation
Introduced some years ago, navigation systems, integrating individual brain imaging data and dedicated to TMS practice, serve several objectives (Lefaucheur, 2010): (i) to determine the exact cortical location of a TMS target; (ii) to ensure the reproducibility of TMS targeting during repeated sessions or follow-up studies; (iii) to improve the accuracy of TMS motor mapping methods; (iv) to determine the functional involvement of a cortical region (e.g., motor ability or speech), especially in the context of presurgical mapping. However, various approaches have been proposed before image-guided systems became available.
A common method to identify a brain area uses its spatial relationship with functional criteria such as motor responses or phosphenes obtained from stimulation of the primary motor or visual cortices, respectively. The position of the coil over the skull is varied until the desired effect is obtained; so the optimal stimulation site is defined by this effect. This approach was used for the localization of functional responses like speech arrest (Pascual-Leone et al., 1991), and for the detection of visual phenomena like phosphenes over the occipital cortex (Amassian et al., 1998). However, it is important to monitor and to correct the coil position to avoid the risk of not reaching the target area of the subject when non-motor brain areas represent the TMS ‘target’, or during long stimulation sessions such as for the therapeutic applications of repetitive TMS, or during mapping procedures, or when the same target brain area is to be accessed in follow-up sessions. Accurate localization of specific brain areas to stimulate requires a precise online matching system between the orientation of the coil on the scalp and the site of stimulation. To solve this problem several TMS research groups have determined the stimulation site using other strategies such as a standardized function-guided procedure (see Pascual-Leone et al., 1996, for locating the dorsolateral prefrontal cortex) or the international EEG 10–20 system [American Soc. EEG, 1991] (Rossini et al., 1987a, 1987b; Seyal et al., 1992; Walsh et al., 1998). Given the assumption that there is a consistent correlation between scalp locations and underlying brain structures, the coil is placed above a certain 10–20 position to depolarize neurons in the underlying cortex. The accuracy of these methods in finding the exact coil position related to certain cortical areas is, however, in the range of centimeters. Furthermore, these techniques do not take into account inter- and intra-individual variability of cortical anatomy, so it remains difficult to compare results from different subjects in studies using this information on coil positioning.
A more accurate solution to the problem of coil placement is offered by stereotaxic neuronavigation devices that enable the precise location of the magnetic coil with respect to the underlying brain anatomy; moreover, if MRI guided systems utilize individual MR images of that specific subject, individual brain structures can be taken into full account. The frameless stereotaxic neuronavigation system combines magnetic resonance imaging (MRI) data with TMS, guiding the coil to regions selected on the MR images (Krings et al., 1997; Ettinger et al., 1998; Boroojerdi et al., 1999; Paus, 1999; Gugino et al., 2001; Herwig et al., 2001a, 2002). The subject’s head and the MR scan are coregistered in a common reference space using a set of anatomical landmarks (such as the alar wings of the nose, the tragus of the ear and the internal angles of both eyes), visible on both the subjects MRI and on his/her head. This allows a link between MR images and real anatomy and a three-dimensional (3-D) orientation by interactive visual navigation. The 3-D position of the landmarks can be measured with a digitizing pen using a radiofrequency-based, mechanical or optical tracking system. The optical-tracking system uses a camera to measure the 3-D locations of infra-red LEDs attached on the coil and on the subject’s head, giving the possibility of tracking simultaneously the 3-D orientation and the movement of the coil and of the subject’s head.
These systems allow real-time monitoring of coil position without restraining the subject’s head during a TMS experiment in order to preserve the effective stimulation sites on the cortical surface. Stereotaxic neuronavigation can be based on the subject’s structural (anatomical) MRI, on the functional MRI obtained in the same subject, and on the use of functional neuroimaging data from the literature (“probabilistic approach”; Paus et al., 1997) or from a brain “model” based on archives of the brains of healthy subjects (e.g., Talairach and Tournoux, 1988). Stereotaxy achieves greater accuracy, of the order of a few millimeters, compared with some centimeters for the non-navigated techniques (Sparing et al., 2008). Moreover, the trial-to-trial replacement variability is downgraded close to zero. The use of neuronavigation systems reduces the variability of the induced electric fields into the brain from one TMS pulse to another (Cincotta et al., 2010). Such systems are particularly useful to target cortical regions other than the motor cortex, such as the dorsolateral prefrontal cortex (Herwig et al., 2001b; Ahdab et al., 2010; Mylius et al., 2013).
10. Stimulation of nerve roots and peripheral nerve
Conduction across proximal segments of peripheral nerves, plexuses and nerve roots is commonly tested using H reflexes and F waves. This involves peripheral nerve stimulation, with transmission of nerve volleys centrally and then distally across the body segment under study. An alternative technique involves direct stimulation of proximal structures, and this may be preferable:
In corticospinal lesions, when measuring CMCT, as discussed above.
In peripheral nerve pathology, when looking for abnormalities of impulse conduction in motor axons in proximal segments in, e.g., nerve root lesions, multifocal motor neuropathy and acquired inflammatory polyneuropathies/radiculopathies. Here the responses to the peripheral stimuli may be combined with those to TMS.
The demonstration of conduction slowing or conduction block in proximal segments can be problematic with routine diagnostic testing, and a number of techniques have been developed to stimulate motor axons proximal to common sites of pathology. Such testing may be useful particularly in plexus lesions and in radiculopathies (either compressive or inflammatory) (e.g. Fisher, 2002; Alfonsi et al., 2003; Vucic et al., 2006; Incesu et al., 2013).
Unlike the situation with transcranial stimulation, recordings made using peripheral stimulation are best done at rest. Background contraction provides no advantages: the latency and waveform are not altered by background contraction, and the EMG of the contracting muscle would represent unwanted “noise” in such recordings. A comprehensive review of electromagnetic stimulation of motor roots has been published recently, and includes a comparison with other stimulation methods (Matsumoto et al., 2013b).
Stimulation may be electrical or electromagnetic. With the former, the stimuli can be delivered through monopolar needle electrodes inserted close to the targeted structure (e.g., the sciatic nerve at the gluteal fold, plexus or a nerve root; Fisher, 2002; Vucic et al., 2006), or using the high-voltage stimulators developed for transcranial electrical stimulation (Fisher, 2002; Alfonsi et al., 2003). Electromagnetic stimulation has the advantage that it produces minor discomfort, and this can be important with relatively deep nerves, such as the femoral nerve (Bachasson et al., 2014), particularly in obese patients, or patients wearing a cast or those in whom cooperation is likely to be limited. For electrical stimulation, a Teflon-coated monopolar needle electrode is a convenient cathode, but description of this method is beyond the scope of the present document. As mentioned above, neighbors of the target muscle will be activated and will contribute to the CMAP, to a greater extent than with stimulation of the peripheral nerve more distally. Collision techniques may be necessary to eliminate this problem.
Thus, high-voltage or electromagnetic stimulation can be satisfactory alternatives to stimulation through a needle electrode for peripheral nerve abnormalities if latency is not critical and if the site of stimulation is not close to the site of pathology. Uncertainty about precisely where the stimulated axons are activated renders measures of conduction distance unreliable. With diffuse processes, the current required to activate axons will be elevated, and the site of lowest threshold may be some distance from the cathode.
11. Suggested check-list for a routine TMS clinical examination
Record age, height, current medication, and relevant clinical information and complete a check-list for safety (history of epilepsy, metal implants in the skull/scalp/head, cardiac pacemakers, spinal cord stimulators, pregnancy, etc. For a more extensive list of safety questions, see Rossi et al., 2009).
EMG electrode application: ensure a skin-electrode impedance of <10 k Ω.
Supine position (with full muscle relaxation) or seated, with eyes open in a quiet environment (any sudden noise can modify TMS excitability measures).
Demonstrate a few stimuli in the air or to the examiner’s wrist in order to familiarize the subject with stimulus.
Stimulate the scalp, scanning in search for the ‘hot spot’ with optimal coil orientation.
Define the corticomotor threshold for the MEP during relaxation and contraction (if required).
Collect and superimpose 2–3 reproducible MEPs during relaxation/contraction. Take the MEP with largest peak-to-peak amplitude for the MEP/CMAP ratio (usually 20–30% above the resting motor threshold intensity), and the MEP with shortest onset-latency for CMCT calculation.
Perform sustained contraction at about 20% of maximal force for CSP measurements; collect and superimpose 2–3 responses.
Collect CMAP of maximal amplitude during supramaximal electrical peripheral nerve stimulation and calculate the MEP/CMAP amplitude ratio.
Collect and superimpose 2–3 MEPs during spinal root stimulation.
Collect and superimpose the ‘F-waves’ during supramaximal nerve stimulation or record the response to “spinal” stimulation, to allow measurement of CMCT.
Repeat on the other side and note the interside differences of the measured parameters.
Specifically ask about and take note of any side-effect which the subject refers to the TMS procedure at the end of the session.
12. Paired-pulse stimulation
12.1. General principles of paired-pulse TMS studies
Paired-pulse TMS allows assessment of intra-cortical inhibition and facilitation. Several intracortical circuits can be tested and those discussed in this article are summarized in Table 5. It usually involves a conditioning stimulus (CS) followed by a test stimulus (TS), and the MEP amplitudes (usually peak-to-peak) or areas are compared to those produced by the TS alone as a reference (baseline/control) condition. Due to the trial-to-trial variability of MEP induced by TMS, at least 8–10 trials for each combination of CS/TS intensities and interstimulus interval (ISI) between CS and TS should be tested. The studies are usually done with the target muscle at rest. Contraction of the target muscle can strongly alter paired-pulse TMS findings. For example, contraction results in significant reduction of short interval intracortical inhibition (SICI) (Ridding et al., 1995b). Background EMG should be monitored and recorded to determine the state of muscle relaxation or level of muscle activity. In some paradigms, comprehensive examination involves testing a range of CS intensities to determine the thresholds for eliciting inhibition or facilitation. The TS intensity is set to allow observations of inhibition and facilitation, typically at amplitudes of 0.5–1 mV for hand muscles or 110–120% of resting MT. Using a test stimulus that produces MEP at the middle value of the Input–Output curve (S50) is logically optimal and allows equal amounts of inhibition and facilitation (Kukke et al., 2014).
Table 5.
Summary paired-TMS methods.
| Method | Cortical circuit
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| SICI | LICI | SICF | ICF | SIHI | LIHI | SAI | LAI | CBI | |
| Conditioning/first stimulus | Sub-threshold TMS | Supra-threshold TMS | Supra-threshold TMS | Sub-threshold TMS | Supra-threshold TMS–contra M1 | Supra-threshold TMS–contra M1 | Median nerve ES | Median nerve ES | Sub-threshold TMS-contra cerebellum |
| Test stimulus/second stimulus to M1 | Supra-threshold | Supra-threshold | Sub-threshold or threshold | Supra-threshold | Supra-threshold | Supra-threshold | Supra-threshold | Supra-threshold | Supra-threshold |
| Interstimulus interval (ms) | 1–6 | 50–200 | 1.0–1.5, 2.3–3.0, 4.1–5.0 | 8–30 | 8–12 | 40–50 | ~20–25 | ~200 | 5–8 |
| Proposed neurotransmitter/receptor | GABAA DA | GABAB | GLU GABAA | GLU NE | Not known | GABAB | ACh GABAA | Not known | Not known |
ACh = acetylcholine; CBI = cerebellar inhibition; contra = contralateral; DA = dopamine; ES = electrical stimulation; GABA = γ-aminobutyric acid; GLU = glutamate; ICF = intracortical facilitation; LAI = long latency afferent inhibition; LICI = long interval intracortical inhibition; LIHI = long latency interhemispheric inhibition; M1 = primary motor cortex; NE = norepinephrine; SAI = short latency afferent inhibition; SICF = short interval intracortical facilitation; SICI = short interval intracortical inhibition; SIHI = short latency interhemispheric inhibition.
12.2. Short interval intracortical inhibition (SICI) and intracortical facilitation (ICF)
SICI is elicited when a subthreshold CS is followed by a suprathreshold TS at an ISI of 1–6 ms (Kujirai et al., 1993) (Fig. 12) and it is due to cortical inhibition (Nakamura et al., 1997). There are two phases of SICI, peaking at ISIs of ~1 ms and 2.5 ms. SICI at 1 ms may partly be related to neuronal refractoriness but may also involve synaptic inhibition (Fisher et al., 2002; Hanajima et al., 2003; Roshan et al., 2003). SICI at 2.5 ms likely represents post-synaptic inhibition mediated by gamma-aminobutyric acid type A (GABAA) receptors because drugs that enhance GABAAergic neurotransmission increase SICI (Ziemann et al., 1996a; Di Lazzaro et al., 2007). SICI in reality reflects the balance between inhibition and facilitation. The relationship between the degree of SICI and CS intensity is a U-shaped curve (Chen et al., 1998; Ilic et al., 2002). At low CS intensities, increasing the CS intensity leads to greater SICI but further increase leads to reduction of inhibition and eventual facilitation, due to the recruitment of facilitatory circuits (Kujirai et al., 1993; Peurala et al., 2008). Therefore, SICI is a complex measure and the results from SICI studies should be interpreted carefully taking into account the different stimulus parameters. With SICI effects on I3 waves using posteriorly directed induced currents in the brain (see Section 2.2.4), the inhibition continues 20 ms or longer, which is compatible with GABAA inhibition in animals without any contamination by facilitation effects (Hanajima et al., 1998). This procedure has not been used widely because it is more complex than the usual method. There is a further issue: to standardize conditions, the intensity of the subthreshold CS is typically set as a percentage of MT. If there is a decrease in MT because cortical excitability is enhanced, the strength of the conditioning stimulus evoking inhibition will be less, and vice versa. As noted in the preceding paragraph, the relationship between CS intensity and SICI is not linear, and is not easily predicted. These issues need to be considered when comparing data from patients with control data, difference in SICI between groups being due to a CS of different intensity rather than a pathological change. As recommended below, a range of CS intensities should be used in experimental studies, even though the procedure then becomes lengthy.
Fig. 12.
EMG responses to TMS in relaxed first dorsal interosseous muscle are inhibited by a prior, subthreshold, magnetic conditioning stimulus (from Kujirai et al., 1993 –with permission). (A) Shows examples of EMG data from a single subject. The top trace shows absence of any responses to the conditioning stimulus given alone. The lower two records have two superimposed traces, the response to the test stimulus given alone, and the response to the test stimulus when given 3 ms (middle traces) or 2 ms (lower traces) after a conditioning stimulus. The larger of the two traces (dotted line) is the response to the test stimulus alone. It is dramatically suppressed at these two interstimulus intervals. Note the shorter latency of the conditioned response at an ISI = 3 ms. Each trace is the average of 10 sweeps. (B) Shows the mean (+SEM) time course of suppression in 10 subjects. At each interstimulus interval, the size of the conditioned responses is expressed as a percentage of the size of the control response. In both (A) and (B), the conditioning and test stimuli were given through the same figure-of-eight coil oriented so that electric current in the junction region flowed from anterior to posterior over the lateral part of the motor cortex.
Intracortical facilitation (ICF) can be elicited with a similar protocol as SICI but at longer ISIs of 6–30 ms (Kujirai et al., 1993). It reflects a true facilitation rather than a rebound after SICI because it has a higher threshold than SICI and a different sensitivity to change in the CS current direction (Ziemann et al., 1996b). Excitatory glutamatergic circuits in M1 may be involved (Ziemann, 2004) and it has been suggested that this form of facilitation might result from the recruitment of additional cortical circuits separate from those more easily activated by single pulse stimulation (Di Lazzaro and Rothwell, 2014). However, the physiological basis of ICF is still poorly understood (Di Lazzaro et al., 2006).
12.3. Short interval intracortical facilitation (SICF)
SICF can be elicited by a suprathreshold first stimulus followed by a second stimulus, also suprathreshold (Tokimura et al., 1996) or at RMT level (Ziemann et al., 1998). SICF occurs at three distinct phases with ISI at around 1.5, 2.9 and 4.5 ms (Ziemann et al., 1998; Chen and Garg, 2000) and is cortically mediated (Ziemann et al., 1998; Di Lazzaro et al., 1999b). It is likely due to the summation of different I-waves at corticospinal neurons (Ziemann and Rothwell, 2000; Ilic et al., 2002; Hanajima et al., 2002). The TMS intensity and ISI for eliciting SICF partly overlap with those for SICI. This may explain why SICI decreases at higher CS intensities (Peurala et al., 2008; Ni et al., 2013). A comprehensive testing protocol will initially evaluate the time course of SICF with a first stimulus that evokes an MEP of about 1 mV in a hand muscle and a second stimulus near the MT, at ISIs from 1 to 5 ms at steps of 0.2 ms. This identifies the optimal ISI for each SICF peak and trough for each individual. The SICF peaks and troughs may be tested further with a second stimulus at different intensities to identify the intensity required to evoke SICF. SICI should ideally be tested at the trough of SICF to reduce contamination by SICF. A range of CS intensities from about 0.5 active motor threshold (AMT) to about 1.4 AMT can be used to obtain SICI recruitment curve at specific ISIs. However, the testing protocol outlined above is lengthy and abbreviated protocols are needed in many experimental situations. For SICI, ISI 2.0 ms and CS intensity below AMT can generally avoid contamination with SICF. In some studies, the CS intensities may be adjusted to produce 50% of maximum inhibition to avoid floor or ceiling effects of subsequent intervention.
12.4. Long interval intracortical inhibition (LICI)
LICI refers to inhibition of a test MEP with a suprathreshold CS applied 50–200 ms prior to the TS (Valls-Solé et al., 1992; Wassermann et al., 1996; Sanger et al., 2001). LICI derives from cortical inhibition (Nakamura et al., 1997) likely mediated by GABAB receptors (Werhahn et al., 1999; McDonnell et al., 2006; Müller-Dahlhaus et al., 2008). For comprehensive testing of LICI and a subsequent late disinhibition, a range of ISIs from 50 to 300 ms should be tested. The ISIs of particular interest may be tested further with different CS intensities. For abbreviated LICI testing, ISI of 100 or 150 ms may be used. Following LICI a phenomenon of Late Cortical Disinhibition – representing a very robust period of late facilitation – has been described (Cash et al., 2010; Caux-Dedeystére et al., 2014).
12.5. Interhemispheric inhibition (IHI)
IHI is measured by delivering suprathreshold CS to M1 of one hemisphere followed by suprathreshold TS to M1 of the contralateral one. IHI is a cortical phenomenon (Di Lazzaro et al., 1999b) that is likely produced by interhemispheric excitatory pathways through the corpus callosum and synapse onto local inhibitory circuits in the target M1 (Ferbert et al., 1992; Wahl et al., 2007; Ni et al., 2009), although there might be some subcortical contribution (Gerloff et al., 1998). In certain restricted conditions, a homologous interhemispheric facilitation followed by the inhibition can be observed (Ugawa et al., 1993; Bäumer et al., 2006). IHI is most pronounced at ISI of ~10 ms and ~40–50 ms which are referred to as short and long latency IHI (SIHI and LIHI) (Chen et al., 2003; Ni et al., 2009). In addition to the interactions between the homologous M1s, IHI, particularly LIHI, represents a widespread inhibitory system projecting from various motor related cortical areas, including the dorsolateral prefrontal cortex, dorsal premotor cortex and somatosensory cortex, to the contralateral M1 (Ni et al., 2009). Pharmacological studies suggest that LIHI is mediated by post-synaptic GABAB receptors (Irlbacher et al., 2007). Interhemispheric facilitation from the premotor cortex to M1 has also been demonstrated (Bäumer et al., 2006).
12.6. Paired-associative stimulation (PAS)
A further method to study cortical functional connections involves a TMS test stimulus preceded by conditioning electrical stimulation of peripheral nerve at different ISIs.
Mariorenzi et al. (1991) first recorded MEPs from thumb flexor muscles, whilst a conditioning stimulation of median or ulnar nerve randomly preceded TMS of the opposite motor cortex (at ISIs of 10–48 ms). Conditioned MEPs compared to control MEPs were significantly attenuated at ISIs between 16 and 22 ms and were significantly increased at ISIs between 21.7 and 24.8 ms. Such effects were limited to median-innervated muscles and did not occur in ulnar-innervated muscles when the conditioning stimulus was on the median nerve. It has been theorized that the interval between muscle stretch and the onset of the transcortical, long latency electromyographic responses (LLRs) is divided into an afferent time (AT), taken at the peak of wave N20 of somatosensory evoked potentials, an efferent time (ET), calculated by means of CMCT, and a cortical interval (CI) for relaying the signal from somatosensory to motor cortex. Considering this theory, the afferent input from peripheral nerve stimulation changes the excitability of corticospinal neurons of the contralateral sensorimotor cortex during the CI (i.e., the MEP facilitation occurred with nerve stimulation-TMS intervals corresponding to the sum of AT + CI).
Given these basic assumptions, it was hypothesized that lasting excitability changes may be induced in the motor cortex by pairing median nerve stimulation with TMS over the motor cortex, because the magnetic stimulation excites the pyramidal cell indirectly through the axons of excitatory interneurons and because somatosensory inputs converge on pyramidal cells located in the motor cortex. This approach prompted the development of the Paired Associative Stimulation method (PAS), a paradigm consisting of low-frequency repetitive stimulation of the median nerve (typically 90–200 stimuli) combined with time locked TMS over the contralateral motor cortex. PAS with the interval between the two associative stimuli set at ~25 ms (PAS25) led to a strong facilitation of MEPs, whereas inhibition occurred when the interval between peripheral and cortical stimulation was reduced to about 10–15 ms (Stefan et al., 2000; Wolters et al., 2003). This bidirectional PAS-induced plasticity is reminiscent of what is observed in experimental models of associative long-term synaptic plasticity, i.e., long-term potentiation, LTP, and long-term depression, LTD (Stefan et al., 2000, 2002; Wolters et al., 2003, 2005; Classen et al., 2004). In addition, PAS-induced excitability changes followed the rules of homeostatic plasticity (Pötter-Nerger et al., 2009). On motor corticospinal output, the effects of PAS are rapid (within 30 min), persistent (>30–60 min duration), reversible and topographically specific. By investigating the effects of PAS on somatosensory and auditory evoked potentials, it has been shown that similar effects are present in the somatosensory and auditory cortices as in the motor cortex. As a general rule, the interval separating two consecutive pairs of conditioning-test stimuli (PAS frequency) can affect the pattern of motor cortical excitability changes. Usually, PAS is delivered at a relatively low frequency (0.01–0.25 Hz) (Stefan et al., 2000; Ziemann, 2004; Müller et al., 2007), but some authors showed long-lasting changes in motor cortex excitability following PAS25 applied at 5 Hz (Quartarone et al., 2006).
Pharmacological studies have demonstrated the involvement of NMDA receptors in PAS25 (Stefan et al., 2002; Wolters et al., 2003). PAS25 does not change the SICI (Rosenkranz and Rothwell, 2006; Kujirai et al., 2006; Quartarone et al., 2006), suggesting that PAS does not influence inhibition mediated by the GABAA receptor, whereas it increases the duration of the CSP recorded from a contracting muscle (Quartarone et al., 2003; Stefan et al., 2000, 2004), suggesting an influence on GABAB receptor-mediated inhibitory circuits. LTP-like effects of PAS25 were also not associated with enhanced intracortical glutamatergic transmission as revealed by lack of ICF changes (Quartarone et al., 2006). In sum, PAS25 does not affect short-latency intracortical circuits (Ni et al., 2014), but, as demonstrated by Kujirai et al. (2006), those recruited at long latencies are facilitated by PAS. Because these elements would probably correspond to those involved in the generation of late I-waves, PAS-induced plasticity would be different from theta-burst induced plasticity, which is probably based on modulation of the first I-wave (Huang et al., 2005), discussed below.
12.7. Short latency (SAI) and long latency (LAI) afferent inhibition
Cutaneous or mixed afferent input from the hand can inhibit MEPs evoked in resting muscles by TMS of the motor cortex at remarkably short latencies with a reduction of cortical excitability which has been confirmed also by epidural spinal cord recordings (Tokimura et al., 2000).
SAI is elicited if median nerve stimulation precedes contralateral M1 TMS at ISIs around the latency of the N20 component of the somatosensory evoked potential, and is followed by a period of facilitation which is likely related to long latency transcortical responses (Mariorenzi et al., 1991; Tokimura et al., 2000). Pharmacological studies showed that SAI involves cholinergic (Di Lazzaro et al., 2000) and GABAAergic (Di Lazzaro et al., 2005) circuits. SAI may be evoked with median nerve stimulation at the wrist or with digit stimulation, usually of the index or the middle finger. The intensity of sensory stimulation may be adjusted to produce a motor twitch, or at two to three times sensory threshold (Ni et al., 2011a). Maximum inhibition occurs at ISIs of about N20 latency plus 2 ms, or between 20 and 22 ms for median nerve stimulation at the wrist and about 25 ms for digit stimulation (Mariorenzi et al., 1991; Bikmullina et al., 2009a; Ni et al., 2011a). LAI is elicited when median nerve stimulation is applied before TMS at ISI around 200 ms (Chen et al., 1999a).
