Abstract
Mesenchymal stem/stromal cells (MSCs) offer great promise in the treatment of ischemic injuries, including stroke, heart infarction, and limb ischemia. However, poor cell survival after transplantation remains a major obstacle to achieve effective MSC therapies. To improve cell survival and retention, we transplanted human bone marrow MSCs with or without a specific prosurvival factor (PSF) cocktail consisting of IGF1, Bcl-XL, a caspase inhibitor, a mitochondrial pathway inhibitor, and Matrigel into the limbs of immune deficient mice, after induction of hindlimb ischemia. The PSF markedly prolonged the retention of the MSCs in the ischemic limb muscles as demonstrated by bioluminescence imaging. Using microcomputed tomography to image the limb muscle vasculature in the mice 9 weeks after the transplantation, we found that the mice transplanted with MSCs without PSF did not show a significant increase in the blood vessels in the ischemic limb compared with the nontransplanted control mice. In contrast, the mice transplanted with MSCs plus PSF showed a significant increase in the blood vessels, especially the larger and branching vessels, in the ischemic limb compared with the control mice that did not receive MSCs. Thus, we demonstrated that prolonged retention of MSCs using PSF effectively promoted angiogenesis in ischemic animal limbs. This study highlights the importance of enhancing cell survival in the development of effective MSC therapies to treat vascular diseases.
Keywords: MSCs, ischemia, prosurvival, engraftment, vasculature
Introduction
Mesenchymal stem/stromal cells (MSCs) offer great promise in the treatment of ischemic injuries, including stroke, heart infarction, and limb ischemia through secreting angiogenic factors and modulating immune response. A major limitation for the robust clinical application of MSCs is their poor viability and engraftment at the site of injury. Multiple factors can contribute to the death of the transplanted MSCs in ischemic tissues: (1) loss of cell–cell, cell–matrix contact that results in anoikis-mediated death; (2) cytotoxic factors released from ischemia damaged tissues, and (3) low oxygen/nutrient environment. Several approaches have been explored to enhance survival and engraftment of MSCs. For example, preconditioning MSCs with hypoxia has been shown to improve survival [1–4]. Furthermore, MSCs that were genetically modified with antiapoptotic or prosurvival genes, for example, survivin, Bcl2, Bcl-xL, and Akt had shown enhanced viability after being transplanted and improved functional recovery of heart after myocardial infarction [5–8]. The direct genetic modification of cells with prosurvival genes, however, is associated with a potential risk of cancer.
Since multiple factors may contribute to the death of the transplanted MSCs in ischemic tissues, a conditioning regimen that minimizes multiple potential death signals is likely to be most effective in promoting survival. A special cocktail of prosurvival factors (PSF) consisted of Matrigel, Bcl-Xl BH4, ZVAD-fmk, cyclosporine A, pinacidil, and IGF1 has been used to increase the engraftment of cardiomyocytes in infarcted rat heart [9]. Matrigel provides extracellular matrices for cells to attach and thus prevents anoikis. Bcl-Xl BH4, the caspase inhibitor ZVAD-fmk, and cyclosporine A block the mitochondrial death pathway. Pinacidil treatment mimics ischemic preconditioning [10] and IGF1 improves survival by activating the Akt pathway [11].
In this study, we investigated whether the PSF cocktail can be used to enhance survival and engraftment of human MSCs in a mouse model of hindlimb ischemia (HLI). We investigated further whether extending retention of MSCs, if can be achieved by treatment of PSF, leads to enhanced angiogenesis in animal ischemic limbs. Using bioluminescence imaging and microcomputed tomography (micro-CT) imaging, we are able to quantitatively assess the retention of human MSCs and the limb vasculature in the HLI mouse xenograft model.
