Abstract
The presence of dynamic left ventricular outflow tract obstruction (LVOTO) can complicate the postoperative course of patients undergoing surgical aortic valve replacement (AVR). The phenomenon of LVOTO is a consequence of an interplay of various pathoanatomic mechanisms. The prevailing cardiovascular milieu dictates the hemodynamic significance of the resultant LVOTO in addition to the anatomical risk factors. A thorough understanding of the predisposing factors, mechanism, and hemodynamic sequel of the obstruction is pivotal in managing these cases. A comprehensive echocardiographic examination aids in risk prediction, diagnosis, severity characterization, and follow-up of management efficacy in the setting of postoperative LVOTO. The armamentarium of management modalities includes conservative (medical) and surgical options. A stepwise approach should be formulated based on the physiological and anatomical substrates predisposing to LVOTO. The index phenomenon occurs more frequently than appreciated and should be considered when the post-AVR patients exhibit hemodynamic instability unresponsive to conventional supportive measures. The present article provides an overview of various peculiarities of this under-recognized phenomenon in the context of the perioperative management of patients undergoing AVR.
Keywords: Aortic valve replacement, asymmetrical septal hypertrophy, left ventricular outflow tract obstruction, mitral systolic anterior motion
Introduction
Dynamic left ventricular outflow tract obstruction (LVOTO) is one of the potential causes for postoperative hemodynamic compromise after surgical aortic valve replacement (AVR).[1] AVR results in subtle changes in left ventricular loading conditions that may provide a substrate for dynamic LVOTO. Various anatomical and physiological factors determine the hemodynamic significance of the resulting LVOTO. The resultant LVOTO, if not addressed timely, culminates as hypotension, compromised forward stroke volume, increased left ventricular end-diastolic pressure (LVEDP), mitral regurgitation, oxygen supply–demand mismatch, or inability to wean the patient from cardiopulmonary bypass (CPB).
Echocardiographic identification of the patients at high risk of developing significant LVOTO in the postoperative period directs the prophylactic measures at alleviating the obstruction. These measures aim at favorably manipulating the physiology to reduce the tendency to obstruction. The echocardiographic assessment also aids in diagnosing LVOTO and delineating the anatomical causes for the underlying phenomenon. A clear characterization of the pathophysiological basis and severity of the LVOTO is imperative for the successful management. The article aims to review the risk factors, pathoanatomic mechanisms, echocardiographic assessment, and management of the resultant dynamic LVOTO following AVR.
Risk Factors
The left ventricular outflow tract (LVOT) and the midventricular area are the potential sites where a dynamic intraventricular gradient (DIG) can exist post-AVR.[2] The various risk factors contributing to dynamic postoperative LVOTO are enlisted in Table 1.[3,4]
Table 1.
Factors predisposing to post aortic valve replacement left ventricular outflow tract obstruction
| AVR for predominant AS |
| Concentric LVH |
| LV septal hypertrophy (sigmoid septum) |
| Relatively narrow LVOT |
| Increased blood flow velocity in LVOT (use of inotropes) |
| Hypovolemia and/or vasodilation |
| Perioperative use of an intraaortic balloon pump |
| A structurally abnormal mitral apparatus with elongated chordae redundant mitral apparatus and calcified annulus |
| Concomitant MVR with a high-profile bioprosthetic valve disc strut or a preserved mitral cusp causing LVOTO |
AVR: Aortic valve replacement, LVOTO: Left ventricular outflow tract obstruction, AS: Aortic stenosis, LVH: Left ventricular hypertrophy, LV: Left ventricle, LVOT: Left ventricular outflow tract, MVR: Mitral valve replacement
Pathoanatomic and Physiological Basis of Left Ventricular Outflow Tract Obstruction
Dynamic LVOTO following AVR is a consequence of underlying structural and functional pathologies. The patients are anatomically predisposed to post-operative obstruction owing to the ventricular and mitral valve apparatus morphological characteristics. The resultant symptoms get further exaggerated in situations with increased functional demand. Most of the initial studies demonstrated that the hemodynamic changes after AVR may exacerbate preexisting dynamic outflow obstruction with life-threatening consequences. These studies postulated that fixed valvular and dynamic subvalvular obstructions coexist in patients with aortic valve disease.[5]
