Abstract
Today, improvements in diagnostic and therapeutic options allow patients with autoimmune diseases (ADs) to live longer and have more active lives compared with patients receiving conventional anti-inflammatory therapy just two decades ago. Current therapies for ADs aim to inhibit immune cell activation and effector immune pathways, including those activated by cytokines and cytokine receptors. Understandably, such goals become more complicated in patients with long-term established ADs who develop parallel chronic or comorbid conditions, including life-threatening diseases, such as cancer. Compared with the general population, patients with ADs have an increased risk of developing hematological, lymphoproliferative disorders, and solid tumors. However, the aim of current cancer therapies is to activate the immune system to create autoimmune-like conditions and eliminate tumors. As such, their comorbid presentation creates a paradox on how malignancies must be addressed therapeutically in the context of autoimmunity. Because the physiopathology of malignancies is less understood in the context of autoimmunity than it is in the general population, we undertook this review to highlight the peculiarities and mechanisms governing immune cells in established ADs. Moreover, we examined the role of the autoimmune cytokine milieu in the development of immune-related adverse events during the implementation of conventional or immune-based therapy.
Keywords: immunotherapy, autoimmune diseases, cancer, check point inhibitors, immune-related adverse events
Introduction
Cancer is the second leading cause of mortality in the general population (Noone and others 2018). As tumor cells disable and systematically highjack the mechanisms of tolerance and immune surveillance to avoid immune detection, therapy aims to reverse such behavior and elicit autoimmune-like processes against tumors. Compared with current conventional cancer therapies, such as chemotherapy, radiotherapy, and surgery, biological activation of the immune system with immune therapy drives the activation of immune mechanisms to eradicate tumors. Immunotherapy has been demonstrated to significantly improve relapse-free survival and decrease tumor burden, but only in a subpopulation of patients with melanoma, non-small cell lung cancer, Hodgkin's lymphoma, and cancer of head and neck (Wraith 2017).
Paradoxically, activation of the immune system to fight cancer presents a challenge in the context of autoimmune diseases (ADs). Autoimmunity results from the progressive and continuous breakdown of immune check and balance mechanisms protecting host tissues from destruction (Zhang and Vignali 2016). As a preexisting condition, the autoimmune process starts 2–4 years (around 3.5 years on average) before diagnosis (preclinical stage). Most ADs progress either as an organ-specific (ie, multiple sclerosis [MS] and type 1 diabetes [T1D]) or as a multiorgan disease (ie, systemic lupus erythematous [SLE] or rheumatoid arthritis [RA]). Patients with common autoimmune disorders, such as SLE, RA, and inflammatory bowel disease (IBD) have an increased risk of developing cancers, including hematological disorders and some solid tumors (Franks and Slansky 2012; Liu and others 2014; Yu and others 2016) (Table 1).
Table 1.
Most Frequent Associations Between Autoimmune Diseases and Malignancies
| Autoimmune disease | Nonhematological malignancies | Hematological malignancies | References |
|---|---|---|---|
| Systemic lupus erythematous | Cervical, lung, breast | Hodgkin and non-Hodgkin lymphoma | Giat and others (2017); Malaguarnera and others (2012) |
| Rheumatoid arthritis | Lung, breast, ovary | T cell non-Hodgkin lymphoma, lymphoma (follicular or diffuse large B cell) | Giat and others (2017); Malaguarnera and others (2012); Bernatsky and others (2006) |
| Primary Sjogren syndrome | Oropharynx | Mucosa-associated lymphoid tissue type B cell lymphoma | Malaguarnera and others (2012); Bernatsky and others (2006) |
| Inflammatory bowel disease | Colorectal cancer | Non-Hodgkin lymphoma | Franks and Slansky(2012); Axelrad and others (2016) |
| Systemic sclerosis | Lung, skin, esophageal | Malaguarnera and others (2012) | |
| Wagner's granulomatosis | Bladder | Giat and others (2017) |
Several reports concurred that cancer survival decreases significantly in patients with preexisting AD or chronic inflammatory diseases (Hemminki and others 2012; Criscitiello and others 2016). The decrease in survival has been largely associated with a higher risk of developing immune-related adverse events (IrAEs) in response to anticancer therapy than in the overall population. Generally, IrAEs are unwanted inflammatory events caused by the direct use of disease-modifying antirheumatic drugs alone or in combination with adjuvant biologic therapy (Rosman and others 2013). Although any organ system can be affected, IrAEs most commonly involve the gastrointestinal tract, endocrine glands, skin, and liver (Postow and others 2018). IrAEs occur due to therapy-associated cytokine release and T cell-mediated organ infiltration during therapy with immune checkpoint inhibitors (CPIs) (Liu and others 2014).
Hence, cytokines play substantial roles defining the occurrence and severity of IrAEs in a host and disease-specific manner. Because the physiopathology of malignancies is less understood in the context of autoimmunity than it is in the general population, we undertook this review to examine the role of the autoimmune cytokine overlapping milieu or AICOM over the mechanisms defining the biologic response and outcomes of conventional or immune-based therapy in cancer patients with preexisting AD.
The State of Autoimmune Disease When Anticancer Immunotherapy Is Required
Knowing that cancer patients with preexisting ADs are more likely to be female, older, and with established ADs (Khan and others 2016), it is clear that most initial immune mechanisms have already been established when immunotherapy is required as cancer treatment. While explanation of the initial (onset) mechanisms is not the focus of the present review and has been reviewed extensively elsewhere (Goodnow 2007), disease stages are an important prognostic factor both in ADs and every malignancy. Every disease stage relates with the functional status of the immune system and circulating levels of cytokines. For example, higher cytokine levels are more frequently found in advanced stages than in early stages of either AD or cancer (Seruga and others 2008). At this stage, inflammation and cancers exhibit four identifiable and comparable stages. Stage I (early/onset) is characterized by mild-to-moderate inflammation resulting from recruitment of innate immune cells, such as myeloid cells, to sites of inflammation. At this stage, inflammation and most tumors are contained within a target organ or diseased (in situ) organs, respectively. At stage II (mild course), there is evidence of an activated adaptive immune system featuring T and B cell proliferation and production of autoantibodies compared with stage I in ADs. On the other hand, tumors are larger than in stage I, but tumor cells remain contained within diseased organs or have started to spread locally into lymph nodes. Likely tumor-secreted cytokines, such as granulocyte–macrophage colony-stimulating factor (GM-CSF), macrophage-CSF or interleukin (IL)-12 have started to be detected in serum. These inflammation- or tumor-derived cytokines are shaping the composition of the cytokine milieu and actively influence the functional status of immune cells in the host.
Stage III (moderate course or active) is characterized by an intermittent or progressive course that could include severe episodes of activity. Stage III features predominant recruitment, activation, and proliferation of leukocytes (neutrophils, eosinophils, and basophils) at sites of inflammation, including single or multiple target organs or tumors. For ADs, many stage III patients exhibit comorbid conditions related to advancement of immunopathology such as muscle atrophy in RA or moderate nephritis in SLE. For cancer, it usually means increased tumor burden and metastatic spread into surrounding tissues and distant lymph nodes. Stage IV (severe course) indicates end-stage disease. It results in constantly abnormal inflammatory processes associated with severe comorbid conditions and organ failures such as kidney failure in SLE or widespread metastasis for cancers.
Adaptation Mechanisms Boosting Response in Established Autoimmunity: Priming and Trained Immunity
Based on retrospective epidemiological studies (Johnson and others 2016; Menzies and others 2017; Abdel-Wahab and others 2018), check point immunotherapy was administered to melanoma patients exhibiting AD and cancer at stages II or III (Fig. 1). At those stages, internal organs and primary tumors have been exposed to multiple waves of cytokines, including transforming growth factor-β (TGF-β) as well as type I and II interferons (IFNs). To withstand these different types of stimuli over time, immune and non-immune cells set in place several adaptation mechanisms or countermeasures, including expression of coinhibitory molecules such as programmed cell death-1 (PD-1), lymphocyte activation gene 3 (LAG-3), natural killer (NK) cell receptor 2B4 (CD244), T cell immunoglobulin and mucin-domain containing-3 TIM-3, and cytotoxic T lymphocyte–associated protein 4 (CTLA-4) (Blackburn and others 2008). For the purpose of this review, we will focus on the adaptation mechanisms identified on immune cells such as priming, trained immunity, tolerance, or anergy.
