Abstract
A 47-year-old man was referred to our hospital with a 1-month history of fever and dyspnea after inhalation of insecticide in a confined space. We diagnosed rapidly progressive interstitial pneumonia. High-dose methylprednisolone, tacrolimus, and intermittent infusion of cyclophosphamide were administered. His condition rapidly deteriorated; therefore, extracorporeal membrane oxygenation therapy was performed. Unfortunately, he died 69 days after admission. Although typical skin findings suggestive of dermatomyositis were absent, anti-melanoma differentiation-associate gene (anti-MDA5) antibody was positive. Our findings suggest that in patients with hyperferritinemia and rapidly progressive interstitial lung disease (RP-ILD) demonstrating random ground glass shadows and peripheral consolidations by high-resolution computed tomography (HRCT) even if skin manifestations related to dermatomyositis are not complicated, we should assume anti-MDA5 antibody-positive interstitial pneumonia.
Keywords: Anti-melanoma differentiation-associated gene 5 antibody, Rapidly progressive interstitial pneumonia, Clinical amyopathic dermatomyositis, Extracorporeal membrane oxygenation
Abbreviations: Anti-MDA5, anti-melanoma differentiation-associate gene; ARS, anti-aminoacyl-tRNA synthetase; CADM, clinically amyopathic dermatomyositis; IVCY, intravenous cyclophosphamide; HRCT, high-resolution computed tomography; RIG-I, retinoic acid inducible gene-I; RP-ILD, rapidly progressive interstitial lung disease
1. Introduction
Clinically amyopathic dermatomyositis (CADM) is a subset of dermatomyositis showing typical skin manifestations without myositis. Patients with CADM do not have apparent muscle weakness or elevated serum muscle enzymes. CADM may be complicated by rapidly progressive interstitial lung disease (RP-ILD) [1,2]. Approximately 60–70% of CADM patients have anti-melanoma differentiation-associated gene 5 (anti-MDA5) antibodies. To date, the mechanism of the production of anti-MDA5 antibodies and their pathogenic roles has not been fully elucidated [3]. The mortality rate of anti-MDA5-positive patients with respiratory failure is high. Rapid intervention with immunosuppressive therapy including high-dose corticosteroids, cyclosporine, and intermittent intravenous cyclophosphamide (IVCY) is recommended immediately after diagnosis of RP-ILD with anti-MDA5 antibody [3].
In this case, we report an anti-MDA5 antibody positive patient without the cutaneous symptoms of CADM but exhibiting treatment-resistant RP-ILD.
2. Case report
A 47-year-old Japanese man with a history of bronchial asthma and allergic rhinitis presented with cough and fever. He had no history of smoking and no family history of bronchial asthma or allergy. When helping with house cleaning, he was exposed to a large amount of pyrethroid insecticide in a confined space without a mask. Approximately 1 week after pyrethroid exposure, he complained of cough and fever and visited a neighboring doctor. No person had reported similar symptoms in the surrounding area. He was treated with azithromycin (500 mg/day) and garenoxacin (400 mg/day). His symptoms did not improve; therefore, he visited our hospital 4 weeks after the insecticide exposure. On admission, he was alert. His vital signs were: blood pressure, 110/68 mmHg; pulse rate, 113/min; and body temperature, 37.3 °C. Percutaneous arterial blood oxygen saturation was 98% on room air. Pharyngeal redness and hoarseness were found. Bilateral fine crackles at the lung base were observed. Superficial lymph node swelling was not found. Gottron signs, inverse Gottron signs, swelling, heliotrope rash, purpura, arthritis, and other physical findings suggestive of dermatomyositis were not observed. Muscle weakness was also not revealed according to the manual muscle testing.
On admission, his WBC count was 4900/μL, C-reactive protein was 3.53 mg/dL, and KL-6 was 542 U/mL (normal range, <500 U/mL) (Table 1).
Table 1.