12.8. Thalamo-cortical inhibition induced by cerebellar stimulation (CBI)
CBI refers to inhibition of M1 when preceded by stimulation over the contralateral cerebellar hemisphere, as seen in pioneering studies with cerebellar TES. It is thought to be mediated by activation of cerebellar Purkinje cells which inhibit M1 via a disynaptic pathway through relays in the deep cerebellar nuclei and in the ventro-lateral thalamus (Ugawa et al., 1991a, 1995b; Pinto and Chen, 2001; Groiss and Ugawa, 2012). This may be related to a potential of positive polarity recorded by scalp EEG in the con-tralateral central-frontal area from cerebellar stimulation (Amassian et al., 1992). Cerebellar stimulation can be performed with a double cone coil (Ugawa et al., 1995b; Werhahn et al., 1996) placed 3 cm lateral to the inion on a line joining the inion and the external auditory meatus. The intensity of cerebellar stimulation is set at 5% below the AMT (for stimulating the pyramidal tract over the cerebellum), while M1 stimulation is performed with a figure-of-eight coil adjusted to produce MEP of about 0.5 mV in hand muscles. CBI is maximum at ISIs of 5–8 ms (Amassian et al., 1992; Ugawa et al., 1995b).
12.9. Interactions between cortical circuits
Interactions between different cortical circuits can be tested with triple stimulation paradigms. For example, LICI inhibits SICI and this interaction is likely mediated by presynaptic GABAB receptors (Sanger et al., 2001). Details of the method are described elsewhere (for a review see Ni et al., 2011b). Inputs from other cortical areas such as the premotor cortex, supplementary motor area and parietal cortices also modulate M1 excitability. Whether the effects are inhibitory or facilitatory to the M1 depends on the stimulation site, CS intensity and ISI. Neuro-navigation techniques may improve the accuracy identifying the target area outside the M1. Detailed description of these techniques are available elsewhere (Civardi et al., 2001; Koch et al., 2007; Bäumer et al., 2009; Arai et al., 2012).
13. TMS–EEG evoked cortical responses
TMS has been combined with different neuroimaging techniques (Siebner et al., 2009; Ziemann, 2011). For example, a promising tool has been introduced that permits the co-registration of the EEG activity – which has a temporal resolution of a few milliseconds and can be simultaneously sampled from a large number of scalp sites – during TMS (Fig. 13), thus providing the possibility to noninvasively probe the brain’s cortical excitability and time-resolved connectivity (Ilmoniemi et al., 1997; Virtanen et al., 1999; Ilmoniemi and Kicic, 2010). This means that combined with EEG, TMS is developing towards a brain research method in which stimulation is navigated into a desired brain area and the concurrently recorded EEG scalp potentials are processed into source images of the TMS-evoked neuronal activation (Komssi et al., 2004). Needless to say, such an approach is quite promising also for clinical applications (Julkunen et al., 2013; Ragazzoni et al., 2013; Frantseva et al., 2014; Napolitani et al., 2014; Sarasso et al., 2014).
Fig. 13.
TMS–EEG co-registration (Bonato et al., 2006 – with permission). (A) Grand average of the electroencephalographic (EEG) responses from 100 ms pre to 300 ms post-transcranial magnetic stimulation (TMS) at all scalp locations recorded during real-TMS and Sham-TMS. This figure refers to stimulation of the left primary motor cortex (MI) performed with the coil oriented 45° away from the midline and with the handle pointing backwards and laterally. The grey point indicates the site of stimulation (between F3 and C3), while the arrow indicates the orientation of the coil in respect to the stimulation site (45° to the sagittal plane). The electrode montage used for the experiment is shown at the bottom. Polarity of the waveforms is plotted with negative values upward in this and subsequent figures. The two Sham-TMS conditions (Sham 1-TMS and Sham 2-TMS) have been averaged. (B) Grand average of the EEG responses recorded at the vertex (Cz) during the real-TMS (thick solid line) and the Sham-TMS (thin solid line) conditions of the left MI performed with the coil oriented 45° away the midline and with the handle pointing backwards and laterally. Standard deviation of real TMS is also shown (dashed line). The onset of the TMS stimulus (at 0 ms) is labelled. Main features are marked in these sample waveforms for orientation. The two Sham-TMS conditions (Sham 1-TMS and Sham 2-TMS) have been averaged.
The first published attempt to measure TMS-evoked brain responses was made in 1989 by Cracco et al. (1989); in their setup, one scalp electrode was used to record EEG responses to TMS at the homologous cortical area contra-lateral to the stimulation site and it was possible to record cortico-cortically mediated activity with an onset latency of 9–12 ms (for a review see Komssi and Kähkönen, 2006). However, this approach at that time was substantially hampered by severe technical limitations related to the coupling of a strong stimulation artefact to the recording system, known already from studies with electrical stimuli (Freeman, 1971). It was necessary to overcome this difficulty. One way to suppress the stimulus artifact was to use a sample-and-hold circuit able to block the EEG signal for several milliseconds immediately adjacent to the TMS pulse, as previously suggested by electrical stimulation experiments (Freeman, 1971). This method, avoiding saturation of the recording amplifiers by the magnetic stimuli, allowed for the first time the accurate recording of multichannel EEG activity in response to TMS. With this type of amplifier TMS-evoked brain EEG responses were successfully measured in 1997 (Ilmoniemi et al., 1997; Virtanen et al., 1999). The propagation of TMS-evoked brain activity was then traced between brain areas starting a few milliseconds post-stimulus. In the following years, TMS–EEG studies have started to describe the scalp topography and investigated the possible generator sources of the TMS-evoked EEG potentials (TEPs) in order to extend our understanding of the activation mechanisms of TMS. Moreover, they have confirmed the potential of TMS–EEG as a tool for basic neuro-physiological research and possibly for diagnostic purposes (for a review see Ferreri and Rossini, 2013).
The electric currents induced in the brain by TMS can depolarize cell membranes so that voltage-sensitive ion channels are opened and action potentials are initiated. Subsequent synaptic activation is directly reflected in the EEG (Ilmoniemi et al., 1997), which records a linear projection of the postsynaptic current distribution on the lead fields of its measurement channels. EEG is not very sensitive to action potentials because of their symmetric current distribution and short duration, so it is believed that postsynaptic currents generate most of the EEG signals. If the conductivity structure of the head is taken into account, the EEG signals can be used to locate and quantify these synaptic current distributions and to make inferences on local excitability and area-to-area functional connectivity in the nervous system (Komssi et al., 2002, 2004, 2007; Massimini et al., 2005; Ferreri et al., 2011). The initial TMS-evoked response, although difficult to measure uncontaminated by artifact (Veniero et al., 2009), appears to result from the activation of the target area whereas later deflections are partially due to activity triggered by axon-propagated signals. How the signals are transmitted strongly depends on the state of the simultaneous and/or in-series firing of distributed neuromodulatory neural network (Kähkönen et al., 2001; Massimini et al., 2005), and also on local activation at the time of stimulus delivery (Ilmoniemi and Kicic, 2010; Veniero et al., 2013).
Understanding the TMS-evoked activity that is elicited at sites distant from the TMS target can benefit from knowledge of the anatomical connectivity of the brain as seen by diffusion tensor imaging (DTI) studies with MRI (Ilmoniemi and Kicic, 2010; Niskanen et al., 2011; Peters et al., 2013). TEPs are generally highly reproducible, provided that the delivery and targeting of TMS is well controlled and stable from pulse to pulse and between experiments (Casarotto et al., 2010). Several components of the EEG response to single-pulse TMS in the motor cortex have been clearly identified (for review see Komssi and Kähkönen, 2006; Ilmoniemi and Kicic, 2010; Ferreri and Rossini, 2013). In particular, single-pulse TMS over M1 is able to evoke EEG activity lasting up to 300 ms composed at the vertex of a sequence of deflections of negative polarity peaking at approximately 7, 18, 44, 100, and 280 ms, alternating with positive polarity peaks at approximately 13, 30, 60 and 190 ms post-TMS (Ilmoniemi et al., 1997; Paus et al., 2001; Komssi et al., 2002, 2004, 2007; Nikulin et al., 2003; Kähkönen et al., 2004, 2005; Kähkönen and Wilenius, 2007; Bonato et al., 2006; Daskalakis et al., 2008; Farzan et al., 2009; Lioumis et al., 2009; Mäki and Ilmoniemi, 2010a; Veniero et al., 2010; Ferreri et al., 2011, 2012; Premoli et al., 2014; Casula et al., 2014). However, these components are not invariable, the most reproducible being N7, P30, N44, P60, N100 and P180 (Table 6; Fig. 13). In addition to some inter-individual differences, the responses depend on the exact coil location and orientation (Komssi et al., 2002), on the state of the cortex (Nikulin et al., 2003; Ferreri et al., 2014b; Kundu et al., 2014) and on the vigilance of the subject (Massimini et al., 2005, 2012). In addition to standard evoked responses, TMS may also trigger oscillatory activity or perturb ongoing rhythms (Paus et al., 2001; Fuggetta et al., 2005; Van Der Werf and Paus, 2006; Van Der Werf et al., 2006; Rosanova et al., 2009; Maki and Ilmoniemi, 2010b; Veniero et al., 2011; Thut et al., 2011; Vernet et al., 2013; Garcia Dominguez et al., 2014; Shafi et al., 2014), eliciting event-related EEG synchronization/desynchronization (Pfurtscheller and Lopes da Silva, 1999).
Table 6.
TMS-evoked EEG potentials (modified and extended from Ferreri et al., 2011).
| Component | TEPs after M1 stimulation
|
|||||
|---|---|---|---|---|---|---|
| N7 | P30 | N44 | P60 | N100 | P180 | |
| Latency | 7.1 ± 2.5 | 28.8 ± 5.3 | 44.1 ± 5.8 | 62.6 ± 9.5 | 103.3 ± 19.3 | 189.7 ± 24.3 |
| Proposed neurotransmitters/receptors involved | GLU/NMDAR | GABAA | GABAA/alpha1-subunit-GABAARs | GABAB ACh | GABAB/GABABRs | GABAB |
ACh = acetylcholine; GABA = γ-aminobutyric acid; GABAARs = GABAA receptors; GABABRs = GABAB receptors; GLU = glutamate; M1 = primary motor cortex; NMDAR = N-methyl-D-aspartate receptor; TEPs = TMS-evoked cortical potentials.
13.1. TMS–EEG in studying cortical excitability, connectivity and plasticity
The usual purpose of a topographic plot of TEPs is to detect both local and distant effects of TMS, i.e., to measure both local excitability of the stimulated patch of the cortex and the propagation of TMS-evoked activity in a broader cortical network. The overall response amplitudes are usually highest under the coil and diminish with increasing distance from the stimulation point. Locally, within one hemisphere, increased EEG activity can be seen in a number of neighboring electrodes, suggesting the spread of TMS-evoked activity to anatomically interconnected cortical areas (Ilmoniemi et al., 1997; Paus et al., 2001; Ferreri et al., 2011). An important feature of TEP topography is that even though only one cortical hemisphere is stimulated, bihemispheric EEG responses are evoked with different features. TMS-evoked activity propagates from the stimulation site ipsilaterally via association fibers and contralaterally via transcallosal fibers and to subcortical structures via projection fibers (Ilmoniemi et al., 1997; Komssi et al., 2002, 2004; Ilmoniemi and Kicic, 2010; Ferreri et al., 2011, 2012, 2014b). Therefore, EEG as a measurement of cortical activity after the TMS pulse makes possible to study cortico-cortical interactions by applying TMS to one area and observing responses in remote, but interconnected areas, or to study how the activity in one area affects the ongoing activity in other areas (Ilmoniemi and Kicic, 2010; Ferreri et al., 2014b). Post-synaptic effects of the inhibitory interneurons are considered to be represented as the TMS-evoked N44 and N100 components (Nikulin et al., 2003; Bender et al., 2005; Bonato et al., 2006; Ferreri et al., 2011, 2012; Premoli et al., 2014), as it has been seen shown that inhibitory processes in deeper cortical layers can produce surface-negative potentials preferentially (Caspers et al., 1980). An important challenge in interpreting TMS results comes from the fact that the effects of brain stimulation propagate from the target site ortho-and anti-dromically in the neuronal network (Ilmoniemi and Kicic, 2010) and similarly, the stimulated area is influenced by connected areas. Because area-to-area modulation is often inhibitory, corticospinal excitability (as reflected by the magnitude of the descending volley elicited by a given cortical stimulus) does not necessarily increase with the general level of cortical activity (Ilmoniemi and Kicic, 2010).
There is growing evidence, also from stimulation of cortical areas other than the M1, that the impact of TMS on the EEG response is not only determined by the properties of the stimulus alone, but also by the initial state of the activated brain region (Ferrarelli et al., 2008; Casarotto et al., 2011). This was preliminarily demonstrated by the analysis of the features of the EEG-based pre-stimulus spectral EEG profile to test the hypothesis that fluctuations in neuronal activity have a functional significance and may account for the variability in neuronal or behavioral responses to physically identical external stimuli, such as TMS (Ilmoniemi and Kicic, 2010). Besides assessment of the general state of the brain (Kähkönen et al., 2001; Massimini et al., 2005; for a review see Massimini et al., 2012), concurrent TMS and EEG have the potential to offer insights into how brain areas interact during sensory processing (Bikmullina et al., 2009b; Ferreri et al., 2012, 2014a), cognition (Bonnard et al., 2009; Miniussi and Thut, 2010) or motor control (Nikulin et al., 2003; Kičić et al., 2008; Ferreri et al., 2011). Several recent findings open up promising possibilities to use this technique to assess directly whether and where in the cortex LTP or LTD plasticity phenomena can be induced with several different paradigms (Esser et al., 2006; Huber et al., 2008; Veniero et al., 2013). Detection of the natural frequencies of TEPs with TMS–EEG may also have diagnostic potential and clinical applications, as it opens up possibilities to map the natural frequency of different cortical areas in various neuropsychiatric conditions such as depression, schizophrenia, epilepsy, dementia or disorders of consciousness (for review see Massimini et al., 2012; Daskalakis et al., 2012; Ferreri and Rossini, 2013; Kimiskidis et al., 2014). Since natural frequencies reflect relevant circuit properties, TMS-evoked EEG may radically extend the window opened by conventional MEP recordings (Maki and Ilmoniemi, 2010a). Whereas TMS-MEP is limited to motor areas, TMS–EEG can access any cortical region (primary and associative) both in healthy and diseased subjects, and may offer a straightforward and flexible way to detect and follow-up the state of corticothalamic circuits and cortico-cortical connections.
14. Repetitive TMS
Repetitive TMS (rTMS) was introduced in 1989 using consecutive stimuli with a progressively shorter interstimulus interval as short as 10 ms (Rossini and Caramia, 1992). It needs a specific set of stimulators able to overcome the recharging time to maintain the same stimulus output with extremely brief interstimulus intervals (i.e. 10 or 20 ms). rTMS has a modulatory effect on cortical excitability, which outlasts the stimulation period and can be used in a variety of ways both in motor and non-motor brain regions and with local and nonlocal effects on brain activity.
The majority of research which has studied the effects of rTMS on cortical activity has focused on the effects of stimulation in M1, as the cortical excitability of this brain region can be easily measured. There is relative consensus that stimulation frequencies below 1 Hz are mainly inhibitory, while repetition rates of 5 Hz and beyond are mainly facilitatory at least regard to corticospinal motor output. Limited evidence as to the effect of rTMS in other brain regions has come from neuroimaging and EEG studies. These studies have been conducted predominantly in clinical groups where changes induced by stimulation may not necessarily reflect changes seen in healthy subjects. rTMS paradigms used to induce changes in cortical excitability include conventional rTMS techniques involving high (>3 Hz) and low (<1 Hz) frequency stimulation as well as newer patterned protocols such as theta burst stimulation (TBS) or quadripulse stimulation (QPS).
A substantial number of studies have explored the effects of conventional high and low frequency rTMS on cortical excitability, and there is an emerging body of research characterising the response to TBS.
14.1. High frequency rTMS
A large number of studies, especially in the early 2000s explored the effects of “high-frequency” rTMS on M1 cortical excitability (Pascual-Leone et al., 1994; see review by Fitzgerald et al. (2006)). A typical experiment would assess cortical excitability before and after the repeated application of brief, high-frequency stimulation trains, often with assessment of excitability during stimulation trials as well. These studies employed intensities of stimulation ranging from well below the resting MT to substantially above it (~150% of the resting MT). They also employed a diverse range of stimulus frequencies from 2 to 20 Hz or higher. Overall, this literature suggests that most high-frequency stimulation protocols produce an increase in cortical excitability, as measured by the size of induced MEPs within the rTMS stimulation train (Fitzgerald et al., 2006). The majority of studies also report increases in cortical excitability outlasting repeated stimulation trains although a number of studies (for example Peinemann et al., 2004), which used lower stimulus intensities, have reported no persistent post-TMS changes in cortical excitability.
There are also conflicting reports as to the effects of high-frequency rTMS on parameters of cortical inhibition (Fitzgerald et al., 2006; Jung et al., 2008). When cortical inhibition has been assessed with paired pulse TMS methods, the majority of studies have reported a decrease in SICI after high-frequency rTMS trains in healthy subjects (for example, Peinemann et al., 2000). However, the reverse (SICI increase) can be observed in pathological conditions, especially in patients with reduced SICI at baseline (Lefaucheur et al., 2006a). The CSP duration appears to be relatively increased during the stimulation train, but there is less consistency in reports on the after-effects of rTMS on CSP (Fitzgerald et al., 2006). A recent study found that greater effects on CSP duration were produced with a greater number of applied stimuli at high frequencies (i.e. 20 Hz) (de Jesus et al., 2014). Recently comments on non-linear effects or rTMS could potentially avoid the frequent assumption the more (intensity and number of stimuli) the better (Gamboa et al., 2010).
14.2. Low-frequency rTMS
In the majority of studies, low-frequency stimulation has been applied at 1 Hz, although a smaller number of studies have utilized frequencies between 0.1 and 0.9 Hz (Chen et al., 1997; Fitzgerald et al., 2006). Low frequency rTMS is often employed as a single, low-frequency stimulation train lasting 10–20 min. There have been some reports in which repeated briefer low-frequency stimulation trains were applied. Few effects of this form of low frequency stimulation have been reported within the stimulation trains but there is a moderately consistent effect of low-frequency stimulation on post-train cortical excitability, typically characterised by a reduction in MEP size (Fitzgerald et al., 2006). More reliable effects on cortical excitability are induced with higher intensities of stimulation and where stimulation trains are repeated on more than one occasion.
The effects of low frequency rTMS on cortical inhibition are not consistent (Fitzgerald et al., 2006). A number of studies have reported no changes in SICI with 1 Hz stimulation (for example Gilio et al., 2003). A large number of studies have examined the effect of 1 Hz stimulation on the CSP with highly contradictory effects reported across studies (Fitzgerald et al., 2006). Recently, de Jesus et al. (2014) reported no effect of low frequency stimulation on intracortical excitability, including inhibition, in the motor cortex.
14.3. Patterned stimulation
The most well established patterned form of TMS is the application of theta burst stimulation (TBS) (Huang et al., 2005). Other approaches, including the use of quadripulse stimuli or the inhibitory effect of continuous theta burst (Rothkegel et al., 2010), have been less evaluated even if quite promising and, for certain aspects, more stable and reliable. These also include the application of repetitive pairs of TMS pulses and the combination of high and low frequency stimulation in a ‘priming stimulation’ paradigm (Hamada et al., 2013).
TBS involves TMS. TBS consists of TMS pulses delivered as a 3-pulse 50-Hz burst applied at 5 Hz (i.e., 50 Hz burst of 3 pulses delivered every 200 ms). Intermittent TMS (iTBS) involves 600 pulses delivered as 2-s trains of TBS repeated every 10 s (i.e. 2 s of TBS followed by an 8 s rest) for about 3 min. iTBS was initially considered as one of the most effective and reliable methods of producing LTP-like plasticity in the cortex (Huang et al., 2005), By contrast, continuous application of TBS trains for 40 s (cTBS) resulted in a LTD-like decrease in motor cortex excitability, as revealed by prolonged reduction of MEP size (Huang et al., 2005). Studies of both types of patterned stimuli have suggest that they produce lasting effects on cortical excitability that exceed those seen with standard rTMS protocols (Iezzi et al., 2011). Most studies which have explored the effect of iTBS have found that it produces an increase in cortical excitability that persists in some cases considerably longer than the effects seen with high frequency rTMS (Di Lazzaro et al., 2011). In contrast, cTBS has been shown to produce long-lasting reductions in cortical excitability, although these are seen with less consistency than the effects of iTBS. Few studies have explored the effect of TBS on cortical inhibition, and these have showed heterogeneous results (Suppa et al., 2008; McAllister et al., 2009; Doeltgen and Ridding, 2011). Recently, there has also been an increasing interest in exploring alternative stimulation parameters for the application of TBS, with stimulation bursts of 3 pulses applied at intervals of 33.3 ms (30 Hz), repeating every 200 ms (5 Hz) or every 167 ms (6 Hz) (Goldsworthy et al., 2012a; Wu et al., 2012). In addition, as TBS sessions are short, there is increasing interest in exploring whether providing multiple sessions with some time spacing between them can produce greater effects on cortical excitability than single sessions alone. The application of repeated cTBS sessions at 10 min intervals appears to produce greater reductions in cortical excitability than single sessions (Goldsworthy et al., 2012b). However, this effect was not seen with iTBS or TBS at a number of time intervals in a second study (Gamboa et al., 2010, 2011). It is also important to note that there are a number of significant variables that modulate the effects of TBS (and other forms of rTMS) on cortical excitability. For example, the effect of 1 Hz stimulation appears to be modulated by time of day and cortisol secretion (Clow et al., 2014). The effect of this type of stimulation is also dependent on the type of TMS coil, the cortical induced current direction and the stimulation intensity utilized (Lang et al., 2006; Talelli et al., 2007). In addition, the induction of changes in cortical excitability appears to be substantially influenced by the induction of intrinsic cortical activity prior to or during the period of stimulation (Gentner et al., 2008). Finally, limited research has explored the reliability of induction of cortical excitability changes with TBS at different points in time: this appears to be better with iTBS (Hinder et al., 2014) than cTBS (Vernet et al., 2014). It is also worth noting that there may well be a variable response to TBS and other forms of non-invasive brain stimulation such that the expected change in cortical excitability will only be reproduced in a subset of subjects (Lopez-Alonso et al., 2014). Indeed, there is considerable intersubject variability in response to cTBS and iTBS (Hamada et al., 2013). Moreover, one must emphasize that, as for any other rTMS method, the resulting effect of a TBS protocol on cortical excitability and neural function of different areas is difficult to generalize from results obtained to the stimulation of the motor cortex in healthy subjects: TBS effects other than the “classical” iTBS/cTBS antagonism may be observed on non-motor function in patients by stimulating motor or extra-motor cortical regions (Grossheinrich et al., 2009; Borckardt et al., 2011; Lefaucheur et al., 2012). However, the promising effect of such short-duration stimulation protocols on synaptic plasticity should be really toned down at present, since it was recently demonstrated that TBS effects were highly variable between individuals, depending on differences in the interneuronal cortical networks that are preferentially recruited by the TMS pulse (Hamada et al., 2013).
Quadripulse stimulation (QPS) is another promising approach of patterned rTMS, which is thought to induce stable and reliable long-term effects (Hamada et al., 2008, 2009; Nakatani-Enomoto et al., 2012). QPS facilitates MEPs for interstimuli intervals (ISIs) of 1.5–10 ms and suppresses MEPs for ISIs of 30–100 ms. Its bidirectional effects on cortical excitability were confirmed by a neuroimaging study (Watanabe et al., 2014). The use of monophasic TMS pulses and the relatively long duration of the session (30 min) may explain its more stable effect. QPS should be a promising stimulation method for long lasting effect induction even though a few investigators have used it; more replication studies are needed.
14.4. General comments and practical considerations
Repetitive TMS is usually not a routine clinical tool; the interested Reader is referred for technical details to recent review publications (Rossi et al., 2009; Lefaucheur et al., 2014). Safety recommendations should be adhered to for the use of various forms of rTMS and patterned TMS (Rossi et al., 2009).