Bartunek et al. presented the largest prospective study on the index phenomenon.[5] They studied 100 patients undergoing AVR for aortic stenosis (AS) and discovered postoperative LVOT gradients in 14 patients, worsened by vasodilation or beta agonists. The proposed mechanism was a combination of a muscular cavity and systolic anterior motion (SAM) of the anterior mitral valve leaflet, with the LVOTO being more pronounced in small, hyperdynamic, and asymmetrically hypertrophied ventricles. However, there are sporadic case reports of LVOTO following AVR for predominant regurgitant lesions of the aortic valve.[6]
Asymmetric septal hypertrophy (ASH) and SAM of the mitral valve have been described as the causes of high intracavitary pressures in three out of the six patients with aortic valve disease by Nanda et al.[7] Interestingly, two out of the three patients had pure aortic incompetence. In the individual case reports of AS complicated with postoperative LVOTO as reported by Schwinger et al.[8] and Cutrone et al.,[9] the left ventricle (LV) was described as having severe concentric hypertrophy. The combination of the venturi and the drag forces forms the basis of LVOTO due to the complex interaction between the altered LV geometry and mitral valve components.
Disproportionate septal thickening, although a characteristic feature of hypertrophic cardiomyopathy, may also be evident in other pathological conditions characterized by pressure-overload hypertrophy. The septal hypertrophy results as a consequence of an exaggerated remodeling response of the ventricle to the pressure overload. Lewis and Maron reported an evidence of ASH in as high as almost one-third of the total patients in their series of elderly hypertensive cohort. ASH was defined by the ratio of wall thickness of any two LV segments exceeding 1.5.[10] The reported incidence of ASH is approximately 10% in various series involving patients with unspecified aortic valve disease. ASH was described in 9% of patients with critical AS at catheterization in a study by Hess et al.[11]
Patients can also have dynamic LVOTO without the evidence of SAM. The mechanism responsible for this obstruction is LV septal hypertrophy, especially involving the basal area of the septum (“sigmoid” septum) resulting in a relatively narrow LVOT.[1] The elevated end-systolic LVOT pressure owing to the aortic valve disease is responsible for the “splinting” effect on the LVOT walls, thereby preventing LVOTO. The removal of a fixed obstruction post-AVR “unmasks” the underlying dynamic obstruction, in face of a decline in the LV end-systolic pressure.[12]
In addition, several physiological factors such as filling state, ventricular contractility, systemic vascular resistance, and LV volume determine the hemodynamic significance of the resultant obstruction. The above-mentioned factors predispose to flow acceleration and abnormally high DIG in the background of a hyperdynamic state in a small LV cavity and signify abnormally turbulent ejection dynamics.
Echocardiographic Prediction of Postoperative Left Ventricular Outflow Tract Obstruction
Various echocardiographic parameters in the preoperative as well as in the immediate postoperative period can predict the subsequent development of LVOTO after AVR. Figure 1[3,13,14,15,16] depicts the various anatomical, physiological, and geometrical echocardiographic findings which present an elevated risk of postoperative LVOTO.
Figure 1.
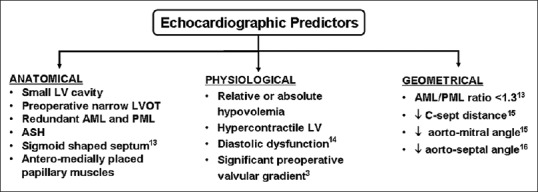
Echocardiographic findings which anatomically, physiologically, or geometrically predispose a post aortic valve replacement patient to the development of LVOTO. LVOTO: Left ventricular outflow tract obstruction, ASH: Asymmetric septal hypertrophy, LVOT: Left ventricular outflow tract, LV: Left ventricle, AML: Anterior mitral leaflet, PML: Posterior mitral leaflet
A hypertrophied septum or a “sigmoid”-shaped septum bulging into the LVOT can be readily appreciated in the midesophageal (ME) aortic valve long-axis (LAX) view with the transesophageal echocardiography (TEE) probe angle at 110°–120° as shown in Figure 2.[17] Any features of ASH in the preoperative TEE as evidenced by a ratio of septal wall to free wall thickness >1.4 in a transgastric short-axis view or a thick basal interventricular septum >15 mm constitute an independent risk predictor for LVOTO postoperatively.[9,10]
Figure 2.