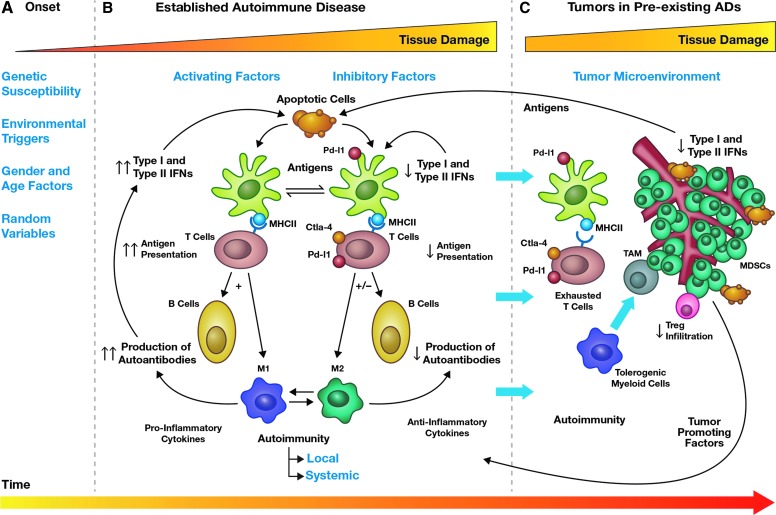
FIG. 1.
Preexisting autoimmune mechanisms modulating tumor growth in the context of autoimmunity. The pathogenesis of cancer in ADs can be divided into three distinct stages (A–C, top) over time of disease (bottom arrow). In the first stage or onset (A), autoimmunity develops in healthy individuals triggered by unknown environmental, genetic, or random factors. This stage can last for 3 to 4 years before clinical diagnosis. In the second stage (B), the AD is diagnosed, and chronic inflammatory mechanisms are established. Based on the balance between activating factors (ie, type I and II IFNs) and inhibitory factors (ie, expression of the coinhibitory molecules such as PD-L1 or CTLA-4) organ or tissue damage ensues. At this stage, responsive feedback loops are established to withstand changes in the AICOM resulting from activation of T cells, B cells, dendritic cells, and macrophages. In the third stage, tumors develop and start gradually modifying the ongoing AICOM through production of tumor-promoting factors to evade immune surveillance. However, immune cells already battling to control ADs showed signs of exhaustion or tolerogenic functional states evidence by the expression of coinhibitory molecules (arrows crossing from stage B, C). Under conditions different from cancer patients without autoimmunity, immunotherapy is required to contain tumor progression. ADs, autoimmune diseases; AICOM, autoimmune cytokine milieu; CTLA-4, cytotoxic T lymphocyte–associated protein 4; IFNs, interferons; MDSCs, myeloid-derived suppressor cells; MHC, major histocompatibility complex; PD-L1, programmed cell death-1 ligands; TAM, tumor-associated macrophages.
Priming represents the response of the adaptive immune system, involving T or B cells, after recognition of specific antigens presented by antigen-presenting cells (APCs) of the innate system, most likely dendritic cells (DCs) and in lesser extend monocytes/macrophages. However, in the context of autoimmunity, monocytes/macrophages aberrantly expressing major histocompatibilty complex II (MHC II) molecules also increase their ability as APCs.
During priming, innate and adaptive immune cells establish cytokine-mediated feedback mechanisms through secretion of IL-1α, IL-6, IL-12, IFN-γ, and tumor necrosis factor-α (TNF-α) (Deng and others 2013; Crowley and others 2017). For example, apart from T cell cross-priming, recent evidence shows that NK cells require IL-18 priming in vivo to produce IFN-γ upon subsequent stimulation with IL-12 (Chaix and others 2008). Thus, the orderly action of two cytokines, first IL-18 then IL-12, are necessary to fully produce IFN-γ by NK cells, thereby establishing a temporal and hierarchical activation sequence. As a result, primed immune cells exhibit stronger responses than naive cells based on stimuli thresholds. The concept of cross-priming is the basis of anticancer vaccination and immunotherapy, as well as some chemotherapy options and radiotherapy. For example, immunotherapy based on agonist stimulation of IFN genes (STING) requires STING expression in Batf3-dependent DCs to enhance cross-priming with checkpoint blockade against murine B16F10 melanoma (Barber 2014; Corrales and Gajewski 2015).
Like priming, trained immunity represent the long-lasting capacity to respond more strongly to stimuli through epigenetic reprogramming, which does not involve gene mutation or recombination, by innate immune cells (such as monocytes, macrophages, or NK cells) (Netea and others 2016). Trained immunity is a nonspecific immunologic memory resulting from rewiring the epigenetic program and the functional state of the innate immune system, eventually resulting in protection against secondary infections (Netea and others 2016). An important difference between classical immunological memory and trained immunity is that the latter has a longer duration than the former (Mitroulis and others 2018). Specifically, functional reprogramming of monocytes for either enhanced (training) or decreased (tolerance) cytokine production impacts the outcome of inflammation (Ifrim and others 2014). The functional phenotype of a trained monocyte has been defined with the following characteristics: (1) increased cytokine production, (2) changes in cellular metabolism (mainly increased glycolysis and lactate production), and (3) epigenetic rewiring (Arts and others 2018). Noticeably, training represents epigenetic manifestations of long-term processes for the adaptive and innate immune systems after encountering a stimulus.
Adaptation Mechanisms Limiting Response in Established Autoimmunity: Tolerance, Anergy, and Exhaustion
Opposite to priming and trained immunity, the terms tolerance and anergy have often been used interchangeably, to describe processes that limit immune cell activation. However, these terms are not equivalent, given the existence of significant differences in functional characteristics and underlying molecular programs (Schietinger and Greenberg 2014). Tolerance is a dynamic and active process through which innate and adaptive immune cells limit the immune system activation and prevent tissue damage. For example, tolerance of self-reactive T cells ensues in both a central mode occurring in the thymus, and in a peripheral mode occurring at the site of peripheral lymphoid organs (Singh and others 2013).
Different from central tolerance, peripheral tolerance inactivates self-reactive T cells through induction of an imprinted cell-intrinsic program mediating a state of functional unresponsiveness (Schietinger and Greenberg 2014). Constant exposure to both endogenous cytokines, such as TNF-α and IL-1, and/or exogenous stimuli, such as low levels of lipopolysaccharide can induce a tolerogenic or dysfunctional state on immune cells (Crowley and others 2017). Similarly, the tolerogenic state in macrophages is characterized by low production of cytokines, such as IL-1, IL-6, and TNF-α (Salim and others 2016). Noticeably, additional evidence suggests that mature DCs can limit effector T cell responses and promote immune tolerance in response to signaling triggered by cytokines IL-27 and IL-10 (Mascanfroni and others 2013; Takenaka and Quintana 2017). Recent reports indicate that the cytokine milieu resulting from tolerogenic immune cells could also prime microenvironments to regulate adaptive responses for T helper 17 (Th17) cells over time (Hu and others 2011).
Exhaustion is a state acquired progressively over a period of weeks or months depending on the chronic stimulus, such as sepsis or autoimmunity. Generally, immune cell exhaustion is associated with impaired, rather than lost, cell-specific functions, such as proliferation (T cells), cytokine production (T-, B-, NK-cells, and macrophages), cytotoxicity (NK cells), and phagocytosis (macrophages) (Huang and others 2009; Kardava and others 2011; Bi and Tian 2017). As the high expression of coinhibitory molecules is already in place to limit autoimmune damage (Nishimura and others 1999; Klocke and others 2016), PD-1 expression in combination with reduced proliferation is commonly used to identify exhausted immune cells (Wherry and Kurachi 2015). So far, STAT3, STAT4, and SMAD transcription factors, which are also involved in regulation of chronic inflammation, seem to control expression of most of the inhibitory receptors (Shalapour and Karin 2015). For example, STAT and SMAD molecules are involved in the regulation of coinhibitory receptors through activation of cytokines such as IL-10 and TGF-β, or through prolonged activation of type I IFNs (IFN-α/β) (Shalapour and Karin 2015).
Chronic activation of the above described mechanisms contributes to the maintenance of T cell exhaustion during chronic infections (Schietinger and Greenberg 2014). Noticeably, the strategy to use monoclonal antibodies (mAbs) targeting inhibitory molecules can successfully reinvigorate both tumor- and nontumor-infiltrating immune cells. As a result, the use of blocking mAbs against coinhibitory receptors can elicit both antitumor and autoimmune effects. Indeed, clinical data demonstrated that 40%–60% of cancer patients with preexisting ADs receiving antibodies against CTLA-4 developed some form of IrAEs, and only 20% of cancer patients with ADs exhibited mostly partial antitumor responses (Johnson and others 2016; Menzies and others 2017). Consequently, therapeutic strategies aiming to reactivate dysfunctional or exhausted immune cells, specifically T cells, need to consider the inflammatory feedback that could exacerbate systemic autoimmunity and induce immune tolerance in the tumor microenvironment (TME).
Different from exhaustion, anergy describes the state by which lymphocytes are functionally unresponsive after antigen encounter, but remain alive for extended periods in a hyporesponsive state (Singh and others 2013). Mostly described for T and B cells, anergy could be established through a generalized inhibition of proliferation (clonal anergy) or through inhibition of effector functions (adaptive tolerance or in vivo anergy). Anergic phenotypes have been described in chronic infection and ADs. For example, a significant number of HIV-specific T cells circulate in an anergic state that could be reversed by immune modulator cytokines, such as IL-2, IL-7, and particularly IL-15 (Gu and others 2007). Similarly, in SLE, chronic engagement leads to an eventual reduction in signaling capacity of B cells and T cells (Foster 2007).
