Abstract
Objective:
A growing body of research indicates that there exists a correlation between Vit D deficiency and cardiovascular diseases (CVD). In addition to being genetically determined, it is strongly influenced by lifestyle factors. In this study, Vit D and its interrelated factors have been studied as profile marker for identifying the risk of CVD in patients.
Methods:
The present study includes comparison of a total 200 adults CVD patients with the healthy patients as control, by measuring their serum lipid levels and Vit D concentrations with other CVD risk factors.
Results:
The average serum Vit D in CVD patients and controls are found to be 22.55±6.2 ng/ml and 37.62±3.2 ng/ml respectively, showing that 63% of CVD patients and 35% of controls are Vit D deficient. Serum lipids levels were considered as marker for patients having CVD which include high levels of total cholesterol, triglycerides, and low-density lipoprotein-cholesterol while low levels of high-density lipoproteins-cholesterol levels. Other risk factors like hypertension, lifestyle, smoking, dietary factors and nutritional status shows significantly correlation for CVD patients compared to controls.
Conclusion:
Literature supports the relationship between lipid profile and Vit D level by using this as a profile marker for CVD patients. Our study also suggests the same that vitamin D can be used as profile marker for cardiovascular diseases.
Keywords: Cardiovascular diseases, hypertension, lipid profile, Vitamin D
Introduction
The World Health Organization (WHO) estimates that 54% of deaths from noncommunicable diseases are due to cardiovascular diseases (CVD), which involve various anomalies in the heart or the coronary arteries and other blood vessels. The various manifestations of CVD include angina pectoris, myocardial infarction, cardiac failure, hypertensive heart disease, cardiomyopathies, valvular heart diseases, cardiac arrhythmias, aortic aneurysms, peripheral arterial diseases, and various arterial and venous thromboembolic diseases.[1]
The Framingham Heart Study conducted by the National Heart, Lung, and Blood Institute reported that elevation in blood pressure (BP) and cholesterol level is associated with an increased incidence of coronary artery disease. CVD risk is partly genetic but strongly influenced by lifestyle factors such as obesity, increased body fat mass, adipocyte differentiation and proliferation, insulin secretion and sensitivity, BP modulation, change in blood lipids, and inhibition of atherogenesis. The above-mentioned factors are known to cause both Vitamin D deficiency and CVD.[2]
Vitamin D receptors (VDRs) are found in the myocardium and involved in the pathogenesis of many cardiovascular problems. The mechanism of Vitamin D deficiency leading to atherosclerotic vascular disease is complex. It involves raised parathyroid hormone (PTH) levels leading to heightened renin–angiotensin–aldosterone system (RAAS) activity, insulin resistance, and inflammation as shown in Figure 1.
Figure 1.
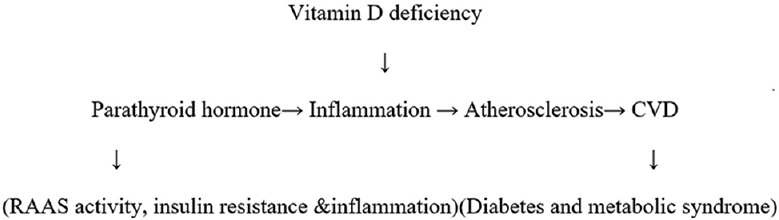
Mechanism of Vitamin D deficiency
At the cellular level, Vitamin D acts through VDR, which is found in virtually all tissues of the body, including cardiovascular tissues such as cardiomyocytes and endothelial and vascular smooth muscle cells. Cardiovascular effects of Vitamin D share the common initial steps of nuclear and plasma membrane VDR activation at the cellular level.
