Abstract
Interleukin-33 (IL-33) is one of the members of the IL-1 family of cytokines and a ligand of ST2 and IL-1 receptor accessory protein (IL-1RAcP) that is known to affect Th2 inflammatory response with partial effects on Th1 responses. This cytokine is released by epithelial and smooth muscle cells of the airway system during their injury by several environmental stimuli, such as allergens, viruses, helminths, and pollutants. IL-33 is an alarmin that acts as an endogenous danger signal, and it has been known to affect various types of cells, such as mast cells, basophils, eosinophils, T cells, and specific subsets of innate lymphoid cells (ILCs). In recent findings, this cytokine is believed to have a critical role in several types of cancers, such as lung cancer, liver cancer, and head and neck squamous cell cancer. The expression of IL-33/ST2 in cancer tissues shows a close association with tumor growth and tumor progression in several types of cancer, suggesting the IL-33/ST2 pathway as a potential target for therapy.
Keywords: tumorigenesis, interleukin-33, ST2, cancer, tumor microenvironment
Interleukin-33, an IL-1 Family Cytokine with Different Manners of Activation
Interleukin-33 (IL-33) is a ligand of ST2 (also known as IL-1R4, IL-1RL1, T1, Der4, and fit-1) (Schmitz and others 2005). This cytokine is produced by various types of immune cells, such as mast cells, macrophages, and dendritic cells, and also by nonimmune cells, such as endothelial cells, epithelial cells, smooth muscle cells, and fibroblasts (Oboki and others 2010).
This cytokine is identical to the previously reported DVS27 and the nuclear factor of high endothelial venules and is increased in vasospastic cerebral arteries in the presence of subarachnoid hemorrhage (Onda and others 1999; Baekkevold and others 2003). It was revealed that IL-33 is associated with heterochromatin via a helix-turn-helix motif of the N-terminal part and acts as a transcriptional repressor in vitro (Carriere and others 2007; Roussel and others 2008). Furthermore, IL-33 also serves the function of a nuclear factor-like IL-1α and HMGB1 (Werman and others 2004; Andersson and Tracey 2011).
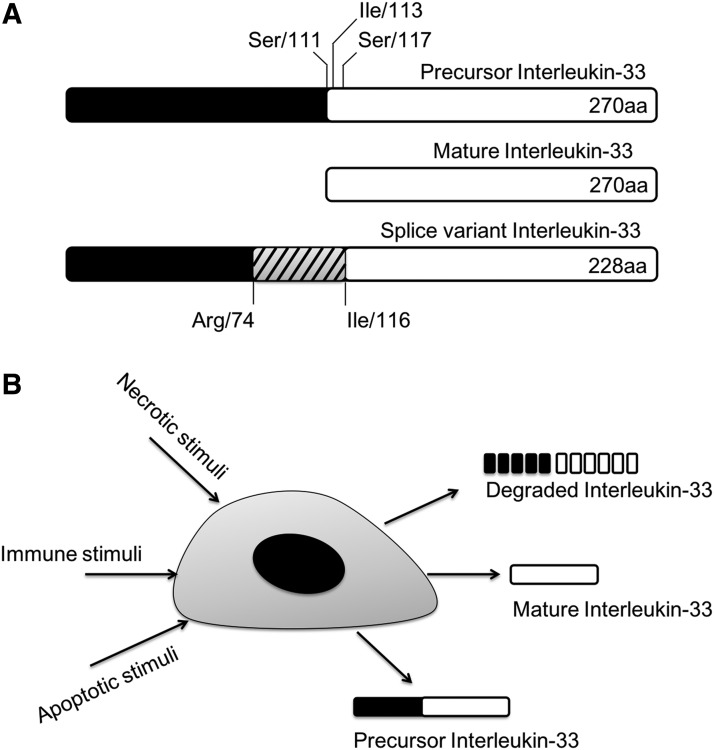
The IL-1 family cytokines such as IL-1β and IL-18 are absent from hydrophobic signal peptides for protein secretion, which keeps the precursor proteins in an inactive form in the cytoplasm (Dinarello 1998, 2009). Following the activation of caspase-1 (also known as IL-1-converting enzyme) by inflammasomes, IL-1β and IL-18 precursors mature by cleavage to be secreted through a unique mechanism (Dinarello 1998, 2009). When IL-33 was first identified, it was considered that IL-33 experiences an analogous process of other IL-1 family cytokines to be mature and secreted (Schmitz and others 2005). In the case of this cytokine, however, even the full-length protein without maturation has a biological function (Fig. 1A) (Cayrol and Girard 2009; Luthi and others 2009; Talabot-Ayer and others 2009). Also, the caspase-mediated proteolysis that occurs during apoptosis and activation of inflammasomes is not necessary for activation or secretion of IL-33.
FIG. 1.
The activation of precursor IL-33. (A) It is believed that IL-33 precursor protein undergoes a certain maturation process to acquire activity, but the rigorous cleavage site is still controversial. First, IL-33 was believed to be activated by the cleavage of caspase-1 at Ser 111 (Schmitz and others, 2005). Commercially available IL-33 recombinant proteins are expressed as Ile113-Thr270. The other site, Ser 117, was also proposed following the study with PR3 protease (Bae and others, 2012). The short splice variant of IL-33, which lacks exon 3 (Arg74-Gly116), is also a biologically active form. (B) IL-33 can be released by several stimuli under certain circumstances, and this can be both precursor form and mature form and even undergoes a degradation process. IL-33, interleukin-33.
In another study on IL-33, the cleavage of pro-IL-33 in apoptotic cells leads to inactivation of IL-33, meanwhile full-length pro-IL-33 proteins released from damaged cells are active (Cayrol and Girard 2009; Luthi and others 2009; Ohno and others 2009). These authors reported that the caspase-1 cleavage site is not the initially proposed Asp110-Ser111 but Asp178-Gly179 of IL-33 protein, which is a consensus sequence of caspase-3 cleavage. Caspase-3 and -7 cleaves Asp178-Gly179 site, which destroys the biological function of pro-IL-33 and leads to apoptosis (Schroder and Kaufman 2005; Cayrol and Girard 2009; Luthi and others 2009; Hong and others 2011).
A study showed calpain-dependent processing of pro-IL-33 in the similar manner of IL-1α. Calpain cleavage processes IL-33 maturation and releases this cytokine from human epithelial and endothelial cells. Nevertheless, the functional consequence of this cleavage is still unclear (Hayakawa and others 2009). In human macrophages and mast cells, caspase-1/8 and calpain are unessential for the secretion of IL-33 (Ohno and others 2009). Peritoneal macrophages of caspase-1 knockout mice released IL-33, and stimulation augments the secretion with lipopolysaccharide (LPS) or phorbol 12-myristate 13-acetate plus ionomycin (Ohno and others 2009). These 2 studies show conflicting results on cleavage and secretion of IL-33. The study of calpain-dependent IL-33 maturation was performed in cells originated from humans, whereas Ohno and others (2009) revealed the process of IL-33 with mouse peritoneal neutrophils. The homology of human and mouse IL-33 sequence is significant; however, in amino acid level, the identity is 55%.
Studies have identified splice variants of IL-33. One of the splice variants of IL-33 that lacks exon 3 was revealed to be more active than the full-length form of IL-33 (Hong and others 2011). The cleaved form of IL-33 by neutrophil elastase, cathepsin G, or PR3 shows 10-fold higher activity than that of full-length IL-33 (Bae and others 2012; Lefrancais and others 2012). Fascinatingly, PR3 has functions of both activation and inactivation of precursor IL-33. PR3 cleaves the precursor IL-33 at the N-terminus, and the mature IL-33 is highly active and sufficient to induce inflammatory cytokines (Bae and others 2012). However, prolonged incubation of IL-33 with PR3 led to degradation of IL-33 (Fig. 1B) (Bae and others 2012). IL-33 has a consensus PR3 cleavage motif as ABZ-X3X2X1-ANB-NH2. Cleavage at this site by PR3 activates the cytokine (Wysocka and others 2008; Bae and others 2012). The full mapping of the IL-33 fragments by the cleavage of PR3 is not known yet since PR3 cleaves PR3 at specific sites first but chops up IL-33 after all (Bae and others 2012).
There is a report that IL-33 can be leaked from damaged cells instead of other classic secretion mechanisms of usual cytokines. In their findings, IL-33 works as a damage-associated molecular pattern molecule or as an alarmin like IL-1α and HMGB1 that are leaked from necrotic cells following tissue damage (Moussion and others 2008).