15. TMS in cognitive neuroscience
TMS has gained a stable and unique place among the non-invasive exploratory functional techniques for in vivo human brain investigation outside the classical motor cortex target. TMS can infer causality of a given cognitive/behavioral phenomenon, but this requires a number of “control” conditions including site (i.e., beside the “sham” stimulation of the target brain region, also the real stimulation of a non-target brain area), time and context specific effect on performance.
15.1. The “online interference” approach
The “online” concept is here developed in a wide sense, which includes both facilitation and depression of a given task performance. The unique feature of TMS in the field of cognitive neuroscience depends mainly on its ability to interact transiently with the stimulated brain area, modifying its activity and therefore interrogating its function. Its functional impact is due to the ability to impinge on neuronal function transiently (Miniussi et al., 2013), modifying information processing dependent on the activity of the involved neurons (Silvanto et al., 2008; Siebner et al., 2009). Interference with TMS is therefore complementary to more traditional neuroimaging studies based on hemodynamic (fMRI) or metabolic (PET) approaches to cognitive challenges, irrespective of the neocortical region that is being targeted. TMS has been considered an advantageous alternative to classical lesion studies in patients not only because it can be applied in healthy controls but also for a number of additional reasons, given that the use of TMS follows the rule of inference. If cortical area A is involved in cognitive process B and is not involved in process C, the alteration of the activity of area A will result in altered performance in B and not C. Thus, for deductive reasoning, area A plays a causal role in the performance of B. Additionally, discrete lesions are either chronic processes, or have chronic consequences after acute presentation. The resulting behavioral effects thus reflect the specific information provided both by the lesion itself and by the plastic adaptive changes of the surviving brain areas. Moreover, TMS can be safely repeated in subjects on different occasions, eventually allowing an intra-lab or between-lab retest of a given experimental hypothesis. Finally, in some specific fields, as memory tasks requiring a two-stage cognitive process (i.e., encoding and later retrieval of items), TMS allows teasing apart the effects on one of these two tasks more easily than in the case of lesion studies (e.g. Rossi et al., 2001, 2004). Because information processing of higher brain functions is distributed along several parallel distributed networks involving “nodes” in many cortical areas, a single pulse is often inadequate to interact with the brain activity at a behaviorally relevant level. However, the first example of TMS outside the motor cortex used single pulses to transiently suppress visual perception by stimulating the occipital cortex about 80–100 ms after the presentation of the visual stimulus (Amassian et al., 1989). Single pulses can also produce a transient “neglect” of a sensory stimulus (Oliveri et al., 1999, 2000). In this context TMS may be used as a tool to investigate and understand the role and timing of the involvement of a target area in a specific performance (Walsh and Cowey, 2000), the contribution of different sites to different aspects of a cognitive function (Robertson et al., 2003), the relative timing of the contribution of two or more areas to task performance and the function of intracortical and transcallosal connectivity (Jahanshahi and Rothwell, 2000). In short, TMS can be used to investigate what information is processed in a given brain structure, and when this processing occurs. Therefore TMS is a technique that can be used to investigate brain–behavior relationships. In general, the possibility of understanding the location, timing (i.e. cognitive chronometry) (Walsh and Pascual-Leone, 2003) and functional relevance of the neuronal activity underlying cortical functions makes TMS an essential technique mainly in perception and cognitive research. Nevertheless several clinical applications are also possible.
15.2. Cognitive mapping
Similarly to the motor cortex non-motor areas can also be mapped; for example, one can produce phosphenes in different regions of the visual field with an accuracy of 1–2 degrees of visual angle by stimulating appropriate parts of the occipital cortex (Kammer, 1999; Merabet et al., 2003).
For clinical application, navigated TMS (navigated brain stimulation, NBS) (Ruohonen and Kahru, 2010) may allow an interference-based interrogation of a specific function. In this context it has been shown that presurgical mapping of the motor homunculus is sometimes used for localization when there are tumors that can distort brain architecture. There are some data to indicate that such mapping can improve outcome (Krieg et al., 2014). NBS mapping has been used and approved by the FDA, and in this context it has been shown that NBS can be used for mapping speech-sensitive cortical areas as a valuable tool for in preoperative assessment (Picht et al., 2013). These methods are not standardized, and various language tasks can be used as well as various patterns of TMS stimulation (Tarapore et al., 2013; Rogić et al., 2014; Rösler et al., 2014). However, attention should be paid not to misinterpret the data. For example, true speech arrest of cortical origin should be different from the spreading of the induced electric field to the facial nerve, which may induce transient dysarthria (Stewart et al., 2001a).
15.3. Stimulation characteristics (frequency, intensity)
In the online application, the faster the rTMS frequency, the greater the disruption of the activity of the targeted brain region, and the greater the final behavioral effects will be (that is, unless single pulse TMS is applied at the optimal time, e.g., Amassian et al., 1989). However, with rTMS the potential risks will be greater and more prominent nonspecific behavior and attention effects will be observed, which can make the results more difficult to interpret (Rossi et al., 2009). Moreover, the effects induced by online stimulation are generally short-lived, lasting approximately a few hundred milliseconds to a few seconds. In this area, traditional methods of determining stimulation threshold have usually been used (Rossini et al., 1994). Nevertheless, in this field considering that often a non-motor area is under study, stimulation intensity is usually established as the lowest stimulation intensity that can successfully affect behavior when TMS is delivered over the area of interest, based on the literature and/or the researcher’s previous experience (Stewart et al., 2001b; Sandrini et al., 2011).
15.4. TMS as a therapeutic tool
In contrast to online and short-lived rTMS effects, rTMS may be applied for several minutes with the aim of enhancing or inhibiting cortical reactivity and to modulate the hypo- or hyperfunction of a given brain network. This approach is called offline stimulation. TMS has been suggested to facilitate recovery from several cognitive deficits in post-stroke aphasia, neglect, Alzheimer and psychiatric patients (Miniussi et al., 2008; Miniussi and Rossini, 2011; Barr et al., 2013). All these applications need further confirmation to establish the exact boundaries of their practical utility in larger cohorts of patients and following the rules of Evidence Based Medicine in double-blind, placebo-controlled and cross-over trials. The principle underlying behind these applications is that inducing changes in cortical excitability leads to a recovery or reorganisation of the functional network – including balancing the hemispheres when bihemispheric mutual control is normally present. Nevertheless, so far, further investigations are needed to determine efficacy, and whether TMS can be used to ameliorate deficits in the cognitive domain with long-lasting, clinically exploitable, effects. Moreover, an important aspect is that in some cases TMS cannot be considered the treatment by itself, but as an adjunct to other rehabilitative interventions to reduce the treatment time and potentiate the final effects.
16. Therapeutic applications of rTMS
In the next sections, several paradigmatic examples of clinical applications with rTMS for disease treatment are outlined. These are ones for which a recent evidence-based review (Lefaucheur et al., 2014) demonstrated level “A” efficacy.
17. rTMS in depression
Current State of rTMS Clinical Practice for Depression
To summarize the acute antidepressant effectiveness data, over 89 individual trials have been conducted, and 4 different large multisite trials have all found statistically and clinically significant effects of daily prefrontal rTMS for 3–6 weeks compared to sham in patients affected by major depression who did not respond to at least two antidepressant drugs. 12 meta-analyses confirm these trials. In some studies, however, non significant effects were reported. Two different devices currently have US Food and Drug (FDA) clearance for treating depression, with several others in some form of FDA pre-review.
Who should deliver TMS in Depression?
Because it is a medical therapeutic treatment with a risk of seizure, rTMS should only be performed in a medical setting under the guidance and supervision of a licensed physician (Rossi et al., 2009); meanwhile, national regulations are determining these legal boundaries. When it is being used to treat acute depression it is suggested that the medical team should include a psychiatrist (Schlaepfer et al., 2010; Carpenter et al., 2012), and that the psychiatrists who use TMS should receive training in brain stimulation methods.
Unresolved Issues
Although the literature suggests that daily prefrontal rTMS has an antidepressant effect greater than sham TMS (placebo), and that the magnitude of this effect is at least as large as antidepressant drug treatments, many issues remain unresolved. For example, it is unclear how best to deliver rTMS to treat depression. Most, but not all (Klein et al., 1999), studies have used focal coils positioned over the left prefrontal cortex. It is still not known whether rTMS over one hemisphere is better than another, or whether there are better methods for placing the coil. For the most part, the coil has been positioned using a rule-based algorithm to find the prefrontal cortex, which was adopted in the early studies (George et al., 1995). However, this method was shown to be imprecise in the particular prefrontal regions stimulated directly underneath the coil, depending largely on the subject’s head size (Herwig et al., 2001b). Many studies now employ a positioning system based on EEG coordinates, or placing the treatment coil at least 6 cm anterior to the thumb motor area, rather than the “standard” 5 cm (Johnson et al., 2013). It may be that 7 cm is even better (Beam et al., 2009). Two retrospective analyses of clinical trials where brain imaging was performed to document the coil location have independently confirmed that a coil position that is anterior and lateral is associated with a better clinical response (Herbsman et al., 2009; Johnson et al., 2013). Another group has performed a randomized controlled trial examining different prefrontal locations and a more anterior and lateral location did indeed produce a superior antidepressant response (Fitzgerald et al., 2009). These findings suggest that the location of the coil matters, even within broad boundaries of a specific lobe. It is not clear whether individualized location via neuronavigated methods will be needed for optimizing response, or whether general algorithms will suffice for a probabilistic positioning for most patients. Stimulation intensity and duration of series are other unresolved parameters in rTMS for depression. There is now increasing recognition that higher intensities of stimulation are needed to reach the prefrontal cortex, especially in elderly patients, where atrophy of the prefrontal cortex may outpace that of the motor cortex. Stimulation intensity is often based on MT even when a systematic study on the intensity (dose)/effect has not been carried out (Kozel et al., 2000; McConnell et al., 2001; Mosimann et al., 2002; Padberg et al., 2002). Data indicate that rTMS therapeutic effects likely develop over several weeks. Consequently, many of the initial trials, which lasted only 1–2 weeks, were probably too brief to generate maximum clinical antidepressant effects.
There is limited data on using rTMS as a maintenance treatment in depression (Nahas et al., 2000). Pilot findings suggest that rTMS might eventually be used as a maintenance tool in depression, and that one treatment per week might be a good first attempt at a maintenance schedule. Several groups have performed maintenance rTMS, but there have been no controlled clinical trials, and optimal ways of using rTMS to prevent relapse remain to be defined (Li et al., 2004; O’Reardon et al., 2005). Another interesting concept is the use of maintenance TMS following acute series or maintenance Electro Convulsive Therapy (ECT) in patients who require maintenance treatment above psychopharmacology, but for whom adverse cognitive effects, procedural side effects, fear of or intolerance of anesthesia, concurrent medication limitations, geographic distance or lifestyle infringements make ECT difficult or impossible.
18. rTMS in neuropathic pain
A large review was recently published (see Lefaucheur et al., 2014). The present section only concerns chronic neuropathic or non-neuropathic pain and not the use of rTMS to relieve provoked acute or experimental pain, which has also been reviewed elsewhere (Mylius et al., 2012). Because epidural stimulation of the motor cortex through surgically-implanted electrodes was used in the early nineties to treat drug-resistant neuropathic pain (Tsubokawa et al., 1991), rTMS therapy for pain relief was also targeted to the motor cortex (M1), or more precisely the precentral gyrus. Since the first report (Lefaucheur et al., 2001a), about 20 original placebo-controlled studies including at least 10 patients who received active low frequency (LF) or high frequency (HF) rTMS of M1 have been published to date, covering about 700 patients. Stimulation was always applied to the motor (precentral) cortex of the hemisphere contralateral to pain, usually the area corresponding homotopically to the painful zone. First, all studies consistently reported the absence of any significant analgesic effect of LF rTMS of M1 delivered contralateral to the pain side (Lefaucheur et al., 2001a, 2006b, 2008, 2014; André-Obadia et al., 2006; Saitoh et al., 2007). Conversely, HF rTMS delivered over the same target was found to produce significant pain-relieving effects (pain relief >30% in 46–62% of patients and >50% pain relief in 29%). These effects are optimal 2–4 days after a single rTMS session (Lefaucheur et al., 2001b). However, for a therapeutic application, repeated rTMS sessions are needed. Two studies clearly showed long-lasting pain relief following a 5-day protocol of 20 Hz rTMS of M1 in patients with post-stroke pain (Khedr et al., 2005), trigeminal neuropathic pain (Khedr et al., 2005), and phantom limb pain due to amputation (Ahmed et al., 2011). Finally, the largest study to date, by Hosomi et al. (2013), was based on a 10-day protocol of 5 Hz rTMS of M1 in a multicenter series of 64 patients with chronic neuropathic pain of various origins. They found modest but significant pain reduction following active vs. sham rTMS, but they used a rather low frequency of stimulation (5 Hz) and a limited number of pulses (500) per session.
Although an analgesic effect of rTMS of M1 has been demonstrated, there are still many open questions before rTMS can be used in the treatment of chronic pain in daily clinical practice. The two main issues are the design of a maintenance protocol for long-term therapy and the method of determining the optimal target within the precentral gyrus; in fact, the location of the best target over the precentral gyrus with respect to the clinical presentation remains challenging (Lefaucheur et al., 2004b, 2006b). Only a few studies have used neuronavigation in this context (Hirayama et al., 2006; Lefaucheur et al., 2012), and there are arguments to suggest that diffusion tensor fiber tracking, in particular, could be of interest for this purpose (Goto et al., 2008; Ohn et al., 2012). Several rTMS target locations have been assessed for their ability to produce neuropathic pain relief, but M1 was found to be preferable to premotor or primary somatosensory cortical regions (Hirayama et al., 2006; Saitoh et al., 2006). However, stimulation of the dorsolateral prefrontal cortex (DLPFC) remains to be studied, according to the proven efficacy of this target in depression and the well-known relation between depression and chronic pain. Apart from being a therapeutic tool for neuropathic pain, rTMS can also be used as a method to select candidates for neurosurgically implanted cortical stimulation. It has been shown that a response to HF rTMS of M1 can predict a positive outcome after implantation (Lefaucheur et al., 2004a, 2011; André-Obadia et al., 2008, 2014; Hosomi et al., 2008). Therefore, it is good practice to perform rTMS tests before considering chronic cortical stimulation.
Regarding non-neuropathic pain conditions, sham-controlled results obtained in large series of patients and replicated by independent groups are lacking. In complex regional pain syndrome type 1, there are two sham-controlled studies showing a significant reduction of pain intensity following HF rTMS of M1, but outlasting stimulation for only a short time on average (Pleger et al., 2004; Picarelli et al., 2010). In fibromyalgia, two groups have reported prolonged effects of repeated HF rTMS sessions with a maintenance protocol, both on pain and quality of life, up to several months, but the target was the left M1 in one group (Mhalla et al., 2011) and the left DLPFC in the other group (Short et al., 2011). In migraine, HF rTMS of the left M1 (Misra et al., 2012, 2013) or the left DLPFC (Brighina et al., 2004) has also been assessed in sham-controlled studies showing a significant decrease in the frequency and intensity of migraine attacks. However, these promising results remain to be replicated by other groups (Conforto et al., 2014). It should also be noted that “conventional” rTMS protocols must be distinguished from devices delivering single or double TMS shocks over the occipital cortex during the aura of a migraine attack (Lipton et al., 2010). Despite rather debatable results, favorable recommendations have been recently issued on these devices for clinical application with FDA approval this year. Finally, one group showed that LF rTMS (1 Hz) delivered to the right secondary somatosensory cortex could provide pain relief in patients with visceral pain secondary to chronic pancreatitis (Fregni et al., 2005, 2011). Again, these results await replication in larger placebo-controlled studies.
Although rTMS represents a promising therapeutic tool in chronic pain, with proven efficacy, especially for M1 stimulation in neuropathic origin, further research is still needed to compare the respective value of various cortical targets, depending on the side and frequency of stimulation and the clinical presentation, with respect to the location and the respective sensory-discriminant and affective-emotional components of pain. A personalized approach should reduce the very high variability in rTMS analgesic response between individuals.
19. Conclusions and final remarks
This report aimed to provide the Reader with a comprehensive and updated knowledge on the basic mechanisms and practical applications of various forms of non-invasive, electromagnetic stimulation of the central and peripheral nervous systems and on the use of TMS as an adjunctive therapy of diseases resistant or only partly responding to drug treatments. An extended bibliography is also listed.
The same philosophy of the previous 1994 document has been implemented in the attempt to create a handout of value both for routine daily examinations and for building a research protocol. Other valuable reviews have been published in recent years on several of the topics covered in this publication. This document will expand these offerings by taking advantage of a worldwide panel of experts covering all the various facets of the complex mosaic of non-invasive brain stimulation. We hope that reading this document will be as interesting and as much fun as it was for the authors to assemble it in its present form.
HIGHLIGHTS.
This review is an up-date document on basic aspects of non-invasive stimulation of brain, spinal cord and nerve roots.
The main physiological, theoretical and methodological features of transcranial magnetic stimulation (TMS) are described.
Instructions for practical use of non-invasive stimulation in clinical applications and research are provided.
Footnotes
Conflict of interest
This work was not sponsored.
None of the authors have declared any conflict of interest.