Materials and Methods
Preparation and transduction of MSCs
MSCs were obtained from human bone marrow aspirate from a single donor (All Cells, Emeryville, CA) and cultured in minimum essential medium alpha (HyClone Thermo Scientific, Waltham, MA) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Flowery Branch, GA) and glutamine (HyClone) as previously described [12]. MSCs at passage 4 were transduced with a lentiviral vector encoding luciferase (LUC) and enhanced green fluorescent protein (EGFP) as previously described [13]. These cells were further passaged 2 times before use. Only one batch of the transduced MSCs was used.
Flow cytometry
Cells grown on culture plates were washed with phosphate-buffered saline (PBS) and then lifted with TrypLE Express (Life Technologies). Cells were washed with PBS again, centrifuged at 335 g for 5 min, and resuspended in PBS. Following filtered through a 70 μm nylon mesh (Fisher Scientific), the cells were subjected to flow cytometry analysis using a Beckman Coulter Cytomics FC 500. All these processes were performed at room temperature. Data were analyzed with CXP Analysis Software.
HLI mouse model and cell transplantation
All animal experiments were approved by the Institutional Animal Care and Use Committee at University of California Davis. Unilateral HLI was surgically created in immune deficient NOD/SCID/IL2Rγ−/− (NSG) mice through femoral artery ligation followed by a cut at the femoral artery as previously described [4]. Two days after the surgery, these mice were divided into three groups for intramuscular injections at the ischemic site: (1) 20 μL of PBS (n = 3); (2) MSCs (2 × 105/mouse) in 20 μL of PBS (n = 5); and (3) MSCs (2 × 105/mouse) in 20 μL of PBS with a PSF cocktail (100 μM ZVAD, 100 nM Bcl-XI BH4, 100 ng/mL IGF1, 50 μM Pinacidil, and 50% Matrigel) (n = 5) [9]. The MSCs in group 3 were pretreated with 100 ng/mL IGF1 and 0.2 μM cyclosporine A for 2 days.
Bioluminescence imaging
Animals were injected with 100 μL of 20 mg/mL D-luciferin intraperitoneally and left for 5 min, and were then anesthetized for 5 min using 2%–3% isofluorane. The mice were imaged with the IVIS Spectrum (PerkinElmer, Richmond, CA) for 5 min under anesthesia. Bioluminescence images of the mice were taken the day of cell transplantation and weekly thereafter. The bioluminescence signal was presented as total photons of the region of interest.
Micro-CT imaging
Nine weeks after cell transplantation, these mice were euthanized and a catheter was inserted into the left ventricle of the mice. The mouse vasculature was flushed with PBS supplemented with 100 U/mL heparin sodium (Fresenius Kabi USA) and then 4% paraformaldehyde. One to two milliliters of Microfil MV-120 (Flow Tech, Inc., MA) was injected into the left ventricle to increase contrast of vasculature. The leg muscles from both limbs were separately collected and imaged with a micro-CT imaging system (VivaCT40; Scano Medical, Bassersdorf, Switzerland) using a voxel size of 10.5 μm, X-ray source voltage of 55 kVp, and 145 μA current. The Micro-CT images were analyzed using a matching three-dimensional segmentation algorithm of thresholding, 150–200, sigma 1, and support 2. The vessel density in a limb was calculated by the vessel volume divided by the tissue volume, and the relative vessel density in the ischemic limb was normalized to the contralateral control limb.
Immunostaining
The mouse muscles from the ischemic limbs were frozen in O.C.T compound (Sakura Finetek USA, CA) and sectioned using a Cryotome FSE (Thermo Scientific) at 10 μm. The sections were mounted on microscope slides and fixed in 4% paraformaldehyde for 20 min. Following extensive wash with PBS, the sections were subjected to immunostaining as we previously described [14]. The goat anti-GFP antibodies (Novus Biologicals) and mouse anti-myosin heavy chain (MHC; BD Biosciences) antibodies were diluted to 100-fold in 1% bovine serum albumin (BSA) and incubated with the tissue sections overnight at 4°C. AlexaFluor488-conjugated donkey anti-goat IgG antibodies and AlexaFluor546-conjugated donkey anti-mouse IgG antibodies were diluted to 500-fold in 1% BSA and incubated with the tissue section for 1 h at room temperature. Fluorescence images were captured using a Nikon Eclipse Ti-U Inverted Microscope.