Blood biochemical examination on admission.
| Admission blood test | |||
|---|---|---|---|
| Hematology | Biochemistry | ||
| WBC | 4900/μ L | AST | 84 U/L |
| Neutrophil | 89.10% | ALT | 32 U/L |
| Lymphocyte | 7.60% | LDH | 598 U/L |
| Monocyte | 3.30% | CK | 673 IU/L |
| Eosinophil | 0% | ALP | 242 U/L |
| Hb | 15.0 g/dL | Na | 132 mmol/L |
| Pit | 16.3 × 104 | K | 3.6 mmol/L |
| Cl | 97 mmol/L | ||
| BUN | 20.0 mg/dL | ||
| Cre | 1.08 mg, dL | ||
| CRP | 3.53 mg/dL | ||
| Arterial blood gas | Anti–nuclear antibody | x 80 | |
| (O2 2L nasal) | Anti–aminoacyl–tRNA Synthetase | negative | |
| pH | 7.454 | KL–6 | 542 U/mL |
| PaO2 | 125.2 Torr | SP–D | 30.6 ng/mL |
| PaCo2 | 31.8 Torr | Aldolase | 11.3 IU/L |
| HCO3- | 21.8 mmol/L | Ferritin | 1122.0 ng/mL |
| Bronchoalveolar lavage | |||
| Total cell number | 2.3 × 105/mL | ||
| Macrophage | 89% | ||
| Lymphocyte | 10% | ||
| Neutrophil | 1% | ||
| Eosinophil | 0% | ||
Plt: platelet; AST: aspartate aminotransferase; ALT: alanine transaminase; LDH: lactate dehydrogenase; CK: creatine kinase; ALP: alkaline phosphatase; BUN: blood urea nitrogen; Cre: creatinine; CRP: C-reactive protein; KL-6: sialylated carbohydrate antigen KL-6; SP-D: pulmonary surfactant protein-D.
Creatine kinase was 673 IU/L (normal range 38–196 IU/L); lactic dehydrogenase was 598 U/L (normal range 121–245 U/L); and ferritin was 1122.0 ng/mL (normal range 13–277 ng/mL) all of which were elevated.
In this case, we could not perform MRI or confirm myogenic change in EMG against the high serum CK value.
Aldolase was within the normal range. Antinuclear antibody was positive (homogeneous pattern, 1:80) and anti-aminoacyl-tRNA synthetase (ARS) antibody was negative. Anti-MDA5 antibody was not measured upon admission because of health insurance regulations.
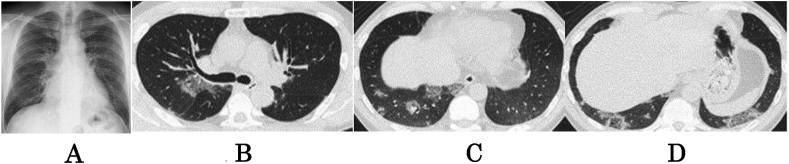
Chest radiographs showed bilateral ground glass opacities (Fig. 1A). High-resolution computed tomography (HRCT) revealed random focal areas of grand glass opacities. Honeycombing and pleural effusion were not observed (Fig. 1B, C, D).
Fig. 1.
Chest X-ray and chest plain computed tomography. A: Chest radiograph on admission shows bilateral ground glass opacities. B, C, D. Chest CT scan shows random ground glass opacities in the lower lung fields. Honeycombing and pleural effusion are not present.
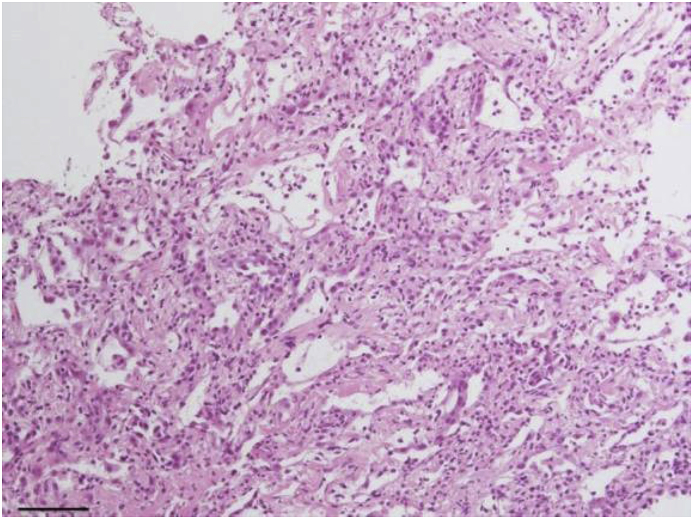
Transbronchial lung biopsy from left lower lobe S8 and S9 revealed alveolar structure failure. The alveolar wall was enlarged with the infiltration of inflammatory cells and epithelial atypia and exfoliation, suggesting severe epithelial injury (Fig. 2).