References
- Abbruzzese G, Schenone A, Scramuzza G. Impairment of central motor conduction in diabetic patients. Electroencephalogr Clin Neurophysiol. 1993;89:335–40. doi: 10.1016/0168-5597(93)90073-x. [DOI] [PubMed] [Google Scholar]
- Adrian ED, Moruzzi G. Impulses in the pyramidal tract. J Physiol. 1939;97:153–99. doi: 10.1113/jphysiol.1939.sp003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahdab R, Ayache SS, Brugières P, Goujon C, Lefaucheur JP. Comparison of “standard” and “navigated” procedures of TMS coil positioning over motor, premotor and prefrontal targets in patients with chronic pain and depression. Neurophysiol Clin. 2010;40:27–36. doi: 10.1016/j.neucli.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Ahmed MA, Mohamed SA, Sayed D. Long-term antalgic effects of repetitive transcranial magnetic stimulation of motor cortex and serum beta endorphin in patients with phantom pain. Neurol Res. 2011;33:953–8. doi: 10.1179/1743132811Y.0000000045. [DOI] [PubMed] [Google Scholar]
- Alfonsi E, Merlo IM, Clerici AM, Candeloro E, Marchioni E, Moglia A. Proximal nerve conduction by high-voltage electrical stimulation in S1 radiculopathies and acquired demyelinating neuropathies. Clin Neurophysiol. 2003;114:239–47. doi: 10.1016/s1388-2457(02)00331-0. [DOI] [PubMed] [Google Scholar]
- Amassian VE, Stewart M, Quirk GJ, Rosenthal JL. Physiological basis of motor effects of a transient stimulus to cerebral cortex. Neurosurgery. 1987;20:74–93. [PubMed] [Google Scholar]
- Amassian VE, Cracco RQ, Maccabee PJ, Cracco JB, Rudell A, Eberle L. Suppression of visual perception by magnetic coil stimulation of human occipital cortex. Electroencephalogr Clin Neurophysiol. 1989;74:458–62. doi: 10.1016/0168-5597(89)90036-1. [DOI] [PubMed] [Google Scholar]
- Amassian VE, Cracco RQ, Maccabee PJ, Cracco JB. Cerebello-frontal cortical projections in humans studied with the magnetic coil. Electroencephalogr Clin Neurophysiol. 1992;85:265–72. doi: 10.1016/0168-5597(92)90115-r. [DOI] [PubMed] [Google Scholar]
- Amassian VE, Cracco RQ, Maccabee PJ, Cracco JB, Rudell AP, Eberle L. Transcranial magnetic stimulation in study of the visual pathway. J Clin Neurophysiol. 1998;15:288–304. doi: 10.1097/00004691-199807000-00002. [DOI] [PubMed] [Google Scholar]
- American Electroencephalographic Society guidelines for standard electrode position nomenclature. J Clin Neurophysiol. 1991;8:200–2. [PubMed] [Google Scholar]
- André-Obadia N, Peyron R, Mertens P, Mauguiere F, Laurent B, Garcia-Larrea L. Transcranial magnetic stimulation for pain control. Double-blind study of different frequencies against placebo, and correlation with motor cortex stimulation efficacy. Clin Neurophysiol. 2006;117:1536–44. doi: 10.1016/j.clinph.2006.03.025. [DOI] [PubMed] [Google Scholar]
- André-Obadia N, Mertens P, Gueguen A, Peyron R, Garcia-Larrea L. Pain relief by rTMS: differential effect of current flow but no specific action on pain subtypes. Neurology. 2008;71:833–40. doi: 10.1212/01.wnl.0000325481.61471.f0. [DOI] [PubMed] [Google Scholar]
- André-Obadia N, Mertens P, Lelekov-Boissard T, Afif A, Magnin M, Garcia-Larrea L. Is Life better after motor cortex stimulation for pain control? Results at long-term and their prediction by preoperative rTMS. Pain Physician. 2014;17:53–62. [PubMed] [Google Scholar]
- Arai N, Okabe S, Furubayashi T, Mochizuki H, Iwata NK, Hanajima R, et al. Differences in after-effect between monophasic and biphasic high-frequency rTMS of the human motor cortex. Clin Neurophysiol. 2007;118:2227–33. doi: 10.1016/j.clinph.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Arai N, Lu MK, Ugawa Y, Ziemann U. Effective connectivity between human supplementary motor area and primary motor cortex: a paired-coil TMS study. Exp Brain Res. 2012;220:79–87. doi: 10.1007/s00221-012-3117-5. [DOI] [PubMed] [Google Scholar]
- Awiszus F. TMS and threshold hunting. Suppl Clin Neurophysiol. 2003;56:13–23. doi: 10.1016/s1567-424x(09)70205-3. [DOI] [PubMed] [Google Scholar]
- Awiszus F. Fast estimation of transcranial magnetic stimulation motor threshold: is it safe? Brain Stimul. 2011;4:58–9. doi: 10.1016/j.brs.2010.09.004. discussion 60–63. [DOI] [PubMed] [Google Scholar]
- Awiszus F. On relative frequency estimation of transcranial magnetic stimulation motor threshold. Clin Neurophysiol. 2012;123:2319–20. doi: 10.1016/j.clinph.2012.04.014. [DOI] [PubMed] [Google Scholar]
- Bachasson D, Temesi J, Bankole C, Lagrange E, Boutte C, Millet GY, et al. Assessement of quadriceps strength, endurance and fatigue in FSHD and CMT: benefits and limits of femoral nerve magnetic stimulation. Clin Neurophysiol. 2014;125:396–405. doi: 10.1016/j.clinph.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Baker SN, Curio G, Lemon RN. EEG oscillations at 600 Hz are macroscopic markers for cortical spike bursts. J Physiol. 2003;550:529–34. doi: 10.1113/jphysiol.2003.045674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1:1106–7. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- Barker AT, Freeston IL, Jalinous R, Jarrat JA. Magnetic stimulation of the human brain and peripheral nervous system: an introduction and the results of an initial clinical evaluation. Neurosurgery. 1987;20:100–9. doi: 10.1097/00006123-198701000-00024. [DOI] [PubMed] [Google Scholar]
- Barr MS, Farzan F, Rajji TK, Voineskos AN, Blumberger DM, Arenovich T, et al. Can repetitive magnetic stimulation improve cognition in schizophrenia? Pilot data from a randomized controlled trial. Biol Psychiatry. 2013;73:510–7. doi: 10.1016/j.biopsych.2012.08.020. [DOI] [PubMed] [Google Scholar]
- Bashir S, Perez JM, Horvath JC, Pascual-Leone A. Differentiation of motor cortical representation of hand muscles by navigated mapping of optimal TMS current directions in healthy subjects. J Clin Neurophysiol. 2013;30:390–5. doi: 10.1097/WNP.0b013e31829dda6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäumer T, Bock F, Koch G, Lange R, Rothwell JC, Siebner HR, et al. Magnetic stimulation of human premotor or motor cortex produces interhemispheric facilitation through distinct pathways. J Physiol. 2006;572:857–68. doi: 10.1113/jphysiol.2006.104901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäumer T, Schippling S, Kroeger J, Zittel S, Koch G, Thomalla G, et al. Inhibitory and facilitatory connectivity from ventral premotor to primary motor cortex in healthy humans at rest–a bifocal TMS study. Clin Neurophysiol. 2009;120:1724–31. doi: 10.1016/j.clinph.2009.07.035. [DOI] [PubMed] [Google Scholar]
- Beam W, Borckardt JJ, Reeves ST, George MS. An efficient and accurate new method for locating the F3 position for prefrontal TMS applications. Brain Stimul. 2009;2:50–4. doi: 10.1016/j.brs.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender S, Basseler K, Sebastian I, Resch F, Kammer T, Oelkers-Ax R, et al. Electroencephalographic response to transcranial magnetic stimulation in children: Evidence for giant inhibitory potentials. Ann Neurol. 2005;58:58–67. doi: 10.1002/ana.20521. [DOI] [PubMed] [Google Scholar]
- Berardelli A, Inghilleri M, Cruccu G, Manfredi M. Descending volley after electrical and magnetic transcranial stimulation in man. Neurosci Lett. 1990;112:54–8. doi: 10.1016/0304-3940(90)90321-y. [DOI] [PubMed] [Google Scholar]
- Bergmann TO, Mölle M, Schmidt MA, Lindner C, Marshall L, Born J, et al. EEG-guided transcranial magnetic stimulation reveals rapid shifts in motor cortical excitability during the human sleep slow oscillation. J Neurosci. 2012;32:243–53. doi: 10.1523/JNEUROSCI.4792-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J. Functional MRI of the immediate impact of transcranial magnetic stimulation on cortical and subcortical motor circuits. Eur J Neurosci. 2004;19:1950–62. doi: 10.1111/j.1460-9568.2004.03277.x. [DOI] [PubMed] [Google Scholar]
- Bikmullina R, Bäumer T, Zittel S, Munchau A. Sensory afferent inhibition within and between limbs in humans. Clin Neurophysiol. 2009a;120:610–8. doi: 10.1016/j.clinph.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Bikmullina R, Kičić D, Carlson S, Nikulin VV. Electrophysiological correlates of short-latency afferent inhibition: a combined EEG and TMS study. Exp Brain Res. 2009b;194:517–26. doi: 10.1007/s00221-009-1723-7. [DOI] [PubMed] [Google Scholar]
- Bonato C, Miniussi C, Rossini PM. Transcranial magnetic stimulation and cortical evoked potentials: a TMS/EEG co-registration study. Clin Neurophysiol. 2006;117:1699–707. doi: 10.1016/j.clinph.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Bonnard M, Spieser L, Meziane HB, de Graaf JB, Pailhous J. Prior intention can locally tune inhibitory processes in the primary motor cortex: direct evidence from combined TMS–EEG. Eur J Neurosci. 2009;30:913–23. doi: 10.1111/j.1460-9568.2009.06864.x. [DOI] [PubMed] [Google Scholar]
- Borckardt JJ, Reeves ST, Beam W, Jensen MP, Gracely RH, Katz S, et al. A randomized, controlled investigation of motor cortex transcranial magnetic stimulation (TMS) effects on quantitative sensory measures in healthy adults: evaluation of TMS device parameters. Clin J Pain. 2011;27:486–94. doi: 10.1097/AJP.0b013e31820d2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boroojerdi B, Foltys H, Krings T, Spetzger U, Thron A, Topper R. Localization of the motor hand area using transcranial magnetic stimulation and functional magnetic resonance imaging. Clin Neurophysiol. 1999;110:699–704. doi: 10.1016/s1388-2457(98)00027-3. [DOI] [PubMed] [Google Scholar]
- Boroojerdi B, Battaglia F, Muellbacher W, Cohen LG. Mechanisms influencing stimulus-response properties of the human corticospinal system. Clin Neurophysiol. 2001;112:931–7. doi: 10.1016/s1388-2457(01)00523-5. [DOI] [PubMed] [Google Scholar]
- Boyd SG, Rothwell JC, Cowan JM, Webb PJ, Morley T, Asselman P, et al. A method of monitoring function in corticospinal pathways during scoliosis surgery with a note on motor conduction velocities. J Neurol Neurosurg Psychiatry. 1986;49:251–7. doi: 10.1136/jnnp.49.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil-Neto JP, Cammarota A, Valls-Solé J, Pascual-Leone A, Hallett M, Cohen LG. Role of intracortical mechanisms in the late part of the silent period to transcranial stimulation of the human motor cortex. Acta Neurol Scand. 1995;92:383–6. doi: 10.1111/j.1600-0404.1995.tb00151.x. [DOI] [PubMed] [Google Scholar]
- Brighina F, Piazza A, Vitello G, Aloisio A, Palermo A, Daniele O, et al. RTMS of the prefrontal cortex in the treatment of chronic migraine: a pilot study. J Neurol Sci. 2004;227:67–71. doi: 10.1016/j.jns.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Burke D, Hicks RG, Stephen JPH. Corticospinal volleys evoked by anodal and cathodal stimulation of the human motor cortex. J Physiol. 1990;425:283–99. doi: 10.1113/jphysiol.1990.sp018103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Hicks R, Gandevia SC, Stephen J, Woodforth I, Crawford M. Direct comparison of corticospinal volleys in human subjects to transcranial magnetic and electrical stimulation. J Physiol (Lond) 1993;470:383–93. doi: 10.1113/jphysiol.1993.sp019864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Bartley K, Woodforth IJ, Yakoubi A, Stephen JPH. The effects of a volatile anaesthetic on the excitability of human corticospinal axons. Brain. 2000;123:992–1000. doi: 10.1093/brain/123.5.992. [DOI] [PubMed] [Google Scholar]
- Caramia MD, Desiato MT, Cicinelli P, Iani C, Rossini PM. Latency jump of “relaxed” versus “contracted” motor evoked potentials as a marker of cortico-spinal maturation. Electroencephalogr Clin Neurophysiol. 1993;89:616. doi: 10.1016/0168-5597(93)90086-5. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Janicak PG, Aaronson ST, Boyadjis T, Brock DG, Cook IA, et al. Transcranial Magnetic Stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depress Anxiety. 2012;29:587–96. doi: 10.1002/da.21969. [DOI] [PubMed] [Google Scholar]
- Casarotto S, Romero Lauro LJ, Bellina V, Casali AG, Rosanova M, Pigorini A, et al. EEG responses to TMS are sensitive to changes in the perturbation parameters and repeatable over time. PLoS One. 2010;5:e10281. doi: 10.1371/journal.pone.0010281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casarotto S, Määttä S, Herukka SK, Pigorini A, Napolitani M, Gosseries O, et al. Transcranial magnetic stimulation-evoked EEG/cortical potentials in physiological and pathological aging. Neuroreport. 2011;22:592–7. doi: 10.1097/WNR.0b013e328349433a. [DOI] [PubMed] [Google Scholar]
- Cash RF, Ziemann U, Murray K, Thickbroom GW. Late cortical disinhibition in human motor cortex: a triple-pulse transcranial magnetic stimulation study. J Neurophysiol. 2010;103:511–8. doi: 10.1152/jn.00782.2009. [DOI] [PubMed] [Google Scholar]
- Caspers H, Speckmann EJ, Lehmenkuhler A. Electrogenesis of cortical DC potentials. Prog Brain Res. 1980;54:3–15. doi: 10.1016/S0079-6123(08)61603-9. [DOI] [PubMed] [Google Scholar]
- Casula EP, Tarantino V, Basso D, Arcara G, Marino G, Toffolo GM, et al. Low-frequency rTMS inhibitory effects in the primary motor cortex: insights from TMS-evoked potentials. Neuroimage. 2014;98:225–32. doi: 10.1016/j.neuroimage.2014.04.065. [DOI] [PubMed] [Google Scholar]
- Caux-Dedeystère A, Rambour M, Duhamel A, Cassim F, Derambure P, Devanne H. Task-dependent changes in late inhibitory and disinhibitory actions within the primary motor cortex in humans. Eur J Neurosci. 2014;39:1485–90. doi: 10.1111/ejn.12505. [DOI] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, et al. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- Chen R, Tam A, Bütefisch C, Corwell B, Ziemann U, Rothwell JC, et al. Intracortical inhibition and facilitation in different representations of the human motor cortex. J Neurophysiol. 1998;80:2870–81. doi: 10.1152/jn.1998.80.6.2870. [DOI] [PubMed] [Google Scholar]
- Chen R, Corwell B, Hallett M. Modulation of motor cortex excitability by median nerve and digit stimulation. Exp Brain Res. 1999a;129:77–86. doi: 10.1007/s002210050938. [DOI] [PubMed] [Google Scholar]
- Chen R, Lozano AM, Ashby P. Mechanism of the silent period following transcranial magnetic stimulation. Evidence from epidural recordings. Exp Brain Res. 1999b;128:539–42. doi: 10.1007/s002210050878. [DOI] [PubMed] [Google Scholar]
- Chen R, Garg R. Facilitatory I wave interaction in proximal arm and lower limb muscle representations of the human motor cortex. J Neurophysiol. 2000;83:1426–34. doi: 10.1152/jn.2000.83.3.1426. [DOI] [PubMed] [Google Scholar]
- Chen R, Yung D, Li J-Y. Organization of ipsilateral excitatory and inhibitory pathways in the human motor cortex. J Neurophysiol. 2003;89:1256–64. doi: 10.1152/jn.00950.2002. [DOI] [PubMed] [Google Scholar]
- Chu NS. Motor evoked potentials with magnetic stimulation: correlations with height. Electroencephalogr Clin Neurophysiol. 1989;74:481–5. doi: 10.1016/0168-5597(89)90039-7. [DOI] [PubMed] [Google Scholar]
- Cicinelli P, Traversa R, Bassi A, Scivoletto G, Rossini PM. Interhemispheric differences of hand muscle representation in human motor cortex. Muscle Nerve. 1997;20:535–42. doi: 10.1002/(sici)1097-4598(199705)20:5<535::aid-mus1>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Cicinelli P, Mattia D, Spanedda F, Traversa R, Marciani MG, Pasqualetti P, et al. Transcranial magnetic stimulation reveals an interhemispheric asymmetry of cortical inhibition in focal epilepsy. Neuroreport. 2000;11:701–7. doi: 10.1097/00001756-200003200-00010. [DOI] [PubMed] [Google Scholar]
- Cincotta M, Giovannelli F, Borgheresi A, Balestrieri F, Toscani L, Zaccara G, et al. Optically tracked neuronavigation increases the stability of hand-held focal coil positioning: evidence from “transcranial” magnetic stimulation-induced electrical field measurements. Brain Stimul. 2010;3:119–23. doi: 10.1016/j.brs.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Civardi C, Cantello R, Asselman P, Rothwell JC. Transcranial magnetic stimulation can be used to test connections to primary motor areas from frontal and medial cortex in humans. Neuroimage. 2001;14:1444–53. doi: 10.1006/nimg.2001.0918. [DOI] [PubMed] [Google Scholar]
- Classen J, Wolters A, Stefan K, Wycislo M, Sandbrink F, Schmidt A, et al. Paired associative stimulation. Suppl Clin Neurophysiol. 2004;57:563–9. [PubMed] [Google Scholar]
- Claus D. Central motor conduction: method and normal results. Muscle Nerve. 1990;13:1125–32. doi: 10.1002/mus.880131207. [DOI] [PubMed] [Google Scholar]
- Clow A, Law R, Evans P, Vallence AM, Hodyl NA, Goldsworthy MR, et al. Day differences in the cortisol awakening response predict day differences in synaptic plasticity in the brain. Stress. 2014;17:219–23. doi: 10.3109/10253890.2014.905533. [DOI] [PubMed] [Google Scholar]
- Conforto AB, Amaro E, Jr, Gonçalves AL, Mercante JP, Guendler VZ, Ferreira JR, et al. Randomized, proof-of-principle clinical trial of active transcranial magnetic stimulation in chronic migraine. Cephalalgia. 2014;34:464–72. doi: 10.1177/0333102413515340. [DOI] [PubMed] [Google Scholar]
- Cowan JM, Rothwell JC, Dick JP, Thompson PD, Day BL, Marsden CD. Abnormalities in central motor pathway conduction in multiple sclerosis. Lancet. 1984;2:304–7. doi: 10.1016/s0140-6736(84)92683-7. [DOI] [PubMed] [Google Scholar]
- Cowan JM, Day BL, Marsden C, Rothwell JC. The effect of percutaneous motor cortex stimulation on H reflexes in muscles of the arm and leg in intact man. J Physiol (Lond) 1986;377:333–47. doi: 10.1113/jphysiol.1986.sp016190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cracco RQ, Amassian VE, Maccabee PJ, Cracco JB. Comparison of human transcallosal responses evoked by magnetic coil and electrical stimulation. Electroencephalogr Clin Neurophysiol. 1989;74:417–24. doi: 10.1016/0168-5597(89)90030-0. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Farzan F, Barr MS, Maller JJ, Chen R, Fitzgerald PB. Long-interval cortical inhibition from the dorsolateral prefrontal cortex: a TMS–EEG study. Neuropsychopharmacology. 2008;33:2860–9. doi: 10.1038/npp.2008.22. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Farzan F, Radhu N, Fitzgerald PB. Combined transcranial magnetic stimulation and electroencephalography: its past, present and future. Brain Res. 2012;1463:93–107. doi: 10.1016/j.brainres.2012.04.045. [DOI] [PubMed] [Google Scholar]
- Davey NJ, Smith HC, Savic G, Maskill DW, Ellaway PH, Frankel HL. Comparison of input–output patterns in the corticospinal system of normal subjects and incomplete spinal cord injured patients. Exp Brain Res. 1999;127:382–90. doi: 10.1007/s002210050806. [DOI] [PubMed] [Google Scholar]
- Day BL, Rothwell JC, Thompson PD, Dick JP, Cowan JM, Berardelli A, et al. Motor cortex stimulation in intact man. [2] Multiple descending volleys. Brain. 1987;110:1191–209. doi: 10.1093/brain/110.5.1191. [DOI] [PubMed] [Google Scholar]
- Day BL, Dressler D, Maertens de Noordhout A, Marsden CD, Nakashima K, Rothwell JC, et al. Electric and magnetic stimulation of human motor cortex: surface EMG and single motor unit responses. J Physiol (Lond) 1989;412:449–73. doi: 10.1113/jphysiol.1989.sp017626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan E, Censor N, Buch ER, Sandrini M, Cohen LG. Noninvasive brain stimulation: from physiology to network dynamics and back. Nat Neurosci. 2013;16:838–44. doi: 10.1038/nn.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jesus DR, Favalli GP, Hoppenbrouwers SS, Barr MS, Chen R, Fitzgerald PB, et al. Determining optimal rTMS parameters through changes in cortical inhibition. Clin Neurophysiol. 2014;125:755–62. doi: 10.1016/j.clinph.2013.09.011. [DOI] [PubMed] [Google Scholar]
- Deng ZD, Lisanby SH, Peterchev AV. Coil design considerations for deep transcranial magnetic stimulation. Clin Neurophysiol. 2014;125:1202–12. doi: 10.1016/j.clinph.2013.11.038. See comment in PubMed Commons below. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmedt JE. Size principle of motoneuron recruitment and the calibration of muscle force and speed in man. Adv Neurol. 1983;39:227–51. [PubMed] [Google Scholar]
- Devanne H, Lavoie BA, Capaday C. Input–output properties and gain changes in the human corticospinal pathway. Exp brain res. 1997;114:329–38. doi: 10.1007/pl00005641. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Molinari M, Leggio MG, Nardone R, Fogli D, et al. Excitability of the motor cortex to magnetic stimulation in patients with cerebellar lesions. J Neurol Neurosurg Psychiatry. 1994;57:108–10. doi: 10.1136/jnnp.57.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, et al. Effects of voluntary contraction on descending volleys evoked by transcranial stimulation in conscious humans. J Physiol. 1998;508:625–33. doi: 10.1111/j.1469-7793.1998.625bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Ferrara L, Saturno E, Pilato F, et al. The diagnostic value of motor evoked potentials. Clin Neurophysiol. 1999a;110:1297–307. doi: 10.1016/s1388-2457(99)00060-7. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Rothwell JC, Oliviero A, Profice P, Insola A, Mazzone P, et al. Intracortical origin of the short latency facilitation produced by pairs of threshold magnetic stimuli applied to human motor cortex. Exp Brain Res. 1999b;129:494–9. doi: 10.1007/s002210050919. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Pennisi MA, Di Giovanni S, Zito G, et al. Muscarinic receptor blockade has differential effects on the excitability of intracortical circuits in the human motor cortex. Exp Brain Res. 2000;135:455–61. doi: 10.1007/s002210000543. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Mazzone P, Insola A, Pilato F, Saturno E, et al. Comparison of descending volleys evoked by monophasic and biphasic magnetic stimulation of the motor cortex in conscious humans. Exp Brain Res. 2001a;141:121–7. doi: 10.1007/s002210100863. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Meglio M, Cioni B, Tonali P, et al. Descending spinal cord volleys evoked by transcranial magnetic and electrical stimulation of the motor cortex leg area in conscious humans. J Physiol. 2001b;537:1047–58. doi: 10.1111/j.1469-7793.2001.01047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Saturno E, Pilato F, Insola A, Mazzone P, et al. The effect on corticospinal volleys of reversing the direction of current induced in the motor cortex by transcranial magnetic stimulation. Exp Brain Res. 2001c;138:268–73. doi: 10.1007/s002210100722. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Mazzone P, et al. The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol. 2004a;115:255–66. doi: 10.1016/j.clinph.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Meglio M, et al. Direct recording of the output of the motor cortex produced by transcranial magnetic stimulation in a patient with cerebral cortex atrophy. Clin Neurophysiol. 2004b;115:112–5. doi: 10.1016/s1388-2457(03)00320-1. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Oliviero A, Saturno E, Dileone M, Tonali PA. Role of motor evoked potentials in diagnosis of cauda equine and lumbosacral cord lesions. Neurology. 2004c;63:2266–71. doi: 10.1212/01.wnl.0000147296.97980.ca. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, Tonali PA, Ziemann U. Dissociated effects of diazepam and lorazepam on short latency afferent ì inhibition. J Physiol. 2005;569:315–23. doi: 10.1113/jphysiol.2005.092155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Oliviero A, Dileone M, Saturno E, Mazzone P, et al. Origin of facilitation of motor evoked potentials after paired magnetic stimulation: direct recording of epidural activity in conscious humans. J Neurophysiol. 2006;96:1765–71. doi: 10.1152/jn.00360.2006. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, Profice P, Ranieri F, Ricci V, et al. Segregating two inhibitory circuits in human motor cortex at the level of GABAA receptor subtypes: a TMS study. Clin Neurophysiol. 2007;118:2207–14. doi: 10.1016/j.clinph.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Dileone M, Pilato F, Capone F, Musumeci G, Ranieri F, et al. Modulation of motor cortex neuronal networks by rTMS: comparison of local and remote effects of six different protocols of stimulation. J Neurophysiol. 2011;105:2150–6. doi: 10.1152/jn.00781.2010. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Ziemann U. The contribution of transcranial magnetic stimulation in the functional evaluation of microcircuits in human motor cortex. Front Neural Circ. 2013;7:18. doi: 10.3389/fncir.2013.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Rothwell JC. Corticospinal activity evoked and modulated by non-invasive stimulation of the intact human motor cortex. J Physiol. 2014;592:4115–28. doi: 10.1113/jphysiol.2014.274316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doeltgen SH, Ridding MC. Low-intensity, short-interval theta burst stimulation modulates excitatory but not inhibitory motor networks. Clin Neurophysiol. 2011;122:1411–6. doi: 10.1016/j.clinph.2010.12.034. [DOI] [PubMed] [Google Scholar]