Data representation and statistical analysis
Data are presented as average ± standard deviation. Student t-test was performed to detect statistical difference between two groups, and one-way analysis of variance was performed to detect statistical difference among groups of three or more.
Results
Expression of EGFP in human MSCs
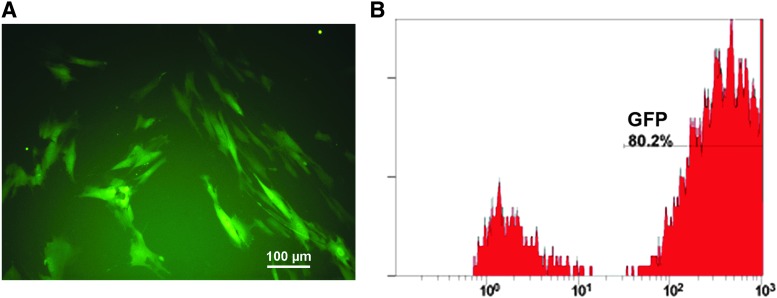
Multiple markers, such as CD105, CD90, CD73, and CD146, have been used to characterize human MSCs. However, no single marker has been found to be specific for these MSCs. Therefore, it is not a trivial challenge to histologically trace these cells in vivo. To facilitate quantitative assessment of survival of human MSCs over time as well as histological identification of the MSCs in vivo, we transduced human bone barrow-derived MSCs with a lentiviral vector encoding the luciferase gene and EGFP. The expression of EGFP was detected in the majority of the transduced cells using fluorescence microscopy (Fig. 1A). Flow cytometric analysis revealed that the transduction efficiency was 80.2% (Fig. 1B). These transduced MSCs were used for in vivo evaluation of their retention and effectiveness on promoting angiogenesis in this study.
FIG. 1.
Evaluation of the expression of GFP in the transduced MSCs. Human bone marrow MSCs were transduced with a lentiviral vector carrying the luciferase gene and EGFP. These cells were assessed for their expression of GFP by fluorescence microscopy (A) and flow cytometry (B). Scale bar: 100 μm. EGFP, enhanced green fluorescent protein; GFP, green fluorescent protein; MSCs, mesenchymal stem/stromal cells.
The PSF cocktail enhanced retention of MSCs in ischemic mouse limbs
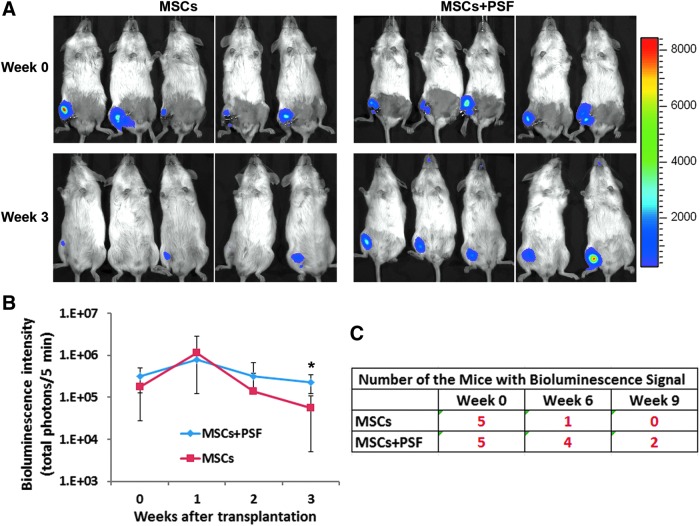
We surgically created HLI in immune deficient NSG mice by unilaterally ligating femoral arteries. The MSCs that were transduced to express luciferase and EGFP were transplanted into the ischemic limbs of the mice with or without the PSF cocktail. The bioluminescent signals from the transplanted MSCs were well maintained in both experimental groups within the first 2 weeks of transplantation as revealed by IVIS imaging (Fig. 2). However, 3 weeks after the transplantation, the majority of the transplanted MSCs without PSF were lost, but the MSCs with the PSF cocktail were retained in the mice at a significantly higher level than MSCs without the PSF cocktail (Fig. 2A, B). At 6 weeks after transplantation, four out of the five mice transplanted with MSCs plus PSF still retained bioluminescence signals, while only one out of the five mice transplanted with MSCs without PSF retained bioluminescence signals (Fig. 2C). At 9 weeks after transplantation, none of the mice transplanted with MSC without PSF retained bioluminescence signals, but two out of the five mice transplanted with MSC plus PSF still retained bioluminescence signals.