As with T cells, B cell anergy results from B cell binding to low avidity or soluble antigens without receiving adequate additional signals to support their activation (Tsubata 2017). Anergic B cells are unable to interact effectively with helper T cells and do not participate in immune responses against their cognate antigen (Mauri and others 2014). Anergic B cells are characterized by a short half-life (<5 days) and low expression of B cell receptor on their cell surface (Andrews and Wilson 2010). Interestingly, it was reported that NK-cells infiltrating MHC class I deficient tumors acquired an anergic state (Ardolino and others 2014). Moreover, the authors reported that cytokine therapy with IL-12 and IL-18 reversed the anergic state in MHC-deficient tumors suggesting that modulating cytokine levels could enhance immune response. Thus, recognizing the training status of immune cells could impact the type of therapeutic intervention and the time before adjustment needed for patients with ADs based on their disease state.
The AICOM
Even at preclinical stages, patients with autoimmune disorders exhibit increased serum levels of certain inflammatory cytokines. For example, SLE patients exhibit increased levels of IFN-γ, IL-5, and IL-6 (Lu and others 2016). The specific set of cytokines present in a nascent autoimmune environment could be called the “autoimmune cytokine overlapping milieu” or AICOM. Under AICOM, autoimmunity evolves as a continuously progressive condition reflecting the host-attempted response to balance external and internal stimuli.
It is widely believed that over time a mixture of deregulated pro- and anti-inflammatory set of events characterize human ADs. Most human established ADs exhibit their own mix of local and systemic inflammatory events during disease progression. Consequently, instead of one AICOM, several different and comparable AICOMs are established throughout different body or tissue locations. Generally, systemic engagement of multiple AICOMs result in high systemic cytokine levels and are associated with relapses or active states (also called flares), whereas engagement of local AICOMs result in relatively lower cytokine levels, although high compared with healthy subjects, are associated with remitting or inactive states (Wildner and Kaufmann 2013). Consequently, the group of pro- and anti-inflammatory cytokines in AICOMs are indicators of the immune process. Over time, those cytokines define the temporal and hierarchical establishment of costimulatory and coinhibitory pathways balancing the effects of autoimmunity (Zhang and Vignali 2016).
Similarly, some myeloid leukemias exhibit a consistent history of relapse and remission stages during the course of disease (Sasine and Schiller 2015). In such cases, the inability to resolve chronic inflammation is widely considered one of the primary causes of carcinogenesis and tumor progression (Aggarwal and others 2009). The underlying deregulated cytokine production and aberrant cytokine signaling correspond to the predominance of one or a mixture of cytokines associated with the activation of three main subsets of T cells: T helper 1 (Th1), T helper 2 (Th2), or Th17. Classically, Th1 cytokines are associated with the induction of cytotoxic functions from Th1 cells (ie, IL-2, IFN-γ, and TNF-α) and macrophages (ie, IL-1, IL-6, IL-12, and TNF-α). By contrast, Th2 cytokines are associated with chronic and repair processes carried out by the following cytokines IL-4, IL-5, IL-6, IL-10, IL-13, and IL-25 (Leung and others 2010; Moudgil and Choubey 2011).
More recently, a new subset of T cells called Th17 cells producing mainly IL-17 along with other cytokines, such as IL-6, IL-21, IL-22, and IL-26, have been recognized to play roles in ADs and cancer. The development of Th17 cells is supported by other cytokines, such as IL-6, TGF-β, and IL-23, produced primarily by APC such as DCs and monocytes (Harrington and others 2005; Park and others 2005; Bettelli and others 2006). Nevertheless, heterogeneous rather than homogenous activation patterns featuring increased ratios of blood Th2 and Th17 cells over Th1 cells have been observed in Ads, such as SLE or RA (Ueno and others 2015). Recent evidence using fate mapping experiments found that subsets of Th17 cells also express IFN-γ, which negatively affected the severity of experimental autoimmune encephalomyelitis (EAE) (Hirota and others 2011). Moreover, human Th17 cells can preferentially produce IL-10 and IL-17 to respond against bacterial infection (eg, Staphylococcus aureus) or IL-17 and IFN-γ to respond to fungi (eg, Candida albicans) (Burkett and others 2015).
IL-17 is known to be produced alternatively by activated CD8+ T cells, TCRγδ+ T cells, and neutrophils (Stark and others 2005) suggesting that immune cells exhibit increased plasticity to respond against different stimuli. Considering that chronic exposure to IFN-γ is associated with autoimmunity, recent evidence linking increased levels of IL-17 along with IFN-γ to IBD (Harbour and others 2015) suggests that composition of the cytokine milieu involve a variety of immune cells responding to autoimmunity or chronic inflammation. Indeed, AICOMs contain a variety of cytokine activating subsets likely coming from multiple immune cell types, including IFN-α, IFN-β, B cell-activating factor of the TNF family (BAFF/TNFSF13B), IL-12p40, and SCF/c-kit ligand (Slight-Webb and others 2016). Under such circumstances, Th17 differentiation is being regulated by some of those cytokines such as IL-12, IFN-γ, or TGF-β plus IL-6 (McGeachy and Cua 2008). Therefore, the ability of immune cells to form heterogeneous populations complicate the traditional approach to targeting unspecific cell types, especially when heterogeneous subsets of immune cells coproduce cytokines traditionally categorized as Th1, Th2, or Th17.
Targeting Tregs and Th17 Cells in Established ADs and Cancer
Historically, the use of cytokines as adjuvant cancer therapy has been associated with moderate responses and severe adverse events, especially for type I IFN (IFN-α/β), type II IFN, and IL-2 (Gogas and others 2010; Ascierto and others 2013). Different from other cytokines, IFNs are consistently elevated both in preclinical and clinical stages of ADs, such as in RA or in SLE. Paradoxically, IFN-γ-null−/− mice were found to be highly susceptible to multiorgan-specific ADs, including EAE (Willenborg and others 1996). Moreover, blockade of IFN-γ or its signaling pathways exacerbated the severity of disease in murine EAE and collagen type II-induced arthritis (CIA) (Leung and others 2010). Indeed, failure to produce IFN-γ and the resulting deficit in CD4+CD25+ regulatory T cells (Treg) function during acute inflammation provided evidence that IFN-γ through induction of Forkhead box P3 (FOXP3) is essential in self-regulatory mechanisms (Wang and others 2006). This finding led researchers to investigate the nature of Treg and Th17 cells. Tregs help maintain immune self-tolerance through limiting the activation and expansion of autoreactive immune clones. There are two types of FOXP3-expressing Tregs: one identified as CD25+FOXP3+ natural or Thymic-derived (nTreg) cells that prevent autoimmunity, and the second is identified as post-thymic-induced Treg (iTreg) cells that maintain a noninflammatory environment in the gut (Leung and others 2010).
Several reports concur that loss of peripheral tolerance due to deficient or dysfunctional Tregs contribute to the development of various ADs such as RA and SLE (Chavele and Ehrenstein 2011). Common gamma chain cytokines such as IL-2, IL-7, and IL-15, are required for FOXP3 expression and Treg cell development in the thymus. By contrast, cytokines that induce other Th cell differentiation fates such as IL-4, IFN-γ, and IL-6 restrain or limit iTreg development (Zhou and others 2009). Although Treg cells exert their suppressive functions in a cell–cell contact manner in vitro, it should not be ruled out that Tregs secrete soluble factors (Leung and others 2010). For example, Treg-mediated suppression is mediated by TGF-β and IL-10 in murine models of T1D and EAE, respectively (Leung and others 2010).
The predominance of proinflammatory cytokines (eg, IL-6, IL-1b, and IL-21) in ADs restrain Treg development and support Th17 differentiation (Wang and others 2006). Indeed, expansion of double-negative T cells (CD4− and CD8−) capable of producing both IFN-γ and IL-17 in patients with SLE, as well as in lupus-prone mice, relates to progressive lupus nephritis and AD (Tsokos and others 2016). Collectively, the persistence of inflammation in lesion sites is significantly associated with reduced levels and impaired function of CD4+CD25+FOXP3+ Treg cells induced by proinflammatory cytokines. Thus, biologic therapies in ADs aim to inhibit Th17 and promote Treg cell development to reverse the altered balance between Treg and Th17 cell subsets.