- Donoghue JP, Sanes JN. Motor areas of the cerebral cortex. J Clin Neurophysiol. 1994;11:382–96. [PubMed] [Google Scholar]
- Duron B, Khater-Boidin J. Activation of motor pathways in neonates at term by percutaneous stimulation of the motor cortex and spinal cord. C R Acad Sci III. 1988;306:495–500. [PubMed] [Google Scholar]
- Edgley SA, Eyre JA, Lemon RN, Miller S. Excitation of the corticospinal tract by electromagnetic and electrical stimulation of the scalp in the macaque monkey. J Physiol. 1990;425:301–20. doi: 10.1113/jphysiol.1990.sp018104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Eyre JA, Lemon RN, Miller S. Comparison of activation of corticospinal neurons and spinal motor neurons by magnetic and electrical transcranial stimulation in the lumbosacral cord of the anaesthetized monkey. Brain. 1997;120:839–53. doi: 10.1093/brain/120.5.839. [DOI] [PubMed] [Google Scholar]
- Eisen AA, Shtybel W. Clinical experience with transcranial magnetic stimulation. Muscle Nerve. 1990;13:995–1011. doi: 10.1002/mus.880131102. [DOI] [PubMed] [Google Scholar]
- Esser SK, Hill SL, Tononi G. Modeling the effects of transcranial magnetic stimulation on cortical circuits. J Neurophysiol. 2005;94:622–39. doi: 10.1152/jn.01230.2004. [DOI] [PubMed] [Google Scholar]
- Esser SK, Huber R, Massimini M, Peterson MJ, Ferrarelli F, Tononi G. A direct demonstration of cortical LTP in humans: a combined TMS/EEG study. Brain Res Bull. 2006;69:86–94. doi: 10.1016/j.brainresbull.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Ettinger GJ, Leventon ME, Grimson WE, Kikinis R, Gugino L, Cote W, et al. Experimentation with a transcranial magnetic stimulation system for functional brain mapping. Med Image Anal. 1998;2:133–42. doi: 10.1016/s1361-8415(98)80008-x. [DOI] [PubMed] [Google Scholar]
- Farzan F, Barr MS, Wong W, Chen R, Fitzgerald PB, Daskalakis ZJ. Suppression of gamma-oscillations in the dorsolateral prefrontal cortex following long interval cortical inhibition: a TMS–EEG study. Neuropsychopharmacology. 2009;34:1543–51. doi: 10.1038/npp.2008.211. [DOI] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol (Lond) 1992;453:525–46. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarelli F, Massimini M, Peterson MJ, Riedner BA, Lazar M, Murphy MJ, et al. Reduced evoked gamma oscillations in the frontal cortex in schizophrenia patients: a TMS/EEG study. Am J Psychiatry. 2008;165:996–1005. doi: 10.1176/appi.ajp.2008.07111733. [DOI] [PubMed] [Google Scholar]
- Ferreri F, Pasqualetti P, Määttä S, Ponzo D, Ferrarelli F, Tononi G, et al. Human brain connectivity during single and paired pulse transcranial magnetic stimulation. Neuroimage. 2011;54:90–102. doi: 10.1016/j.neuroimage.2010.07.056. [DOI] [PubMed] [Google Scholar]
- Ferreri F, Ponzo D, Hukkanen T, Mervaala E, Könönen M, Pasqualetti P, et al. Human brain cortical correlates of short-latency afferent inhibition: a combined EEG–TMS study. J Neurophysiol. 2012;108:314–23. doi: 10.1152/jn.00796.2011. [DOI] [PubMed] [Google Scholar]
- Ferreri F, Rossini PM. TMS and TMS–EEG techniques in the study of the excitability, connectivity, and plasticity of the human motor cortex. Rev Neurosci. 2013;24:431–42. doi: 10.1515/revneuro-2013-0019. [DOI] [PubMed] [Google Scholar]
- Ferreri F, Ponzo D, Vollero L, Guerra A, Di Pino G, Petrichella S, et al. Does an intraneural interface short-term implant for robotic hand control modulate sensorimotor cortical integration? An EEG–TMS co-registration study on a human amputee. Restor Neurol Neurosci. 2014a;32:281–92. doi: 10.3233/RNN-130347. [DOI] [PubMed] [Google Scholar]
- Ferreri F, Vecchio F, Ponzo D, Pasqualetti P, Rossini PM. Time-varying coupling of EEG oscillations predicts excitability fluctuations in the primary motor cortex as reflected by motor evoked potentials amplitude: an EEG–TMS study. Hum Brain Mapp. 2014b;35:1969–80. doi: 10.1002/hbm.22306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fietzek UM, Heinen F, Berweck S, Maute S, Hufschmidt A, Schulte-Monting J, et al. Development of the corticospinal system and hand motor function: central conduction times and motor performance tests. Dev Med Child Neurol. 2000;42:220–7. doi: 10.1017/s0012162200000384. [DOI] [PubMed] [Google Scholar]
- Filmer HL, Dux PE, Mattingley JB. Applications of transcranial direct current stimulation for understanding brain function. Trends Neurosci. 2014;37:742–53. doi: 10.1016/j.tins.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Fisher J, Nakamura Y, Bestmann S, Rothwell C, Bostock H. Two phases of intracortical inhibition revealed by transcranial magnetic threshold tracking. Exp Brain Res. 2002;143:240–8. doi: 10.1007/s00221-001-0988-2. [DOI] [PubMed] [Google Scholar]
- Fisher MA. Electrophysiology of radiculopathies. Clin Neurophysiol. 2002;113:317–35. doi: 10.1016/s1388-2457(02)00018-4. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117:2584–96. doi: 10.1016/j.clinph.2006.06.712. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Hoy K, McQueen S, Maller JJ, Herring S, Segrave R, et al. A randomized trial of rTMS targeted with MRI based neuro-navigation in treatment-resistant depression. Neuropsychopharmacology. 2009;34:1255–62. doi: 10.1038/npp.2008.233. [DOI] [PubMed] [Google Scholar]
- Fox PT, Narayana S, Tandon N, Sandoval H, Fox SP, Kochunov P, et al. Column-based model of electric field excitation of cerebral cortex. Hum Brain Mapp. 2004;22:1–14. doi: 10.1002/hbm.20006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantseva M, Cui J, Farzan F, Chinta LV, Perez Velazquez JL, Daskalakis ZJ. Disrupted cortical conductivity in schizophrenia: TMS–EEG study. Cereb Cortex. 2014;24:211–21. doi: 10.1093/cercor/bhs304. [DOI] [PubMed] [Google Scholar]
- Freeman JA. An electronic stimulus artifact suppressor. Electroencephalogr Clin Neurophysiol. 1971;31:170–2. doi: 10.1016/0013-4694(71)90188-x. [DOI] [PubMed] [Google Scholar]
- Fregni F, DaSilva D, Potvin K, Ramos-Estebanez C, Cohen D, Pascual-Leone A, et al. Treatment of chronic visceral pain with brain stimulation. Ann Neurol. 2005;58:971–2. doi: 10.1002/ana.20651. [DOI] [PubMed] [Google Scholar]
- Fregni F, Potvin K, DaSilva D, Wang X, Lenkinski RE, Freedman SD, et al. Clinical effects and brain metabolic correlates in non-invasive cortical neuromodulation for visceral pain. Eur J Pain. 2011;15:53–60. doi: 10.1016/j.ejpain.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuggetta G, Fiaschi A, Manganotti P. Modulation of cortical oscillatory activities induced by varying single-pulse transcranial magnetic stimulation intensity over the left primary motor area: a combined EEG and TMS study. Neuroimage. 2005;27:896–908. doi: 10.1016/j.neuroimage.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Fuhr P, Agostino R, Hallett M. Spinal motor neuron excitability during the silent period after cortical stimulation. Electroencephalogr Clin Neurophysiol. 1991;81:257–62. doi: 10.1016/0168-5597(91)90011-l. [DOI] [PubMed] [Google Scholar]
- Furby A, Bourriez JL, Jacquesson JM, Mounier-Vehier F, Guieu JD. Motor evoked potentials to magnetic stimulation: technical considerations and normative data from 50 subjects. J Neurol. 1992;239:152–6. doi: 10.1007/BF00833916. [DOI] [PubMed] [Google Scholar]
- Gamboa OL, Antal A, Moliadze V, Paulus W. Simply longer is not better: reversal of theta burst after-effect with prolonged stimulation. Exp Brain Res. 2010;204:181–7. doi: 10.1007/s00221-010-2293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamboa OL, Antal A, Laczo B, Moliadze V, Nitsche MA, Paulus W. Impact of repetitive theta burst stimulation on motor cortex excitability. Brain Stimul. 2011;4:145–51. doi: 10.1016/j.brs.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Garassus P, Charles N, Mauguere F. Assessment of motor conduction times using magnetic stimulation of brain, spinal cord and peripheral nerve. Electromyogr Clin Neurophysiol. 1993;33:3–10. [PubMed] [Google Scholar]
- Garcia Dominguez L, Radhu N, Farzan F, Daskalakis ZJ. Characterizing long interval cortical inhibition over the time-frequency domain. PLoS One. 2014;9:e92354. doi: 10.1371/journal.pone.0092354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentner R, Wankerl K, Reinsberger C, Zeller D, Classen J. Depression of human corticospinal excitability induced by magnetic theta-burst stimulation: evidence of rapid polarity-reversing metaplasticity. Cereb Cortex. 2008;18:2046–53. doi: 10.1093/cercor/bhm239. [DOI] [PubMed] [Google Scholar]
- Gerloff C, Cohen LG, Floeter MK, Chen R, Corwell B, Hallett M. Inhibitory influence of the ipsilateral motor cortex on responses to stimulation of the human cortex and pyramidal tract. J Physiol (Lond) 1998;510:249–59. doi: 10.1111/j.1469-7793.1998.249bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George MS, Wassermann EM, Williams WA, Callahan A, Ketter TA, Basser P, et al. Daily repetitive Transcranial Magnetic Stimulation (rTMS) improves mood in depression. NeuroReport. 1995;6:1853–6. doi: 10.1097/00001756-199510020-00008. [DOI] [PubMed] [Google Scholar]
- Ghezzi A, Callea L, Zaffaroni M, Zibetti A, Montanini R. Study of central and peripheral motor conduction in normal subjects. Acta Neurol Scand. 1991;84:503–6. doi: 10.1111/j.1600-0404.1991.tb05003.x. [DOI] [PubMed] [Google Scholar]
- Gilio F, Rizzo V, Siebner HR, Rothwell JC. Effects on the right motor hand-area excitability produced by low-frequency rTMS over human contralateral homologous cortex. J Physiol. 2003;551:563–73. doi: 10.1113/jphysiol.2003.044313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsworthy MR, Pitcher JB, Ridding MC. A comparison of two different continuous theta burst stimulation paradigms applied to the human primary motor cortex. Clin Neurophysiol. 2012a;123:2256–63. doi: 10.1016/j.clinph.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Goldsworthy MR, Pitcher JB, Ridding MC. The application of spaced theta burst protocols induces long-lasting neuroplastic changes in the human motor cortex. Eur J Neurosci. 2012b;35:125–34. doi: 10.1111/j.1460-9568.2011.07924.x. [DOI] [PubMed] [Google Scholar]
- Goto T, Saitoh Y, Hashimoto N, Hirata M, Kishima H, Oshino S, et al. Diffusion tensor fiber tracking in patients with central post-stroke pain; correlation with efficacy of repetitive transcranial magnetic stimulation. Pain. 2008;140:509–18. doi: 10.1016/j.pain.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Groiss SJ, Ugawa Y. Cerebellar stimulation in ataxia. Cerebellum. 2012;11:440–2. doi: 10.1007/s12311-011-0329-3. [DOI] [PubMed] [Google Scholar]
- Groppa S, Oliviero A, Eisen A, Quartarone A, Cohen LG, Mall V, et al. A practical guide to diagnostic transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol. 2012;123:858–82. doi: 10.1016/j.clinph.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossheinrich N, Rau A, Pogarell O, Hennig-Fast K, Reinl M, Karch S, et al. Theta burst stimulation of the prefrontal cortex: safety and impact on cognition, mood, and resting electroencephalogram. Biol Psychiatry. 2009;65:778–84. doi: 10.1016/j.biopsych.2008.10.029. [DOI] [PubMed] [Google Scholar]
- Gugino LD, Romero JR, Aglio L, Titone D, Ramirez M, Pascual-Leone A, et al. Transcranial magnetic stimulation coregistered with MRI: a comparison of a guided versus blind stimulation technique and its effect on evoked compound muscle action potentials. Clin Neurophysiol. 2001;112:1781–92. doi: 10.1016/s1388-2457(01)00633-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guleyupoglu B, Schestatsky P, Edwards D, Fregni F, Bikson M. Classification of methods in transcranial electrical stimulation (tES) and evolving strategy from historical approaches to contemporary innovations. J Neurosci Methods. 2013;219:297–311. doi: 10.1016/j.jneumeth.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55:187–99. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Hamada M, Terao Y, Hanajima R, Shirota Y, Nakatani-Enomoto S, Furubayashi T, et al. Bidirectional long term motor cortical plasticity and metaplasticity induced by quadripulse transcranial magnetic stimulation. J Physiol. 2008;586:3927–47. doi: 10.1113/jphysiol.2008.152793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada M, Hanajima R, Terao Y, Okabe S, Nakatani-Enomoto S, Furubayashi T, et al. Primary motor cortical metaplasticity induced by priming over the supplementary motor area. J Physiol. 2009;587:4845–62. doi: 10.1113/jphysiol.2009.179101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada M, Murase N, Hasan A, Balaratnam M, Rothwell JC. The role of interneuron networks in driving human motor cortical plasticity. Cereb Cortex. 2013;23:1593–605. doi: 10.1093/cercor/bhs147. [DOI] [PubMed] [Google Scholar]
- Han TR, Kim JH, Lim JY. Optimization of facilitation related to threshold in transcranial magnetic stimulation. Clin Neurophysiol. 2001;112:593–9. doi: 10.1016/s1388-2457(01)00471-0. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Sakai K, Furubayashi T, Machii K, et al. Paired-pulse magnetic stimulation of the motor cortex: differences among I waves. J Physiol. 1998;509:607–18. doi: 10.1111/j.1469-7793.1998.607bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Enomoto H, Shiio Y, Mochizuki H, et al. Mechanisms of intracortical I-wave facilitation elicited by paired-pulse magnetic stimulation in humans. J Physiol. 2002;538:253–61. doi: 10.1113/jphysiol.2001.013094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanajima R, Furubayashi T, Iwata NK, Shiio Y, Okabe S, Kanazawa I, et al. Furhter evidence to support different mechanisms underlying intracortical inhibition of the motor cortex. Exp Brain Res. 2003;151:427–34. doi: 10.1007/s00221-003-1455-z. [DOI] [PubMed] [Google Scholar]
- Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motoneurons. J Neurophysiol. 1965;28:560–80. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- Henneman E, Clamann HP, Gillies JD, Skinner RD. Rank order of motoneurons within a pool: law of combination. J Neurophysiol. 1974;37:1338–49. doi: 10.1152/jn.1974.37.6.1338. [DOI] [PubMed] [Google Scholar]
- Henneman E. The size-principle: a deterministic output emerges from a set of probabilistic connections. J Exp Biol. 1985;115:105–12. doi: 10.1242/jeb.115.1.105. [DOI] [PubMed] [Google Scholar]
- Herbsman T, Avery D, Ramsey D, Holtzheimer P, Wadjik C, Hardaway F, et al. More lateral and anterior prefrontal coil location is associated with better repetitive transcranial magnetic stimulation antidepressant response. Biol Psychiatry. 2009;66:509–15. doi: 10.1016/j.biopsych.2009.04.034. [DOI] [PubMed] [Google Scholar]
- Herwig U, Schonfeldt-Lecuona C, Wunderlich AP, von Tiesenhausen C, Thielscher A, Walter H, et al. The navigation of transcranial magnetic stimulation. Psychiatry Res. 2001a;108:123–31. doi: 10.1016/s0925-4927(01)00121-4. [DOI] [PubMed] [Google Scholar]
- Herwig U, Padberg F, Unger J, Spitzer M, Schonfeldt-Lecuona C. Transcranial magnetic stimulation in therapy studies: examination of the reliability of “standard” coil positioning by neuronavigation. Biol Psychiatry. 2001b;50:58–61. doi: 10.1016/s0006-3223(01)01153-2. [DOI] [PubMed] [Google Scholar]
- Herwig U, Kolbel K, Wunderlich AP, Thielscher A, von Tiesenhausen C, Spitzer M, et al. Spatial congruence of neuronavigated transcranial magnetic stimulation and functional neuroimaging. Clin Neurophysiol. 2002;113:462–8. doi: 10.1016/s1388-2457(02)00026-3. [DOI] [PubMed] [Google Scholar]
- Hess CW, Mills KR, Murray NM. Responses in small hand muscles from magnetic stimulation of the human brain. J Physiol. 1987;388:397–419. doi: 10.1113/jphysiol.1987.sp016621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinder MR, Goss EL, Fujiyama H, Canty AJ, Garry MI, Rodger J, et al. Inter- and intra-individual variability following intermittent theta burst stimulation: implications for rehabilitation and recovery. Brain Stimul. 2014;7:365–71. doi: 10.1016/j.brs.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Hirayama A, Saitoh Y, Kishima H, Shimokawa T, Oshino S, Hirata M, et al. Reduction of intractable deafferentation pain by navigation-guided repetitive transcranial magnetic stimulation of the primary motor cortex. Pain. 2006;122:22–7. doi: 10.1016/j.pain.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Hosomi K, Saitoh Y, Kishima H, Oshino S, Hirata M, Tani N, et al. Electrical stimulation of primary motor cortex within the central sulcus for intractable neuropathic pain. Clin Neurophysiol. 2008;119:993–1001. doi: 10.1016/j.clinph.2007.12.022. [DOI] [PubMed] [Google Scholar]
- Hosomi K, Shimokawa T, Ikoma K, Nakamura Y, Sugiyama K, Ugawa Y, et al. Daily repetitive transcranial magnetic stimulation of primary motor cortex for neuropathic pain: a randomized, multicenter, double-blind, crossover, sham-controlled trial. Pain. 2013;154:1065–72. doi: 10.1016/j.pain.2013.03.016. [DOI] [PubMed] [Google Scholar]
- Haug BA, Schönle PW, Knobloch C, Köhne M. Silent period measurement revives as a valuable diagnostic tool with transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1992;85:158–60. doi: 10.1016/0168-5597(92)90081-l. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–6. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Huber R, Määttä S, Esser SK, Sarasso S, Ferrarelli F, Watson A, et al. Measures of cortical plasticity after transcranial paired associative stimulation predict changes in electroencephalogram slow-wave activity during subsequent sleep. J Neurosci. 2008;28:7911–8. doi: 10.1523/JNEUROSCI.1636-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iezzi E, Suppa A, Conte A, Li Voti P, Bologna M, Berardelli A. Short-term and long-term plasticity interaction in human primary motor cortex. Eur J Neurosci. 2011;33:1908–15. doi: 10.1111/j.1460-9568.2011.07674.x. [DOI] [PubMed] [Google Scholar]
- Ilic TV, Meintzschel F, Cleff U, Ruge D, Kessler KR, Ziemann U. Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J Physiol (Lond) 2002;545:153–67. doi: 10.1113/jphysiol.2002.030122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilmoniemi RJ, Virtanen J, Ruohonen J, Karhu J, Aronen HJ, Naatanen R, et al. Neuronal responses to magnetic stimulation reveal cortical reactivity and connectivity. Neuroreport. 1997;8:3537–40. doi: 10.1097/00001756-199711100-00024. [DOI] [PubMed] [Google Scholar]
- Ilmoniemi RJ, Kičić D. Methodology for combined TMS and EEG. Brain Topogr. 2010;22:233–48. doi: 10.1007/s10548-009-0123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incesu TK, Secil Y, Tokucoglu F, Gurgor N, Özdemirkiran T, Akhan G, et al. Diagnostic value of lumbar root stimulation at the early stage of Guillain-Barré syndrome. Clin Neurophysiol. 2013;124:197–203. doi: 10.1016/j.clinph.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Inghilleri M, Berardelli A, Cruccu G, Manfredi M. Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. J Physiol. 1993;466:521–34. [PMC free article] [PubMed] [Google Scholar]
- Inghilleri M, Berardelli A, Marchetti P, Manfredi M. Effects of diazepam, baclofen and thiopental on the silent period evoked by transcranial magnetic stimulation in humans. Exp Brain Res. 1996;109:467–72. doi: 10.1007/BF00229631. [DOI] [PubMed] [Google Scholar]
- Irlbacher K, Brocke J, Mechow JV, Brandt SA. Effects of GABA(A) and GABA(B) agonists on interhemispheric inhibition in man. Clin Neurophysiol. 2007;118:308–16. doi: 10.1016/j.clinph.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Rothwell J. Transcranial magnetic stimulation studies of cognition: an emerging field. Exp Brain Res. 2000;131:1–9. doi: 10.1007/s002219900224. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Baig M, Ramsey D, Lisanby SH, Avery D, McDonald WM, et al. Prefrontal rTMS for treating depression: location and intensity results from the OPT TMS multi-site clinical trial. Brain Stimul. 2013;6:108–17. doi: 10.1016/j.brs.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SH, Shin JE, Jeong YS, Shin HI. Changes in motor cortical excitability induced by high-frequency repetitive transcranial magnetic stimulation of different stimulation durations. Clin Neurophysiol. 2008;119:71–9. doi: 10.1016/j.clinph.2007.09.124. [DOI] [PubMed] [Google Scholar]
- Julkunen P, Säisänen L, Könönen M, Vanninen R, Kälviäinen R, Mervaala E. TMS–EEG reveals impaired intracortical interactions and coherence in Unverricht-Lundborg type progressive myoclonus epilepsy (EPM1) Epilepsy Res. 2013;106:103–12. doi: 10.1016/j.eplepsyres.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Kähkönen S, Kesäniemi M, Nikouline VV, Karhu J, Ollikainen M, Holi M, et al. Ethanol modulates cortical activity: direct evidence with combined TMS and EEG. Neuroimage. 2001;14:322–8. doi: 10.1006/nimg.2001.0849. [DOI] [PubMed] [Google Scholar]
- Kähkönen S, Wilenius J, Komssi S, Ilmoniemi RJ. Distinct differences in cortical reactivity of motor and prefrontal cortices to magnetic stimulation. Clin Neurophysiol. 2004;115:583–8. doi: 10.1016/j.clinph.2003.10.032. [DOI] [PubMed] [Google Scholar]
- Kähkönen S, Komssi S, Wilenius J, Ilmoniemi RJ. Prefrontal transcranial magnetic stimulation produces intensity-dependent EEG responses in humans. Neuroimage. 2005;24:955–60. doi: 10.1016/j.neuroimage.2004.09.048. [DOI] [PubMed] [Google Scholar]
- Kähkönen S, Wilenius J. Effects of alcohol on TMS-evoked N100 responses. J Neurosci Methods. 2007;166:104–8. doi: 10.1016/j.jneumeth.2007.06.030. [DOI] [PubMed] [Google Scholar]
- Kammer T. Phosphenes and transient scotomas induced by magnetic stimulation of the occipital lobe: their topographic relationship. Neuropsychologia. 1999;37:191–8. doi: 10.1016/s0028-3932(98)00093-1. [DOI] [PubMed] [Google Scholar]
- Kammer T, Beck S, Thielscher A, Laubis-Herrmann U, Topka H. Motor thresholds in humans: a transcranial magnetic stimulation study comparing different pulse waveforms, current directions and stimulator types. Clin Neurophysiol. 2001;112:250–8. doi: 10.1016/s1388-2457(00)00513-7. [DOI] [PubMed] [Google Scholar]
- Keil J, Timm J, Sanmiguel I, Schulz H, Obleser J, Schönwiesner M. Cortical brain states and corticospinal synchronization influence TMS-evoked motor potentials. J Neurophysiol. 2014;111:513–9. doi: 10.1152/jn.00387.2013. [DOI] [PubMed] [Google Scholar]
- Kernell D, Chien-Ping WU. Responses of the pyramidal tract to stimulation of the baboon’s motor cortex. J Physiol. 1967;191:653–72. doi: 10.1113/jphysiol.1967.sp008273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khedr EM, Kotb H, Kamel NF, Ahmed MA, Sadek R, Rothwell JC. Longlasting antalgic effects of daily sessions of repetitive transcranial magnetic stimulation in central and peripheral neuropathic pain. J Neurol Neurosurg Psychiatry. 2005;76:833–8. doi: 10.1136/jnnp.2004.055806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kičić D, Lioumis P, Ilmoniemi RJ, Nikulin VV. Bilateral changes in excitability of sensorimotor cortices during unilateral movement: combined electroencephalographic and transcranial magnetic stimulation study. Neuroscience. 2008;152:1119–29. doi: 10.1016/j.neuroscience.2008.01.043. [DOI] [PubMed] [Google Scholar]
- Kimiskidis VK, Papagiannopoulos S, Sotirakoglou K, Kazis DA, Kazis A, Mills KR. Silent period to transcranial magnetic stimulation: construction and properties of stimulus-response curves in healthy volunteers. Exp Brain Res. 2005;163:21–31. doi: 10.1007/s00221-004-2134-4. [DOI] [PubMed] [Google Scholar]
- Kimiskidis VK, Papagiannopoulos S, Kazis DA, Sotirakoglou K, Vasiliadis G, Zara F, et al. Lorazepam-induced effects on silent period and corticomotor excitability. Exp Brain Res. 2006;173:603–11. doi: 10.1007/s00221-006-0402-1. [DOI] [PubMed] [Google Scholar]
- Kimiskidis VK, Valentin A, Kälviäinen R. Transcranial magnetic stimulation for the diagnosis and treatment of epilepsy. Curr Opin Neurol. 2014;27:236–41. doi: 10.1097/WCO.0000000000000071. [DOI] [PubMed] [Google Scholar]