Fig. 2.
Cellulo-myxomatous alveolitis. The photomicrograph with HE staining shows notable inflammatory cell infiltration of the alveolar wall with reactive pneumocyte hyperplasia (scale bar, 10μm).
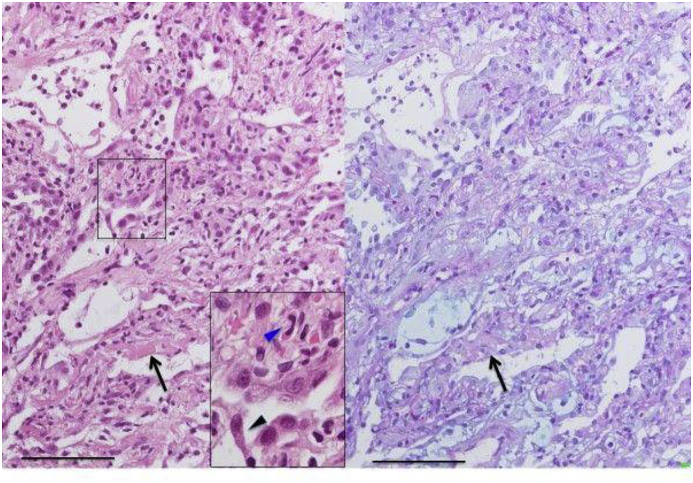
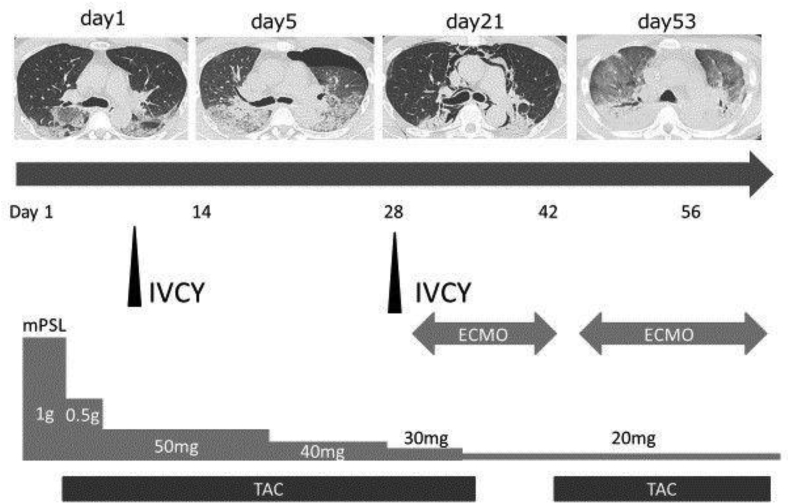
No neoplastic changes or honeycomb lung were found. Alcian blue Periodic acid-Schiff staining revealed collections of fibroblasts stained blue suggesting early fibrosis (Fig. 3). The patient's clinical course is shown in (Fig. 4). His condition worsened 3 days after admission and 10 L/min oxygen was required. Methylprednisolone (1000 mg/day) was administered for 3 days for acute respiratory failure and was continued at a reduced dose (500 mg/day) without improvement. We did not identify physical findings such as purpura, inverse Gottron signs, or vascular injury. However, the high level of ferritin, rapidly progressive respiratory failure, and findings on chest CT suggested the possibility of rapidly progressive interstitial pneumonia associated with anti-MDA5 antibody. Therefore, in addition to steroids, IVCY (500 mg/body) and oral tacrolimus (5 mg/day) were added. The trough tacrolimus level was maintained at 5–10 ng/ml. Intravenous corticosteroid pulse therapy followed 0.75 mg/kg body weight (50 mg/day) prednisolone.
Fig. 3.
Cellulo-myxomatous alveolitis with desquamative pneumocytes and fibrinous exudates. Photomicrograph of lung sections with HE and Alcian blue/PAS staining shows mixed cellulo-myxomatous change of alveolar wall with pneumocyte hyperplasia and fibrinous exudates (arrows). High power view shows findings of desquamative and atypical pneumocytes (black arrow head) with some eosinocyte infiltrations (blue arrow head) (scale bar, 100μm).