In cancer, it has been shown that CD4+CD25+FOXP3+ Treg cells increase in several solid and hematological malignancies (Wang and others 2006). The frequency of Tregs has been associated with mechanisms of tumor immune escape and poor prognosis, as Tregs recruited to tumors are involved in the inhibition of effector functions in both inflammation and cancer (Criscitiello and others 2016). Tumor-recruited Tregs release chemoattractant cytokines (CCL2 and CCL22), immunosuppressive cytokines (TGF-β and IL-10), and upregulate indoleamine-pyrrole 2,3-dioxygenase (IDO) expression leading to T cell anergy (Gyorki and others 2013). In mouse models, Treg depletion can enhance melanoma immunity. By contrast, a significantly greater number of Th17 cells infiltrate tumors at early stages. However, at late stages, the number of intratumoral Th17 cells decreased compared with the density of Th17 cells in the adjacent, nontumor tissue of patients (Takanori and others 2010). This heightened early presence of Th17 cells in tumor tissue holds true for a vast range of malignancies, implying that tumors themselves produce factors that promote Th17 cell trafficking to the diseased site (Bailey and others 2014). Inflammatory Th17 cells and their associated cytokines (ie, IL-17A, IL-17F, IL-21, IL-22, etc.) mediate tumor growth in two distinct ways—by driving angiogenesis and by suppressing antitumor immunity (Bailey and others 2014). The ability of Th17 cells to express several effector cytokines, including IL-2, IL-17, GM-CSF, IFN-γ, and TNF-α define the TME (Zou and Restifo 2010). Nevertheless, tumor-mediated mechanisms exploit the high interconvertible plasticity among Tregs and Th17 cells leading to phenotype changes, including an intermediate phenotype that coexpresses FOXP3 and retinoic acid-related orphan receptor gamma T (RORγT) (Du and others 2008). Different from ADs, cancer therapies aim to increase the antitumor effects of Th17 cells enhancing cytotoxic T cell activity in tumors, while limiting its proangiogenic/protumoral function creating a paradox for treating cancer in the context of autoimmunity.
Chemotherapy in the Treatment of Cancer in Patients with Preexisting ADs
Cancer patients with preexisting ADs are usually excluded from clinical trials with checkpoint inhibitors due to increased risk of toxicity. Consequently, standard cancer therapy options, such as radiation therapy or chemotherapy are the alternative to immunotherapy for these patients. Nevertheless, these interventions have both indirect and direct effects on the immune system depending on the underlying AD. For example, TGF-β is a key mediator of tissue fibrosis and tissue repair in systemic sclerosis (SS) (Lafyatis 2014). SS is associated with increased risk to develop lung, breast, and hematological malignancies (Zeineddine and others 2016). Combined analysis of two studies of women with breast cancer showed that the 8% of women homozygous for the TGF-β1 (-509T) allele, which increases circulating levels of TGF-β1, had a 15-fold increased risk of fibrosis following radiotherapy (Seruga and others 2008). As a result, the use of chemotherapy with agents such as cyclophosphamide is recommended in patients with SS overradiation therapy, which enhances TGF-β (Meng and others 2016).
Like SS, SLE patients have increased risk for hematological malignancies, such as non-Hodgkin's lymphoma, and some solid tumors in lung, liver, vulvar/vaginal, and thyroid areas (Goobie and others 2015). However, SLE patients have lower serum TGF-β1 levels than healthy control individuals. The low levels of TGF-β are associated with both increased disease severity and autoreactive lymphocyte subsets (Becker-Merok and others 2010). For cancer patients with SLE, the release of TGF-β as a result of radiation therapy could be beneficial to the underlying SLE. Consequently, the use of radiotherapy in cancer patients with lupus could restrict underlying autoimmunity but the same treatment could pose a risk for patients with SS.
Chemotherapy-induced death of cancer cells can cause the release of immunogenic antigens, and the emission of danger-associated molecular patterns, which result in cell-mediated immune responses to the tumor (Seruga and others 2008). Nevertheless, patients with AD commonly use several chemotherapy drugs as treatment for autoimmunity, such as cyclophosphamide, mercaptopurine, methotrexate, or mitoxantrone (Ben-Ari 2004). In fact, the expression of IFN-related signature genes in autoimmunity, such as levels of CD8a, CD8b, and IFN-γ can improve clinical response to anthracycline chemotherapy (Mattarollo and others 2011). Moreover, when used in the context of breast cancer tumors, anthracyclines activate a type I IFN gene signature, including the rapid secretion of type I IFN and the release of the chemokine CXCL10/IP-10. Eliciting a type I signature through activation of the pattern recognition receptor Toll-like receptor-3 can predict response to anthracycline therapy in breast cancer patients (Sistigu and others 2014). Consequently, the use of anthracyclines could have negative consequences with type I or type II IFN-driven autoimmune conditions, such as RA, SLE, or MS, especially during relapsing states.
On the other hand, cyclophosphamide and methotrexate have specific uses as immune suppressors either in severe or nonsevere stages, respectively. Cyclophosphamide is used in combination with glucocorticoids to preserve organ function in severe lupus nephritis, severe RA, or severe MS. Because of toxicity, the use of cyclophosphamide is limited to severe autoimmune events or to advanced stages of lymphomas, multiple myeloma, ovarian cancer, breast cancer, or sarcomas. Conversely, methotrexate is tolerated at both low and high doses and approved to treat RA, psoriasis, MS, lupus, sarcoidosis, and ectopic pregnancy. It is used at different higher doses and schedules in cancer patients without autoimmunity, but liver toxicity remains the main complication (Emens and Middleton 2015). Also, methotrexate can inhibit cell replication and recruitment of immature and inflammatory monocytes to sites of inflammation. Collectively, there are concerns that chronic immunosuppression from chemotherapies conceivably increase the risk of malignancy, or tumor progression in patients with autoimmunity.
Immune Biological Therapies in ADs and Cancer
Immune biological therapies carry the risk to increase their toxicity or worsen the severity of underlying AD by increasing the occurrence of IrAEs. Specifically, IrAEs grade III or IV are a major cause of concern as a proportion of patients discontinue treatment and fatalities can occur if not promptly treated. The most prominent CPI-blocking strategies with antibodies are those targeting CTLA-4 and PD-1 or its ligand, programmed cell death ligand 1 (PD-L1). For patients receiving combination immunotherapy with anti-PD-1 and anti-CTLA-4, ∼50% developed grade III or IV irAEs compared with patients treated with anti-PD-1 alone (14%) or anti-CTLA-4 (20%–25%) alone (Stucci and others 2017). In fact, just monotherapy with anti-CTLA-4 or anti-PD-1 induced IrAEs or flares in ∼50% of melanoma patients with preexisting ADs. Noticeably, the response rates for anti-CTLA-4 (20%) and anti-PD-1 (33%), were comparable to those reported in melanoma patients without autoimmunity with or without autoimmunity. Thus, the focus of immunotherapy in the context of autoimmunity is to avoid or minimize the occurrence of IrAEs.
Noticeably, genes encoding CTLA-4 (Ctla4) and PD-1 (pdcd1) have IFN-stimulated response elements making them subject to cytokine regulation, especially for type I and II IFNs (Zhang and Vignali 2016). Recent advances in transcriptional regulation have shown that IFN-stimulated gene factor 3, a complex composed of STAT1, STAT2, and IFN regulatory factor 9 regulates expression of PD-1 (Garcia-Diaz and others 2017). As the loss of IFN production is a hallmark of tumor-infiltrating lymphocytes (TILs), the role of other STAT-activating cytokines, such as IL-10, IL-12, IL-27, and TNF have become centrally important to our understanding of how TILs express coinhibitory molecules as part of the mechanism of response and resistance to immunotherapy (Burkholder and others 2014). Despite their apparent similar regulation, CTLA-4 and PD-1 have distinct roles in the regulation of immunity. CTLA-4 regulates the amplitude of early activation of naive and memory T cells, whereas PD-1 is responsible for the corresponding upregulation of its ligands PD-L1 and PD-L2 that limit the activity of T cells in the periphery during an inflammatory response (Yuan and others 2016).
Similar to their use in cancer, antibodies targeting coinhibitory and costimulatory pathways are used in AD. Most biologic therapies target effector cytokines using antibodies against TNF-α, anti-IL-1, and anti-IL-6 molecules. Historically, the use of antibodies targeting the cytokine TNFSF13B or BAFF have been successful in modifying multiple functions of B cells, such as maturation, proliferation, affinity maturation, and immunoglobulin class switching (Mackay and Mackay 2002). Interestingly, the definition of BAFF cytokine systems provides a major advance in understanding molecular mechanisms of B lymphocyte and tumor cell survival pathways. The results raise additional but addressable questions that could generate a clearer picture of fundamental processes in autoimmunity and cancer, as well as new therapeutic targets (Ware 2000). An alternative to targeting B cell development is to target specific B cell subsets using depleting mAbs, such as anti-CD20 mAbs. CD20 is a transmembrane protein that is expressed on pre-B cells and mature B cells, but not on plasma cells. These anti-CD20 antibodies attenuate humoral autoimmunity and CD4+ T cell autoimmunity by limiting essential ongoing autoantigen presentation by CD20+ B cells, but host defenses can still persist as plasma (effector) cells remain functional (Holdsworth and others 2016). Similarly, anti-CD20 used to target CD20-positive melanoma stem cells, which make up as little as 2% of a tumor, was reported to eradicate melanoma in mice (Schmidt and others 2011). These results provide further evidence for using biological therapies both in autoimmunity and cancer. Considering that these alternative approaches avoid direct T cell targeting, there is a lesson to be learned regarding future approaches that combine cancer and autoimmune biological therapies to possibly treat cancer in patients with preexisting ADs.