IL-33 Receptor
The IL-33 receptor (IL-33R) is a heterodimeric complex of ST2 and IL-1 receptor accessory protein (IL-1RAcP) (Ali and others 2007; Chackerian and others 2007; Palmer and others 2008). ST2 was known as an orphan receptor for >10 years before the discovery of IL-33 (Trajkovic and others 2004). Over the years, ST2 has been shown to be a selective marker for murine and humans (Trajkovic and others 2004). This molecule is expressed in mast cells (Allakhverdi and others 2007), eosinophils (Cherry and others 2008; Suzukawa and others 2008b), and even in basophils (Suzukawa and others 2008a) without known ligands. These cells produce inflammatory cytokines and chemokines IL-4, IL-5, IL-6, IL-13, and IL-8 in response to IL-33 (Allakhverdi and others 2007; Iikura and others 2007; Cherry and others 2008; Pecaric-Petkovic and others 2009). In a recent study, circulating CD34+ hematopoietic progenitors respond to IL-33 by expressing ST2 and releasing high levels of Th2-associated cytokines (Allakhverdi and others 2009). Such observations suggest what the potential role of IL-33 is in Th2 immune responses and the relevance to allergic inflammatory diseases, such as asthma and atopic dermatitis.
Two splice variants of ST2 have been reported: transmembranous ST2L and soluble sST2. The alternative splicing is regulated under the control of 2 distinct promoters of ST2 DNA (Oboki and others 2010; Smith 2010; Ohno and others 2012). The transmembrane form, ST2L, is the functional component of IL-33R, whereas the soluble form, sST2, is the decoy receptor of IL-33 in the similar manner of IL-1 receptor antagonist (Oboki and others 2010; Smith 2010; Ohno and others 2012). In humans, a third variant, ST2V, is also reported from the gastrointestinal tract, but the function is still unclear (Tago and others 2001).
IL-1RAcP is a co-receptor for IL-33 signaling, and this molecule acts as a shared co-receptor for other IL-1 family members (Ali and others 2007; Chackerian and others 2007; Palmer and others 2008). IL-1RAcP forms a complex with ST2L in a ligand-dependent manner, and the affinity of mouse IL-33 for ST2L is increased in complex with IL-1RAcP (Palmer and others 2008). The action of IL-33 requires IL-1RAcP, and this can be experimentally inhibited by dominant-negative IL-1RAcP mutants or by anti-IL-1RAcP antibodies. It is also known that sST2 and soluble IL-1RAcP, a splice variant of IL-1RAcP, inhibit the activity of IL-33 synergistically (Palmer and others 2008).
IL-33 Signaling
In mast cells of the mouse, IL-33 binds to ST2L and recruits IL-1RAcP, which induces the activation of common signaling pathways of the Toll/interleukin-1 receptor (TIR) family. This leads to the recruitment of MyD88 and the activation of ERK, JNK, p38 MAPK, and NF-κB pathways (Schmitz and others 2005; Ho and others 2007; Baumann and others 2015). These pathways were also confirmed in other types of cells, such as basophils, eosinophils, epithelial cells, endothelial cells, myocytes, and monocytes (Pecaric-Petkovic and others 2009; Chow and others 2010; Yagami and others 2010; Yndestad and others 2010). Although IL-33 activates NF-κB in cardiomyocytes by itself, IL-33 reduces angiotensin II and phenylephrine-induced NF-κB activity, which infers that IL-33 signal is dependent on cellular environment (Sanada and others 2007).
It was reported that the activation of NF-κB, p38, and JNK signaling was dependent on TNF receptor-associated factor 6 (TRAF6), whereas ERK activation is TRAF6 independent from ST2L-overexpressed fibroblasts (Funakoshi-Tago and others 2008). Besides, the activation of NF-κB was Janus kinase 2-dependent unlike ERK, p38, or JNK in the ST2L-overexpressed fibroblasts (Funakoshi-Tago and others 2011). Thus, SIGIRR (single Ig IL-1-related receptor) has a negative regulation on the IL-33-ST2L signaling pathway, which inhibits the activation of ERK, JNK, and NF-κB like other TIR family members (Bulek and others 2009).
It has also been reported that IL-33 crosstalks with c-kit, which is related to the activation of PKB, ERK, JNK, and STAT3 in mast cells, unlike classical TIR signals. This induces the optimal cellular responses to IL-33 (Drube and others 2010). Thus, IL-33 activates GRB2-associated-binding protein 2 (GAB2), phospholipase C-γ2, and Syk during the differentiation of osteoclasts. This means that IL-33 and immunoreceptor tyrosine-based activation motif (ITAM)-bearing receptors crosstalk potentially (Mun and others 2010). Overall, these findings are relevant to the cross talk between receptor activator of NF-κB ligand (RANKL) and signaling through the ITAM in Fc receptor-γ or DAP12 during osteoclastogenesis and of other directions of TLR-ITAM cross talk (Takayanagi 2007; Ivashkiv 2008; Palmer and Gabay 2011; Baumann and others 2015).
However, 1 caveat is that most data related to IL-33 signaling via ST2L were conducted using recombinant 18 kDa IL-33 that is now believed to be an artificially truncated form of IL-33. Although the full-length IL-33 binds to ST2L, recruits IL-1RAcP, and activates NF-κB (Ali and others 2007; Cayrol and Girard 2009; Luthi and others 2009; Talabot-Ayer and others 2009), the 18 kDa IL-33 and full-length IL-33 have differences in specific activities (Luthi and others 2009; Smith 2011).
Cellular Targets of IL-33
IL-33 is released from damaged structural cells and activates mast cells. IL-33 binds to ST2L on either mature or precursor mast cells (Allakhverdi and others 2007) and starts subsequent activation of NF-κB and transcription of proinflammatory cytokines, such as IL-1β, IL-6, IL-13, TNF-α, chemokines, and prostaglandins (Ali and others 2007; Haenuki and others 2012; Ohno and others 2012). IL-33 induces cytokine production in the presence or absence of co-stimulation of mast cells via IgE/antigen–FɛRI signals (Iikura and others 2007; Haenuki and others 2012). IL-33 primes mast cells for activation by IgG immune complexes and increases their survival and adhesion (Kaieda and others 2012).
IL-33/ST2L interactions induce degranulation in response to IgE cross-linking stimuli, and it enhances basophil migration to eotaxin without effect on CCR3 expression (Chan and others 2001; Smithgall and others 2008). Thus, IL-33 synergizes with IL-3 to induce IL-4 expression and CD11b production by basophils, which enhance basophil adhesiveness (Smithgall and others 2008). Like mast cells, basophils also produce proinflammatory cytokines, such as IL-1β, IL-4, IL-5, IL-6, IL-13, and GM-CSF (Smithgall and others 2008; Suzukawa and others 2008a; Pecaric-Petkovic and others 2009).
IL-33 regulates blood eosinophil infiltration in airway inflammation in mice (Stolarski and others 2010). IL-33 stimulates IL-5-dependent eosinophil differentiation from CD117+ progenitors. In human eosinophils, IL-33 mediates survival, upregulates ICAM-1 on the cell surface, and suppresses ICAM-3 and selectin. IL-33 induces the release of proinflammatory cytokine IL-6 and chemokines IL-8 and CCL2 and promotes Siglec-8-mediated apoptosis of eosinophils (Chow and others 2010; Na and others 2012). When mice are treated with IL-33, CCR3, and CC4, their ligands, CCL11, CCL17, and CCL22, increase. These results implicate eosinophil chemotaxis in lung tissue (Louten and others 2011). In IL-33 null mice, eosinophil infiltration and cytokine production were decreased (Louten and others 2011). IL-33 stimulates eosinophils to produce superoxide and degranulation (Cherry and others 2008). In humans, the correlation between blood and pulmonary eosinophilia with elevated IL-33 serum level was reported (Kim and others 2010). Macrophages and dendritic cells also express ST2 but in low levels. ST2 stimulation in dendritic cells increases IL-4, IL-5, IL-13, CCL17, TNF-α, and IL-1β (Su and others 2013). In macrophages, IL-33 amplifies the expression of M2 markers (Espinassous and others 2009). Furthermore, IL-33/ST2 signaling enhances the activation of components of LPS receptor, TLR4, and MD2 (Joshi and others 2010).