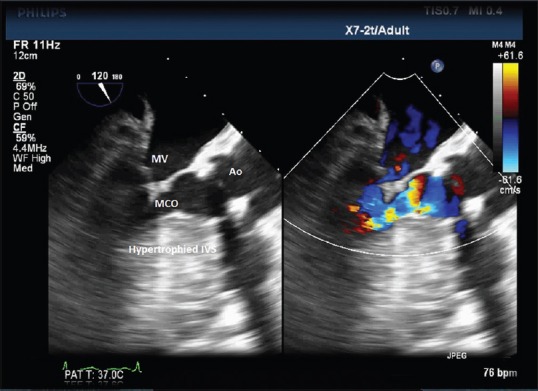
A midesophageal aortic valve long-axis TEE view at 120° showing the hypertrophied interventricular septum predisposing a patient of severe AS to midcavity obstruction. TEE: Transesophageal echocardiography, AS: Aortic stenosis, MV: Mitral valve, Ao: Aorta
A narrow LV cavity can occur secondary to hypovolemia or due to hypertrophied cavity. This can result in an obstruction at the level of midventricular area. The preoperative LV volumes can be estimated in ME views by modified Simpson's method, particularly in the setting of asymmetric hypertrophy involving the midcavity of the LV.
A preoperative documentation of a narrow LVOT is another strong predictor for a postoperative high gradient in the LVOT.[3] The normal LVOT diameter is usually 20 ± 2 mm and can vary in accordance with the body surface area.[17] LVOT diameter should be routinely assessed in the preoperative TEE in the ME aortic valve LAX view by the measurement of the endocardium to endocardium distance of the LVOT within 1 cm of the insertion point of the aortic valve leaflets in midsystole.
Various additional features in the preoperative echocardiographic assessment can predict the occurrence of SAM postoperatively, which can aggravate the LVOTO in already hypertrophied ventricles with a narrow outflow tract.[12,13,14,15,16] These include the presence of redundant or floppy mitral valve leaflets, posterior mitral leaflet length >15 mm, anterior to posterior leaflet ratio <1.3, and a reduced C-sept distance of <25 mm.[15] The leaflet length measurements should be performed during diastole in the ME LAX view with measurements of each leaflet done from the base of the respective leaflet at the mitral annular level to their tips. C-sept distance is measured as the shortest distance from the septum to the point of coaptation of the leaflets and measured in ME LAX view during systole [Figures 3a and 3b depicting a reduced C-sept distance in a patient with septal hypertrophy].
Figure 3.
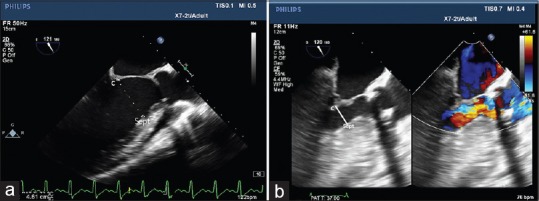
A midesophageal long-axis transesophageal echocardiography view at 120° depicting a normal C-sept distance (a) and a markedly reduced C-sept distance (b) in a patient with septal hypertrophy. C: Coaptation of mitral valve leaflets, Sept: Interventricular septum
Due to the hypertrophy and remodeling, the geometry of the mitral apparatus may also get altered in AS which may result in a more anteriorly and medially placed anterior papillary muscle causing chordal displacement into LVOT.[16] A reduced angle between the aortic and mitral valves can be an additional risk factor for postoperative LVOTO.[15,16]
Echocardiographic Diagnosis of Left Ventricular Outflow Tract Obstruction Post Aortic Valve Replacement
After AVR with a relief in the valvular stenosis and the subsequent reduction in SVR, the subvalvular obstruction may become evident as explained earlier. This may manifest as difficulty in weaning from the CPB in view of decreased LV stroke volume, elevated LVEDP, and varying degree of mitral regurgitation with a subsequent deterioration of the hemodynamics. Figure 4 illustrates a case of post-AVR LVOTO characterized by a combination of SAM and interventricular septal hypertrophy as the etiologies for the resultant turbulent flow in the LVOT.