FIG. 2.
Effects of the PSF cocktail on retention of MSCs in ischemic mouse limbs. (A): Bioluminescent images of mice transplanted with MSCs with or without PSF at the day of transplantation (week 0) and 3 weeks after transplantation. (B) Bioluminescent signal intensity for the mice over time. *P < 0.05 between these two groups. (C) The number of mice in the indicated group that retained bioluminescent signal over time. PSF, prosurvival factor.
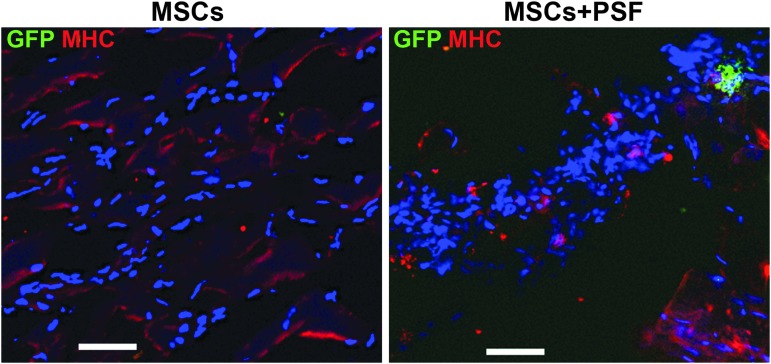
Consistent with the bioluminescence data collected at 9 weeks after cell transplantation, the presence of the transplanted MSCs was detected in the mice transplanted with the MSCs plus PSF using immunostaining of the animal ischemic tissues with GFP antibodies (Fig. 3). We noticed that these GFP-positive cells were in clusters, and many of them lacked intact nuclei, suggesting that these cells were in the process of dying. Since the half-life of GFP is within a few hours [15], the GFP-positive signal is unlikely to be from cells that died some days ago. In contrast, no GFP-positive cells were detected in the mice transplanted with the MSCs without PSF at this time point (Fig. 3). These GFP-positive cells were stained negative for MHC (Fig 3), suggesting that they were not differentiated into myocytes. Our data demonstrate that the PSF cocktail markedly prolonged the retention of MSCs in ischemic limb muscles.
FIG. 3.
Histological detection of the transplanted MSCs in the ischemic limbs. The ischemic limb tissues from the mice transplanted with MSCs without or with PSF were immunostained with antibodies against GFP (green fluorescence) and MHC (red fluorescence) after 9 weeks of cell transplantation. Nuclei were shown in blue (DAPI stain). Scale bar: 50 μm. MHC, myosin heavy chain.