Mouse Models of ADs
The lack of knowledge about the mechanisms of AD concomitant with cancer make preclinical mouse models necessary for the analysis of anticancer therapies in the context of an autoimmune environment. To the best of our knowledge, there are no murine models of autoimmunity that develop cancer spontaneously or that contain cancer oncogenes. Considering that women represent 80% of all cases of autoimmunity in the United States (Klein and Flanagan 2016), it is imperative to find well-established preclinical mouse models of autoimmunity with female sex bias to examine the efficacy of experimental cancer therapies in the context of autoimmunity. Fortunately, there are trusted experimental mouse models mimicking human ADs (Wagner and others 2002; Webb 2014) that show female gender bias (Hodge and others 2014), although with minor caveats and considerations. In this section, we will overview the most commonly used mouse models of autoimmunity.
Rheumatoid arthritis
In the case of RA, there are several mouse models, but the first animal model for RA was an adjuvant-induced rat model easy to use and highly reproducible (Table 2). The CIA model arose in mouse and replaced the rat model as the standard. The arthritogenic response is generated through immunization with type II collagen emulsified in complete Freund's adjuvant in a DBA/1 mouse. A well-controlled model based on monoclonal anti-collagen type II antibodies has also gained popularity. More recently, transgenic mouse models have been leveraged to study RA. The new models of spontaneous RA include the human T cell leukemia virus (HTLV)-induced arthritis mice that carries the genome of HTLV-1; K/BxN arthritis mice that produces autoantibodies against glucose-6-phosphate isomerase; SKG arthritis mice that possess a point mutation in ZAP-70 and are dependent upon environmental stimuli (Asquith and others 2009; Benson and others 2018). Several other spontaneous models of arthritis involve deficiencies in cytokine signaling pathways, such as mice with deficiency of IL-1 receptor antagonist (IL-1ra−/−) causing unopposed excess in IL-1 signaling (Koenders and others 2008); mice with point inactivating mutation at F759 in the gp130 IL-6 receptor subunit causing STAT3-mediated proliferation of CD4+ T cells (Sawa and others 2006)or the mice with a double mutation in IFN-1R and DNase II (DNase−/− IFN-1R−/−) that impairs macrophage clearance of phagocytosed DNA from apoptotic cells and results in the production of TNF-α (Kawane and others 2006).
Table 2.
Preclinical Mouse Models for RA and IBD
| Model | Features | Type | References |
|---|---|---|---|
| Collagen type II-induced arthritis (CIA) | Induced by collagen type II emulsified in complete Freund's adjuvant. Incidence and chronicity depend on susceptibility of mouse strain and collagen being used. | Induced | Luross and Williams (2001); Labelle and Hynes (2012) |
| KBxN arthritis (KBN) | Mice display T and B cell responses to glucose-6-phosphate isomerase on cartilage surface. | Spontaneous | Kyburz and Corr (2003) |
| SKG arthritis (SKG) | Inflammatory arthritis associated with a point mutation in ZAP-70. Microbiota can affect disease presentation. | Spontaneous | Sakaguchi and others (2003) |
| IL-1 receptor antagonist−/− arthritis (IL-1ra−/−) | Lack of IL-1 receptor antagonist results in spontaneous destructive arthritis. IL-17 dependent. | Spontaneous | Horai and others (2000) |
| AOM/DSS (IBD) | Chemical induction of DNA damage by AOM combined with repeated epithelial damage by DSS mimic key features of CA-CRC. | Induced | Parang and others (2016) |
AOM, azoxymethane; CA-CRC, colitis-associated colorectal cancer; DSS, dextran sodium sulfate; IBD, inflammatory bowel disease; IL, interleukin.
Systemic lupus erythematosus
Several mouse models have been developed that mimic variable aspects of SLE (Table 3). Recently, Hodge and others (2014) developed the AU-rich element (ARE) deletion (ARE-Del−/−) mouse model of lupus. The deletion in the 3′ untranslated region of the IFN-γ gene increased messenger RNA stability, subsequently increasing the serum levels of IFN-γ. The circulating levels of IFN-γ in the ARE-del model are comparable to levels found in human SLE patients. Moreover, the ARE-del heterozygous mice demonstrate serum levels of IFN-γ that are approximately half the levels observed in the homozygous ARE-del mice. Noticeably, the heterozygous ARE-del mice exhibit moderate renal lesions and elevated blood counts of monocytes and eosinophils. These data suggest a dose-dependent effect of IFN-γ as a driver of disease progression for SLE. Apart from type I IFNs, IFN-γ is also recognized as a significant contributor to SLE pathogenesis (Harigai and others 2008; Pollard and others 2013). Similar to SLE patients, the ARE-del mice exhibit a female-bias phenotype along with neutrophilia, monocytosis, serum low complement supply, glomerulonephritis, and glomerular complement deposition. Another well-established mouse model of lupus is the Murphy Roths Large/lymphoproliferative (MRL/lpr) mouse (Chan and Shlomchik 2000). The MRL/lpr model has a loss-of-function mutation within the gene encoding the Fas protein that bypasses the need for an initiating IFN signature. However, the MRL/lpr model lacks gender bias. Morphologically, the MRL model displays a severe SLE-like disease progression with enlarged lymph nodes, splenomegaly, glomerulonephritis, and in some cases arthritis. SLE morphology and serology can also be seen in the New Zealand Black × White F1 mice affecting only female mice (Wong and others 2013). However, a male bias in SLE onset can be seen in the C57BL/6 BXSB mouse [a cross of C57BL/6J × SB, followed by selection of the satin, nonbeige phenotype (Staats 1985)]. All lupus models described herein exhibit serum antinuclear antibodies and anti-double-stranded DNA antibodies (Table 3).
Table 3.
Phenotype Comparison of Available Preclinical Mouse Models of Systemic Lupus Erythematous
| Phenotype | SLE patients | NZBWF1* | MRL/MPJ-faslpr/J* | ARE-Del−/− | ARE-Del+/− |
|---|---|---|---|---|---|
| Disease onset/severity | Gradual | Severe | Severe | Gradual/moderate | Gradual/mild |
| IFN signature | Stronga/type I and II | Weaka/type I | Absenta | Strong/type II (≥20 pg/mL) | Moderate/type II (≤20 pg/mL) |
| Antinuclear antibodies | Yes | Yes | Yes | Yes | Yes |
| Lymphadenopathy | Yes | Yes | Yes | Yes | Yes |
| Splenomegaly | Yes | Yes | Yes | Yes | Yes |
| Glomerulonephritis | Yes | Yes | Yes | Yes | Yes |
| SLE genes | Yes | Yes | No | Yes | Yes |
| Gender bias (F/M) | F/M 9:1 | Only females | No | Both F/M | Both F/M |
| Age of onset | 16–55 years | 29 weeks | 10 weeks | 10–12 weeks | 10–12 weeks |
| 50% survival age | NA | 45 weeks | 28 weeks | >52 weeks | >52 weeks |
| 50% mortality in females | NA | 7–8 months | 5 months | >10 months | >10 months |
| Breeding performance | NA | Fair | Fair | Poor | Fair |
Inflammatory bowel disease
Like SLE, IBD is complicated by environmental and genetic contributions leading to different clinical presentations. IBD encompasses both ulcerative colitis, limited to the large intestine and rectum, and Crohn's disease, which can present along the gastrointestinal tract. Patients with IBD are at increased risk for colorectal cancer (CRC) (Danese and Mantovani 2010). There has been a large research effort to investigate ulcerative colitis-associated CRC (CA-CRC) in an animal model. The dextran sodium sulfate (DSS)-induced colitis model is the standard mouse model. The addition of the procarcinogen azoxymethane (AOM/DSS) accelerates onset of tumors in mice and has been at the forefront of exploration of the role of gut AD in tumorigenesis. A recent report indicated the potential for natural products as chemopreventive agents in the AOM/DSS mouse model of CA-CRC (Barker and others 2018). As a dietary supplement, the natural triterpenoid celastrol has been associated with significant suppression of inflammatory cytokines TNF-α, IL-6, and IL-1β, as well as the reduction of inducible nitric oxide synthase and cyclooxygenase-2 (Barker and others 2018).
Summary
With recent clinical focus on immune checkpoint inhibitors in combination with traditional therapeutic approaches to treat cancer, cancer specifically in the context of autoimmunity presents targeting paradoxes that need to be addressed both in AD and host-dependent manners. Indeed, the conditions in which cancer arises in established ADs call for novel therapeutic options somewhat different to the use of checkpoint inhibitors as a first line of attack. We believe that those strategies should be tested thoroughly in preclinical models of autoimmunity and cancer to diminish the already high risk of developing IrAEs facing cancer patients with preexisting ADs.