It has been known that IL-33 induces Th2 cytokines and chemotaxis of in vitro polarized Th2 cells (Schmitz and others 2005; Komai-Koma and others 2007; Ohno and others 2012). In mouse respiratory system, antigen-specific Th2 cells stimulated by IL-33 induce IL-5 and IL-13, but not IL-4, and these cells are called atypical Th2 cells (Kurowska-Stolarska and others 2008). This was also observed from BAL fluid that IL-5 and IL-13 levels were increased while IL-4 did not change following intranasal administration of IL-33, which suggests that IL-33 is involved in IL-4-independent Th2 cell differentiation (Louten and others 2011). IL-33 induces IL-5 and IL-13 and impressively interferon-γ (IFN-γ), a Th1 cytokine. It also enhances Th2 cytokines in vitro among skewed cells in HDM-specific T cell culture (Smithgall and others 2008). IL-33 is involved in Th2 responses to allergens such as house dust mites, peanuts in experimental animal models of asthma, and food allergy (Chu and others 2013). Interestingly, ST2 or IL-33 null mice have normal levels of Th2 differentiation (Hoshino and others 1999; Townsend and others 2000). The expression level of ST2 is low in CD8 T cells; however, IL-33 enhances the antitumor activity of CD8 T cells (Gao and others 2015). Furthermore, loss of ST2 impaired response to LCMV infection in CD8 T cells (Bonilla and others 2012). iNKT cells have membranous ST2 and express both Th1 and Th2 cytokines under stimulation of IL-33 (Smithgall and others 2008). These results suggest that IL-33 promotes Th2 cytokines in a particular condition as well as Th1 cytokines. NK cells express ST2, and the combination of IL-33 and IL-12 increases IFN-γ levels (Smithgall and others 2008; Bourgeois and others 2009). These data have yet to translate to human disease. These phenomena imply possible existence of another component that supports IL-33R complex, which transduces Th1 signal following the stimulation by IL-33. Recent studies show that T cell subset is not the only type of lymphoid cells with ST2. B1 B cells express ST2, which enhances proliferation capacity, and IgM, IL-5, and IL-13 production (Komai-Koma and others 2011).
In recent studies, various IL-5 and IL-13 producing Lin− c-kit+ Sca-1+ innate lymphoid cells (ILCs) have been reported as a distinct subset of cells from lymphoid progenitors, lymphoid tissue inducer cells, and RORγt+ ILCs (Moro and others 2010; Neill and others 2010; Price and others 2010; Saenz and others 2010; Barlow and McKenzie 2011; Koyasu and Moro 2011b). Thus, GATA-3 was identified as a critical transcription factor for their development (Hoyler and others 2012). Lin− c-kit+ Sca-1+ ILC2 cells were found in fat-associated lymphoid clusters in visceral adipose tissue. IL-33 activates ST2L on these cells, which induces the production of IL-5 and IL-6, but not IL-4, more than basophils or mast cells. This induction is independent of the combination of IL-2 and IL-25. However, IL-2 and IL-25 enhance the activity of IL-33 in this regard.
ILC2 is involved in defense mechanism against helminths via IL-5- and IL-13-dependent eosinophilia and goblet cell hyperplasia (Moro and others 2010; Koyasu and Moro 2011a). ST2-expressing ILC2 is present in mesenteric lymph nodes, spleen, and liver of IL-25 or IL-33 injected or helminth-infected mice. ILC2 is essential for host defense mechanism against Nippostrongylus brasiliensis infection (Neill and others 2010; Price and others 2010; Liang and others 2011) and the onset of allergic airway inflammation (Wilhelm and others 2011; Barlow and others 2012). However, there are ST2− Lin− c-kit+ Sca-1+ MPPtype2 cells present in the mesenteric lymph nodes and GALT of IL-25 or helminth-infected mice (Saenz and others 2010). It is believed that IL-33 and ST2 are vital factors for the expansion of IL-5- and IL-13-producing ILCs, but it is still controversial that ILCs express IL-5 and/or IL-13 under stimulation of IL-33 or IL-25.
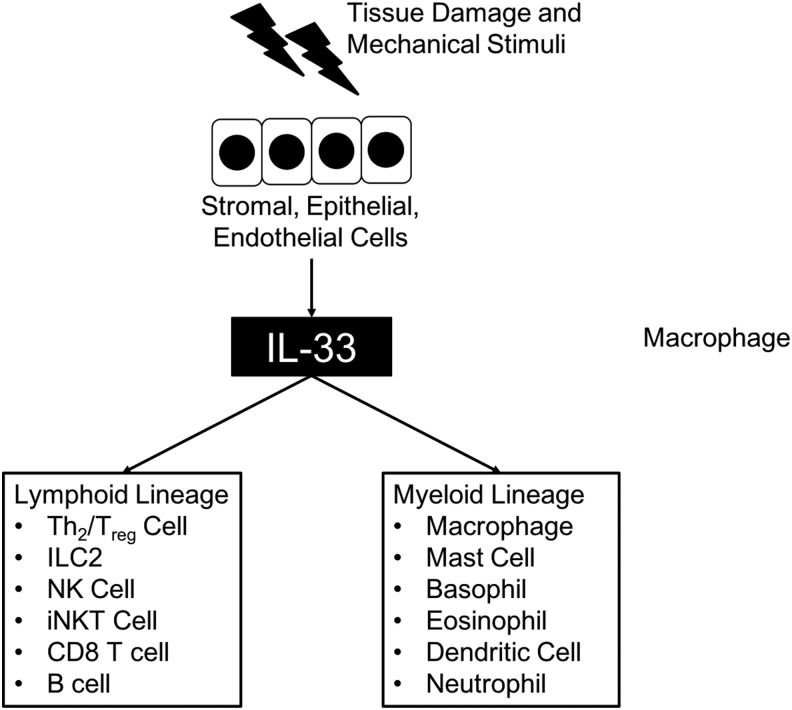
Meanwhile, IL-33-responsive c-kit− Sca-1+ CD25+ cells were revealed as a distinct subset of cells from c-kit+ NH cells (Brickshawana and others 2011). In human and mouse lungs, Lin− c-kit+ Sca-1+ CD90+ CD25+ IL-7Rα+ ST2+ residential cells differentiated by a transcription factor, Id2, express IL-5 and IL-13 in response to IL-33 (Monticelli and others 2011). Two subsets of ILCs that solely express IL-5 or IL-13 in the presence of IL-33 or IL-25 in the peritoneal cavity, lungs, and gut of mice were also reported (Ikutani and others 2012). A subset of Lin− c-kit+ Sca-1+ CD25+ ILCs that expresses IL-5 and IL-13 in response to IL-33, but not to IL-25, from mouse lungs was also reported (Bartemes and others 2012) (Fig. 2).
FIG. 2.
Cellular targets of IL-33. Stromal, epithelial, and endothelial cells secrete IL-33 after tissue damage or mechanical stress. Secreted IL-33 stimulates several subsets of both lymphoid and myeloid lineages of immune cells.
IL-33 and Cancers
Cytokines play central roles in cellular interaction in the inflammatory tumor microenvironment (Candido and Hagemann 2013). In recent reports, IL-33 is highly involved in several cancers where this cytokine exerts protumorigenic and antitumorigenic functions up to the environment (Lu and others 2016).
Breast cancer
Breast cancer is a primary cause of women's death by malignant tumors (Ferlay and others 2010). The reason for fatalness from breast cancer is extensive metastasis to the bones, brain, liver, and lungs (Lu and Kang 2007). The paradoxical roles of the immune system lead to the promotion or prevention of tumorigenesis. Leukocytes infiltrate in tumor tissues and have a substantial consequence on cancer development. CTLs and NK cells have antitumorigenic functions; meanwhile, Tregs and myeloid-derived suppressor cells have immunosuppressive functions in the tumor microenvironment (Jiang and Shapiro 2014; Carrega and others 2016).
IL-33/ST2 signaling has been reported to promote breast cancer in several studies. IL-33 and ST2 levels are increased in breast tissues with cancer than in healthy breast tissue (Liu and others 2014a; Kim and others 2015). Also, IL-33 and its decoy receptor ST2 levels are increased in breast cancer patients and correlated with the markers of poor prognosis, such as VEGF, MMP-11, and platelet-derived growth factor-C (Yang and others 2015).
When ST2 is deficient, it leads to delayed tumorigenesis and metastasis through downregulated tumor cell proliferation in a syngeneic 4T1 breast cancer model. Besides, the administration of IL-33 increased tumor cell proliferation in wild-type mice in this model (Jovanovic and others 2011, 2014). There is also a report that IL-33 has a straight effect on malignant breast cancer cells. Direct stimulation of ST2-positive breast cancer cells with IL-33 enhanced the size, proliferation, and colony formation. This happened via phosphorylation and activation of p38 MAPK pathway through IL-33/ST2 signaling (Kim and others 2015).
These findings show that the IL-33/ST2 pathway enhances breast cancer progression directly and indirectly. This noticeable change suggests that IL-33/ST2 can be a strong candidate for diagnosis and therapy of breast cancer.
Colorectal cancer
Colorectal cancer is one of the most common cancers and in many cases has a fatal prognosis (Brenner and others 2014; Wasmer and Krebs 2016). Colorectal cancer shows the classic developmental stages of epithelial cancer progression, adenoma-carcinoma consequence, followed by the accumulation of mutations in critical tumor genes and tumor suppressor genes such as Wnt/β-catenin, TP53, MAPK, KRAS, myc, and TGF-β/bone morphogenetic protein signaling pathways (Vogelstein and others 1988; Terzic and others 2010; Fearon 2011). Chronic inflammation like inflammatory bowel disease is a crucial factor of colorectal cancer (Grivennikov and others 2010; Grivennikov and Cominelli 2016; Robles and others 2016; Wasmer and Krebs 2016). Furthermore, several proinflammatory cytokines and inflammatory mediators are involved in colorectal cancer progression (Mager and others 2016; Wasmer and Krebs 2016).