Vitamin D deficiency has been associated with several risk factors for CVD, namely, hypertension, hyperlipidemia, and metabolic syndrome and hence plays an important role in modulating CVD. According to Holick, Vitamin D deficiency and its consequences are extremely subtle but have enormous implications for human health and disease.[3] Optimal Vitamin D levels are achieved by environmental factors such as exposure to ultraviolet light and consumption of foods rich in fat-soluble Vitamin D (dairy products, eggs, meat, and oily sea fish) and are measured by concentration of serum 25-hydroxyvitamin D (25[OH]D).[4]
Geographically, mountain regions, poor socioeconomic state, and culturally diverse conditions are responsible for low levels of Vitamin D in population residing there. These extreme conditions have been responsible for certain negative lifestyle practices, thus predisposing the population to hypertension, diabetes, decreased Vitamin D levels, chronic obstructive pulmonary disease, and tuberculosis. Information indicates that socioeconomic factors such as unemployment, education, and poverty are common in the above region that causes CVD and Vitamin D deficiency. This has been shown in many studies undertaken in various population groups and geographical locations, stating that there is Vitamin D deficiency in 70%–80% of population. Although India has abundant sunshine,[5,6,7] it is still an unresolved issue whether Vitamin D deficiency is involved in the pathogenesis of CVD or vice versa.
There are few reports indicating association of CVD and serum Vitamin D levels, stating that this relationship is multifactorial. This study has been designed to find the association of serum Vitamin D levels with multifactorial risk factors of CVD mentioned above and whether serum Vitamin D can be used as profile marker for CVD.
Materials and Methods
This study was conducted for a period June 2016 to April 2017. We compared the Vitamin D levels in 100 adult CVD patients (CVD group) and 100 healthy participants (control group) of both genders and aged between 30 and 70 years. The patients in CVD group were identified using the WHO criteria based on clinical symptoms and electrocardiogram changes along with alterations in cardiac enzyme levels as per their clinical file records. The participants having conditions such as diabetes mellitus, liver or renal disease, malignancy, hyper- or hypoparathyroidism, Vitamin D supplements, or drugs that accelerate or absorb metabolism of Vitamin D and CVD patients using lipid-lowering medication were excluded from the study. Pregnant females and lactating mothers were also excluded from the study.
All participants provided their written consent and completed a questionnaire regarding personal demographic, socioeconomic, dietary behaviors, lifestyle factors, Vitamin D supplement intake, and sunlight exposure along with biochemical tests (25[OH] D and lipid profile). Vitamin D status was evaluated by measuring the concentration of the major circulating Vitamin D metabolite 25(OH)D (calcidiol), which was investigated in serum for qualitative determination using ELISA technique.[8] The ranges for Vitamin D were taken as sufficient (serum level 30–60 ng/ml) and deficient (serum level <30 ng/ml).
Statistics
One-way analysis of variance and the student's paired t-test were performed to compare both groups for Vitamin D concentrations with P ≤ 0.05, which was considered statistically significant.
Lipid profile included determination of serum total cholesterols (TCs), high-density lipoprotein (HDL) concentration, and triglyceride (TG). Low-density lipoprotein-cholesterol (LDL-C) concentrations were calculated using Friedewald equation: LDL = TC−HDL−TG × (0.2).
Hyperlipidemia was taken as TG >150 mg/dl, HDL <35 mg/dl, and LDL >100 mg/dl while hypertension was taken as considering the systolic BP >140 mmHg and diastolic BP ≥90 mmHg.[9,10] Obesity was considered if the waist circumference was ≥90 cm at the level of the anterior superior iliac spine.
Results
During the study period (June 2016 to April 2107), 100 registered adult cases of CVD and 100 healthy controls of both genders, aged between 30 and 70 years, were studied. In both groups, male/female ratio was 66:34 (statistically nonsignificant). The mean age of men was 46 years and women was 39 years. In CVD group, 66% of men and 75% of women were educated (graduates), whereas in control group, 48% of men and 56% of women were educated. This was a statistically significant variation (P = 0.001). In both the groups, 75% of men and 34% of women were working. Sixty-eight percent of participants in the CVD group were not satisfied with their financial status, whereas this number was 28% in control group. This difference between the two groups was statistically significant (P = 0.001).
Among the CVD category, 65% were hypertensive, 54% were obese, 86% were associated with hyperlipidemia, and 60% suffered from depressive disorder, while in the control group, only 12% were obese, none were hypertensive and hyperlipidemic, and no one was suffering from depressive disorder. This difference of clinical diseases between two groups was statistically significant (P = 0.001). In CVD group, 50% of the patients had a family history of hyperlipidemia, while in the control group, this was only 10%.