Acknowledgments
This work was supported in part by the Intramural Research Program of the NCI, Cancer and Inflammation Program, Center for Cancer Research, and National Institutes of Health.
Author Disclosure Statement
The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Abdel-Wahab N, Shah M, Lopez-Olivo MA, Suarez-Almazor ME. 2018. Use of immune checkpoint inhibitors in the treatment of patients with cancer and preexisting autoimmune disease: a systematic review. Ann Intern Med 168:121–130 [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Vijayalekshmi RV, Sung B. 2009. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res 15(2):425–430 [DOI] [PubMed] [Google Scholar]
- Andrews SF, Wilson PC. 2010. The anergic B cell. Blood 115(24):4976–4978 [DOI] [PubMed] [Google Scholar]
- Ardolino M, Azimi CS, Iannello A, Trevino TN, Horan L, Zhang L, Deng W, Ring AM, Fischer S, Garcia KC, Raulet DH. 2014. Cytokine therapy reverses NK cell anergy in MHC-deficient tumors. J Clin Invest 124(11):4781–4794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arts RJW, Joosten LAB, Netea MG. 2018. The potential role of trained immunity in autoimmune and autoinflammatory disorders. Front Immunol 9:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascierto PA, Gogas HJ, Grob JJ, Algarra SM, Mohr P, Hansson J, Hauschild A. 2013. Adjuvant interferon alfa in malignant melanoma: an interdisciplinary and multinational expert review. Crit Rev Oncol Hematol 85(2):149–161 [DOI] [PubMed] [Google Scholar]
- Asquith DL, Miller AM, McInnes IB, Liew FY. 2009. Animal models of rheumatoid arthritis. Eur J Immunol 39(8):2040–2044 [DOI] [PubMed] [Google Scholar]
- Axelrad JE, Lichtiger S, Yajnik V. 2016. Inflammatory bowel disease and cancer: the role of inflammation, immunosuppression, and cancer treatment. World J Gastroenterol 22:4794–4801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey SR, Nelson MH, Himes RA, Li Z, Mehrotra S, Paulos CM. 2014. Th17 cells in cancer: the ultimate identity crisis. Front Immunol 5:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber GN. 2014. STING-dependent cytosolic DNA sensing pathways. Trends Immunol 35(2):88–93 [DOI] [PubMed] [Google Scholar]
- Barker EC, Kim BG, Yoon JH, Tochtrop GP, Letterio JJ, Choi SH. 2018. Potent suppression of both spontaneous and carcinogen-induced colitis-associated colorectal cancer in mice by dietary celastrol supplementation. Carcinogenesis 39(1):36–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker-Merok A, Eilertsen Gø, Nossent JC. 2010. Levels of transforming growth factor-β are low in systemic lupus erythematosus patients with active disease. J Rheumatol 37(10):2039–2045 [DOI] [PubMed] [Google Scholar]
- Ben-Ari ET. 2004. Dual purpose: some cancer therapies used to treat autoimmune diseases. J Natl Cancer Inst 96(8):577–579 [DOI] [PubMed] [Google Scholar]
- Benson RA, McInnes IB, Garside P, Brewer JM. 2018. Model answers: rational application of murine models in arthritis research. Eur J Immunol 48(1):32–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernatsky S, Ramsey-Goldman R, Clarke A. 2006. Malignancy and autoimmunity. Curr Opin Rheumatol 18:129–134 [DOI] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441(7090):235–238 [DOI] [PubMed] [Google Scholar]
- Bi J, Tian Z. 2017. NK cell exhaustion. Front Immunol 8:760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DAA, Wherry EJ. 2008. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol 10:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett PR, Meyer zu Horste G, Kuchroo VK. 2015. Pouring fuel on the fire: Th17 cells, the environment, and autoimmunity. J Clin Invest 125(6):2211–2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkholder B, Huang R-Y, Burgess R, Luo S, Jones VS, Zhang W, Lv Z-Q, Gao C-Y, Wang B-L, Zhang Y-M, Huang R-P. 2014. Tumor-induced perturbations of cytokines and immune cell networks. Biochim Biophys Acta 1845(2):182–201 [DOI] [PubMed] [Google Scholar]
- Chaix J, Tessmer MS, Hoebe K, Fuséri N, Ryffel B, Dalod M, Alexopoulou L, Beutler B, Brossay L, Vivier E, Walzer T. 2008. Priming of natural killer cells by interleukin-18. J Immunol 181(3):1627–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan OTM, Shlomchik MJ. 2000. Cutting edge: B cells promote CD8+ T cell activation in MRL-Faslpr mice independently of MHC class I antigen presentation. J Immunol 164(4):1658–1662 [DOI] [PubMed] [Google Scholar]
- Chavele KM, Ehrenstein MR. 2011. Regulatory T-cells in systemic lupus erythematosus and rheumatoid arthritis. FEBS Lett 585(23):3603–3610 [DOI] [PubMed] [Google Scholar]
- Corrales L, Gajewski TF. 2015. Molecular pathways: targeting the stimulator of interferon genes (STING) in the immunotherapy of cancer. Clin Cancer Res 21(21):4774–4779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criscitiello C, Bagnardi V, Esposito A, Gelao L, Santillo B, Viale G, Rotmensz N, Goldhirsch A, Curigliano G. 2016. Impact of autoimmune diseases on outcome of patients with early breast cancer. Oncotarget 7(32):51184–51192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley T, O'Neil JD, Adams H, Thomas AM, Filer A, Buckley CD, Clark AR. 2017. Priming in response to pro-inflammatory cytokines is a feature of adult synovial but not dermal fibroblasts. Arthritis Res Ther 19:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese S, Mantovani A. 2010. Inflammatory bowel disease and intestinal cancer: a paradigm of the Yin-Yang interplay between inflammation and cancer. Oncogene 29(23):3313–3323 [DOI] [PubMed] [Google Scholar]
- Deng H, Maitra U, Morris M, Li L. 2013. Molecular mechanism responsible for the priming of macrophage activation. J Biol Chem 288(6):3897–3906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Huang C, Zhou B, Ziegler SF. 2008. Isoform-specific inhibition of RORα-mediated transcriptional activation by human FOXP3. J Immunol 180(7):4785–4792 [DOI] [PubMed] [Google Scholar]
- Emens LA, Middleton G. 2015. The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res 3(5):436–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster MH. 2007. T cells and B cells in Lupus Nephritis. Semin Nephrol 27(1):47–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks AL, Slansky JE. 2012. Multiple associations between a broad spectrum of autoimmune diseases, chronic inflammatory ciseases and cancer. Anticancer Res 32(4):1119–1136 [PMC free article] [PubMed] [Google Scholar]
- Garcia-Diaz A, Shin DS, Moreno BH, Saco J, Escuin-Ordinas H, Rodriguez GA, Zaretsky JM, Sun L, Hugo W, Wang X, Parisi G, Saus CP, Torrejon DY, Graeber TG, Comin-Anduix B, Hu-Lieskovan S, Damoiseaux R, Lo RS, Ribas A. 2017. Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. Cell Rep 19(6):1189–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giat E, Ehrenfeld M, Shoenfeld Y. 2017. Cancer and autoimmune diseases. Autoimmun Rev 16:1049–1057 [DOI] [PubMed] [Google Scholar]
- Gogas H, Kirkwood JM, Falk CS, Dafni U, Sondak VK, Tsoutsos D, Stratigos A, Markopoulos C, Pectasides D, Spyropoulou-Vlachou M. 2010. Correlation of molecular human leukocyte antigen typing and outcome in high-risk melanoma patients receiving adjuvant interferon. Cancer 116(18):4326–4333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goobie GC, Bernatsky S, Ramsey-Goldman R, Clarke AE. 2015. Malignancies in systemic lupus erythematosus: a 2015 update. Curr Opin Rheumatol 27(5):454–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodnow CC. 2007. Multistep pathogenesis of autoimmune disease. Cell 130(1):25–35 [DOI] [PubMed] [Google Scholar]
- Gu XXJ, Yue FY, Kovacs CM, Ostrowski MA. 2007. The role of cytokines which signal through the common γ chain cytokine receptor in the reversal of HIV specific CD4+ and CD8+ T cell anergy. PLoS One 2(3):e300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyorki DE, Callahan M, Wolchok JD, Ariyan CE. 2013. The delicate balance of melanoma immunotherapy. Clin Trans Immunol 2:e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbour SN, Maynard CL, Zindl CL, Schoeb TR, Weaver CT. 2015. Th17 cells give rise to Th1 cells that are required for the pathogenesis of colitis. Proc Natl Acad Sci USA 112(22):7061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harigai M, Kawamoto M, Hara M, Kubota T, Kamatani N, Miyasaka N. 2008. Excessive production of IFN-gamma in patients with systemic lupus erythematosus and its contribution to induction of B lymphocyte stimulator/B cell-activating factor/TNF ligand superfamily-13B. J Immunol 181(3):2211–2219 [DOI] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. 2005. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 6(11):1123–1132 [DOI] [PubMed] [Google Scholar]