Fig. 4.
Disease course of the patient over time.
He developed pneumothorax and pneumomediastinum, requiring insertion of a thoracic drainage. His clinical condition deteriorated progressively with severe desaturation of 60% at 15 L/min oxygen, and extra-corporeal membrane oxygenation was introduced in the intensive care room on hospital day 31. However, he died on hospital day 69 without improvement of his respiratory condition. Anti-MDA5 antibody measured by an in-house enzyme-linked immunosorbent assay [4] using stored serum obtained at the hospital after death was strongly positive at 126 units (normal value < 8.0 units).
His condition worsened 3 days after admission and 10 L/min oxygen was required. Methylprednisolone (1000 mg/day) was administered for 3 days for acute respiratory failure and was continued at a reduced dose (500 mg/day) without improvement suggested the possibility of rapidly progressive interstitial pneumonia associated with anti-MDA5 antibody. Therefore, in addition to steroids, IVCY (500 mg/body) and oral tacrolimus (5 mg/day) were added. Intravenous corticosteroid pulse therapy followed 0.75 mg/kg body weight (50 mg/day) prednisolone. He developed pneumothorax and pneumomediastinum, requiring insertion of a thoracic drainage. His clinical condition deteriorated progressively with severe desaturation of 60% at 15 L/min oxygen, and extra-corporeal membrane oxygenation was introduced in the intensive care room on hospital day 31. However, he died on hospital day 69 without improvement of his respiratory condition. Anti-MDA5 antibody measured by an in-house enzyme-linked immunosorbent assay [4] using stored serum obtained at the hospital after death was strongly positive at 126 units (normal value < 8.0 units).
3. Discussion
In this case, rapidly progressive interstitial pneumonia induced by anti-MDA5 antibody developed after insecticide inhalation. There was no rash of dermatomyositis. David et al. [5] reported that patients with anti-MDA5 antibody and skin rash were more likely to exhibit have swelling of the hands, arthralgia/arthritis, skin ulcers, palmar papules, mechanical hands, hair loss, and oral mucosal ulcers. Furthermore, purpura and inverse Gottron signs suggestive of vascular injury are characteristic in patients with anti-MDA5 antibodies [6].
In cases of CADM with anti-MDA5 antibody, the characteristic skin findings described above are regarded as mandatory. However, in recent years, some case reports have described RP-ILD without cutaneous manifestations related to CADM [[7], [8], [9]]. This may be due to increasing opportunities to measure anti-MDA5 antibody in patients with RP-ILD regardless of a diagnosis of dermatomyositis. These reports found random peripheral ground-glass opacities predominantly in the lung bases on HRCT, which is consistent with findings of HRCT revealed in CADM with anti-MDA5.
In this case, we did not reach a definite diagnosis of CADM due to a lack of physical findings in the skin. We suggest the possibility that ILD could precede skin manifestations related to CADM, similar to the situations in patients with anti-ARS; ILD occasionally precedes skin rash or myositis in patients with anti-ARS.
Furthermore, CADM cases with anti-MDA5 antibody develop predominantly between autumn and winter, suggesting the involvement of environmental factors such as viral infection [10]. MDA5, the antigen of anti-MDA5 antibody, is a member of the retinoic acid inducible gene-I (RIG-I) family of proteins and is involved in the prevention of viral infection [11]. RIG-I is a receptor that recognizes intracellular viral RNA [12]. An in vivo study using MDA5 knockout mice demonstrated that RIG-I recognizes viral RNA and increases the production of interferons [13,14]. While the innate immune response involving interferons is involved in viral infections, the excessive production of interferons may induce autoimmune diseases [15]. According to previous reports [16,17], high levels of serum interferon-α are observed in patients with anti-MDA5-positive RP-ILD.
There is no literature on the clinical features of high-CK value anti-MDA5 antibody positive cases. However, currently, CK values are reportedly significantly lower in anti-MDA5 antibody positive cases than in negative ones [18]. In contrast case reports of high-CK anti-MDA5 antibody positive cases were also reported [19]. DM was originally reported to exhibit an extremely variegated disease image. Even at the high CK values, when RP-ILD is clinically observed, the possibility of positive anti-MDA5 antibody must be considered.