- Klein E, Kreinin I, Chistyakov A, Koren D, Mecz L, Marmur S, et al. Therapeutic efficacy of right prefrontal slow repetitive transcranial magnetic stimulation in major depression: a double-blind controlled study. Arch Gen Psychiatry. 1999;56:315–20. doi: 10.1001/archpsyc.56.4.315. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003;2:145–56. doi: 10.1016/s1474-4422(03)00321-1. [DOI] [PubMed] [Google Scholar]
- Koch G, Fernandez Del Olmo M, Cheeran B, Ruge D, Schippling S, Caltagirone C, et al. Focal stimulation of the posterior parietal cortex increases the excitability of the ipsilateral motor cortex. J Neurosci. 2007;27:6815–22. doi: 10.1523/JNEUROSCI.0598-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh TH, Eyre JA. Maturation of corticospinal tracts assessed by electromagnetic stimulation of the motor cortex. Arch Dis Child. 1988;63:1347–52. doi: 10.1136/adc.63.11.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komssi S, Aronen HJ, Huttunen J, Kesäniemi M, Soinne L, Nikouline VV, et al. Ipsi-and contralateral EEG reactions to transcranial magnetic stimulation. Clin Neurophysiol. 2002;113:175–84. doi: 10.1016/s1388-2457(01)00721-0. [DOI] [PubMed] [Google Scholar]
- Komssi S, Kähkönen S, Ilmoniemi RJ. The effect of stimulus intensity on brain responses evoked by transcranial magnetic stimulation. Hum Brain Mapp. 2004;21:154–64. doi: 10.1002/hbm.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komssi S, Kähkönen S. The novelty value of the combined use of electroencephalography and transcranial magnetic stimulation for neuroscience research. Brain Res Rev. 2006;52:183–92. doi: 10.1016/j.brainresrev.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Komssi S, Savolainen P, Heiskala J, Kähkönen S. Excitation threshold of the motor cortex estimated with transcranial magnetic stimulation electroencephalography. Neuroreport. 2007;18:13–6. doi: 10.1097/WNR.0b013e328011b89a. [DOI] [PubMed] [Google Scholar]
- Kozel FA, Nahas Z, DeBrux C, Molloy M, Lorberbaum JP, Bohning DE, et al. How the distance from coil to cortex relates to age, motor threshold and possibly the antidepressant response to repetitive transcranial magnetic stimulation. J Neuropsychiatry Clin Neurosci. 2000;12:376–84. doi: 10.1176/jnp.12.3.376. [DOI] [PubMed] [Google Scholar]
- Krieg SM, Sabih J, Bulubasova L, Obermueller T, Negwer C, Janssen I, et al. Preoperative motor mapping by navigated transcranial magnetic brain stimulation improves outcome for motor eloquent lesions. Neuro Oncol. 2014;16:1274–82. doi: 10.1093/neuonc/nou007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krings T, Buchbinder BR, Butler WE, Chiappa KH, Jiang HJ, Cosgrove GR, et al. Functional magnetic resonance imaging and transcranial stimulation: complementary approaches in the evaluation of cortical motor function. Neurology. 1997;48:1406–16. doi: 10.1212/wnl.48.5.1406. [DOI] [PubMed] [Google Scholar]
- Kujirai K, Kujirai T, Sinkjaer T, Rothwell JC. Associative plasticity in human motor cortex during voluntary muscle contraction. J Neurophysiol. 2006;96:1337–46. doi: 10.1152/jn.01140.2005. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, et al. Corticocortical inhibition in human motor cortex. J Physiol (Lond) 1993;471:501–19. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukke SN, Paine RW, Chao CC, de Campos AC, Hallett M. Efficient and reliable characterization of the corticospinal system using transcranial magnetic stimulation. J Clin Neurophysiol. 2014;31:246–52. doi: 10.1097/WNP.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu B, Johnson JS, Postle BR. Pre-stimulation phase predicts the TMS-evoked response. J Neurophysiol. 2014;112:1885–93. doi: 10.1152/jn.00390.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakso I, Hirata A, Ugawa Y. Effects of coil orientation on the electric field induced by TMS over the hand motor area. Phys Med Biol. 2014a;59:203–18. doi: 10.1088/0031-9155/59/1/203. [DOI] [PubMed] [Google Scholar]
- Laakso I, Matsumoto H, Hirata A, Terao Y, Hanajima R, Ugawa Y. Multi-scale simulations predict responses to non invasive nerve root stimulation. J Neural Eng. 2014b;11:056013. doi: 10.1088/1741-2560/11/5/056013. [DOI] [PubMed] [Google Scholar]
- Lang N, Harms J, Weyh T, Lemon RN, Paulus W, Rothwell JC, et al. Stimulus intensity and coil characteristics influence the efficacy of rTMS to suppress cortical excitability. Clin Neurophysiol. 2006;117:2292–301. doi: 10.1016/j.clinph.2006.05.030. [DOI] [PubMed] [Google Scholar]
- Liepert J, Oreja-Guevara C, Cohen LG, Tegenthoff M, Hallett M, Malin JP. Plasticity of cortical hand muscle representation in patients with hemifacial spasm. Neurosci Lett. 1999;272:33–6. doi: 10.1016/s0304-3940(99)00574-1. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, Drouot X, Keravel Y, Nguyen JP. Pain relief induced by repetitive transcranial magnetic stimulation of precentral cortex. Neuroreport. 2001a;12:2963–5. doi: 10.1097/00001756-200109170-00041. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, Drouot X, Nguyen JP. Interventional neurophysiology for pain control: duration of pain relief following repetitive transcranial magnetic stimulation of the motor cortex. Neurophysiol Clin. 2001b;31:247–52. doi: 10.1016/s0987-7053(01)00260-x. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, Drouot X, Ménard-Lefaucheur I, Nguyen JP. Neuropathic pain controlled for more than a year by monthly sessions of repetitive transcranial magnetic stimulation of the motor cortex. Neurophysiol Clin. 2004a;34:91–5. doi: 10.1016/j.neucli.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, Drouot X, Menard-Lefaucheur I, Zerah F, Bendib B, Cesaro P, et al. Neurogenic pain relief by repetitive transcranial magnetic cortical stimulation depends on the origin and the site of pain. J Neurol Neurosurg Psychiatry. 2004b;75:612–6. doi: 10.1136/jnnp.2003.022236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefaucheur JP, Drouot X, Menard-Lefaucheur I, Keravel Y, Nguyen JP. Motor cortex rTMS restores defective intracortical inhibition in chronic neuropathic pain. Neurology. 2006a;67:1568–74. doi: 10.1212/01.wnl.0000242731.10074.3c. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, Hatem S, Nineb A, Ménard-Lefaucheur I, Wendling S, Keravel Y, et al. Somatotopic organization of the analgesic effects of motor cortex rTMS in neuropathic pain. Neurology. 2006b;67:1998–2004. doi: 10.1212/01.wnl.0000247138.85330.88. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, Drouot X, Menard-Lefaucheur I, Keravel Y, Nguyen JP. Motor cortex rTMS in chronic neuropathic pain: pain relief is associated with thermal sensory perception improvement. J Neurol Neurosurg Psychiatry. 2008;79:1044–9. doi: 10.1136/jnnp.2007.135327. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP. Why image-guided navigation becomes essential in the practice of transcranial magnetic stimulation. Neurophysiol Clin. 2010;40:1–5. doi: 10.1016/j.neucli.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, Ménard-Lefaucheur I, Goujon C, Keravel Y, Nguyen JP. Predictive value of rTMS in the identification of responders to epidural motor cortex stimulation therapy for pain. J Pain. 2011;12:1102–11. doi: 10.1016/j.jpain.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, Ayache SS, Sorel M, Farhat WH, Zouari HG, Ciampi de Andrade D. Analgesic effects of repetitive transcranial magnetic stimulation of the motor cortex in neuropathic pain: influence of theta burst stimulation priming. Eur J Pain. 2012;16:1403–13. doi: 10.1002/j.1532-2149.2012.00150.x. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP. Pain. Handb Clin Neurol. 2013;116:423–40. doi: 10.1016/B978-0-444-53497-2.00035-8. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, André-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS) Clin Neurophysiol. 2014;125:2150–206. doi: 10.1016/j.clinph.2014.05.021. [DOI] [PubMed] [Google Scholar]
- Lemon RN, Baker SN, Davis JA, Kirkwood PA, Maier MA, Yang HS. The importance of the cortico-motoneuronal system for control of grasp. Novartis Found Symp. 1998;218:202–15. doi: 10.1002/9780470515563.ch11. [DOI] [PubMed] [Google Scholar]
- Li X, Nahas Z, Anderson B, Kozel FA, George MS. Can left prefrontal rTMS be used as a maintenance treatment for bipolar depression? Depress Anxiety. 2004;20:98–100. doi: 10.1002/da.20027. [DOI] [PubMed] [Google Scholar]
- Liddell EG, Phillips CG. The cortical representation of motor units. Brain. 1952;75:510–25. doi: 10.1093/brain/75.4.510. [DOI] [PubMed] [Google Scholar]
- Limoge A, Robert C, Stanley TH. Transcutaneous cranial electrical stimulation (TCES): a review 1998. Neurosci Biobehav Rev. 1999;23:529–38. doi: 10.1016/s0149-7634(98)00048-7. [DOI] [PubMed] [Google Scholar]
- Lipton RB, Dodick DW, Silberstein SD, Saper JR, Aurora SK, Pearlman SH, et al. Single-pulse transcranial magnetic stimulation for acute treatment of migraine with aura: a randomised, double-blind, parallel-group, sham controlled trial. Lancet Neurol. 2010;9:373–80. doi: 10.1016/S1474-4422(10)70054-5. [DOI] [PubMed] [Google Scholar]
- Lioumis P, Kičić D, Savolainen P, Makela JP, Kähkönen S. Reproducibility of TMS-evoked EEG responses. Hum Brain Mapp. 2009;30:1387–96. doi: 10.1002/hbm.20608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Alonso V, Cheeran B, Rio-Rodriguez D, Fernandez-Del-Olmo M. Inter-individual variability in response to non-invasive brain stimulation paradigms. Brain Stimul. 2014;7:372–80. doi: 10.1016/j.brs.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Ludolph A, Wenning G, Masur H, Füratsch N, Elger C. Electromagnetic stimulation of the nervous system. I. Normal values in the central nervous system and a comparison with electric stimulation. EEG–EMG. 1989;20:153. [PubMed] [Google Scholar]
- Maccabee PJ, Amassian VE, Eberle LP, Cracco RQ. Magnetic coil stimulation of straight and bent amphibian and mammalian peripheral nerve in vitro: locus of excitation. J Physiol. 1993;460:201–19. doi: 10.1113/jphysiol.1993.sp019467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccabee PJ, Lipitz ME, Desudchit T, Golub RW, Nitti VW, Bania JP, et al. A new method using neuromagnetic stimulation to measure conduction time within the cauda equina. Electroencephalogr Clin Neurophysiol. 1996;101:153–66. doi: 10.1016/0924-980x(95)00264-l. [DOI] [PubMed] [Google Scholar]
- Macdonell RA, Donnan GA. Magnetic cortical stimulation in acute spinal cord injury. Neurology. 1995;45:303–6. doi: 10.1212/wnl.45.2.303. [DOI] [PubMed] [Google Scholar]
- Maegaki Y, Maeoka Y, Takeshita K. Magnetic stimulation of the lumbosacral vertebral column in children: normal values and possible sites of stimulation. Electroencephalogr Clin Neurophysiol. 1997;105:102–8. doi: 10.1016/s0924-980x(97)96017-3. [DOI] [PubMed] [Google Scholar]
- Maertens de Noordhout A, Pepin JL, Schoenen J, Delwaide PJ. Percutaneous magnetic stimulation of the motor cortex in migraine. Electroencephalogr Clin Neurophysiol. 1992;85:110–5. doi: 10.1016/0168-5597(92)90076-n. [DOI] [PubMed] [Google Scholar]
- Magistris MR, Rosler KM, Truffert A, Myers JP. Transcranial stimulation excites virtually all motor neurons supplying the target muscle. A demonstration and a method improving the study of motor evoked potentials. Brain. 1998;121:437–50. doi: 10.1093/brain/121.3.437. [DOI] [PubMed] [Google Scholar]
- Magistris MR, Rösler KM, Truffert A, Landis T, Hess CW. A clinical study of motor evoked potentials using a triple stimulation technique. Brain. 1999;122:265–79. doi: 10.1093/brain/122.2.265. [DOI] [PubMed] [Google Scholar]
- Mano Y, Nakamuro T, Ikoma K, Sugata T, Morimoto S, Takayanagi T, et al. Central motor conductivity in aged people. Intern Med. 1992;31:1084–7. doi: 10.2169/internalmedicine.31.1084. [DOI] [PubMed] [Google Scholar]
- Mäki H, Ilmoniemi RJ. The relationship between peripheral and early cortical activation induced by transcranial magnetic stimulation. Neurosci Lett. 2010a;478:24–8. doi: 10.1016/j.neulet.2010.04.059. [DOI] [PubMed] [Google Scholar]
- Mäki H, Ilmoniemi RJ. EEG oscillations and magnetically evoked motor potentials reflect motor system excitability in overlapping neuronal populations. Clin Neurophysiol. 2010b;121:492–501. doi: 10.1016/j.clinph.2009.11.078. [DOI] [PubMed] [Google Scholar]
- Mariorenzi R, Zarola F, Caramia MD, Paradiso C, Rossini PM. Non-invasive evaluation of central motor tract excitability changes following peripheral nerve stimulation in healthy humans. Electroencephalogr Clin Neurophysiol. 1991;81:90–101. doi: 10.1016/0168-5597(91)90002-f. [DOI] [PubMed] [Google Scholar]
- Massimini M, Ferrarelli F, Huber R, Esser SK, Singh H, Tononi G. Breakdown of cortical effective connectivity during sleep. Science. 2005;309:2228–32. doi: 10.1126/science.1117256. [DOI] [PubMed] [Google Scholar]
- Massimini M, Ferrarelli F, Sarasso S, Tononi G. Cortical mechanisms of loss of consciousness: insight from TMS/EEG studies. Cortical mechanisms of loss of consciousness: insight from TMS/EEG studies. Arch Ital Biol. 2012;150:44–55. doi: 10.4449/aib.v150i2.1361. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Ugawa Y. Clinical signs, neurophysiological evaluation, and medication of spasticity–review. Brain Nerve. 2008;60:1409–14. [PubMed] [Google Scholar]
- Matsumoto H, Octaviana F, Hanajima R, Terao Y, Yugeta A, Hamada M, et al. Magnetic lumbosacral motor root stimulation with a flat, large round coil. Clin Neurophysiol. 2009a;120:770–5. doi: 10.1016/j.clinph.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Octaviana F, Terao Y, Hanajima R, Yugeta A, Hamada M, et al. Magnetic stimulation of the cauda equina in the spinal canal with a flat, large round coil. J Neurol Sci. 2009b;284:46–51. doi: 10.1016/j.jns.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Hanajima R, Shirota Y, Hamada M, Terao Y, Ohminami S, et al. Cortico-conus motor conduction time (CCCT) for leg muscles. Clin Neurophysiol. 2010a;121:1930–3. doi: 10.1016/j.clinph.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Hanajima R, Terao Y, Hamada M, Yugeta A, Shirota Y, et al. Efferent and afferent evoked potentials in patients with adrenomyeloneuropathy. Clin Neurol Neurosurg. 2010b;112:131–6. doi: 10.1016/j.clineuro.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Hanajima R, Terao Y, Yugeta A, Hamada M, Shirota Y, et al. Prominent cauda equina involvement in patients with chronic inflammatory demyelinating polyradiculoneuropathy. J Neurol Sci. 2010c;290:112–4. doi: 10.1016/j.jns.2009.10.014. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Shirota Y, Ugawa Y. Magnetic augmented translumbosacral stimulation coil stimulation method for accurate evaluation of corticospinal tract function in peripheral neuropathy. Neurol India. 2010d;58:796–7. doi: 10.4103/0028-3886.72183. [DOI] [PubMed] [Google Scholar]
- Matsumoto L, Hanajima R, Matsumoto H, Ohminami S, Terao Y, Tsuji S, et al. Supramaximal responses can be elicited in hand muscles by magnetic stimulation of the cervical motor roots. Brain Stimul. 2010e;3:153–60. doi: 10.1016/j.brs.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Konoma Y, Shimizu T, Okabe S, Shirota Y, Hanajima R, et al. Aging influences central motor conduction less than peripheral motor conduction: a transcranial magnetic stimulation study. Muscle Nerve. 2012;46:932–6. doi: 10.1002/mus.23430. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Hanajima R, Terao Y, Hashida H, Ugawa Y. Neurophysiological analysis of the cauda equina in POEMS syndrome. Neurol Sci. 2013a;34:121–2. doi: 10.1007/s10072-012-0950-z. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Hanajima R, Terao Y, Ugawa Y. Magnetic-motor-root stimulation: review. Clin Neurophysiol. 2013b;124:1055–67. doi: 10.1016/j.clinph.2012.12.049. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Konoma Y, Fujii K, Hanajima R, Terao Y, Ugawa Y. A conduction block in sciatic nerves can be detected by magnetic motor root stimulation. J Neurol Sci. 2013c;331:174–6. doi: 10.1016/j.jns.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Tokushige S, Hashida H, Hanajima R, Terao Y, Ugawa Y. Focal lesion in upper part of brachial plexus can be detected by magnetic cervical motor root stimulation. Brain Stimul. 2013d;6:538–40. doi: 10.1016/j.brs.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Mazevet D, Pierrot-Deseilligny E, Rothwell JC. A propriospinal-like contribution to electromyographic responses evoked in wrist extensor muscles by transcranial stimulation of the motor cortex in man. Exp Brain Res. 1996;109:495–9. doi: 10.1007/BF00229634. [DOI] [PubMed] [Google Scholar]
- McAllister SM, Rothwell JC, Ridding MC. Selective modulation of intracortical inhibition by low-intensity theta burst stimulation. Clin Neurophysiol. 2009;120:820–6. doi: 10.1016/j.clinph.2009.02.003. [DOI] [PubMed] [Google Scholar]
- McConnell KA, Nahas Z, Shastri A, Lorberbaum JP, Kozel FA, Bohning DE, et al. The transcranial magnetic stimulation motor threshold depends on the distance from coil to underlying cortex: a replication in healthy adults comparing two methods of assessing the distance to cortex. Biol Psychiatry. 2001;49:454–9. doi: 10.1016/s0006-3223(00)01039-8. [DOI] [PubMed] [Google Scholar]
- McDonnell MN, Orekhov Y, Ziemann U. The role of GABA(B) receptors in intracortical inhibition in the human motor cortex. Exp Brain Res. 2006;173:86–93. doi: 10.1007/s00221-006-0365-2. [DOI] [PubMed] [Google Scholar]
- Melgari JM, Pasqualetti P, Pauri F, Rossini PM. Muscles in “concert”: study of primary motor cortex upper limb functional topography. PLoS One. 2008;3:e3069. doi: 10.1371/journal.pone.0003069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merabet LB, Theoret H, Pascual-Leone A. Transcranial magnetic stimulation as an investigative tool in the study of visual function. Optom Vis Sci. 2003;80:356–68. doi: 10.1097/00006324-200305000-00010. [DOI] [PubMed] [Google Scholar]
- Merton PA, Morton HB. Stimulation of the cerebral cortex in the intact human subject. Nature. 1980;285:227. doi: 10.1038/285227a0. [DOI] [PubMed] [Google Scholar]
- Merton PA, Hill DK, Morton HB, Marsden CD. Scope of a technique for electrical stimulation of human brain, spinal cord and muscle. Lancet. 1982;2:597–600. doi: 10.1016/s0140-6736(82)90670-5. [DOI] [PubMed] [Google Scholar]
- Mhalla A, Baudic S, Ciampi de Andrade D, Gautron M, Perrot S, Teixeira MJ, et al. Long-term maintenance of the analgesic effects of transcranial magnetic stimulation in fibromyalgia. Pain. 2011;152:1478–85. doi: 10.1016/j.pain.2011.01.034. [DOI] [PubMed] [Google Scholar]
- Mills KR, Murray NM. Corticospinal tract conduction time in multiple sclerosis. Ann Neurol. 1985;18:601–5. doi: 10.1002/ana.410180514. [DOI] [PubMed] [Google Scholar]
- Mills KR, Murray NM. Electrical stimulation over the human vertebral column: which neural elements are excited? Electroencephalogr Clin Neurophysiol. 1986;63:582–9. doi: 10.1016/0013-4694(86)90145-8. [DOI] [PubMed] [Google Scholar]
- Mills KR, Murray NM, Hess CW. Magnetic and electrical transcranial brain stimulation: physiological mechanisms and clinical applications. Neurosurgery. 1987;20:164–8. doi: 10.1097/00006123-198701000-00033. [DOI] [PubMed] [Google Scholar]
- Mills KR, Nithi KA. Corticomotor threshold to magnetic stimulation: normal values and repeatability. Muscle Nerve. 1997;20:570–6. doi: 10.1002/(sici)1097-4598(199705)20:5<570::aid-mus5>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Miniussi C, Cappa SF, Cohen LG, Floel A, Fregni F, Nitsche MA, et al. Efficacy of repetitive transcranial magnetic stimulation/transcranial direct current stimulation in cognitive neurorehabilitation. Brain Stimul. 2008;4:326–36. doi: 10.1016/j.brs.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Miniussi C, Thut G. Combining TMS and EEG offers new prospects in cognitive neuroscience. Brain Topogr. 2010;22:249–56. doi: 10.1007/s10548-009-0083-8. [DOI] [PubMed] [Google Scholar]
- Miniussi C, Rossini PM. Transcranial magnetic stimulation in cognitive rehabilitation. Neuropsychol Rehabil. 2011;21:579–601. doi: 10.1080/09602011.2011.562689. [DOI] [PubMed] [Google Scholar]
- Miniussi C, Harris JA, Ruzzoli M. Modelling non-invasive brain stimulation in cognitive neuroscience. Neurosci Biobehav Rev. 2013;37:1702–12. doi: 10.1016/j.neubiorev.2013.06.014. [DOI] [PubMed] [Google Scholar]
- Mishory A, Molnar C, Koola J, Li X, Kozel FA, Myrick H, et al. The maximum-likelihood strategy for determining transcranial magnetic stimulation motor threshold, using parameter estimation by sequential testing is faster than conventional methods with similar precision. J ECT. 2004;20:160–5. doi: 10.1097/00124509-200409000-00007. [DOI] [PubMed] [Google Scholar]
- Misra UK, Kalita J, Bhoi SK. High frequency repetitive transcranial magnetic stimulation (rTMS) is effective in migraine prophylaxis: an open labeled study. Neurol Res. 2012;34:547–51. doi: 10.1179/1743132812Y.0000000045. [DOI] [PubMed] [Google Scholar]
- Misra UK, Kalita J, Bhoi SK. High-rate repetitive transcranial magnetic stimulation in migraine prophylaxis: a randomized, placebo-controlled study. J Neurol. 2013;260:2793–801. doi: 10.1007/s00415-013-7072-2. [DOI] [PubMed] [Google Scholar]
- Möller C, Arai N, Lücke J, Ziemann U. Hysteresis effects on the input–output curve of motor evoked potentials. Clin Neurophysiol. 2009;120:1003–8. doi: 10.1016/j.clinph.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Mosimann UP, Marré SC, Werlen S, Schmitt W, Hess CW, Fisch HU, et al. Antidepressant effects of repetitive transcranial magnetic stimulation in the elderly: correlation between effect size and coil-cortex distance. Arch Gen Psychiatry. 2002;59:560–1. doi: 10.1001/archpsyc.59.6.560. [DOI] [PubMed] [Google Scholar]
- Müller JF, Orekhov Y, Liu Y, Ziemann U. Homeostatic plasticity in human motor cortex demonstrated by two consecutive sessions of paired associative stimulation. Eur J Neurosci. 2007;25:3461–8. doi: 10.1111/j.1460-9568.2007.05603.x. [DOI] [PubMed] [Google Scholar]
- Mueller JK, Grigsby EM, Prevosto V, Petraglia FW, 3rd, Rao H, Deng ZD, et al. Simultaneous transcranial magnetic stimulation and single-neuron recording in alert non human primates. Nat Neurosci. 2014;17:1130–6. doi: 10.1038/nn.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller K, Hömberg V, Lenard HG. Magnetic stimulation of motor cortex and nerve roots in children. Maturation of cortico motoneuronal projections. Electroencephalogr Clin Neurophysiol. 1991;81:63–70. doi: 10.1016/0168-5597(91)90105-7. [DOI] [PubMed] [Google Scholar]
- Müller-Dahlhaus F, Liu Y, Ziemann U. Inhibitory circuits and the nature of their interactions in the human motor cortex a pharmacological TMS study. J Physiol. 2008;586:495–514. doi: 10.1113/jphysiol.2007.142059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylius V, Borckardt JJ, Lefaucheur JP. Noninvasive cortical modulation of experimental pain. Pain. 2012;153:1350–63. doi: 10.1016/j.pain.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Mylius V, Ayache SS, Ahdab R, Farhat WH, Zouari HG, Belke M, et al. Definition of DLPFC and M1 according to anatomical landmarks for navigated brain stimulation: inter-rater reliability, accuracy, and influence of gender and age. Neuroimage. 2013;78:224–32. doi: 10.1016/j.neuroimage.2013.03.061. [DOI] [PubMed] [Google Scholar]
- Nahas Z, Oliver NC, Johnson M, Molloy M, Hughes PL, Ballenger JC, et al. Feasibility and efficacy of left prefrontal rTMS as a maintenance antidepressant. Biol Psychiatry. 2000;47:156–7. [Google Scholar]
- Najib U, Bashir S, Edwards D, Rotenberg A, Pascual-Leone A. Transcranial brain stimulation: clinical applications and future directions. Neurosurg Clin N Am. 2011;22:233–51. doi: 10.1016/j.nec.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. J Physiol (Lond) 1997;498:817–23. doi: 10.1113/jphysiol.1997.sp021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani-Enomoto S, Hanajima R, Hamada M, Terao Y, Matusmoto H, Shirota Y, et al. Bidirectional modulation of sensory cortical excitability by quadripulse magnetic stimulation (QPS) in humans. Clin Neurophysiol. 2012;123:1415–21. doi: 10.1016/j.clinph.2011.11.037. [DOI] [PubMed] [Google Scholar]
- Napolitani M, Bodart O, Canali P, Seregni F, Casali A, Laureys S, et al. Transcranial magnetic stimulation combined with high-density EEG in altered states of consciousness. Brain Inj. 2014;28:1180–9. doi: 10.3109/02699052.2014.920524. [DOI] [PubMed] [Google Scholar]