Unlike other studies that show protumorigenic functions of the IL-33/ST2 pathway, there is a recent report that suggests a protective role of IL-33. IL-33 and ST2 levels are upregulated in adenoma and low-grade adenocarcinoma of patients with colorectal cancer (Cui and others 2015; Mertz and others 2016). Also, IL-33 messenger RNA (mRNA) level was upregulated in patients with stage I–III colorectal cancer, showing protective features (Zhang and others 2017). The high expression of IL-33 and ST2 is also reported in mouse colitis-related models. ST2-deficient mice showed delayed tumor growth and fewer lesions of colorectal cancer, whereas IL-33-overexpressed animals showed enhanced tumorigenesis and metastasis (Maywald and others 2015; Mertz and others 2016; Zhang and others 2017).
Metastatic SW620 human colorectal cancer cell line shows increased invasive potential not only with the stimulation of exogenous IL-33 but also with overexpression of IL-33. Furthermore, the overexpression of IL-33 in SW620 cells increased tumor growth, metastasis, and reduced survival when they were injected into nude mice (Liu and others 2014b).
Interestingly, in a recent finding, the expression of ST2 in tumor tissues was lower than the adjacent nontumor sites, which accompanied with a slowed tumor progression. This is because of reduced CCL2 production by tumor cells due to downregulated IL-33/ST2 signal (O'Donnell and others 2016). Also, IL-33-mediated IgA production leads to the prevention of microbial dysbiosis and IL-1α-dependent inflammation in the intestine. In AOM/DSS-treated IL-33-deficient mice, the secretion of inflammatory cytokine was increased, and tumor number, size, and grade were also increased compared with wild-type mice (Malik and others 2016). However, this does not necessarily mean that IL-33 has a similar function in humans. In mouse, manipulation of microbiota alone is sufficient to impair colorectal cancer progression, regardless of IL-33 (Mager and others 2016).
From these findings, it is not easy to determine if IL-33/ST2 signaling promotes or protects from colorectal cancer. It will be necessary to compare each mouse models' characteristics and their mechanisms that show protumorigenic or protective functions. Especially in human patients, chemotherapy may regulate the expression of ST2 and IL-33 levels, which likely influence tumor progression (O'Donnell and others 2016). More investigations are needed to understand the role and mechanism of IL-33/ST2 signaling in tumorigenesis to target this signaling pathway for efficient cancer therapy.
Non-small-cell lung cancer
Non-small-cell lung cancer (NSCLC) is the leading cause of lung cancer, 85% of lung cancers. The prognosis of NSCLC has not changed dramatically, but the underlying mechanisms have been understood prominently (Chen and others 2014). IL-33/ST2 in respiratory diseases was mainly studied regarding allergy, asthma, and chronic obstructive pulmonary diseases (Liew and others 2010, 2016; Byers and others 2013; Makrinioti and others 2014), whereas it has been studied much less in cancer.
Patients with NSCLC have enhanced expression of IL-33 and ST2 in cancer tissue than in adjacent healthy tissue, and the expression level is correlated with the clinical stage (Wang and others 2016). In opposition, another study showed decreased levels of IL-33 and ST2 mRNA in lung cancer tissue compared with healthy tissue, and the transcribed mRNA levels were reverse-correlated with cancer stages and survival rate. Along the same line, the protein level of ST2 was increased in low metastatic cancer cells than in highly metastatic 3LL Lewis lung carcinoma cells (Akimoto and others 2016).
The potential function of IL-33/ST2 in lung cancer remains controversial. Different patient cohorts, and the size of the cohort, might contribute to the discrepancy. There could be other possible variations like radiotherapy or surgery of the tumor tissue. Chemotherapy and radiotherapy can distort the molecular panel quite rapidly, which may also lead to the change of IL-33 level (Ha and others 2014; Tan and others 2016). Therefore, additional studies regarding IL-33/ST2 in lung cancer with primary NSCLC cells are needed to clarify its role and precise mechanism.
Other cancers
In hepatocellular carcinoma (HCC), many patients have a history of chronic hepatitis B or C infections followed by the progression of fibrosis or cirrhosis with high mortality. The multistep process of HCC involves the role of angiogenesis (Raza and Sood 2014; Waller and others 2015). Compared with healthy liver tissue, IL-33 expression was positive in the liver tissue of patients with HCC. Besides, serum IL-33 levels were higher in both preoperative and postoperative serum samples of HCC patients compared with healthy controls (Zhang and others 2012). The mechanism of IL-33/ST2 signaling in HCC is still unclear. However, in HCC tissues, effector-memory CD8+ T cells were increased, which may be associated with prolonged patient survival (Brunner and others 2015).
Among primary hepatic malignancies, cholangiocarcinoma (CCA) is the second most common that affects biliary duct system (Tyson and El-Serag 2011). CCA is closely associated with chronic inflammation, parasite infestation, primary sclerosing cholangitis, hepatolithiasis, biliary duct cysts, and poisonings (Tyson and El-Serag 2011). In mouse models, the ST2 levels increased in CCA, which enhanced IL-33 signaling and resulted in the elevation of IL-6 expression, as well as enhanced mitotic signals and cancer cell survival (Kobayashi and others 2005; Wehbe and others 2006; Yamada and others 2015). Also, IL-33-administered mice showed enhanced proliferation of cholangiocytes through an increased number of type-2 ILCs that produce IL-13. Activation of these ILCs activates AKT and YAP constitutively in bile ducts to promote liver metastasis (Li and others 2014). Moreover, external administration of IL-33 encouraged CCA development in mouse CCA models (Yamada and others 2015).
Head and neck squamous cell cancer (HNSCC) has high mortality and difficulties for surgical resection because of the anatomical complicity. The etiology of HNSCC includes smoking, alcohol, and human papillomavirus (Leemans and others 2011). Cancer-associated fibroblasts (CAFs) are critical participants in the HNSCC microenvironment promoting tumor growth, invasion, and metastasis, which means that CAF is a decisive factor in the severity of the disease. CAFs release IL-33 that induces epithelial-to-mesenchymal transition of cancer cells and cell aggressiveness. Besides, IL-33 induces its own expression and forms positive feedback. The expression level of IL-33 in CAFs is reversely correlated with the survival of patients. A poor prognosis is primarily indicated for patients with HNSCC in tongues with high expression of IL-33 and enhanced microvessel formation in the stroma (Chen and others 2013; Ishikawa and others 2014; Wasmer and Krebs 2016).
Gastric cancer is the fourth most common cancer with the second highest cause of death due to cancer throughout the world. The primary etiology factors of gastric cancer are diet, Helicobacter pylori infection, and gastroesophageal reflux and obesity (Carcas 2014). Meanwhile, inflammation is an essential factor in gastric cancer formation (Peek and Crabtree 2006; Fox and Wang 2007).
Renal cell carcinoma is the ninth most common cancer in men. In a recent report, IL-33 expression is tightly correlated with the poor prognosis for patients with renal cell carcinoma. The tumor cells have enhanced signaling of JNK, which has been stimulated by IL-33/ST2 signaling pathway (Wu and others 2018).
Conclusion
Many studies have investigated the role of IL-33, which provides novel insights into the mechanism of cancers. IL-33 is released from various types of cells during inflammation-mediated tissue injury. IL-33 signaling promotes the severity of allergic responses and cancer progression since IL-33 is an important factor to increase allergy-mediated inflammation that can worsen many types of cancer diseases.
The tumor microenvironment is a central operating system for the progression of tumor development, local invasion, and metastasis (Hanahan and Coussens 2012). One of the main regulators of the tumor microenvironment is pro- or anti-inflammatory cytokines, such as IL-1β, IL-6, and TNF-α (Candido and Hagemann 2013). As an alarmin, IL-33 amplifies innate immune responses that can contribute to several different types of inflammatory disorders as well as modulation of tumorigenesis (Oboki and others 2010; Liew and others 2016; Lu and others 2016). Numerous studies using patient samples, in vitro experiments, and in vivo mouse models reveal multiple roles of the IL-33-ST2 pathway in the tumor microenvironment regarding tumor initiation, development, and resistance to therapy. In general, IL-33 has a protumorigenic feature in different types of cancers; however, contradictory findings of IL-33 were also reported in several other types of cancers (Wasmer and Krebs 2016). It is not easy to develop a new therapy before all research processes, including animal experiments and clinical trials, have been explored. However, patient- or disease-specific therapy using IL-33 or the antagonist (ST2) can be an additive solution for cancer treatment.