The incidence of smoking and psychological stress observed in the CVD category was higher as compared to the control group. The level of physical activity was low in both groups with no statistical significance.
In CVD group, 64% of the patients had Vitamin D deficiency, whereas in control group, this value was 35%. The level of 25(OH)D as measured ranged from a minimum of 27.62 ± 7.2 ng/ml and maximum of 48 ± 3.2 ng/ml. In CVD group, the mean 25(OH)D level was 22.55 ± 6.2 ng/ml, and in control group, the mean 25(OH)D level was 37.62 ± 7.2 ng/ml. This was statistically significance (P = 0.001).
In analysis of the serum lipid profile, the average TC was 213 ± 47.2 and 185 ± 17.6 mg/dl, HDL was 44.2 ± 7.2 and 39 ± 10.2 mg/dl, TG was 189 ± 77.2 and 135 ± 17.6 mg/dl, and LDL was 113 ± 35.2 and 100 ± 17.6 mg/dl in CVD and control categories, respectively. The study reveals that 40% of the participants in the CVD category had higher TC (≥240 mg/dl) as compared to 15% in the control group, while LDL was very high (≥160 mg/dl) among 32% of the participants in the CVD category, as compared to only 6% in the control category. High serum TG levels (≥200 mg/dl) and low serum HDL levels (≤35 mg/dl) were found in 48% in the CVD category and in only 10% of the participants in the control category. These differences reached statistical significance (P = 0.001).
Discussion
The main objective of our study was to find Vitamin D as a molecular marker of CVD and to evaluate about Vitamin D, lipid status, and other CVD risk factors. All the participants in our study were married with a mean age of 42 ± 5 years. The conclusions of our study were in coherence with the results of other studies, stating that incidence of CVD increases with aging, i.e., men >45 years and in women >55 years of age (postmenopausal).[11] Age is a dominant influence in the pathogenesis of atherosclerosis. The accumulation of atherosclerotic plaque is a progressive process and does not manifest clinically until lesions reach a critical threshold and begin to precipitate organ injury.[12] Thus, between the ages of 40–60 years, the incidence of CVD in men increases fivefold. Premenopausal women are relatively protected against CVD and its consequences compared with age-matched men. This has been attributed to the protective role of estrogen in females.[13] Myocardial infarction and other complications of CVD are uncommon in premenopausal women (unless otherwise predisposed by diabetes, hyperlipidemia, or severe hypertension). In females, after menopause, the incidence of CVD or its related diseases exceeds that of men.[14]
In this study, difference in hypertension, obesity, and hyperlipidemia between CVD and control categories had statistical significance. CVD category had a very high incidence of familial history of hyperlipidemia as compared to control category which is attributed to well-defined genetic derangements in lipoprotein metabolism, such as familial hypercholesterolemia, which result in excessively high lipid levels.[15,16]
In our study, the impact of education and financial status was significant and inversely related with the incidence of CVD. The study suggested that low education level and low income were factors associated with adverse cardiac events. The result of our study on smoking and physical activity were in-agreement with other studies which suggests that regular exercise has been recognized as a central element in CVD prevention.[17,18,19]
As observed by others, our study also shows an inverse association between CVD levels and Vitamin D levels. This association is a result of reverse caution, whereby the deficiency of Vitamin D seen in persons with CVD is a result of these persons being less healthy and less likely to go outdoors and be exposed to sunlight or may be mediated by a biological mechanism, whereby low Vitamin D causes increased risk of CVD events. Norman and Powell described several mechanisms by which Vitamin D may be associated with atherosclerosis and CVD events.[20,21]
A study by Anderson et al. also provided evidence from participants with stable CVD that association between Vitamin D and CVD risk may be mediated by PTH. Although PTH level may be one mechanism through which low Vitamin D concentration leads to increase CVD risk through increased vascular remodeling, there are many other pathways such as RAAS, elevation of BP, adverse glucose metabolic profile, increased inflammation, and increased atherogenesis.[22]