In this case, the respiratory symptoms appeared insecticide inhalation, although the presence of prior infection remained unknown. This was the first occurrence of insecticide inhalation in this patient. The involvement of inhalant might have injured the alveolar epithelial cells and stimulated the innate immune response via MDA5 in the lungs. However, the underlying mechanism remains unclear, and there are no previous reports demonstrating a relationship between pyrethroid insecticide and lung injury, as far as we search. The timing of disease onset might be by chance identical to the period of the inhalation, which not might have been involved in the development of anti-MDA5-positive RP-ILD.
In Japan, testing for anti-MDA5 antibody in patients who have received a definitive diagnosis of dermatomyositis was approved by health insurance since October 2016. This test might be beneficial for the selection of therapeutic strategies even in cases of RP-ILD without dermatomyositis associated skin manifestations.
In summary, we report a case of anti-MDA5 antibody-positive rapidly progressive interstitial pneumonia without cutaneous manifestations. The present case strongly indicates the necessity to assume anti-MDA5 antibody-positive interstitial pneumonia in cases of high ferritin and RP-ILD development, and random ground glass shadows and peripheral consolidations on HRCT even if the dermatomyositis-associated skin symptoms are not observed. Additionally, multiple immunosuppressive therapy should be initiated immediately if the anti-MDA5 antibody is positive.
Disclosure statement
Declarations of interest: none.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
The authors would like to thank Surgical intensive care unit; Dr. Nao Umei, and, Professor Shingo Ichiba, for their valuable opinions on this case.
References
- 1.Sato S., Murakami A., Kuwajima A., Takehara K., Mimori T., Kawakami A., Mishima M., Suda T., Seishima M., Fujimoto M., Kuwana M. Clinical Utility of an Enzyme-Linked Immunosorbent Assay for Detecting Anti-Melanoma Differentiation-Associated Gene 5 Autoantibodies. PLoS One. 2016;11 doi: 10.1371/journal.pone.0154285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagata K., Tomii K., Nanjo S., Kubota M., Tachikawa R., Nishino M. Four cases of interstitial pneumonia associated with amyopathic dermatomyositis characterized by the anti-CADM-140 antibody. Ann. Jpn. Respir. Soc. 2011;49:30–36. (in Japanese) [PubMed] [Google Scholar]
- 3.Nakashima R., Mimori T. Anti-MDA5(melanoma differentiation-associated gene 5) antibody and dermatomyositis with rapidly progressive interstitial pneumonia. Jpn. J. Clin. Immunol. 2013;36:71–76. doi: 10.2177/jsci.36.71. (in Japanese) [DOI] [PubMed] [Google Scholar]
- 4.Sato S., Hoshino K., Satoh T., Fujita T., Kawakami Y., Kuwana M. RNA helicase encoded by melanoma differentiation-associated gene 5 is a major autoantigen in patients with clinically amyopathic dermatomyositis: association with rapidly progressive interstitial lung disease. Arthritis Rheum. 2009;60:2193–2200. doi: 10.1002/art.24621. [DOI] [PubMed] [Google Scholar]
- 5.Fiorentino D., Chung L., Zwerner J., Rosen A., Casciola-Rosen L. The mucocutaneous and systemic phenotype of dermatomyositis patients with antibodies to MDA5 (CADM-140): a retrospective study. J. Am. Acad. Dermatol. 2011;65:25–34. doi: 10.1016/j.jaad.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muro Y., Sugiura K., Akiyama M. Cutaneous manifestations in dermatomyositis: key clinical and serological features-a comprehensive review. Clin. Rev. Allergy Immunol. 2016;51:293–302. doi: 10.1007/s12016-015-8496-5. [DOI] [PubMed] [Google Scholar]