The mechanism of IL-33 in cancer is still indistinct. It is always controversial in that IL-33 maturation is obtained by particular enzymatic activity. Nevertheless, this cytokine may be activated by multiple activating processes. In addition, the various effects of IL-33 in T cell immunity suggest the possible existence of another component of IL-33R complexes. Unlike the identified function of ST2 and IL-1RAcP complex, another member of the IL-33R complex may be the clue to explaining the effect of IL-33 on both Th1 and Th2 immunity regarding inflammation-mediated cancers.
Acknowledgments
J.H. and P.C.L. are supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health. S.K. was supported by the National Research Foundation of Korea Grants NRF-2015R1A2A2A01003472, -2014M3A6A4075058, and -2015R1A2A1A15051472.
Author Disclosure Statement
No competing financial interests exist.
References
- Akimoto M, Hayashi JI, Nakae S, Saito H, Takenaga K. 2016. Interleukin-33 enhances programmed oncosis of ST2L-positive low-metastatic cells in the tumour microenvironment of lung cancer. Cell Death Dis 7:e2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S, Huber M, Kollewe C, Bischoff SC, Falk W, Martin MU. 2007. IL-1 receptor accessory protein is essential for IL-33-induced activation of T lymphocytes and mast cells. Proc Natl Acad Sci U S A 104(47):18660–18665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allakhverdi Z, Comeau MR, Smith DE, Toy D, Endam LM, Desrosiers M, Liu YJ, Howie KJ, Denburg JA, Gauvreau GM, Delespesse G. 2009. CD34+ hemopoietic progenitor cells are potent effectors of allergic inflammation. J Allergy Clin Immunol 123(2):472–478 [DOI] [PubMed] [Google Scholar]
- Allakhverdi Z, Smith DE, Comeau MR, Delespesse G. 2007. Cutting edge: the ST2 ligand IL-33 potently activates and drives maturation of human mast cells. J Immunol 179(4):2051–2054 [DOI] [PubMed] [Google Scholar]
- Andersson U, Tracey KJ. 2011. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol 29:139–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae S, Kang T, Hong J, Lee S, Choi J, Jhun H, Kwak A, Hong K, Kim E, Jo S, Kim S. 2012. Contradictory functions (activation/termination) of neutrophil proteinase 3 enzyme (PR3) in interleukin-33 biological activity. J Biol Chem 287(11):8205–8213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baekkevold ES, Roussigne M, Yamanaka T, Johansen FE, Jahnsen FL, Amalric F, Brandtzaeg P, Erard M, Haraldsen G, Girard JP. 2003. Molecular characterization of NF-HEV, a nuclear factor preferentially expressed in human high endothelial venules. Am J Pathol 163(1):69–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow JL, Bellosi A, Hardman CS, Drynan LF, Wong SH, Cruickshank JP, McKenzie AN. 2012. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J Allergy Clin Immunol 129(1):191–198.e1–e4 [DOI] [PubMed] [Google Scholar]
- Barlow JL, McKenzie AN. 2011. Nuocytes: expanding the innate cell repertoire in type-2 immunity. J Leukoc Biol 90(5):867–874 [DOI] [PubMed] [Google Scholar]
- Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. 2012. IL-33-responsive lineage- CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol 188(3):1503–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann C, Bonilla WV, Frohlich A, Helmstetter C, Peine M, Hegazy AN, Pinschewer DD, Lohning M. 2015. T-bet- and STAT4-dependent IL-33 receptor expression directly promotes antiviral Th1 cell responses. Proc Natl Acad Sci U S A 112(13):4056–4061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla WV, Frohlich A, Senn K, Kallert S, Fernandez M, Johnson S, Kreutzfeldt M, Hegazy AN, Schrick C, Fallon PG, Klemenz R, Nakae S, Adler H, Merkler D, Lohning M, Pinschewer DD. 2012. The alarmin interleukin-33 drives protective antiviral CD8(+) T cell responses. Science 335(6071):984–989 [DOI] [PubMed] [Google Scholar]
- Bourgeois E, Van LP, Samson M, Diem S, Barra A, Roga S, Gombert JM, Schneider E, Dy M, Gourdy P, Girard JP, Herbelin A. 2009. The pro-Th2 cytokine IL-33 directly interacts with invariant NKT and NK cells to induce IFN-gamma production. Eur J Immunol 39(4):1046–1055 [DOI] [PubMed] [Google Scholar]
- Brenner H, Kloor M, Pox CP. 2014. Colorectal cancer. Lancet 383(9927):1490–1502 [DOI] [PubMed] [Google Scholar]
- Brickshawana A, Shapiro VS, Kita H, Pease LR. 2011. Lineage(−)Sca1+c-Kit(−)CD25+ cells are IL-33-responsive type 2 innate cells in the mouse bone marrow. J Immunol 187(11):5795–5804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner SM, Rubner C, Kesselring R, Martin M, Griesshammer E, Ruemmele P, Stempfl T, Teufel A, Schlitt HJ, Fichtner-Feigl S. 2015. Tumor-infiltrating, interleukin-33-producing effector-memory CD8(+) T cells in resected hepatocellular carcinoma prolong patient survival. Hepatology 61(6):1957–1967 [DOI] [PubMed] [Google Scholar]
- Bulek K, Swaidani S, Qin J, Lu Y, Gulen MF, Herjan T, Min B, Kastelein RA, Aronica M, Kosz-Vnenchak M, Li X. 2009. The essential role of single Ig IL-1 receptor-related molecule/Toll IL-1R8 in regulation of Th2 immune response. J Immunol 182(5):2601–2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers DE, Alexander-Brett J, Patel AC, Agapov E, Dang-Vu G, Jin X, Wu K, You Y, Alevy Y, Girard JP, Stappenbeck TS, Patterson GA, Pierce RA, Brody SL, Holtzman MJ. 2013. Long-term IL-33-producing epithelial progenitor cells in chronic obstructive lung disease. J Clin Invest 123(9):3967–3982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candido J, Hagemann T. 2013. Cancer-related inflammation. J Clin Immunol 33 (Suppl. 1):S79–S84 [DOI] [PubMed] [Google Scholar]
- Carcas LP. 2014. Gastric cancer review. J Carcinog 13:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrega P, Campana S, Bonaccorsi I, Ferlazzo G. 2016. The yin and yang of innate lymphoid cells in cancer. Immunol Lett 179:29–35 [DOI] [PubMed] [Google Scholar]
- Carriere V, Roussel L, Ortega N, Lacorre DA, Americh L, Aguilar L, Bouche G, Girard JP. 2007. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci U S A 104(1):282–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayrol C, Girard JP. 2009. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc Natl Acad Sci U S A 106(22):9021–9026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chackerian AA, Oldham ER, Murphy EE, Schmitz J, Pflanz S, Kastelein RA. 2007. IL-1 receptor accessory protein and ST2 comprise the IL-33 receptor complex. J Immunol 179(4):2551–2555 [DOI] [PubMed] [Google Scholar]
- Chan WL, Pejnovic N, Lee CA, Al-Ali NA. 2001. Human IL-18 receptor and ST2L are stable and selective markers for the respective type 1 and type 2 circulating lymphocytes. J Immunol 167(3):1238–1244 [DOI] [PubMed] [Google Scholar]
- Chen SF, Nieh S, Jao SW, Wu MZ, Liu CL, Chang YC, Lin YS. 2013. The paracrine effect of cancer-associated fibroblast-induced interleukin-33 regulates the invasiveness of head and neck squamous cell carcinoma. J Pathol 231(2):180–189 [DOI] [PubMed] [Google Scholar]
- Chen Z, Fillmore CM, Hammerman PS, Kim CF, Wong KK. 2014. Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer 14(8):535–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry WB, Yoon J, Bartemes KR, Iijima K, Kita H. 2008. A novel IL-1 family cytokine, IL-33, potently activates human eosinophils. J Allergy Clin Immunol 121(6):1484–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow JY, Wong CK, Cheung PF, Lam CW. 2010. Intracellular signaling mechanisms regulating the activation of human eosinophils by the novel Th2 cytokine IL-33: implications for allergic inflammation. Cell Mol Immunol 7(1):26–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu DK, Llop-Guevara A, Walker TD, Flader K, Goncharova S, Boudreau JE, Moore CL, Seunghyun In T, Waserman S, Coyle AJ, Kolbeck R, Humbles AA, Jordana M. 2013. IL-33, but not thymic stromal lymphopoietin or IL-25, is central to mite and peanut allergic sensitization. J Allergy Clin Immunol 131(1):187–200.e1–e8 [DOI] [PubMed] [Google Scholar]