- Ni Z, Gunraj C, Nelson AJ, Yeh IJ, Castillo G, Hoque T, et al. Two phases of interhemispheric inhibition between motor related cortical areas and the primary motor cortex in human. Cereb Cortex. 2009;19:1654–65. doi: 10.1093/cercor/bhn201. [DOI] [PubMed] [Google Scholar]
- Ni Z, Charab S, Gunraj C, Nelson AJ, Udupa K, Yeh IJ, et al. Transcranial magnetic stimulation in different current directions activates separate cortical circuits. J Neurophysiol. 2011a;105:749–56. doi: 10.1152/jn.00640.2010. [DOI] [PubMed] [Google Scholar]
- Ni Z, Müller-Dahlhaus F, Chen R, Ziemann U. Triple-pulse TMS to study interactions between neural circuits in human cortex. Brain Stimul. 2011b;4:281–93. doi: 10.1016/j.brs.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Ni Z, Bahl N, Gunraj C, Mazzella F, Chen R. Increased motor cortical facilitation and decreased inhibition in Parkinson’s Disease. Neurology. 2013;80:1746–53. doi: 10.1212/WNL.0b013e3182919029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Z, Gunraj C, Kailey P, Cash RF, Chen R. Heterosynaptic modulation of motor cortical plasticity in human. J Neurosci. 2014;34:7314–21. doi: 10.1523/JNEUROSCI.4714-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J, Petersen N, Ballegaard M. Latency of effects evoked by electrical and magnetic brain stimulation in lower limb motoneurones in man. J Physiol Lond. 1995;484(Pt 3):791–802. doi: 10.1113/jphysiol.1995.sp020704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikulin VV, Kičić D, Kähkönen S, Ilmoniemi RJ. Modulation of electroencephalographic responses to transcranial magnetic stimulation: evidence for changes in cortical excitability related to movement. Eur J Neurosci. 2003;18:1206–12. doi: 10.1046/j.1460-9568.2003.02858.x. [DOI] [PubMed] [Google Scholar]
- Niskanen E, Könönen M, Määttä S, Hallikainen M, Kivipelto M, Casarotto S, et al. New insights into Alzheimer’s disease progression: a combined TMS and structural MRI study. PLoS One. 2011;6:e26113. doi: 10.1371/journal.pone.0026113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527:633–9. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Transcranial direct current stimulation–update 2011. Restor Neurol Neurosci. 2011;29:463–92. doi: 10.3233/RNN-2011-0618. [DOI] [PubMed] [Google Scholar]
- Ohn SH, Chang WH, Park CH, Kim ST, Lee JI, Pascual-Leone A, et al. Neural correlates of the antinociceptive effects of repetitive transcranial magnetic stimulation on central pain after stroke. Neurorehabil Neural Repair. 2012;26:344–52. doi: 10.1177/1545968311423110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveri M, Rossini PM, Traversa R, Cicinelli P, Filippi MM, Pasqualetti P, et al. Left frontal transcranial magnetic stimulation reduces contralesional extinction in patients with unilateral right brain damage. Brain. 1999;122:1731–9. doi: 10.1093/brain/122.9.1731. [DOI] [PubMed] [Google Scholar]
- Oliveri M, Rossini PM, Cicinelli P, Traversa R, Pasqualetti P, Filippi MM, et al. Neurophysiological evaluation of tactile space perception deficits through transcranial magnetic stimulation. Brain Res Protoc. 2000;5:25–9. doi: 10.1016/s1385-299x(99)00055-0. [DOI] [PubMed] [Google Scholar]
- Opitz A, Legon W, Rowlands A, Bickel WK, Paulus W, Tyler WJ. Physiological observations validate finite element models for estimating subject specific electric field distributions induced by transcranial magnetic stimulation of the human motor cortex. Neuroimage. 2013;81:253–64. doi: 10.1016/j.neuroimage.2013.04.067. [DOI] [PubMed] [Google Scholar]
- Opitz A, Zafar N, Bockermann V, Rohde V, Paulus W. Validating computationally predicted TMS stimulation areas using direct electrical stimulation in patients with brain tumors near precentral regions. Neuroimage Clin. 2014;4:500–7. doi: 10.1016/j.nicl.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reardon JP, Blumner KH, Peshek AD, Pradilla RR, Pimiento PC. Long-term maintenance therapy for major depressive disorder with rTMS. J Clin Psychiatry. 2005;66:1524–8. doi: 10.4088/jcp.v66n1205. [DOI] [PubMed] [Google Scholar]
- Orth M, Rothwell JC. The cortical silent period: intrinsic variability and relation to the waveform of the transcranial magnetic stimulation pulse. Clin Neurophysiol. 2004;115:1076–82. doi: 10.1016/j.clinph.2003.12.025. [DOI] [PubMed] [Google Scholar]
- Osei-Lah AD, Mills KR. Optimising the detection of upper motor neuron fuction dysfunction in amyotrophic lateral sclerosis: a transcranial magnetic stimulation study. J Neurol. 2004:1364–9. doi: 10.1007/s00415-004-0545-6. [DOI] [PubMed] [Google Scholar]
- Ozaki I, Hashimoto I. Exploring the physiology and function of high-frequency oscillations (HFOs) from the somatosensory cortex. Clin Neurophysiol. 2011;122:1908–23. doi: 10.1016/j.clinph.2011.05.023. [DOI] [PubMed] [Google Scholar]
- Padberg F, Zwanzger P, Keck ME, Kathmann N, Mikhaiel P, Ella R, et al. Repetitive Transcranial Magnetic Stimulation (rTMS) in major depression: relation between efficacy and stimulation intensity. Neuropsychopharmacology. 2002;27:638–45. doi: 10.1016/S0893-133X(02)00338-X. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Gates JR, Dhuna A. Induction of speech arrest and counting errors with rapid-rate transcranial magnetic stimulation. Neurology. 1991;41:697–702. doi: 10.1212/wnl.41.5.697. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Cammarota A, Wassermann EM, Brasil-Neto JP, Cohen LG, Hallett M. Modulation of motor cortical outputs to the reading hand of braille readers. Ann Neurol. 1993;34:33–7. doi: 10.1002/ana.410340108. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Solé J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117:847–58. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Rubio B, Pallardo F, Catala MD. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet. 1996;348:233–7. doi: 10.1016/s0140-6736(96)01219-6. [DOI] [PubMed] [Google Scholar]
- Patton HD, Amassian VE. Single- and multiple-unit analysis of cortical stage of pyramidal tract activation. J Neurophysiol. 1954;17:345–63. doi: 10.1152/jn.1954.17.4.345. [DOI] [PubMed] [Google Scholar]
- Paulus W, Peterchev AV, Ridding M. Transcranial electric and magnetic stimulation: technique and paradigms. Handb Clin Neurol. 2013;116:329–42. doi: 10.1016/B978-0-444-53497-2.00027-9. See comment in PubMed Commons below. [DOI] [PubMed] [Google Scholar]
- Paus T, Jech R, Thompson CJ, Comeau R, Peters T, Evans AC. Transcranial magnetic stimulation during positron emission tomography: a new method for studying connectivity of the human cerebral cortex. J Neurosci. 1997;17:3178–84. doi: 10.1523/JNEUROSCI.17-09-03178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Imaging the brain before, during, and after transcranial magnetic stimulation. Neuropsychologia. 1999;37:219–24. doi: 10.1016/s0028-3932(98)00096-7. [DOI] [PubMed] [Google Scholar]
- Paus T, Sipila PK, Strafella AP. Synchronization of neuronal activity in the human primary motor cortex by transcranial magnetic stimulation: an EEG study. J Neurophysiol. 2001;86:1983–90. doi: 10.1152/jn.2001.86.4.1983. [DOI] [PubMed] [Google Scholar]
- Peinemann A, Lehner C, Mentschel C, Munchau A, Conrad B, Siebner HR. Subthreshold 5-Hz repetitive transcranial magnetic stimulation of the human primary motor cortex reduces intracortical paired-pulse inhibition. Neurosci Lett. 2000;296:21–4. doi: 10.1016/s0304-3940(00)01616-5. [DOI] [PubMed] [Google Scholar]
- Peinemann A, Reimer B, Loer C, Quartarone A, Munchau A, Conrad B, et al. Long-lasting increase in corticospinal excitability after 1800 pulses of subthreshold 5 Hz repetitive TMS to the primary motor cortex. Clin Neurophysiol. 2004;115:1519–26. doi: 10.1016/j.clinph.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Person RS, Kozhina GV. Study of orthodromic and antidromic effects of nerve stimulation on single motoneurones of human hand muscles. Electromyogr Clin Neurophysiol. 1978;18:437–56. [PubMed] [Google Scholar]
- Peterchev AV, Goetz SM, Westin GG, Luber B, Lisanby SH. Pulse width dependence of motor threshold and input–output curve characterized with controllable pulse parameter transcranial magnetic stimulation. Clin Neurophysiol. 2013;124:1364–72. doi: 10.1016/j.clinph.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JC, Reithler J, Schuhmann T, de Graaf T, Uludag K, Goebel R, et al. On the feasibility of concurrent human TMS–EEG–fMRI measurements. J Neurophysiol. 2013;109:1214–27. doi: 10.1152/jn.00071.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peurala SH, Muller-Dahlhaus JF, Arai N, Ziemann U. Interference of short-interval intracortical inhibition (SICI) and short-interval intracortical facilitation (SICF) Clin Neurophysiol. 2008;119:2291–7. doi: 10.1016/j.clinph.2008.05.031. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999;110:1842–57. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Picarelli H, Teixeira MJ, de Andrade DC, Myczkowski ML, Luvisotto TB, Yeng LT, et al. Repetitive transcranial magnetic stimulation is efficacious as an add-on to pharmacological therapy in complex regional pain syndrome (CRPS) type I. J Pain. 2010;11:1203–10. doi: 10.1016/j.jpain.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Picht T, Krieg SM, Sollmann N, Rösler J, Niraula B, Neuvonen T, et al. A comparison of language mapping by preoperative navigated transcranial magnetic stimulation and direct cortical stimulation during awake surgery. Neurosurgery. 2013;72:808–19. doi: 10.1227/NEU.0b013e3182889e01. [DOI] [PubMed] [Google Scholar]
- Pierantozzi M, Marciani MG, Palmieri MG, Brusa L, Galati S, Caramia MD, et al. Effect of Vigabatrin on motor responses to transcranial magnetic stimulation: an effective tool to investigate in vivo GABAergic cortical inhibition in humans. Brain Res. 2004;1028:1–8. doi: 10.1016/j.brainres.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Bussel B, Held JP, Katz R. Excitability of human motoneurones after discharge in a conditioning reflex. Electroencephalogr Clin Neurophysiol. 1976;40:279–87. doi: 10.1016/0013-4694(76)90151-6. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Burke D. Spinal and supraspinal control of movement. New York: Cambridge University Press; 2012. The circuitry of the human spinal cord; p. 606. [Google Scholar]
- Pinto AD, Chen R. Suppression of the motor cortex by magnetic stimulation of the cerebellum. Exp Brain Res. 2001;140:505–10. doi: 10.1007/s002210100862. [DOI] [PubMed] [Google Scholar]
- Pitcher JB, Ogston KM, Miles TS. Age and sex differences in human motor cortex input–output characteristics. J Physiol. 2003;546:605–13. doi: 10.1113/jphysiol.2002.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassman BL, Gandevia SC. High-voltage stimulation over the human spinal cord: sources of latency variation. J Neurol Neurosurg Psychiatry. 1989;52:213–7. doi: 10.1136/jnnp.52.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleger B, Janssen F, Schwenkreis P, Volker B, Maier C, Tegenthoff M. Repetitive transcranial magnetic stimulation of the motor cortex attenuates pain perception in complex regional pain syndrome type I. Neurosci Lett. 2004;356:87–90. doi: 10.1016/j.neulet.2003.11.037. [DOI] [PubMed] [Google Scholar]
- Pötter-Nerger M, Fischer S, Mastroeni C, Groppa S, Deuschl G, Volkmann J, et al. Inducing homeostatic-like plasticity in human motor cortex through converging corticocortical inputs. J Neurophysiol. 2009;102:3180–90. doi: 10.1152/jn.91046.2008. [DOI] [PubMed] [Google Scholar]
- Premoli I, Castellanos N, Rivolta D, Belardinelli P, Bajo R, Zipser C, et al. TMS–EEG signatures of GABAergic neurotransmission in the human cortex. J Neurosci. 2014;34:5603–12. doi: 10.1523/JNEUROSCI.5089-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priori A, Bertolasi L, Dressler D, Rothwell JC, Day BL, Thompson PD, et al. Transcranial electric and magnetic stimulation of the leg area of the human motor cortex: single motor unit and surface EMG responses in the tibialis anterior muscle. Electroencephalogr Clin Neurophysiol. 1993;89:131–7. doi: 10.1016/0168-5597(93)90095-7. [DOI] [PubMed] [Google Scholar]
- Qi F, Wu AD, Schweighofer N. Fast estimation of transcranial magnetic stimulation motor threshold. Brain Stimul. 2011;4:50–7. doi: 10.1016/j.brs.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Bagnato S, Rizzo V, Siebner HR, Dattola V, Scalfari A, et al. Abnormal associative plasticity of the human motor cortex in writer’s cramp. Brain. 2003;126:2586–96. doi: 10.1093/brain/awg273. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Rizzo V, Bagnato S, Morgante F, Sant’Angelo A, Girlanda P, et al. Rapid-rate paired associative stimulation of the median nerve and motor cortex can produce long-lasting changes in motor cortical excitability in humans. J Physiol. 2006;575:657–70. doi: 10.1113/jphysiol.2006.114025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragazzoni A, Pirulli C, Veniero D, Feurra M, Cincotta M, Giovannelli F, et al. Vegetative versus minimally conscious states: a study using TMS–EEG, sensory and event-related potentials. PLoS One. 2013;8:e57069. doi: 10.1371/journal.pone.0057069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranck JB., Jr Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res. 1975;98:417–40. doi: 10.1016/0006-8993(75)90364-9. [DOI] [PubMed] [Google Scholar]
- Ravnborg M, Blinkenberg M, Dahl K. Standardization of facilitation of compound muscle action potentials evoked by magnetic stimulation of the cortex. Results in healthy volunteers and in patients with multiple sclerosis. Electroencephalogr Clin Neurophysiol. 1991;81:195–201. doi: 10.1016/0168-5597(91)90072-6. [DOI] [PubMed] [Google Scholar]
- Reato D, Rahman A, Bikson M, Parra LC. Effects of weak transcranial alternating current stimulation on brain activity-a review of known mechanisms from animal studies. Front Hum Neurosci. 2013;7:687. doi: 10.3389/fnhum.2013.00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reutens DC, Berkovic SF. Increased cortical excitability in generalised epilepsy demonstrated with transcranial magnetic stimulation [Letter] Lancet. 1992;339:362–3. doi: 10.1016/0140-6736(92)91679-3. [DOI] [PubMed] [Google Scholar]
- Reutens DC, Puce A, Berkovic SF. Cortical hyperexcitability in progressive myoclonus epilepsy: a study with transcranial magnetic stimulation. Neurology. 1993;43:186–92. doi: 10.1212/wnl.43.1_part_1.186. [DOI] [PubMed] [Google Scholar]
- Richter L, Neumann G, Oung S, Schweikard A, Trillenberg P. Optimal coil orientation for transcranial magnetic stimulation. PLoS One. 2013;8:e60358. doi: 10.1371/journal.pone.0060358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridding MC, Inzelberg R, Rothwell JC. Changes in excitability of motor cortical circuitry in patients with Parkinson’s disease. Ann Neurol. 1995a;37:181–8. doi: 10.1002/ana.410370208. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Taylor JL, Rothwell JC. The effect of voluntary contraction on corticocortical inhibition in human motor cortex. J Physiol (Lond) 1995b;487:541–8. doi: 10.1113/jphysiol.1995.sp020898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson EM, Théoret H, Pascual-Leone A. Studies in cognition: the problems solved and created by transcranial magnetic stimulation. J Cogn Neurosci. 2003;15:948–60. doi: 10.1162/089892903770007344. [DOI] [PubMed] [Google Scholar]
- Rogić M, Deletis V, Fernández-Conejero I. Inducing transient language disruptions by mapping of Broca’s area with modified patterned repetitive transcranial magnetic stimulation protocol. J Neurosurg. 2014;120:1033–41. doi: 10.3171/2013.11.JNS13952. [DOI] [PubMed] [Google Scholar]
- Roick H, von Giesen HJ, Benecke R. On the origin of the postexcitatory inhibition seen after transcranial magnetic brain stimulation in awake human subjects. Exp Brain Res. 1993;94:489–98. doi: 10.1007/BF00230207. [DOI] [PubMed] [Google Scholar]
- Rosanova M, Casali A, Bellina V, Resta F, Mariotti M, Massimini M. Natural frequencies of human corticothalamic circuits. J Neurosci. 2009;29:7679–85. doi: 10.1523/JNEUROSCI.0445-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz K, Rothwell JC. Differences between the effects of three plasticity inducing protocols on the organization of the human motor cortex. Eur J Neurosci. 2006;23:822–9. doi: 10.1111/j.1460-9568.2006.04605.x. [DOI] [PubMed] [Google Scholar]
- Roshan L, Paradiso GO, Chen R. Two phases of short-interval intracortical inhibition. Exp Brain Res. 2003;151:330–7. doi: 10.1007/s00221-003-1502-9. [DOI] [PubMed] [Google Scholar]
- Rösler J, Niraula B, Strack V, Zdunczyk A, Schilt S, Savolainen P, et al. Language mapping in healthy volunteers and brain tumor patients with a novel navigated TMS system: evidence of tumor-induced plasticity. Clin Neurophysiol. 2014;125:526–36. doi: 10.1016/j.clinph.2013.08.015. [DOI] [PubMed] [Google Scholar]
- Rossi S, Cappa SF, Babiloni C, Pasqualetti P, Miniussi C, Carducci F, et al. Prefrontal cortex in long-term memory: an “interference” approach using magnetic stimulation. Nat Neurosci. 2001;4:948–52. doi: 10.1038/nn0901-948. [DOI] [PubMed] [Google Scholar]
- Rossi S, Miniussi C, Pasqualetti P, Babiloni C, Rossini PM, Cappa SF. Age-related functional changes of prefrontal cortex in long-term memory: a repetitive transcranial magnetic stimulation study. J Neurosci. 2004;24:7939–44. doi: 10.1523/JNEUROSCI.0703-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A Safety of TMS consensus group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–39. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Marciani MG, Caramia M, Roma V, Zarola F. Nervous propagation along ‘central’ motor pathways in intact man: characteristics of motor responses to ‘bifocal’ and ‘unifocal’ spine and scalp non-invasive stimulation. Electroencephalogr Clin Neurophysiol. 1985;61:272–86. doi: 10.1016/0013-4694(85)91094-6. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Marciani MG, Caramia M, Hassan NF, Cracco RQ. Transcutaneous stimulation of motor cerebral cortex and spine: non invasive evaluation of central afferent transmission. New York: Alan Liss; 1986. pp. 76–84. [Google Scholar]
- Rossini PM, Caramia M, Zarola F. Central motor tract propagation in man: studies with non-invasive, unifocal, scalp stimulation. Brain Res. 1987a;415:211–25. doi: 10.1016/0006-8993(87)90203-4. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Caramia M, Zarola F. Mechanisms of nervous propagation along central motor pathways: non invasive evaluation in healthy subjects and in patients with neurological disease. Neurosurgery. 1987b;20:183–91. doi: 10.1097/00006123-198701000-00035. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Zarola F, Stalberg E, Caramia M. Pre-movement facilitation of motor-evoked potentials in man during transcranial stimulation of the central motor pathways. Brain Res. 1988;458:20–30. doi: 10.1016/0006-8993(88)90491-x. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Desiato MT, Caramia MD. Age-related changes of motor evoked potentials in healthy humans: noninvasive evaluation of central and peripheral motor tracts excitability and conductivity. Brain Res. 1992;593:14–9. doi: 10.1016/0006-8993(92)91256-e. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Caramia MD. Central conduction studies and magnetic stimulation. Curr Opin Neurol Neurosurg. 1992;5:697–703. [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Caramia MD, Iani C, Desiato MT, Sciarretta G, Bernardi G. Magnetic transcranial stimulation in healthy humans: influence on the behavior of upper limb motor units. Brain Res. 1995;676:314–24. doi: 10.1016/0006-8993(95)00113-5. [DOI] [PubMed] [Google Scholar]
- Rotem A, Neef A, Neef NE, Agudelo-Toro A, Rakhmilevitch D, Paulus W, et al. Solving the orientation specific constraints in transcranial magnetic stimulation by rotating fields. PLoS One. 2014;9:e86794. doi: 10.1371/journal.pone.0086794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothkegel H, Sommer M, Paulus W. Breaks during 5Hz rTMS are essential for facilitatory after effects. Clin Neurophysiol. 2010;121:426–30. doi: 10.1016/j.clinph.2009.11.016. [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Thompson PD, Day BL, Dick JPR, Kachi T, Cowan JMA, et al. Motor cortex stimulation in intact man. General characteristics of EMG responses in different muscles. Brain. 1987;110:1173–90. doi: 10.1093/brain/110.5.1173. [DOI] [PubMed] [Google Scholar]
- Ruohonen J, Karhu J. Navigated transcranial magnetic stimulation. Neurophysiol Clin. 2010;40:7–17. doi: 10.1016/j.neucli.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Rusu CV, Murakami M, Ziemann U, Triesch J. A model of TMS-induced I-waves in motor cortex. Brain Stimul. 2014;7:401–14. doi: 10.1016/j.brs.2014.02.009. [DOI] [PubMed] [Google Scholar]
- Saitoh Y, Hirayama A, Kishima H, Oshino S, Hirata M, Kato A, et al. Stimulation of primary motor cortex for intractable deafferentation pain. Acta Neurochir Suppl. 2006;99:57–9. doi: 10.1007/978-3-211-35205-2_11. [DOI] [PubMed] [Google Scholar]
- Saitoh Y, Hirayama A, Kishima H, Shimokawa T, Oshino S, Hirata M, et al. Reduction of intractable deafferentation pain due to spinal cord or peripheral lesion by high-frequency repetitive transcranial magnetic stimulation of the primary motor cortex. J Neurosurg. 2007;107:555–9. doi: 10.3171/JNS-07/09/0555. [DOI] [PubMed] [Google Scholar]
- Sakai K, Ugawa Y, Terao Y, Hanajima R, Furabayashi T, Kanazawa I. Preferential activation of different I waves by transcranial magnetic stimulation with a figure-of-eight-shaped coil. Exp Brain Res. 1997;113:24–32. doi: 10.1007/BF02454139. [DOI] [PubMed] [Google Scholar]
- Salvador R, Silva S, Basser PJ, Miranda PC. Determining which mechanisms lead to activation in the motor cortex: a modeling study of transcranial magnetic stimulation using realistic stimulus waveforms and sulcal geometry. Clin Neurophysiol. 2011;122:748–58. doi: 10.1016/j.clinph.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrini M, Umiltà C, Rusconi E. The use of transcranial magnetic stimulation in cognitive neuroscience: a new synthesis of methodological issues. Neurosci Biobehav Rev. 2011;35:516–36. doi: 10.1016/j.neubiorev.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Sanger TD, Garg RR, Chen R. Interactions between two different inhibitory systems in the human motor cortex. J Physiol (Lond) 2001;530:307–17. doi: 10.1111/j.1469-7793.2001.0307l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarasso S, Rosanova M, Casali AG, Casarotto S, Fecchio M, Boly M, et al. Quantifying cortical EEG responses to TMS in (un)consciousness. Clin EEG Neurosci. 2014;45:40–9. doi: 10.1177/1550059413513723. [DOI] [PubMed] [Google Scholar]
- Sauve WM, Crowther LJ. The science of transcranial magnetic stimulation. Psychiat Ann. 2014;44:279–83. [Google Scholar]
- Schlaepfer TE, George MS, Mayberg H. WFSBP guidelines on brain stimulation treatments in psychiatry. World J Biol Psychiatry. 2010;11:2–18. doi: 10.3109/15622970903170835. [DOI] [PubMed] [Google Scholar]
- Seyal M, Masuoka LK, Browne JK. Suppression of cutaneous perception by magnetic pulse stimulation of the human brain. Electroencephol Clin Neurophysiol. 1992;85:397–401. doi: 10.1016/0168-5597(92)90053-e. [DOI] [PubMed] [Google Scholar]
- Shafi MM, Brandon Westover M, Oberman L, Cash SS, Pascual-Leone A. Modulation of EEG functional connectivity networks in subjects undergoing repetitive transcranial magnetic stimulation. Brain Topogr. 2014;27:172–91. doi: 10.1007/s10548-013-0277-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirota Y, Hanajima R, Hamada M, Terao Y, Matsumoto H, Tsutsumi R, et al. Inter individual variation in the efficient stimulation site for magnetic brainstem stimulation. Clin Neurophysiol. 2011;122:2044–8. doi: 10.1016/j.clinph.2011.03.025. [DOI] [PubMed] [Google Scholar]