Figure 4.
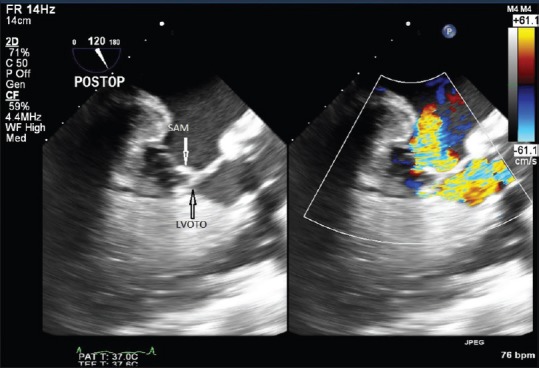
A midesophageal aortic valve long-axis TEE view at 120° demonstrating turbulent flow in the LVOT resulting from the combination of SAM and midventricular hypertrophy, with the resultant mitral regurgitation, in a case of post-AVR patient for predominant AS. TEE: Transesophageal echocardiography, LVOT: Left ventricular outflow tract, SAM: Systolic anterior motion, AVR: Aortic valve replacement, AS: Aortic stenosis, LVOTO: Left ventricular outflow tract obstruction
At this point along with the assessment of prosthetic valve to rule out any paravalvular leak, increased gradient across the valve and patient prosthetic mismatch, a complete evaluation of the subvalvular area and the intraventricular cavity may help to rule out any new-onset obstruction in the outflow tract. This may be evident in the transgastric LAX view at an angle of interrogation 120°–140°.[17]
Advancing the probe further with anteflexion may help in acquisition of deep transgastric LAX view from near the LV apex. Both the LVOT and the aortic valve can be interrogated in these views where the application of continuous-wave Doppler helps in identifying any gradient at the valvular and subvalvular level. Any gradient at the level of prosthetic valve may be evident as a smooth symmetric contour of the flow profile across the valve, whereas the dynamic LVOT gradient may be evident as a “dagger-shaped” flow profile or the late peaking jet due to the increase in gradient in the late systole, as depicted in Figures 5 and 6.[17]
Figure 5.
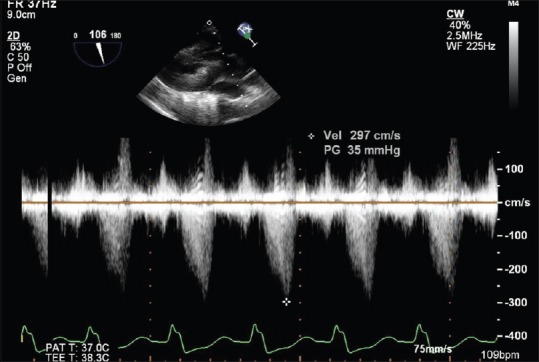
A deep transgastric TEE view at 106° demonstrating a “dagger”-shaped continuous-wave Doppler profile across the LVOT with a characteristic late systolic peaking, in a case of post-AVR patient for predominant AS. TEE: Transesophageal echocardiography, LVOT: Left ventricular outflow tract, AVR: Aortic valve replacement, AS: Aortic stenosis
Figure 6.