MSCs with PSF promoted reconstitution of blood vessels in ischemic limbs
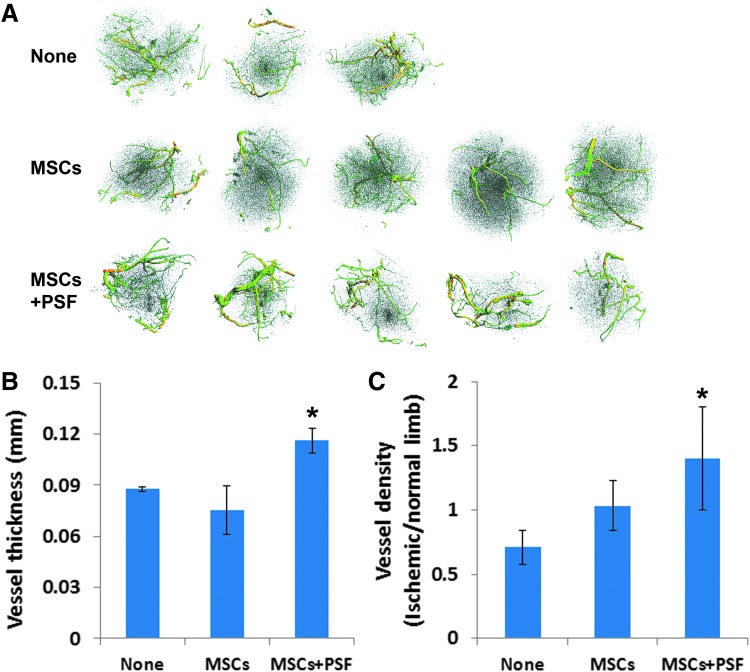
To assess the effect of MSCs with or without PSF on vasculature in ischemic mouse limbs, the experimental mice that had been transplanted with MSCs with or without PSF and the control mice with no cell transplantation were injected with a contrast media, Microfil MV-120, into the left ventricle 9 weeks after the transplantation. Larger and branching vessels in the normal and ischemic limbs from these mice were readily detected by micro-CT imaging. Remarkably, the average vessel thickness in the ischemic legs in the mice that received MSCs with PSF (0.116 ± 0.007 mm) but not MSCs alone (0.075 ± 0.014 mm) showed a significant increase compared with those in the control mice without cell transplantation (0.087 ± 0.001 mm) (Fig. 4). Furthermore, the mice that received MSCs plus PSF had a significant increase in the relative vessel density in the ischemic legs, compared with those in the control mice without cell transplantation (Fig. 4). Therefore, our data demonstrate that while transplantation of MSCs without PSF is not effective in promoting angiogenesis in the ischemic limbs, transplantation of human MSCs with PSF in ischemic mouse limbs is effective in promoting angiogenesis in the ischemic limbs.
FIG. 4.
Evaluation of the vasculature in the ischemic mouse limbs. (A) Microcomputed tomography images of the blood vessels in the ischemic limb muscles from the mice that received MSCs or MSCs with PSF or no MSCs (none) 9 weeks ago. (B) Vessel thickness in the ischemic limbs in the indicated mouse groups. *P < 0.05. (C) Vessel density in the ischemic limb relative to the normal limb in the indicated mouse groups. *P < 0.05.
Discussion
Peripheral vascular disease (PVD) affects over 8 million people in the United States. Angioplasties and bypass surgery are effective treatment for some of the patients. Many patients with severe limb ischemia, however, are not suitable for these treatments. It has been established that MSCs express multiple angiogenic growth factors and can migrate to the hypoxic areas to promote angiogenesis and restoration of vasculature in animal models of HLI [16]. Multiple clinical trials have tested the administration of MSCs for treatment of PVD with moderate or no efficacy [16,17]. Poor posttransplantation viability of MSCs remains a major limitation for the robust clinical application of MSCs and must be overcome to achieve better outcome in treating PVD.