- Cui G, Qi H, Gundersen MD, Yang H, Christiansen I, Sorbye SW, Goll R, Florholmen J. 2015. Dynamics of the IL-33/ST2 network in the progression of human colorectal adenoma to sporadic colorectal cancer. Cancer Immunol Immunother 64(2):181–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. 1998. Interleukin-1 beta, interleukin-18, and the interleukin-1 beta converting enzyme. Ann N Y Acad Sci 856:1–11 [DOI] [PubMed] [Google Scholar]
- Dinarello CA. 2009. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 27:519–550 [DOI] [PubMed] [Google Scholar]
- Drube S, Heink S, Walter S, Lohn T, Grusser M, Gerbaulet A, Berod L, Schons J, Dudeck A, Freitag J, Grotha S, Reich D, Rudeschko O, Norgauer J, Hartmann K, Roers A, Kamradt T. 2010. The receptor tyrosine kinase c-Kit controls IL-33 receptor signaling in mast cells. Blood 115(19):3899–3906 [DOI] [PubMed] [Google Scholar]
- Espinassous Q, Garcia-de-Paco E, Garcia-Verdugo I, Synguelakis M, von Aulock S, Sallenave JM, McKenzie AN, Kanellopoulos J. 2009. IL-33 enhances lipopolysaccharide-induced inflammatory cytokine production from mouse macrophages by regulating lipopolysaccharide receptor complex. J Immunol 183(2):1446–1455 [DOI] [PubMed] [Google Scholar]
- Fearon ER. 2011. Molecular genetics of colorectal cancer. Annu Rev Pathol 6:479–507 [DOI] [PubMed] [Google Scholar]
- Ferlay J, Parkin DM, Steliarova-Foucher E. 2010. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer 46(4):765–781 [DOI] [PubMed] [Google Scholar]
- Fox JG, Wang TC. 2007. Inflammation, atrophy, and gastric cancer. J Clin Invest 117(1):60–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi-Tago M, Tago K, Hayakawa M, Tominaga S, Ohshio T, Sonoda Y, Kasahara T. 2008. TRAF6 is a critical signal transducer in IL-33 signaling pathway. Cell Signal 20(9):1679–1686 [DOI] [PubMed] [Google Scholar]
- Funakoshi-Tago M, Tago K, Sato Y, Tominaga S, Kasahara T. 2011. JAK2 is an important signal transducer in IL-33-induced NF-kappaB activation. Cell Signal 23(2):363–370 [DOI] [PubMed] [Google Scholar]
- Gao X, Wang X, Yang Q, Zhao X, Wen W, Li G, Lu J, Qin W, Qi Y, Xie F, Jiang J, Wu C, Zhang X, Chen X, Turnquist H, Zhu Y, Lu B. 2015. Tumoral expression of IL-33 inhibits tumor growth and modifies the tumor microenvironment through CD8+ T and NK cells. J Immunol 194(1):438–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov SI, Cominelli F. 2016. Colitis-associated and sporadic colon cancers: different diseases, different mutations? Gastroenterology 150(4):808–810 [DOI] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M. 2010. Immunity, inflammation, and cancer. Cell 140(6):883–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha CT, Li XH, Fu D, Moroni M, Fisher C, Arnott R, Srinivasan V, Xiao M. 2014. Circulating interleukin-18 as a biomarker of total-body radiation exposure in mice, minipigs, and nonhuman primates (NHP). PLoS One 9(10):e109249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenuki Y, Matsushita K, Futatsugi-Yumikura S, Ishii KJ, Kawagoe T, Imoto Y, Fujieda S, Yasuda M, Hisa Y, Akira S, Nakanishi K, Yoshimoto T. 2012. A critical role of IL-33 in experimental allergic rhinitis. J Allergy Clin Immunol 130(1):184.e11–194.e11 [DOI] [PubMed] [Google Scholar]
- Hanahan D, Coussens LM. 2012. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21(3):309–322 [DOI] [PubMed] [Google Scholar]
- Hayakawa M, Hayakawa H, Matsuyama Y, Tamemoto H, Okazaki H, Tominaga S. 2009. Mature interleukin-33 is produced by calpain-mediated cleavage in vivo. Biochem Biophys Res Commun 387(1):218–222 [DOI] [PubMed] [Google Scholar]
- Ho LH, Ohno T, Oboki K, Kajiwara N, Suto H, Iikura M, Okayama Y, Akira S, Saito H, Galli SJ, Nakae S. 2007. IL-33 induces IL-13 production by mouse mast cells independently of IgE-FcepsilonRI signals. J Leukoc Biol 82(6):1481–1490 [DOI] [PubMed] [Google Scholar]
- Hong J, Bae S, Jhun H, Lee S, Choi J, Kang T, Kwak A, Hong K, Kim E, Jo S, Kim S. 2011. Identification of constitutively active interleukin 33 (IL-33) splice variant. J Biol Chem 286(22):20078–20086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino K, Kashiwamura S, Kuribayashi K, Kodama T, Tsujimura T, Nakanishi K, Matsuyama T, Takeda K, Akira S. 1999. The absence of interleukin 1 receptor-related T1/ST2 does not affect T helper cell type 2 development and its effector function. J Exp Med 190(10):1541–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyler T, Klose CS, Souabni A, Turqueti-Neves A, Pfeifer D, Rawlins EL, Voehringer D, Busslinger M, Diefenbach A. 2012. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity 37(4):634–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iikura M, Suto H, Kajiwara N, Oboki K, Ohno T, Okayama Y, Saito H, Galli SJ, Nakae S. 2007. IL-33 can promote survival, adhesion and cytokine production in human mast cells. Lab Invest 87(10):971–978 [DOI] [PubMed] [Google Scholar]
- Ikutani M, Yanagibashi T, Ogasawara M, Tsuneyama K, Yamamoto S, Hattori Y, Kouro T, Itakura A, Nagai Y, Takaki S, Takatsu K. 2012. Identification of innate IL-5-producing cells and their role in lung eosinophil regulation and antitumor immunity. J Immunol 188(2):703–713 [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Yagi-Nakanishi S, Nakanishi Y, Kondo S, Tsuji A, Endo K, Wakisaka N, Murono S, Yoshizaki T. 2014. Expression of interleukin-33 is correlated with poor prognosis of patients with squamous cell carcinoma of the tongue. Auris Nasus Larynx 41(6):552–557 [DOI] [PubMed] [Google Scholar]
- Ivashkiv LB. 2008. A signal-switch hypothesis for cross-regulation of cytokine and TLR signalling pathways. Nat Rev Immunol 8(10):816–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Shapiro DJ. 2014. The immune system and inflammation in breast cancer. Mol Cell Endocrinol 382(1):673–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi AD, Oak SR, Hartigan AJ, Finn WG, Kunkel SL, Duffy KE, Das A, Hogaboam CM. 2010. Interleukin-33 contributes to both M1 and M2 chemokine marker expression in human macrophages. BMC Immunol 11:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic I, Radosavljevic G, Mitrovic M, Juranic VL, McKenzie AN, Arsenijevic N, Jonjic S, Lukic ML. 2011. ST2 deletion enhances innate and acquired immunity to murine mammary carcinoma. Eur J Immunol 41(7):1902–1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic IP, Pejnovic NN, Radosavljevic GD, Pantic JM, Milovanovic MZ, Arsenijevic NN, Lukic ML. 2014. Interleukin-33/ST2 axis promotes breast cancer growth and metastases by facilitating intratumoral accumulation of immunosuppressive and innate lymphoid cells. Int J Cancer 134(7):1669–1682 [DOI] [PubMed] [Google Scholar]
- Kaieda S, Wang JX, Shnayder R, Fishgal N, Hei H, Lee RT, Stevens RL, Nigrovic PA. 2012. Interleukin-33 primes mast cells for activation by IgG immune complexes. PLoS One 7(10):e47252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HR, Jun CD, Lee YJ, Yang SH, Jeong ET, Park SD, Park DS. 2010. Levels of circulating IL-33 and eosinophil cationic protein in patients with hypereosinophilia or pulmonary eosinophilia. J Allergy Clin Immunol 126(4):880.e6–882.e6 [DOI] [PubMed] [Google Scholar]
- Kim JY, Lim SC, Kim G, Yun HJ, Ahn SG, Choi HS. 2015. Interleukin-33/ST2 axis promotes epithelial cell transformation and breast tumorigenesis via upregulation of COT activity. Oncogene 34(38):4928–4938 [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Werneburg NW, Bronk SF, Kaufmann SH, Gores GJ. 2005. Interleukin-6 contributes to Mcl-1 up-regulation and TRAIL resistance via an Akt-signaling pathway in cholangiocarcinoma cells. Gastroenterology 128(7):2054–2065 [DOI] [PubMed] [Google Scholar]