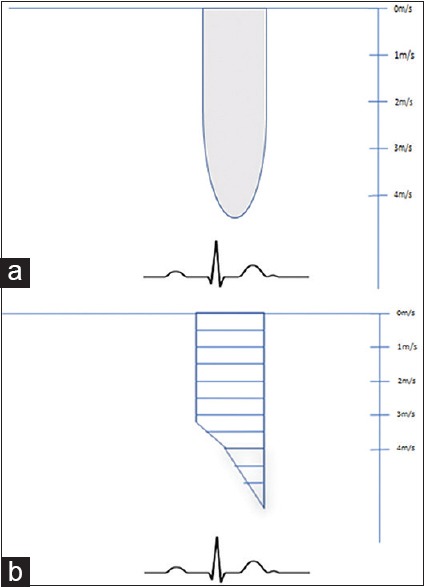
The figure showing the distinguishing features of a valvular gradient (a) and a subvalvular LVOT gradient (b) on continuous-wave Doppler profile, wherein a symmetrical envelope (a) characterizes a valvular obstruction and a “dagger”-shaped envelope (b) signifies a LVOTO, with the gradients peaking in late systole. LVOTO: Left ventricular outflow tract obstruction
Sometimes intracavitary or midcavitary obliteration may be seen following the valve replacement, and this is particularly seen in a globally hypertrophied LV. Hyperkinetic LV may exaggerate the preexisting LVOTO in the postoperative period.
Management
The early diagnosis of LVOTO is essentially critical because the treatment and management are based on a pathophysiological rationale. Patients with preoperative risk factors for LVOTO should be evaluated thoroughly immediately after CPB weaning as they may develop gradient in the outflow tract with subsequent hemodynamic compromise.
The management of dynamic LVOTO post-AVR is complex and further compounded by the hemodynamic profile of the patient.[18] In hemodynamically stable patients, the treatment involves the manipulation of the underlying physiological conditions exacerbating the obstruction. For instance, patients are often volume depleted and on inotropic support in the immediate post-CPB period, which can exaggerate the LVOT gradient. An adequate volume loading of the ventricle along with termination of inotropic agent constitutes the most initial step of management. Titrated doses of β-blockers depress the ventricular contractility, thereby accounting for the decline in the ventricular ejection acceleration, alleviating the aortic outflow obstruction. Another favorable attribute to the β-blocker therapy comprises the effect on heart rate, wherein the decreased heart rate increases the ventricular preload by facilitating improved ventricular relaxation and longer filling time before the ventricular ejection. Vasoconstrictors, primarily α1 agonists, such as phenylephrine are beneficial as they augment the size of the functional LVOT and decrease the LVOT pressure gradient by increasing the systemic vascular resistance. Inodilators, diuretic therapy, or intraaortic balloon pump may worsen the clinical condition by decreasing the afterload; hence, they should be discontinued on suspecting the diagnosis.[19] Some authors have proposed the role of low-rate dual-chamber pacing in the setting of postoperative shock precluding the use of β-blockers.[20] They reported a reduction in LVOT gradients owing to the reduced heart rate and contractility. There exists a school of thought which proposes a prophylactic myectomy during surgery for the patients demonstrating marked septal hypertrophy.[1,2,12,13] The above measures need to be cautiously applied in hemodynamically unstable patients.
If SAM contributes to the LVOTO, a grading of degree of SAM will direct the decision-making. A significant SAM may result in an LVOT gradient >50 mmHg with more than mild MR. A SAM not resolving with the medical management should prompt reinstitution of CPB to address the structural issues contributing to LVOTO. A meticulous repair of the mitral valve leaflets or septal myectomy for the thickened basal septum needs to be considered in such a case scenario.
Post-CPB myocardial stunning can mask the underlying features of LVOTO in the immediate postoperative period owing to the poor ventricular pump function.[21] With the gradual improvement in the myocardial kinetics, LVOTO may become evident. Therefore, an initial absence of LVOT gradient in the immediate post-CPB period in background of a poor LV systolic performance does not rule out post-AVR LVOTO. Hence, serial echocardiographic evaluation of myocardial function and gradient across the valvular and subvalvular areas should be contemplated within the first 48 h after surgery as a routine clinical practice.