- Short EB, Borckardt JJ, Anderson BS, Frohman H, Beam W, Reeves ST, et al. Ten sessions of adjunctive left prefrontal rTMS significantly reduces fibromyalgia pain: a randomized, controlled pilot study. Pain. 2011;152:2477–84. doi: 10.1016/j.pain.2011.05.033. See comment in PubMed Commons below. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebner HR, Dressnandt J, Auer C, Conrad B. Continuous intrathecal baclofen infusions induced a marked increase of the transcranially evoked silent period in a patient with generalized dystonia. Muscle Nerve. 1998;21:1209–12. doi: 10.1002/(sici)1097-4598(199809)21:9<1209::aid-mus15>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Tormos JM, Ceballos-Baumann AO, Auer C, Catala MD, Conrad B, et al. Low-frequency repetitive transcranial magnetic stimulation of the motor cortex in writer’s cramp. Neurology. 1999;52:529–37. doi: 10.1212/wnl.52.3.529. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Rothwell J. Transcranial magnetic stimulation: new insights into representational cortical plasticity. Exp Brain Res. 2003;148:1–16. doi: 10.1007/s00221-002-1234-2. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Bergmann TO, Bestmann S, Massimini M, Johansen-Berg H, Mochizuki H, et al. Consensus paper: combining transcranial stimulation with neuroimaging. Brain Stimul. 2009;2:58–80. doi: 10.1016/j.brs.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Silbert BI, Patterson HI, Pevcic DD, Windnagel KA, Thickbroom GW. A comparison of relative-frequency and threshold hunting methods to determine stimulus intensity in transcranial magnetic stimulation. Clin Neurophysiol. 2013;124:708–12. doi: 10.1016/j.clinph.2012.09.018. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Muggleton N, Walsh V. State-dependency in brain stimulation studies of perception and cognition. Trends Cogn Sci. 2008;12:447–54. doi: 10.1016/j.tics.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Sommer M, Alfaro A, Rummel M, Speck S, Lang N, Tings T, et al. Half sine, monophasic and biphasic transcranial magnetic stimulation of the human motor cortex. Clin Neurophysiol. 2006;117:838–44. doi: 10.1016/j.clinph.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Sommer M, Norden C, Schmack L, Rothkegel H, Lang N, Paulus W. Opposite optimal current flow directions for induction of neuroplasticity and excitation threshold in the human motor cortex. Brain Stimul. 2013;6:363–70. doi: 10.1016/j.brs.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Sparing R, Buelte D, Meister IG, Paus T, Fink FG. Transcranial magnetic stimulation and the challenge of coil placement: a comparison of conventional and stereotaxic neuronavigational strategies. Human Brain Map. 2008;29:82–96. doi: 10.1002/hbm.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Bestmann S, Constantinescu AO, Moreno LM, Allman C, Mekle R, et al. Relationship between physiological measures of excitability and levels of glutamate and GABA in the human motor cortex. J Physiol. 2011;1:5845–55. doi: 10.1113/jphysiol.2011.216978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr A, Caramia M, Zarola F, Rossini PM. Enhancement of motor cortical excitability in humans by non-invasive\electrical stimulation appears prior to voluntary movement. Electroencephalogr Clin Neurophysiol. 1988;70:26–32. doi: 10.1016/0013-4694(88)90191-5. [DOI] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123:572–84. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Benecke R, Cohen LG, Classen J. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J Physiol. 2002;543:699–708. doi: 10.1113/jphysiol.2002.023317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Wycislo M, Classen J. Modulation of associative human motor cortical plasticity by attention. J Neurophysiol. 2004;92:66–72. doi: 10.1152/jn.00383.2003. [DOI] [PubMed] [Google Scholar]
- Stetkarova I, Kofler M. Differential effect of baclofen on cortical and spinal inhibitory circuits. Clin Neurophysiol. 2013;124:339–45. doi: 10.1016/j.clinph.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Stewart L, Walsh V, Frith U, Rothwell JC. TMS produces two dissociable types of speech disruption. Neuroimage. 2001a;13:472–8. doi: 10.1006/nimg.2000.0701. [DOI] [PubMed] [Google Scholar]
- Stewart L, Walsh V, Rothwell JC. Motor and phosphene thresholds: a transcranial magnetic stimulation correlation study. Neuropsychologia. 2001b;39:415–9. doi: 10.1016/s0028-3932(00)00130-5. [DOI] [PubMed] [Google Scholar]
- Suppa A, Ortu E, Zafar N, Deriu F, Paulus W, Berardelli A, et al. Theta burst stimulation induces after-effects on contralateral primary motor cortex excitability in humans. J Physiol. 2008;586:4489–500. doi: 10.1113/jphysiol.2008.156596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Kirimoto H, Onishi H, Yamada S, Tamaki H, Maruyama A, et al. Reciprocal changes in input–output curves of motor evoked potentials while learning motor skills. Brain Res. 2012;1473:114–23. doi: 10.1016/j.brainres.2012.07.043. [DOI] [PubMed] [Google Scholar]
- Tabaraud F, Hugon J, Salle J, Boulesteix J, Vallat J, Dumas M. Study of central motor pathways using cortical magnetic stimulation and spinal electrical stimulation: results in 20 normal subjects. Rev Neurol (Paris) 1989;145:690. [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- Talelli P, Cheeran BJ, Teo JT, Rothwell JC. Pattern-specific role of the current orientation used to deliver Theta Burst Stimulation. Clin Neurophysiol. 2007;118:1815–23. doi: 10.1016/j.clinph.2007.05.062. [DOI] [PubMed] [Google Scholar]
- Tarapore PE, Findlay AM, Honma SM, Mizuiri D, Houde JF, Berger MS, et al. Language mapping with navigated repetitive TMS: proof of technique and validation. Neuroimage. 2013;82:260–72. doi: 10.1016/j.neuroimage.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Loo CK. Stimulus waveform influences the efficacy of repetitive transcranial magnetic stimulation. J Affect Disord. 2007;97:271–6. doi: 10.1016/j.jad.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Terao Y, Ugawa Y, Sakai K, Uesaka Y, Kohara N, Kanazawa I. Transcranial stimulation of the leg area of the motor cortex in humans. Acta Neurol Scand. 1994;89:378–83. doi: 10.1111/j.1600-0404.1994.tb02650.x. [DOI] [PubMed] [Google Scholar]
- Terao Y, Ugawa Y, Hanajima R, Machii K, Furubayashi T, Mochizuki H, et al. Predominant activation of I1-waves from the leg motor area by transcranial magnetic stimulation. Brain Res. 2000;859:137–46. doi: 10.1016/s0006-8993(00)01975-2. [DOI] [PubMed] [Google Scholar]
- Terao Y, Ugawa Y. Basic mechanisms of TMS. J Clin Neurophysiol. 2002;19:322–43. doi: 10.1097/00004691-200208000-00006. [DOI] [PubMed] [Google Scholar]
- Thickbroom GW, Byrnes ML, Mastaglia FL. Methodology and application of TMS mapping. Electroencephalogr Clin Neurophysiol Suppl. 1999;51:48–54. [PubMed] [Google Scholar]
- Thielscher A, Opitz A, Windhoff M. Impact of the gyral geometry on the electric field induced by transcranial magnetic stimulation. NeuroImage. 2011;54:234–43. doi: 10.1016/j.neuroimage.2010.07.061. [DOI] [PubMed] [Google Scholar]
- Thompson PD, Day BL, Crockard HA, Calder I, Murray NM, Rothwell JC, et al. Intra-operative recording of motor tract potentials at the cervico-medullary junction following scalp electrical and magnetic stimulation of the motor cortex. J Neurol Neurosurg Psychiatry. 1991;54:618–23. doi: 10.1136/jnnp.54.7.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G, Veniero D, Romei V, Miniussi C, Schyns P, Gross J. Rhythmic TMS causes local entrainment of natural oscillatory signatures. Curr Biol. 2011;21:1176–85. doi: 10.1016/j.cub.2011.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobimatsu S, Sun SJ, Fukui R, Kato M. Effects of sex, height and age on motor evoked potentials with magnetic stimulation. J Neurol. 1998;245:256–61. doi: 10.1007/s004150050215. [DOI] [PubMed] [Google Scholar]
- Tofts PS, Branston NM. The measurement of electric field, and the influence of surface charge, in magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1991;81:238–9. doi: 10.1016/0168-5597(91)90077-b. [DOI] [PubMed] [Google Scholar]
- Tokimura H, Ridding MC, Tokimura Y, Amassian VE, Rothwell JC. Short latency facilitation between pairs of threshold magnetic stimuli applied to human motor cortex. Electroencephalogr Clin Neurophysiol. 1996;103:263–72. doi: 10.1016/0924-980x(96)95664-7. [DOI] [PubMed] [Google Scholar]
- Tokimura H, Di Lazzaro V, Tokimura Y, Oliviero A, Profice P, Insola A, et al. Short latency inhibition of human hand motor cortex by somatosensory input from the hand. J Physiol (Lond) 2000;523:03–513. doi: 10.1111/j.1469-7793.2000.t01-1-00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toleikis JR, Sloan TB, Ronai AK. Optimal transcranial magnetic stimulation sites for the assessment of motor function. Electroencephalogr Clin Neurophysiol. 1991;81:443–9. doi: 10.1016/0013-4694(91)90006-p. [DOI] [PubMed] [Google Scholar]
- Tomberg C, Caramia MD. Prime mover muscle in finger lift or finger flexion reaction times: identification with transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1991;81:319–22. doi: 10.1016/0168-5597(91)90019-t. [DOI] [PubMed] [Google Scholar]
- Traversa R, Cicinelli P, Bassi A, Rossini PM, Bernardi G. Mapping of motor cortical reorganization after stroke. A brain stimulation study with focal magnetic pulses. Stroke. 1997;28:110–7. doi: 10.1161/01.str.28.1.110. [DOI] [PubMed] [Google Scholar]
- Traversa R, Cicinelli P, Pasqualetti P, Filippi M, Rossini PM. Follow-up of interhemispheric differences of motor evoked potentials from the ‘affected’ and ‘unaffected’ hemispheres in human stroke. Brain Res. 1998;803:1–8. doi: 10.1016/s0006-8993(98)00505-8. [DOI] [PubMed] [Google Scholar]
- Triggs WJ, Calvanio R, Macdonell RA, Cros D, Chiappa KH. Physiological motor asymmetry in human handedness: evidence from transcranial magnetic stimulation. Brain Res. 1994;636:270–6. doi: 10.1016/0006-8993(94)91026-x. [DOI] [PubMed] [Google Scholar]
- Tsubokawa T, Katayama Y, Yamamoto T, Hirayama T, Koyama S. Chronic motor cortex stimulation for the treatment of central pain. Acta Neurochir Suppl (Wien) 1991;52:137–9. doi: 10.1007/978-3-7091-9160-6_37. [DOI] [PubMed] [Google Scholar]
- Ueno S, Matsuda T. Magnetic stimulation of the human brain. Ann NY Acad Sci. 1992;31:366–8. doi: 10.1111/j.1749-6632.1992.tb49632.x. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Kohara N, Shimpo T, Mannen T. Central motor and sensory conduction in adrenoleukomyeloneuropathy, cerebrotendinous xanthomatosis, HTLV-1-associated myelopathy and tabes dorsalis. J Neurol Neurosurg Psychiatry. 1988a;51:1069–74. doi: 10.1136/jnnp.51.8.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugawa Y, Shimpo T, Mannen T. Central motor conduction in cerebrovascular disease and motor neuron disease. Acta Neurol Scand. 1988b;78:297–306. doi: 10.1111/j.1600-0404.1988.tb03660.x. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Genba K, Shimpo T, Mannen T. Physiologic analysis of central motor pathways–simultaneous recording from multiple relaxed muscles. Eur Neurol. 1989a;29:135–40. doi: 10.1159/000116396. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Rothwell JC, Day BL, Thompson PD, Marsden CD. Magnetic stimulation over the spinal enlargements. J Neurol Neurosurg Psychiatry. 1989b;52:1025–32. doi: 10.1136/jnnp.52.9.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugawa Y, Kohara N, Shimpo T, Mannen T. Magneto-electrical stimulation of central motor pathways compared with percutaneous electrical stimulation. Eur Neurol. 1990;30:14–8. doi: 10.1159/000116617. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Day BL, Rothwell JC, Thompson PD, Merton PA, Marsden CD. Modulation of motor cortical excitability by electrical stimulation over the cerebellum in man. J Physiol (Lond) 1991a;441:57–72. doi: 10.1113/jphysiol.1991.sp018738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugawa Y, Rothwell JC, Day BL, Thompson PD, Marsden CD. Percutaneous electrical stimulation of corticospinal pathways at the level of the pyramidal decussation in humans. Ann Neurol. 1991b;29:418–27. doi: 10.1002/ana.410290413. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Genba K, Mannen T, Kanazawa I. Stimulation of corticospinal pathways at the level of the pyramidal decussation in neurological disorders. Brain. 1992;115:1947–61. doi: 10.1093/brain/115.6.1947. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Hanajima R, Kanazawa I. Interhemispheric facilitation of the hand area of the human motor cortex. Neurosci Letts. 1993;160:153–5. doi: 10.1016/0304-3940(93)90401-6. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Uesaka Y, Terao Y, Hanajima R, Kanazawa I. Magnetic stimulation of corticospinal pathways at the foramen magnum level in humans. Ann Neurol. 1994;36:618–24. doi: 10.1002/ana.410360410. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Genba K, Shimizu K, Kanazawa I. Electrical stimulation of the human descending motor tracts at several levels. Can J Neurol Sci. 1995a;22:36–42. doi: 10.1017/s0317167100040476. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Uesaka Y, Terao Y, Hanajima R, Kanazawa I. Magnetic stimulation over the cerebellum in humans. Ann Neurol. 1995b;37:703–13. doi: 10.1002/ana.410370603. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Uesaka Y, Terao Y, Suzuki M, Sakai K, Hanajima R, et al. Clinical utility of magnetic corticospinal tract stimulation at the foramen magnum level. Electroencephalogr Clin Neurophysiol. 1996;101:247–54. doi: 10.1016/0924-980x(96)95150-4. [DOI] [PubMed] [Google Scholar]
- Ugawa Y. Stimulation at the foramen magnum level. Electroencephalogr Clin Neurophysiol Suppl. 1999;51:65–75. [PubMed] [Google Scholar]
- Ugawa Y, Kanazawa I. Motor-evoked potentials: unusual findings. Clin Neurophysiol. 1999;110:1641–5. doi: 10.1016/s1388-2457(99)00103-0. [DOI] [PubMed] [Google Scholar]
- Ugawa Y. Stimulation at the foramen magnum level as a tool to separate cortical from spinal cord excitability changes. Adv Clin Neurophysiol Suppl. 2002;54:216–22. [Google Scholar]
- Valls-Solé J, Pascual-Leone A, Wassermann EM, Hallett M. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol. 1992;85:355–64. doi: 10.1016/0168-5597(92)90048-g. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Pascual-Leone A, Brasil-Neto JP, Cammarota A, McShane L, Hallett M. Abnormal facilitation of the response to transcranial magnetic stimulation in patients with Parkinson’s disease. Neurology. 1994;44:735–41. doi: 10.1212/wnl.44.4.735. [DOI] [PubMed] [Google Scholar]
- Van Der Werf YD, Paus T. The neural response to transcranial magnetic stimulation of the human motor cortex. I. Intracortical and cortico-cortical contributions. Exp Brain Res. 2006;175:231–45. doi: 10.1007/s00221-006-0551-2. [DOI] [PubMed] [Google Scholar]
- Van Der Werf YD, Sadikot AF, Strafella AP, Paus T. The neural response to transcranial magnetic stimulation of the human motor cortex. II. Thalamocortical contributions. Exp Brain Res. 2006;175:246–55. doi: 10.1007/s00221-006-0548-x. [DOI] [PubMed] [Google Scholar]
- Veniero D, Bortoletto M, Miniussi C. TMS–EEG co-registration: on TMS-induced artifact. Clin Neurophysiol. 2009;120:1392–9. doi: 10.1016/j.clinph.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Veniero D, Maioli C, Miniussi C. Potentiation of short-latency cortical responses by high-frequency repetitive transcranial magnetic stimulation. J Neurophysiol. 2010;3:1578–88. doi: 10.1152/jn.00172.2010. [DOI] [PubMed] [Google Scholar]
- Veniero D, Brignani D, Thut G, Miniussi C. Alpha-generation as basic response-signature to transcranial magnetic stimulation (TMS) targeting the human resting motor cortex: a TMS/EEG co-registration study. Psychophysiology. 2011;48:1381–9. doi: 10.1111/j.1469-8986.2011.01218.x. [DOI] [PubMed] [Google Scholar]
- Veniero D, Bortoletto M, Miniussi C. Cortical modulation of short-latency TMS-evoked potentials. Front Hum Neurosci. 2013;6:352. doi: 10.3389/fnhum.2012.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernet M, Bashir S, Yoo WK, Perez JM, Najib U, Pascual-Leone A. Insights on the neural basis of motor plasticity induced by theta burst stimulation from TMS–EEG. Eur J Neurosci. 2013;37:598–606. doi: 10.1111/ejn.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernet M, Bashir S, Yoo WK, Oberman L, Mizrahi I, Ifert-Miller F, et al. Reproducibility of the effects of theta burst stimulation on motor cortical plasticity in healthy participants. Clin Neurophysiol. 2014;125:320–6. doi: 10.1016/j.clinph.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen J, Ruohonen J, Naatanen R, Ilmoniemi RJ. Instrumentation for the measurement of electric brain responses to transcranial magnetic stimulation. Med Biol Eng Comput. 1999;37:322–6. doi: 10.1007/BF02513307. [DOI] [PubMed] [Google Scholar]
- Vucic S, Cairns KD, Black KR, Siao Tick Chong P, Cros D. Cervical nerve root stimulation. Part I: technical aspects and normal data. Clin Neurophysiol. 2006;117:392–7. doi: 10.1016/j.clinph.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Vucic S, Kiernan MC. Abnormalities in cortical and peripheral excitability in flail arm variant amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2007;78:849–52. doi: 10.1136/jnnp.2006.105056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucic S, Ziemann U, Eisen A, Hallett M, Kiernan MC. Transcranial magnetic stimulation and amyotrophic lateral sclerosis: pathophysiological insights. J Neurol Neurosurg Psychiatry. 2013;84:1161–70. doi: 10.1136/jnnp-2012-304019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner T, Valero-Cabre A, Pascual-Leone A. Noninvasive human brain stimulation. Annu Rev Biomed Eng. 2007;9:527–65. doi: 10.1146/annurev.bioeng.9.061206.133100. [DOI] [PubMed] [Google Scholar]
- Wahl M, Lauterbach-Soon B, Hattingen E, Jung P, Singer O, Volz S, et al. Human motor corpus callosum: topography, somatotopy, and link between microstructure and function. J Neurosci. 2007;27:12132–8. doi: 10.1523/JNEUROSCI.2320-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh V, Ellison A, Battelli L, Cowey A. Task-specific impairments and enhancements induced by magnetic stimulation of human visual area V5. Proc Biol Sci. 1998;265:537–43. doi: 10.1098/rspb.1998.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh V, Cowey A. Transcranial magnetic stimulation and cognitive neuroscience. Nat Rev Neurosci. 2000;1:73–9. doi: 10.1038/35036239. [DOI] [PubMed] [Google Scholar]
- Walsh V, Pascual-Leone A. Transcranial magnetic stimulation: a neurochronometrics of mind. Cambridge: MIT Press; 2003. [Google Scholar]
- Ward NS, Newton JM, Swayne OB, Lee L, Thompson AJ, Greenwood RJ, et al. Motor system activation after subcortical stroke depends on corticospinal system integrity. Brain. 2006;129:809–19. doi: 10.1093/brain/awl002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann EM, McShane LM, Hallett M, Cohen LG. Noninvasive mapping of muscle representations in human motor cortex. Electroencephalogr Clin Neurophysiol. 1992;85:1–8. doi: 10.1016/0168-5597(92)90094-r. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Pascual-Leone A, Valls-Sole J, Toro C, Cohen LG, Hallett M. Topography of the inhibitory and excitatory responses to transcranial magnetic stimulation in a hand muscle. Electroencephalogr Clin Neurophysiol. 1993;89:424–33. doi: 10.1016/0168-5597(93)90116-7. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Pascual-Leone A, Hallett M. Cortical motor representation of the ipsilateral hand and arm. Exp Brain Res. 1994;100:121–32. doi: 10.1007/BF00227284. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Samii A, Mercuri B, Ikoma K, Oddo D, Grill SE, et al. Responses to paired transcranial magnetic stimuli in resting, active, and recently activated muscle. Exp Brain Res. 1996;109:158–63. doi: 10.1007/BF00228638. [DOI] [PubMed] [Google Scholar]
- Wassermann EM. Variation in the response to transcranial magnetic brain stimulation in the general population. Clin Neurophysiol. 2002;113:1165–71. doi: 10.1016/s1388-2457(02)00144-x. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Epstein CM, Ziemann U. The oxford handbook of transcranial stimulation. New York: Oxford University Press; 2008. p. 747. [Google Scholar]
- Watanabe T, Hanajima R, Shirota Y, Ohminami S, Tsutsumi R, Terao Y, et al. Bidirectional effects on inter-hemispheric resting state functional connectivity induced by excitatory and inhibitory repetitive transcranial magnetic stimulation. Hum Brain Mapp. 2014;35:1896–905. doi: 10.1002/hbm.22300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werhahn KJ, Fong JK, Meyer BU, Priori A, Rothwell JC, Day BL, et al. The effect of magnetic coil orientation on the latency of surface EMG and single motor unit responses in the first dorsal interosseous muscle. Electroencephalogr Clin Neurophysiol. 1994;93:138–46. doi: 10.1016/0168-5597(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Taylor J, Ridding M, Meyer BU, Rothwell JC. Effect of transcranial magnetic stimulation over the cerebellum on the excitability of human motor cortex. Electroencephalogr Clin Neurophysiol. 1996;101:58–66. doi: 10.1016/0013-4694(95)00213-8. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol (Lond) 1999;517:591–7. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin GG, Bassi BD, Lisanby SH, Luber B New York State Psychiatric Institute, NY, USA. Determination of motor threshold using visual observation overestimates transcranial magnetic stimulation dosage: safety implications. Clin Neurophysiol. 2014;125:142–7. doi: 10.1016/j.clinph.2013.06.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SA, Thickbroom GW, Mastaglia FL. Transcranial magnetic stimulation mapping of the motor cortex in normal subjects. The representation of two intrinsic hand muscles. J Neurol Sci. 1993;118:134–44. doi: 10.1016/0022-510x(93)90102-5. [DOI] [PubMed] [Google Scholar]
- Wochnik-Dyjas D, Glazowski C, Niewiadomska M. Segmental conduction times in the motor nervous system. Electromyogr Clin Neurophysiol. 1997;37:155–67. [PubMed] [Google Scholar]
- Wolters A, Sandbrink F, Schlottmann A, Kunesch E, Stefan K, Cohen LG, et al. A temporally asymmetric Hebbian rule governing plasticity in the human motor cortex. J Neurophysiol. 2003;89:2339–45. doi: 10.1152/jn.00900.2002. [DOI] [PubMed] [Google Scholar]
- Wolters A, Schmidt A, Schramm A, Zeller D, Naumann M, Kunesch E, et al. Timing dependent plasticity in human primary somatosensory cortex. J Physiol. 2005;565:1039–52. doi: 10.1113/jphysiol.2005.084954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SW, Shahana N, Huddleston DA, Gilbert DL. Effects of 30Hz theta burst transcranial magnetic stimulation on the primary motor cortex. J Neurosci Methods. 2012;208:161–4. doi: 10.1016/j.jneumeth.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangen A, Roth Y, Voller B, Hallett M. Transcranial magnetic stimulation of deep brain regions: evidence for efficacy of the H-coil. Clin Neurophysiol. 2005;116:775–9. doi: 10.1016/j.clinph.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lönnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol. 1996a;40:367–78. doi: 10.1002/ana.410400306. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol (Lond) 1996b;496:873–81. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Tergau F, Wassermann EM, Wischer S, Hildebrandt J, Paulus W. Demonstration of facilitatory I wave interaction in the human motor cortex by paired transcranial magnetic stimulation. J Physiol (Lond) 1998;511:181–90. doi: 10.1111/j.1469-7793.1998.181bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Ishii K, Borgheresi A, Yaseen Z, Battaglia F, Hallett M, et al. Dissociation of the pathways mediating ipsilateral and contralateral motor-evoked potentials in human hand and arm muscles. J Physiol. 1999;518:895–906. doi: 10.1111/j.1469-7793.1999.0895p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC. I-waves in motor cortex. J Clin Neurophysiol. 2000;17:397–405. doi: 10.1097/00004691-200007000-00005. [DOI] [PubMed] [Google Scholar]
- Ziemann U. TMS and drugs. Clin Neurophysiol. 2004;115:1717–29. doi: 10.1016/j.clinph.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Paulus W, Nitsche MA, Pascual-Leone A, Byblow WD, Berardelli A, et al. Consensus: motor cortex plasticity protocols. Brain Stimul. 2008;1:164–82. doi: 10.1016/j.brs.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Ziemann U. Transcranial magnetic stimulation at the interface with other techniques: a powerful tool for studying the human cortex. Neuroscientist. 2011;17:368–81. doi: 10.1177/1073858410390225. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Reis J, Schwenkreis P, Rosanova M, Strafella A, Badawy R, et al. TMS & drugs revisited. Clin Neurophysiol. 2014 doi: 10.1016/j.clinph.2014.08.028. pii: S1388-2457(14)00837-2. [DOI] [PubMed] [Google Scholar]