- Hemminki K, Liu X, Ji J, Sundquist J, Sundquist K. 2012. Effect of autoimmune diseases on mortality and survival in subsequent digestive tract cancers. Ann Oncol 23(8):2179–2184 [DOI] [PubMed] [Google Scholar]
- Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, Garefalaki A, Potocnik AJ, Stockinger B. 2011. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol 12:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge DL, Berthet C, Coppola V, Kastenmuller W, Buschman MD, Schaughency PM, Shirota H, Scarzello AJ, Subleski JJ, Anver MR, Ortaldo JR, Lin F, Reynolds DA, Sanford ME, Kaldis P, Tessarollo L, Klinman DM, Young HA. 2014. IFN-gamma AU-rich element removal promotes chronic IFN-gamma expression and autoimmunity in mice. J Autoimmun 53:33–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdsworth SR, Gan P-Y, Kitching AR. 2016. Biologics for the treatment of autoimmune renal diseases. Nat Rev Nephrol 12:217. [DOI] [PubMed] [Google Scholar]
- Horai R, Saijo S, Tanioka H, Nakae S, Sudo K, Okahara A, Ikuse T, Asano M, Iwakura Y. 2000. Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist-deficient mice. J Exp Med 191:313–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Troutman Ty D, Edukulla R, Pasare C. 2011. Priming microenvironments dictate cytokine requirements for T Helper 17 cell lineage commitment. Immunity 35(6):1010–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Venet F, Wang YL, Lepape A, Yuan Z, Chen Y, Swan R, Kherouf H, Monneret G, Chung C-S, Ayala A. 2009. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci USA 106(15):6303–6308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ifrim DC, Quintin J, Joosten LA, Jacobs C, Jansen T, Jacobs L, Gow NA, Williams DL, van der Meer JW, Netea MG. 2014. Trained immunity or tolerance: opposing functional programs induced in human monocytes after engagement of various pattern recognition receptors. Clin Vaccine Immunol 21(4):534–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson Laboratory. Preclinical lupus models and studies. Available at https://www.jax.org/jax-mice-and-services/in-vivo-pharmacology/immunology-services/autoimmune-diseases/lupus-studies (Accessed April16, 2018)
- Johnson DB, Sullivan RJ, Ott PA, Carlino MS, Khushalani NI, Ye F, Guminski A, Puzanov I, Lawrence DP, Buchbinder EI, Mudigonda T, Spencer K, Bender C, Lee J, Kaufman HL, Menzies AM, Hassel JC, Mehnert JM, Sosman JA, Long GV, Clark JI. 2016. Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders. JAMA Oncol 2(2):234–240 [DOI] [PubMed] [Google Scholar]
- Kardava L, Moir S, Wang W, Ho J, Buckner CM, Posada JG, O'Shea MA, Roby G, Chen J, Sohn HW, Chun T-W, Pierce SK, Fauci AS. 2011. Attenuation of HIV-associated human B cell exhaustion by siRNA downregulation of inhibitory receptors. J Clin Invest 121(7):2614–2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawane K, Ohtani M, Miwa K, Kizawa T, Kanbara Y, Yoshioka Y, Yoshikawa H, Nagata S. 2006. Chronic polyarthritis caused by mammalian DNA that escapes from degradation in macrophages. Nature 443:998. [DOI] [PubMed] [Google Scholar]
- Khan SA, Pruitt SL, Xuan L, Gerber DE. 2016. Prevalence of autoimmune disease among patients with lung cancer: implications for immunotherapy treatment options. JAMA Oncol 2(11):1507–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein SL, Flanagan KL. 2016. Sex differences in immune responses. Nat Rev Immunol 16(10):626–638 [DOI] [PubMed] [Google Scholar]
- Klocke K, Sakaguchi S, Holmdahl R, Wing K. 2016. Induction of autoimmune disease by deletion of CTLA-4 in mice in adulthood. Proc Natl Acad Sci USA 113(17):E2383–E2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenders MI, Devesa I, Marijnissen RJ, Abdollahi-Roodsaz S, Boots AM, Walgreen B, di Padova FE, Nicklin MJ, Joosten LA, van den Berg WB. 2008. Interleukin-1 drives pathogenic Th17 cells during spontaneous arthritis in interleukin-1 receptor antagonist-deficient mice. Arthritis Rheum 58(11):3461–3470 [DOI] [PubMed] [Google Scholar]
- Kyburz D, Corr M. 2003. The KRN mouse model of inflammatory arthritis. Springer Semin Immunopathol 25:79–90 [DOI] [PubMed] [Google Scholar]
- Labelle M, Hynes RO. 2012. The initial hours of metastasis: the importance of cooperative host-tumor cell interactions during hematogenous dissemination. Cancer Discov 2:1091–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafyatis R. 2014. Transforming growth factor β—at the centre of systemic sclerosis. Nat Rev Rheumatol 10:706. [DOI] [PubMed] [Google Scholar]
- Leung S, Liu X, Fang L, Chen X, Guo T, Zhang J. 2010. The cytokine milieu in the interplay of pathogenic Th1/Th17 cells and regulatory T cells in autoimmune disease. Cell Mol Immunol 7(3):182–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Blake SJ, Smyth MJ, Teng MW. 2014. Improved mouse models to assess tumour immunity and irAEs after combination cancer immunotherapies. Clin Transl Immunol 3(8):e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R, Munroe ME, Guthridge JM, Bean KM, Fife DA, Chen H, Slight-Webb SR, Keith MP, Harley JB, James JA. 2016. Dysregulation of innate and adaptive serum mediators precedes systemic lupus erythematosus classification and improves prognostic accuracy of autoantibodies. J Autoimmun 74:182–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luross JA, Williams NA. 2001. The genetic and immunopathological processes underlying collagen-induced arthritis. Immunology 103:407–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay F, Mackay CR. 2002. The role of BAFF in B-cell maturation, T-cell activation and autoimmunity. Trends Immunol 23(3):113–115 [DOI] [PubMed] [Google Scholar]
- Malaguarnera M, Cristaldi E, Romano G, Malaguarnera L. 2012. Autoimmunity in the elderly: implications for cancer. J Cancer Res Ther 8:520–527 [DOI] [PubMed] [Google Scholar]
- Mascanfroni ID, Yeste A, Vieira SM, Burns EJ, Patel B, Sloma I, Wu Y, Mayo L, Ben-Hamo R, Efroni S, Kuchroo VK, Robson SC, Quintana FJ. 2013. IL-27 acts on DCs to suppress the T cell response and autoimmunity by inducing expression of the immunoregulatory molecule CD39. Nat Immunol 14:1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattarollo SR, Loi S, Duret H, Ma Y, Zitvogel L, Smyth MJ. 2011. Pivotal role of innate and adaptive immunity in anthracycline chemotherapy of established tumors. Cancer Res 71(14):4809–4820 [DOI] [PubMed] [Google Scholar]
- Mauri C, Reddy V, Blair PA. 2014. Chapter 10—B cell activation and B cell tolerance A2. In: Mackay IR, ed. The autoimmune diseases, 5th ed. Boston, MA: Academic Press, pp 147–158 [Google Scholar]
- McGeachy MJ, Cua DJ. 2008. Th17 cell differentiation: the long and winding road. Immunity 28(4):445–453 [DOI] [PubMed] [Google Scholar]
- Meng XM, Nikolic-Paterson DJ, Lan HY. 2016. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol 12(6):325. [DOI] [PubMed] [Google Scholar]
- Menzies AM, Johnson DB, Ramanujam S, Atkinson VG, Wong AN, Park JJ, McQuade JL, Shoushtari AN, Tsai KK, Eroglu Z, Klein O, Hassel JC, Sosman JA, Guminski A, Sullivan RJ, Ribas A, Carlino MS, Davies MA, Sandhu SK, Long GV. 2017. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol 28(2):368–376 [DOI] [PubMed] [Google Scholar]
- Mitroulis I, Ruppova K, Wang B, Chen L-S, Grzybek M, Grinenko T, Eugster A, Troullinaki M, Palladini A, Kourtzelis I, Chatzigeorgiou A, Schlitzer A, Beyer M, Joosten LAB, Isermann B, Lesche M, Petzold A, Simons K, Henry I, Dahl A, Schultze JL, Wielockx B, Zamboni N, Mirtschink P, Coskun Ü, Hajishengallis G, Netea MG, Chavakis T. 2018. Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell 172(1):147..e12–161.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moudgil KD, Choubey D. 2011. Cytokines in autoimmunity: role in induction, regulation, and treatment. J Interferon Cytokine Res 31(10):695–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea MG, Joosten LAB, Latz E, Mills KHG, Natoli G, Stunnenberg HG, O'Neill LAJ, Xavier RJ. 2016. Trained immunity: a program of innate immune memory in health and disease. Science 352(6284):aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura H, Nose M, Hiai H, Minato N, Honjo T. 1999. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 11(2):141–151 [DOI] [PubMed] [Google Scholar]