- Komai-Koma M, Gilchrist DS, McKenzie AN, Goodyear CS, Xu D, Liew FY. 2011. IL-33 activates B1 cells and exacerbates contact sensitivity. J Immunol 186(4):2584–2591 [DOI] [PubMed] [Google Scholar]
- Komai-Koma M, Xu D, Li Y, McKenzie AN, McInnes IB, Liew FY. 2007. IL-33 is a chemoattractant for human Th2 cells. Eur J Immunol 37(10):2779–2786 [DOI] [PubMed] [Google Scholar]
- Koyasu S, Moro K. 2011a. Innate Th2-type immune responses and the natural helper cell, a newly identified lymphocyte population. Curr Opin Allergy Clin Immunol 11(2):109–114 [DOI] [PubMed] [Google Scholar]
- Koyasu S, Moro K. 2011b. Type 2 innate immune responses and the natural helper cell. Immunology 132(4):475–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurowska-Stolarska M, Kewin P, Murphy G, Russo RC, Stolarski B, Garcia CC, Komai-Koma M, Pitman N, Li Y, Niedbala W, McKenzie AN, Teixeira MM, Liew FY, Xu D. 2008. IL-33 induces antigen-specific IL-5+ T cells and promotes allergic-induced airway inflammation independent of IL-4. J Immunol 181(7):4780–4790 [DOI] [PubMed] [Google Scholar]
- Leemans CR, Braakhuis BJ, Brakenhoff RH. 2011. The molecular biology of head and neck cancer. Nat Rev Cancer 11(1):9–22 [DOI] [PubMed] [Google Scholar]
- Lefrancais E, Roga S, Gautier V, Gonzalez-de-Peredo A, Monsarrat B, Girard JP, Cayrol C. 2012. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc Natl Acad Sci U S A 109(5):1673–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Razumilava N, Gores GJ, Walters S, Mizuochi T, Mourya R, Bessho K, Wang YH, Glaser SS, Shivakumar P, Bezerra JA. 2014. Biliary repair and carcinogenesis are mediated by IL-33-dependent cholangiocyte proliferation. J Clin Invest 124(7):3241–3251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang HE, Reinhardt RL, Bando JK, Sullivan BM, Ho IC, Locksley RM. 2011. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat Immunol 13(1):58–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew FY, Girard JP, Turnquist HR. 2016. Interleukin-33 in health and disease. Nat Rev Immunol 16(11):676–689 [DOI] [PubMed] [Google Scholar]
- Liew FY, Pitman NI, McInnes IB. 2010. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol 10(2):103–110 [DOI] [PubMed] [Google Scholar]
- Liu J, Shen JX, Hu JL, Huang WH, Zhang GJ. 2014a. Significance of interleukin-33 and its related cytokines in patients with breast cancers. Front Immunol 5:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhu L, Lu X, Bian H, Wu X, Yang W, Qin Q. 2014b. IL-33/ST2 pathway contributes to metastasis of human colorectal cancer. Biochem Biophys Res Commun 453(3):486–492 [DOI] [PubMed] [Google Scholar]
- Louten J, Rankin AL, Li Y, Murphy EE, Beaumont M, Moon C, Bourne P, McClanahan TK, Pflanz S, de Waal Malefyt R. 2011. Endogenous IL-33 enhances Th2 cytokine production and T-cell responses during allergic airway inflammation. Int Immunol 23(5):307–315 [DOI] [PubMed] [Google Scholar]
- Lu B, Yang M, Wang Q. 2016. Interleukin-33 in tumorigenesis, tumor immune evasion, and cancer immunotherapy. J Mol Med (Berl) 94(5):535–543 [DOI] [PubMed] [Google Scholar]
- Lu X, Kang Y. 2007. Organotropism of breast cancer metastasis. J Mammary Gland Biol Neoplasia 12(2–3):153–162 [DOI] [PubMed] [Google Scholar]
- Luthi AU, Cullen SP, McNeela EA, Duriez PJ, Afonina IS, Sheridan C, Brumatti G, Taylor RC, Kersse K, Vandenabeele P, Lavelle EC, Martin SJ. 2009. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity 31(1):84–98 [DOI] [PubMed] [Google Scholar]
- Mager LF, Wasmer MH, Rau TT, Krebs P. 2016. Cytokine-induced modulation of colorectal cancer. Front Oncol 6:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makrinioti H, Toussaint M, Jackson DJ, Walton RP, Johnston SL. 2014. Role of interleukin 33 in respiratory allergy and asthma. Lancet Respir Med 2(3):226–237 [DOI] [PubMed] [Google Scholar]
- Malik A, Sharma D, Zhu Q, Karki R, Guy CS, Vogel P, Kanneganti TD. 2016. IL-33 regulates the IgA-microbiota axis to restrain IL-1alpha-dependent colitis and tumorigenesis. J Clin Invest 126(12):4469–4481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maywald RL, Doerner SK, Pastorelli L, De Salvo C, Benton SM, Dawson EP, Lanza DG, Berger NA, Markowitz SD, Lenz HJ, Nadeau JH, Pizarro TT, Heaney JD. 2015. IL-33 activates tumor stroma to promote intestinal polyposis. Proc Natl Acad Sci U S A 112(19):E2487–E2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz KD, Mager LF, Wasmer MH, Thiesler T, Koelzer VH, Ruzzante G, Joller S, Murdoch JR, Brummendorf T, Genitsch V, Lugli A, Cathomas G, Moch H, Weber A, Zlobec I, Junt T, Krebs P. 2016. The IL-33/ST2 pathway contributes to intestinal tumorigenesis in humans and mice. Oncoimmunology 5(1):e1062966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, Kubota M, Turner D, Diamond JM, Goldrath AW, Farber DL, Collman RG, Wherry EJ, Artis D. 2011. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol 12(11):1045–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. 2010. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature 463(7280):540–544 [DOI] [PubMed] [Google Scholar]
- Moussion C, Ortega N, Girard JP. 2008. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel “alarmin”? PLoS One 3(10):e3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mun SH, Ko NY, Kim HS, Kim JW, Kim DK, Kim AR, Lee SH, Kim YG, Lee CK, Kim BK, Beaven MA, Kim YM, Choi WS. 2010. Interleukin-33 stimulates formation of functional osteoclasts from human CD14(+) monocytes. Cell Mol Life Sci 67(22):3883–3892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na HJ, Hudson SA, Bochner BS. 2012. IL-33 enhances Siglec-8 mediated apoptosis of human eosinophils. Cytokine 57(1):169–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE, McKenzie AN. 2010. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464(7293):1367–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell C, Mahmoud A, Keane J, Murphy C, White D, Carey S, O'Riordain M, Bennett MW, Brint E, Houston A. 2016. An antitumorigenic role for the IL-33 receptor, ST2L, in colon cancer. Br J Cancer 114(1):37–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oboki K, Ohno T, Kajiwara N, Saito H, Nakae S. 2010. IL-33 and IL-33 receptors in host defense and diseases. Allergol Int 59(2):143–160 [DOI] [PubMed] [Google Scholar]
- Ohno T, Morita H, Arae K, Matsumoto K, Nakae S. 2012. Interleukin-33 in allergy. Allergy 67(10):1203–1214 [DOI] [PubMed] [Google Scholar]
- Ohno T, Oboki K, Kajiwara N, Morii E, Aozasa K, Flavell RA, Okumura K, Saito H, Nakae S. 2009. Caspase-1, caspase-8, and calpain are dispensable for IL-33 release by macrophages. J Immunol 183(12):7890–7897 [DOI] [PubMed] [Google Scholar]
- Onda H, Kasuya H, Takakura K, Hori T, Imaizumi T, Takeuchi T, Inoue I, Takeda J. 1999. Identification of genes differentially expressed in canine vasospastic cerebral arteries after subarachnoid hemorrhage. J Cereb Blood Flow Metab 19(11):1279–1288 [DOI] [PubMed] [Google Scholar]
- Palmer G, Gabay C. 2011. Interleukin-33 biology with potential insights into human diseases. Nat Rev Rheumatol 7(6):321–329 [DOI] [PubMed] [Google Scholar]
- Palmer G, Lipsky BP, Smithgall MD, Meininger D, Siu S, Talabot-Ayer D, Gabay C, Smith DE. 2008. The IL-1 receptor accessory protein (AcP) is required for IL-33 signaling and soluble AcP enhances the ability of soluble ST2 to inhibit IL-33. Cytokine 42(3):358–364 [DOI] [PubMed] [Google Scholar]
- Pecaric-Petkovic T, Didichenko SA, Kaempfer S, Spiegl N, Dahinden CA. 2009. Human basophils and eosinophils are the direct target leukocytes of the novel IL-1 family member IL-33. Blood 113(7):1526–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek RM, Jr., Crabtree JE. 2006. Helicobacter infection and gastric neoplasia. J Pathol 208(2):233–248 [DOI] [PubMed] [Google Scholar]
- Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, Locksley RM. 2010. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A 107(25):11489–11494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza A, Sood GK. 2014. Hepatocellular carcinoma review: current treatment, and evidence-based medicine. World J Gastroenterol 20(15):4115–4127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles AI, Traverso G, Zhang M, Roberts NJ, Khan MA, Joseph C, Lauwers GY, Selaru FM, Popoli M, Pittman ME, Ke X, Hruban RH, Meltzer SJ, Kinzler KW, Vogelstein B, Harris CC, Papadopoulos N. 2016. Whole-exome sequencing analyses of inflammatory bowel disease-associated colorectal cancers. Gastroenterology 150(4):931–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel L, Erard M, Cayrol C, Girard JP. 2008. Molecular mimicry between IL-33 and KSHV for attachment to chromatin through the H2A-H2B acidic pocket. EMBO Rep 9(10):1006–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenz SA, Noti M, Artis D. 2010. Innate immune cell populations function as initiators and effectors in Th2 cytokine responses. Trends Immunol 31(11):407–413 [DOI] [PubMed] [Google Scholar]
- Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. 2007. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest 117(6):1538–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. 2005. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23(5):479–490 [DOI] [PubMed] [Google Scholar]
- Schroder M, Kaufman RJ. 2005. ER stress and the unfolded protein response. Mutat Res 569(1–2):29–63 [DOI] [PubMed] [Google Scholar]
- Smith DE. 2010. IL-33: a tissue derived cytokine pathway involved in allergic inflammation and asthma. Clin Exp Allergy 40(2):200–208 [DOI] [PubMed] [Google Scholar]
- Smith DE. 2011. The biological paths of IL-1 family members IL-18 and IL-33. J Leukoc Biol 89(3):383–392 [DOI] [PubMed] [Google Scholar]
- Smithgall MD, Comeau MR, Yoon BR, Kaufman D, Armitage R, Smith DE. 2008. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol 20(8):1019–1030 [DOI] [PubMed] [Google Scholar]
- Stolarski B, Kurowska-Stolarska M, Kewin P, Xu D, Liew FY. 2010. IL-33 exacerbates eosinophil-mediated airway inflammation. J Immunol 185(6):3472–3480 [DOI] [PubMed] [Google Scholar]
- Su Z, Lin J, Lu F, Zhang X, Zhang L, Gandhi NB, de Paiva CS, Pflugfelder SC, Li DQ. 2013. Potential autocrine regulation of interleukin-33/ST2 signaling of dendritic cells in allergic inflammation. Mucosal Immunol 6(5):921–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzukawa M, Iikura M, Koketsu R, Nagase H, Tamura C, Komiya A, Nakae S, Matsushima K, Ohta K, Yamamoto K, Yamaguchi M. 2008a. An IL-1 cytokine member, IL-33, induces human basophil activation via its ST2 receptor. J Immunol 181(9):5981–5989 [DOI] [PubMed] [Google Scholar]
- Suzukawa M, Koketsu R, Iikura M, Nakae S, Matsumoto K, Nagase H, Saito H, Matsushima K, Ohta K, Yamamoto K, Yamaguchi M. 2008b. Interleukin-33 enhances adhesion, CD11b expression and survival in human eosinophils. Lab Invest 88(11):1245–1253 [DOI] [PubMed] [Google Scholar]
- Tago K, Noda T, Hayakawa M, Iwahana H, Yanagisawa K, Yashiro T, Tominaga S. 2001. Tissue distribution and subcellular localization of a variant form of the human ST2 gene product, ST2V. Biochem Biophys Res Commun 285(5):1377–1383 [DOI] [PubMed] [Google Scholar]
- Takayanagi H. 2007. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol 7(4):292–304 [DOI] [PubMed] [Google Scholar]
- Talabot-Ayer D, Lamacchia C, Gabay C, Palmer G. 2009. Interleukin-33 is biologically active independently of caspase-1 cleavage. J Biol Chem 284(29):19420–19426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan WL, Jain A, Takano A, Newell EW, Iyer NG, Lim WT, Tan EH, Zhai W, Hillmer AM, Tam WL, Tan DSW. 2016. Novel therapeutic targets on the horizon for lung cancer. Lancet Oncol 17(8):e347–e362 [DOI] [PubMed] [Google Scholar]
- Terzic J, Grivennikov S, Karin E, Karin M. 2010. Inflammation and colon cancer. Gastroenterology 138(6):2101.e5–2114.e5 [DOI] [PubMed] [Google Scholar]
- Townsend MJ, Fallon PG, Matthews DJ, Jolin HE, McKenzie AN. 2000. T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. J Exp Med 191(6):1069–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trajkovic V, Sweet MJ, Xu D. 2004. T1/ST2—an IL-1 receptor-like modulator of immune responses. Cytokine Growth Factor Rev 15(2–3):87–95 [DOI] [PubMed] [Google Scholar]
- Tyson GL, El-Serag HB. 2011. Risk factors for cholangiocarcinoma. Hepatology 54(1):173–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL. 1988. Genetic alterations during colorectal-tumor development. N Engl J Med 319(9):525–532 [DOI] [PubMed] [Google Scholar]
- Waller LP, Deshpande V, Pyrsopoulos N. 2015. Hepatocellular carcinoma: a comprehensive review. World J Hepatol 7(26):2648–2663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Chen Z, Bu X, Han Y, Shan S, Ren T, Song W. 2016. IL-33 signaling fuels outgrowth and metastasis of human lung cancer. Biochem Biophys Res Commun 479(3):461–468 [DOI] [PubMed] [Google Scholar]
- Wasmer MH, Krebs P. 2016. The role of IL-33-dependent inflammation in the tumor microenvironment. Front Immunol 7:682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehbe H, Henson R, Meng F, Mize-Berge J, Patel T. 2006. Interleukin-6 contributes to growth in cholangiocarcinoma cells by aberrant promoter methylation and gene expression. Cancer Res 66(21):10517–10524 [DOI] [PubMed] [Google Scholar]
- Werman A, Werman-Venkert R, White R, Lee JK, Werman B, Krelin Y, Voronov E, Dinarello CA, Apte RN. 2004. The precursor form of IL-1alpha is an intracrine proinflammatory activator of transcription. Proc Natl Acad Sci U S A 101(8):2434–2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm C, Hirota K, Stieglitz B, Van Snick J, Tolaini M, Lahl K, Sparwasser T, Helmby H, Stockinger B. 2011. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat Immunol 12(11):1071–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CW, Wu YG, Cheng C, Hong ZD, Shi ZM, Lin SQ, Li J, He XY, Zhu AY. 2018. Interleukin-33 predicts poor prognosis and promotes renal cell carcinoma cell growth through its receptor ST2 and the JNK signaling pathway. Cell Physiol Biochem 47(1):191–200 [DOI] [PubMed] [Google Scholar]
- Wysocka M, Lesner A, Guzow K, Mackiewicz L, Legowska A, Wiczk W, Rolka K. 2008. Design of selective substrates of proteinase 3 using combinatorial chemistry methods. Anal Biochem 378(2):208–215 [DOI] [PubMed] [Google Scholar]
- Yagami A, Orihara K, Morita H, Futamura K, Hashimoto N, Matsumoto K, Saito H, Matsuda A. 2010. IL-33 mediates inflammatory responses in human lung tissue cells. J Immunol 185(10):5743–5750 [DOI] [PubMed] [Google Scholar]
- Yamada D, Rizvi S, Razumilava N, Bronk SF, Davila JI, Champion MD, Borad MJ, Bezerra JA, Chen X, Gores GJ. 2015. IL-33 facilitates oncogene-induced cholangiocarcinoma in mice by an interleukin-6-sensitive mechanism. Hepatology 61(5):1627–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZP, Ling DY, Xie YH, Wu WX, Li JR, Jiang J, Zheng JL, Fan YH, Zhang Y. 2015. The association of serum IL-33 and sST2 with breast cancer. Dis Markers 2015:516895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yndestad A, Marshall AK, Hodgkinson JD, Tham el L, Sugden PH, Clerk A. 2010. Modulation of interleukin signalling and gene expression in cardiac myocytes by endothelin-1. Int J Biochem Cell Biol 42(2):263–272 [DOI] [PubMed] [Google Scholar]
- Zhang P, Liu XK, Chu Z, Ye JC, Li KL, Zhuang WL, Yang DJ, Jiang YF. 2012. Detection of interleukin-33 in serum and carcinoma tissue from patients with hepatocellular carcinoma and its clinical implications. J Int Med Res 40(5):1654–1661 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Davis C, Shah S, Hughes D, Ryan JC, Altomare D, Pena MM. 2017. IL-33 promotes growth and liver metastasis of colorectal cancer in mice by remodeling the tumor microenvironment and inducing angiogenesis. Mol Carcinog 56(1):272–287 [DOI] [PMC free article] [PubMed] [Google Scholar]