Conclusion
Dynamic LVOTO following AVR remains an under-recognized cause of postoperative hemodynamic compromise. It is important for the attending perioperative physician to be aware of this phenomenon, as the management is peculiar, comprising of fluid therapy, beta-blockade, and removal of inotropes which are usually continued in an effort toward hemodynamic stability. Intraoperative TEE is indispensable in early diagnosis and monitoring the efficacy of the therapy. Thus, the presence of dynamic LVOTO following AVR must be diagnosed as early as possible with a “high index of suspicion” and should be considered as a potentially fatal complication refractory to conventional management and catastrophic, if inappropriately treated.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Bach DS. Subvalvular left ventricular outflow obstruction for patients undergoing aortic valve replacement for aortic stenosis: Echocardiographic recognition and identification of patients at risk. J Am Soc Echocardiogr. 2005;18:1155–62. doi: 10.1016/j.echo.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Aurigemma G, Battista S, Orsinelli D, Sweeney A, Pape L, Cuénoud H, et al. Abnormal left ventricular intracavitary flow acceleration in patients undergoing aortic valve replacement for aortic stenosis. A marker for high postoperative morbidity and mortality. Circulation. 1992;86:926–36. doi: 10.1161/01.cir.86.3.926. [DOI] [PubMed] [Google Scholar]
- 3.Panduranga P, Maddali MM, Mukhaini MK, Valliattu J. Dynamic left ventricular outflow tract obstruction complicating aortic valve replacement: A hidden malefactor revisited. Saudi J Anaesth. 2010;4:99–101. doi: 10.4103/1658-354X.65118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tewari P, Basu R. Left ventricular outflow tract obstruction after mitral valve replacement. Anesth Analg. 2008;106:65–6. doi: 10.1213/01.ane.0000289529.64086.cb. [DOI] [PubMed] [Google Scholar]
- 5.Bartunek J, Sys SU, Rodrigues AC, van Schuerbeeck E, Mortier L, de Bruyne B, et al. Abnormal systolic intraventricular flow velocities after valve replacement for aortic stenosis. Mechanisms, predictive factors, and prognostic significance. Circulation. 1996;93:712–9. doi: 10.1161/01.cir.93.4.712. [DOI] [PubMed] [Google Scholar]
- 6.Sukernik MR, Sumner AD, Pae WE. Systolic anterior motion of the mitral valve after aortic valve replacement for aortic insufficiency. J Cardiothorac Vasc Anesth. 2007;21:574–6. doi: 10.1053/j.jvca.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 7.Nanda NC, Gramiak R, Shah PM, Stewart S, DeWeese JA. Echocardiography in the diagnosis of idiopathic hypertrophic subaortic stenosis co-existing with aortic valve disease. Circulation. 1974;50:752–7. doi: 10.1161/01.cir.50.4.752. [DOI] [PubMed] [Google Scholar]
- 8.Schwinger ME, O’Brien F, Freedberg RS, Kronzon I. Dynamic left ventricular outflow obstruction after aortic valve replacement: A Doppler echocardiographic study. J Am Soc Echocardiogr. 1990;3:205–8. doi: 10.1016/s0894-7317(14)80435-5. [DOI] [PubMed] [Google Scholar]
- 9.Cutrone F, Coyle JP, Novoa R, Stewart R, Currie PJ. Severe dynamic left ventricular outflow tract obstruction following aortic valve replacement diagnosed by intraoperative echocardiography. Anesthesiology. 1990;72:563–6. doi: 10.1097/00000542-199003000-00029. [DOI] [PubMed] [Google Scholar]