- 7.Juan G.M., Manuel R.C., Ines L.L., Ana P.C., Cristina O., Bartomeu C. Rapidly progressive interstitial lung disease due to anti-MDA5 antibodies without skin involvement: a case report and literature review. Rheumatol. Int. 2018;2 doi: 10.1007/s00296-018-3991-7. February 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tamai K., Tachikawa R., Otsuka K., Ueda H., Hosono Y., Tomii K. Early pulmonary involvement of anti-CADM-140 autoantibody-positive rapidly progressive interstitial lung disease preceding typical cutaneous symptoms. Intern. Med. 2014;53:2515–2519. doi: 10.2169/internalmedicine.53.2769. [DOI] [PubMed] [Google Scholar]
- 9.Ortiz S.V., Babot A., Ferrer C. Anti-MDA5-positive dermatomyositis : an emerging entity with a variable clinical presentation. Scand. J. Rheumatol. 2017;10:509–511. doi: 10.1080/03009742.2017.1340512. [DOI] [PubMed] [Google Scholar]
- 10.Nishina N., Sato S., Kawaguchi Y., Kawakami A., Tamura M., Ikeda K., Nunokawa T., Tanino Y., Asakawa K., Kaneko Y., Gono T., Masui K., Kuwana M., JAMI investigators Influence of season and residential environment on development of anti-melanoma differentiation-associated gene 5 antibody-positive dermatomyositis with interstitial lung disease. JADD. 2018;78:776–785. [Google Scholar]
- 11.Yoneyama M., Kikuchi M., Matsumoto K., Imaizumi T., Miyagishi M., Taira K., Foy E., Loo Y.M., Gale M., Jr., Akira S., Yonehara S., Kato A., Fujita T. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J. Immunol. 2005;175:2851–2858. doi: 10.4049/jimmunol.175.5.2851. [DOI] [PubMed] [Google Scholar]
- 12.Yoneyama M., Kikuchi M., Natsukawa T., Shinobu N., Imaizumi T., Miyagishi M., Taira K., Akira S., Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 13.Kato H., Sato S., Yoneyama M., Yamamoto M., Uematsu S., Matsui K., Tsujimura T., Takeda K., Fujita T., Takeuchi O., Akira S. Cell type-specific involvement of RIG-I in antiviral response. Immunity. 2005;23:19–28. doi: 10.1016/j.immuni.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Kato H., Takeuchi O., Sato S., Yoneyama M., Yamamoto M., Matsui K., Uematsu S., Jung A., Kawai T., Ishii K.J., Yamaguchi O., Otsu K., Tsujimura T., Koh C.S., Reis E., Sousa C., Matsuura Y., Fujita T., Akira S. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 15.Trinchieri G. Type I interferon: friend or foe? J. Exp. Med. 2010;207:2053–2063. doi: 10.1084/jem.20101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horai Y., Koga T., Fujikawa K., Takatani A., Nishino A., Nakashima Y., Suzuki T., Kawashiri S.Y., Iwamoto N., Ichinose K., Tamai M., Nakamura H., Ida H., Kakugawa T., Sakamoto N., Ishimatsu Y., Muskae H., Hamaguchi Y., Fujimoto M., Kuwana M., Origuchi T., Kohno S., Kawakami A. Serum interferon-α is a useful biomarker in patients with anti-melanoma differentiation-associated gene 5 (MDA5) antibody-positive dermatomyositis. Mod. Rheumatol. 2015;25:85–89. doi: 10.3109/14397595.2014.900843. [DOI] [PubMed] [Google Scholar]
- 17.Gono T., Kaneko H., Kawaguchi Y., Hanaoka M., Kataoka S., Kuwana M., Takagi K., Ichida H., Katsumata Y., Ota Y., Kawasumi H., Yamanaka H. Cytokine profiles in polymyositis and dermatomyositis complicated by rapidly progressive or chronic interstitial lung disease. Rheumatology. 2014;53:2196–2203. doi: 10.1093/rheumatology/keu258. [DOI] [PubMed] [Google Scholar]
- 18.Nakashima R., Imura Y., Kobayashi S., Yukawa N., Yoshifuji H., Nojima T., Kawabata D., Ohmura K., Usui T., Fuji T., Okawa K., Mimori T. The RIG-I-like receptor IFIH1/MDA5 is a dermatomyositis-specific autoantigen identified by the anti-CADM-140 antibody. Rheumatology (Oxford) 2010;49:433–440. doi: 10.1093/rheumatology/kep375. [DOI] [PubMed] [Google Scholar]
- 19.Sato K., Morozumi Y., Takeuchi Y., Ochiai J., Mabuchi C. A case of dermatomyositis with anti-CADM-140 antibody and alveolar hemorrhage. Clin. Neurol. 2014;54:408–412. doi: 10.5692/clinicalneurol.54.408. [DOI] [PubMed] [Google Scholar]