Using a mouse model of HLI, we showed in this study that prolonged retention of the transplanted MSCs in vivo can be achieved by preconditioning MSCs with PSF. Consistent with our previous report [12], the majority of the transplanted MSCs without PSF was lost in the ischemic limb tissue within 3–4 weeks (Fig. 2). The PSF-treated MSCs, however, showed extended retention for 6–9 weeks (Fig. 2). Kim et al. showed previously that PSF protected MSCs from apoptosis induced by in vitro long-term hypoxic stress and starvation [18]. The potential mechanism for the extended survival of the PSF-treated MSCs in vitro was attributed to elevated Akt activation [18]. To our knowledge, we are the first to show that PSF treatment enhanced MSC retention in vivo. It is worth to mention that the HLI mouse model created by us only spontaneously recovered 20%–35% of perfusion in 10 weeks postsurgery [12], much slower than some other HLI mouse models [19]. Patients with chronic PVD rarely heal spontaneously. The extended retention of MSCs could be especially important in clinical setting.
It was noticed that, although at a slower speed, the loss of MSCs overtime posttransplantation was also observed with PSF treatment (Figs. 2 and 3). We speculate that cell death due to the lack of O2 and nutrients in the ischemic tissue could not be completely prevented by PSF treatment. Further optimization of cell retention is needed to achieve sustained engraftment of MSCs in the future. As for optimizing the components of PSF, Matrigel is not clinically applicable and need to be replaced with a clinically applicable matrix or bioengineered material that supports cell attachment.
Increase in larger vessels in mouse limbs in response to ischemic injury has been reported, likely through vessel remodeling and angiogenesis [20]. We showed that transplantation of MSCs alone did not significantly increase vessel density in our animal model of HLI (Fig. 4). Both significant improvement and no improvement of perfusion in HLI animal model by transplantation of MSCs without any modification had been previously reported [12,16,21]. Our study only tested MSCs from a single donor. Donor differences may explain these apparent inconsistent outcomes. Importantly, we showed that transplantation of MSCs with PSF can promote restoration of blood vessels, especially the larger and branching vessels, in ischemic limbs (Fig. 4). This increase in blood vessels was likely through vessel remodeling and angiogenesis. Thus, our data suggest that prolonged retention of the transplanted MSCs is crucial in promoting angiogenesis in ischemic limb tissues.
In conclusion, the PSF was effective in enhancing retention of human MSCs in ischemic muscles, and transplantation of human MSCs with PSF promoted restoration of blood vessels in the ischemic limbs. This study highlights the importance of preconditioning of MSCs for cell survival after transplantation and for effective restoration of vasculature in ischemic tissues.
Acknowledgments
This work was supported by an Interdepartmental Seed Grant from UC Davis (to P.Z.), NIH R01 R01GM099688 (Nolta), the Wing Fat Endowment, the Milstein Medical Asian American Partnership Foundation (to P.Z.), and California Institute for Regenerative Medicine training grant to California State University Sacramento (TB1-01184, to E.C.-Z. and M.R.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Beegle J, Lakatos K, Kalomoiris S, Stewart H, Isseroff RR, Nolta JA. and Fierro FA. (2015). Hypoxic preconditioning of mesenchymal stromal cells induces metabolic changes, enhances survival, and promotes cell retention in vivo. Stem Cells 33:1818–1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chang CP, Chio CC, Cheong CU, Chao CM, Cheng BC. and Lin MT. (2013). Hypoxic preconditioning enhances the therapeutic potential of the secretome from cultured human mesenchymal stem cells in experimental traumatic brain injury. Clin Sci (Lond) 124:165–176 [DOI] [PubMed] [Google Scholar]
- 3. Hu X, Yu SP, Fraser JL, Lu Z, Ogle ME, Wang JA. and Wei L. (2008). Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg 135:799–808 [DOI] [PubMed] [Google Scholar]