- 10.Lewis JF, Maron BJ. Diversity of patterns of hypertrophy in patients with systemic hypertension and marked left ventricular wall thickening. Am J Cardiol. 1990;65:874–81. doi: 10.1016/0002-9149(90)91429-a. [DOI] [PubMed] [Google Scholar]
- 11.Hess OM, Schneider J, Turina M, Carroll JD, Rothlin M, Krayenbuehl HP, et al. Asymmetric septal hypertrophy in patients with aortic stenosis: An adaptive mechanism or a coexistence of hypertrophic cardiomyopathy? J Am Coll Cardiol. 1983;1:783–9. doi: 10.1016/s0735-1097(83)80191-0. [DOI] [PubMed] [Google Scholar]
- 12.Routledge T, Nashef SA. Severe mitral systolic anterior motion complicating aortic valve replacement. Interact Cardiovasc Thorac Surg. 2005;4:486–7. doi: 10.1510/icvts.2005.111039. [DOI] [PubMed] [Google Scholar]
- 13.Kerut EK, Hanawalt C, Dearstine M, Frank R, Everson C. Mitral systolic anterior motion (SAM) with dynamic left ventricular outflow obstruction following aortic valve replacement. Echocardiography. 2007;24:658–60. doi: 10.1111/j.1540-8175.2007.00444.x. [DOI] [PubMed] [Google Scholar]
- 14.Luckner G, Margreiter J, Jochberger S, Mayr V, Luger T, Voelckel W, et al. Systolic anterior motion of the mitral valve with left ventricular outflow tract obstruction: Three cases of acute perioperative hypotension in noncardiac surgery. Anesth Analg. 2005;100:1594–8. doi: 10.1213/01.ANE.0000152392.26910.5E. [DOI] [PubMed] [Google Scholar]
- 15.Varghese R, Itagaki S, Anyanwu AC, Trigo P, Fischer G, Adams DH, et al. Predicting systolic anterior motion after mitral valve reconstruction: Using intraoperative transoesophageal echocardiography to identify those at greatest risk. Eur J Cardiothorac Surg. 2014;45:132–7. doi: 10.1093/ejcts/ezt234. [DOI] [PubMed] [Google Scholar]
- 16.Critoph CH, Pantazis A, Tome Esteban MT, Salazar-Mendiguchía J, Pagourelias ED, Moon JC, et al. The influence of aortoseptal angulation on provocable left ventricular outflow tract obstruction in hypertrophic cardiomyopathy. Open Heart. 2014;1:e000176. doi: 10.1136/openhrt-2014-000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maslow AD, Regan MM, Haering JM, Johnson RG, Levine RA. Echocardiographic predictors of left ventricular outflow tract obstruction and systolic anterior motion of the mitral valve after mitral valve reconstruction for myxomatous valve disease. J Am Coll Cardiol. 1999;34:2096–104. doi: 10.1016/s0735-1097(99)00464-7. [DOI] [PubMed] [Google Scholar]
- 18.Ibrahim M, Rao C, Ashrafian H, Chaudhry U, Darzi A, Athanasiou T. Modern management of systolic anterior motion of the mitral valve. Eur J Cardiothorac Surg. 2012;41:1260–70. doi: 10.1093/ejcts/ezr232. [DOI] [PubMed] [Google Scholar]
- 19.Coddens J, Van Alphen J, Deloof T, Hendrickx J. Dynamic left ventricular outflow tract obstruction caused by afterload reduction induced by intra-aortic balloon counterpulsation. J Cardiothorac Vasc Anesth. 2002;16:749–51. doi: 10.1053/jcan.2002.128437. [DOI] [PubMed] [Google Scholar]
- 20.Barkman A, McCay J. Cardiogenic shock in a patient with hypertrophic obstructive cardiomyopathy after insertion of a pacemaker. Am J Crit Care. 2002;11:537–42. [PubMed] [Google Scholar]
- 21.Varghese R, Anyanwu AC, Itagaki S, Milla F, Castillo J, Adams DH. Management of systolic anterior motion after mitral valve repair: An algorithm. J Thorac Cardiovasc Surg. 2012;143:S2–7. doi: 10.1016/j.jtcvs.2012.01.063. [DOI] [PubMed] [Google Scholar]