- Noone AM, Howlader N, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. 2018. SEER cancer statistics review, 1975–2015. Bethesda, MD: National Cancer Institute National Cancer Institute, pp 1–45 [Google Scholar]
- Parang B, Barrett CW, Williams CS. 2016. AOM/DSS model of colitis-associated cancer. Methods Mol Biol 1422:297–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. 2005. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 6(11):1133–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard KM, Cauvi DM, Toomey CB, Morris KV, Kono DH. 2013. Interferon-gamma and systemic autoimmunity. Discov Med 16(87):123–131 [PMC free article] [PubMed] [Google Scholar]
- Postow MA, Sidlow R, Hellmann MD. 2018. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 378(2):158–168 [DOI] [PubMed] [Google Scholar]
- Rosman Z, Shoenfeld Y, Zandman-Goddard G. 2013. Biologic therapy for autoimmune diseases: an update. BMC Med 11:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi N, Takahashi T, Hata H, Nomura T, Tagami T, Yamazaki S, Sakihama T, Matsutani T, Negishi I, Nakatsuru S, Sakaguchi S. 2003. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature 426:454–460 [DOI] [PubMed] [Google Scholar]
- Salim T, Sershen CL, May EE. 2016. Investigating the role of TNF-alpha and IFN-gamma activation on the dynamics of iNOS gene expression in LPS stimulated macrophages. PLoS One 11(6):e0153289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasine JP, Schiller GJ. 2015. Emerging strategies for high-risk and relapsed/refractory acute myeloid leukemia: novel agents and approaches currently in clinical trials. Blood Rev 29(1):1–9 [DOI] [PubMed] [Google Scholar]
- Sawa S, Kamimura D, Jin G-H, Morikawa H, Kamon H, Nishihara M, Ishihara K, Murakami M, Hirano T. 2006. Autoimmune arthritis associated with mutated interleukin (IL)-6 receptor gp130 is driven by STAT3/IL-7-dependent homeostatic proliferation of CD4+ T cells. J Exp Med 203(6):1459–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schietinger A, Greenberg PD. 2014. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol 35(2):51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt P, Kopecky C, Hombach A, Zigrino P, Mauch C, Abken H. 2011. Eradication of melanomas by targeted elimination of a minor subset of tumor cells. Proc Natl Acad Sci USA 108(6):2474–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seruga B, Zhang H, Bernstein LJ, Tannock IF. 2008. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer 8:887. [DOI] [PubMed] [Google Scholar]
- Shalapour S, Karin M. 2015. Immunity, inflammation, and cancer: an eternal fight between good and evil. J Clin Invest 125(9):3347–3355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RR, Dubey S, Pinkhasov J. 2013. Chapter 19—Immune tolerance defects in lupus. In: Hahn BH, ed. Dubois' Lupus erythematosus and related syndromes, 8th ed. Philadelphia, PA: W.B. Saunders, pp 256–272 [Google Scholar]
- Sistigu A, Yamazaki T, Vacchelli E, Chaba K, Enot DP, Adam J, Vitale I, Goubar A, Baracco EE, Remedios C, Fend L, Hannani D, Aymeric L, Ma Y, Niso-Santano M, Kepp O, Schultze JL, Tuting T, Belardelli F, Bracci L, La Sorsa V, Ziccheddu G, Sestili P, Urbani F, Delorenzi M, Lacroix-Triki M, Quidville V, Conforti R, Spano JP, Pusztai L, Poirier-Colame V, Delaloge S, Penault-Llorca F, Ladoire S, Arnould L, Cyrta J, Dessoliers MC, Eggermont A, Bianchi ME, Pittet M, Engblom C, Pfirschke C, Preville X, Uze G, Schreiber RD, Chow MT, Smyth MJ, Proietti E, Andre F, Kroemer G, Zitvogel L. 2014. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med 20(11):1301–1309 [DOI] [PubMed] [Google Scholar]
- Slight-Webb S, Lu R, Ritterhouse LL, Munroe ME, Maecker HT, Fathman CG, Utz PJ, Merrill JT, Guthridge JM, James JA. 2016. Autoantibody-positive healthy individuals display unique immune profiles that may regulate autoimmunity. Arthritis Rheumatol 68(10):2492–2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staats J. 1985. Standardized Nomenclature for Inbred Strains of Mice: eighth listing. Cancer Res 45(3):945–977 [PubMed] [Google Scholar]
- Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. 2005. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity 22(3):285–294 [DOI] [PubMed] [Google Scholar]
- Stucci S, Palmirotta R, Passarelli A, Silvestris E, Argentiero A, Lanotte L, Acquafredda S, Todisco A, Silvestris F. 2017. Immune-related adverse events during anticancer immunotherapy: pathogenesis and management. Oncol Lett 14(5):5671–5680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takanori M, Koji K, Yoshiki M, Yoshihiko K, Kousaku M, Mitsuaki W, Shinichirou I, Hideki F. 2010. Distribution of Th17 cells and FoxP3(+) regulatory T cells in tumor-infiltrating lymphocytes, tumor-draining lymph nodes and peripheral blood lymphocytes in patients with gastric cancer. Cancer Sci 101(9):1947–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka MC, Quintana FJ. 2017. Tolerogenic dendritic cells. Semin Immunopathol 39(2):113–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsokos GC, Lo MS, Reis PC, Sullivan KE. 2016. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat Rev Rheumatol 12:716. [DOI] [PubMed] [Google Scholar]
- Tsubata T. 2017. B-cell tolerance and autoimmunity. F1000Res 6:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno H, Banchereau J, Vinuesa CG. 2015. Pathophysiology of T follicular helper cells in humans and mice. Nat Immunol 16:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AH, Gebauer M, Pollok-Kopp B, Hecker M. 2002. Cytokine-inducible CD40 expression in human endothelial cells is mediated by interferon regulatory factor-1. Blood 99(2):520. [DOI] [PubMed] [Google Scholar]
- Wang Z, Hong J, Sun W, Xu G, Li N, Chen X, Liu A, Xu L, Sun B, Zhang JZ. 2006. Role of IFN-gamma in induction of Foxp3 and conversion of CD4+ CD25- T cells to CD4+ Tregs. J Clin Invest 116(9):2434–2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware CF. 2000. April and Baff connect autoimmunity and cancer. J Exp Med 192(11):F35–F38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb DR. 2014. Animal models of human disease: inflammation. Biochem Pharmacol 87(1):121–130 [DOI] [PubMed] [Google Scholar]
- Wherry EJ, Kurachi M. 2015. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 15(8):486–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildner G, Kaufmann U. 2013. What causes relapses of autoimmune diseases? The etiological role of autoreactive T cells. Autoimmun Rev 12(11):1070–1075 [DOI] [PubMed] [Google Scholar]
- Willenborg DO, Fordham S, Bernard CC, Cowden WB, Ramshaw IA. 1996. IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J Immunol 157(8):3223–3227 [PubMed] [Google Scholar]
- Wong M, La Cava A, Hahn BH. 2013. Blockade of programmed death-1 in young (New Zealand Black x New Zealand White) F1 mice promotes the suppressive capacity of CD4+ regulatory T cells protecting from lupus-like disease. J Immunol 190(11):5402–5410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wraith DC. 2017. The future of immunotherapy: a 20-year perspective. Front Immunol 8(1668):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K-H, Kuo C-F, Huang LH, Huang W-K, See L-C. 2016. Cancer risk in patients with inflammatory systemic autoimmune rheumatic diseases: a nationwide population-based dynamic cohort study in Taiwan. Medicine 95(18):e3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Hegde PS, Clynes R, Foukas PG, Harari A, Kleen TO, Kvistborg P, Maccalli C, Maecker HT, Page DB, Robins H, Song W, Stack EC, Wang E, Whiteside TL, Zhao Y, Zwierzina H, Butterfield LH, Fox BA. 2016. Novel technologies and emerging biomarkers for personalized cancer immunotherapy. J Immunother Cancer 4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeineddine N, Khoury LE, Mosak J. 2016. Systemic sclerosis and malignancy: a review of current data. J Clin Med Res 8(9):625–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Vignali DA. 2016. Co-stimulatory and co-inhibitory pathways in autoimmunity. Immunity 44(5):1034–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Chong MM, Littman DR. 2009. Plasticity of CD4+ T cell lineage differentiation. Immunity 30(5):646–655 [DOI] [PubMed] [Google Scholar]
- Zhuang H, Szeto C, Han S, Yang L, Reeves WH. 2015. Animal models of interferon signature positive lupus. Front Immunol 6:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W, Restifo NP. 2010. TH17 cells in tumour immunity and immunotherapy. Nat Rev Immunol 10:248. [DOI] [PMC free article] [PubMed] [Google Scholar]