- 4. Rosová I, Dao M, Capoccia B, Link D. and Nolta JA. (2008). Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells 26:2173–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fan L, Lin C, Zhuo S, Chen L, Liu N, Luo Y, Fang J, Huang Z, Lin Y. and Chen J. (2009). Transplantation with survivin-engineered mesenchymal stem cells results in better prognosis in a rat model of myocardial infarction. Eur J Heart Fail 11:1023–1030 [DOI] [PubMed] [Google Scholar]
- 6. Li W, Ma N, Ong LL, Nesselmann C, Klopsch C, Ladilov Y, Furlani D, Piechaczek C, Moebius JM, et al. (2007). Bcl-2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells 25:2118–2127 [DOI] [PubMed] [Google Scholar]
- 7. Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS. and Dzau VJ. (2003). Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med 9:1195–1201 [DOI] [PubMed] [Google Scholar]
- 8. Xue X, Liu Y, Zhang J, Liu T, Yang Z. and Wang H. (2015). Bcl-xL Genetic Modification Enhanced the Therapeutic Efficacy of Mesenchymal Stem Cell Transplantation in the Treatment of Heart Infarction. Stem Cells Int 2015:176409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, et al. (2007). Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol 25:1015–1024 [DOI] [PubMed] [Google Scholar]
- 10. Ardehali H. and O'Rourke B. (2005). Mitochondrial K(ATP) channels in cell survival and death. J Mol Cell Cardiol 39:7–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davis ME, Hsieh PC, Takahashi T, Song Q, Zhang S, Kamm RD, Grodzinsky AJ, Anversa P. and Lee RT. (2006). Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc Natl Acad Sci U S A 103:8155–8160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Beegle JR, Magner NL, Kalomoiris S, Harding A, Zhou P, Nacey C, White JL, Pepper K, Gruenloh W, et al. (2016). Preclinical evaluation of mesenchymal stem cells overexpressing VEGF to treat critical limb ischemia. Mol Ther Methods Clin Dev 3:16053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou P, Lessa N, Estrada DC, Severson EB, Lingala S, Zern MA, Nolta JA. and Wu J. (2011). Decellularized liver matrix as a carrier for the transplantation of human fetal and primary hepatocytes in mice. Liver Transpl 17:418–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou P, Hohm S, Olusanya Y, Hess DA. and Nolta J. (2009). Human progenitor cells with high aldehyde dehydrogenase activity efficiently engraft into damaged liver in a novel model. Hepatology 49:1992–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Halter M, Tona A, Bhadriraju K, Plant AL. and Elliott JT. (2007). Automated live cell imaging of green fluorescent protein degradation in individual fibroblasts. Cytometry A 71:827–834 [DOI] [PubMed] [Google Scholar]
- 16. Liew A. and O'Brien T. (2012). Therapeutic potential for mesenchymal stem cell transplantation in critical limb ischemia. Stem Cell Res Ther 3:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Peeters Weem SM, Teraa M, de Borst GJ, Verhaar MC. and Moll FL. (2015). Bone marrow derived cell therapy in critical limb ischemia: a meta-analysis of randomized placebo controlled trials. Eur J Vasc Endovasc Surg 50:775–783 [DOI] [PubMed] [Google Scholar]
- 18. Kim S, Chaudhry A, Lee I. and Frank JA. (2014). Effects of long-term hypoxia and pro-survival cocktail in bone marrow-derived stromal cell survival. Stem Cells Dev 23:530–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Helisch A, Wagner S, Khan N, Drinane M, Wolfram S, Heil M, Ziegelhoeffer T, Brandt U, Pearlman JD, Swartz HM. and Schaper W. (2006). Impact of mouse strain differences in innate hindlimb collateral vasculature. Arterioscler Thromb Vasc Biol 26:520–526 [DOI] [PubMed] [Google Scholar]
- 20. Fang JS, Angelov SN, Simon AM. and Burt JM. (2011). Cx37 deletion enhances vascular growth and facilitates ischemic limb recovery. Am J Physiol Heart Circ Physiol 301:H1872–H1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tebebi PA, Kim SJ, Williams RA, Milo B, Frenkel V, Burks SR. and Frank JA. (2017). Improving the therapeutic efficacy of mesenchymal stromal cells to restore perfusion in critical limb ischemia through pulsed focused ultrasound. Sci Rep 7:41550. [DOI] [PMC free article] [PubMed] [Google Scholar]