In the Framingham Offspring Study, the rate of major CVD events was 53%–80% higher among those with low Vitamin D levels, with increased risk magnified among those with hypertension.[23] However, this study suggested a slightly increased risk at higher Vitamin D levels. In an analysis on adult population from the National Health and Nutrition Examination Survey III study followed up for a median of 8.7 years, mortality was inversely associated with Vitamin D levels, with the lowest quartile of Vitamin D having a 26% increased mortality compared with the highest quartile.[24]
In an analysis of 27,000 patients from the Intermountain Healthcare System, the prevalence of Vitamin D deficiency was 60%, and this deficiency was associated with highly significant increases in the prevalence of type 2 diabetes mellitus, hypertension, and dyslipidemia. Moreover, Vitamin D deficiency was highly associated with myocardial infarction, heart failure and stroke, as well as total mortality.[25,26] In a 10-year follow-up of 18,225 men in the Health Professionals Follow-up Study, low levels of Vitamin D were associated with higher risk of myocardial infarction, even after controlling other CVD risk factors.[27] A recently published multicenter study evaluating patients admitted with acute coronary syndromes found that 96% of these patients had abnormal Vitamin D levels.[28]
We also found that men with Vitamin D deficiency and elevated HDL-cholesterol (HDL-C) had higher TG values as compared to men with sufficient Vitamin D. This may also be associated with the increased risk of dyslipidemia. Other studies also found that 25(OH)D level was positively associated with HDL-C and inversely associated with TC, HDL-C, and TC: HDL-C among women.[29] Some studies have suggested that increasing intestinal calcium absorption could reduce synthesis and secretion of hepatic TG, this promotes the conversion of cholesterol into bile acids and thereby reduces the level of serum cholesterol.[30] In addition, Vitamin D has been suggested to be involved in lipid metabolism, such as the synthesis of bile in the liver, which affects the regulation of lipids directly.
Conclusion
The present study suggests that Vitamin D deficiency is a risk factor for developing CVD. The findings show extensive implications of high incidence of Vitamin D deficiency in relation to personal demographic, socioeconomic, dietary behavior, lifestyle factors, Vitamin D supplements intake, and sunlight exposure.
Vitamin D deficiency may be supplemented to the list of causative factors contributing to the CVD and may be used as a profile marker.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.World Health Organization. Cardiovascular Diseases (CVDs). Fact Sheet N°317. Geneva: WHO Media Centre; 2013a. [Last accessed on 2013 Sep 02]. Available from: http://www.who.int/mediacentre/factsheets/fs317/en/index.html . [Google Scholar]
- 2.Nabel EG, Braunwald E. A tale of coronary artery disease and myocardial infarction. N Engl J Med. 2012;366:54–63. doi: 10.1056/NEJMra1112570. [DOI] [PubMed] [Google Scholar]
- 3.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 4.Enko D, Kriegshäuser G, Stolba R, Worf E, Halwachs-Baumann G. Method evaluation study of a new generation of Vitamin D assays. Biochem Med (Zagreb) 2015;25:203–12. doi: 10.11613/BM.2015.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goswami R, Kochupillai N, Gupta N, Goswami D, Singh N, Dudha A, et al. Presence of 25(OH) D deficiency in a rural North Indian village despite abundant sunshine. J Assoc Physicians India. 2008;56:755–7. [PubMed] [Google Scholar]
- 6.Marwaha RK, Tandon N, Garg MK, Kanwar R, Narang A, Sastry A, et al. Vitamin D status in healthy Indians aged 50 years and above. J Assoc Physicians India. 2011;59:706–9. [PubMed] [Google Scholar]
- 7.Harinarayan CV, Ramalakshmi T, Prasad UV, Sudhakar D. Vitamin D status in Andhra Pradesh: A population based study. Indian J Med Res. 2008;127:211–8. [PubMed] [Google Scholar]
- 8.Holick MF. Vitamin D status: Measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19:73–8. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult treatment panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 10.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 11.Amani R, Sharifi N. Cardiovascular disease risk factors. In: Gaze D, editor. The Cardiovascular System – Physiology, Diagnostics and Clinical Implications. London, United Kingdom: InTech; 2012. pp. 280–310. [Google Scholar]
- 12.Antiochos P, Marques-Vidal P, McDaid A, Waeber G, Vollenweider P. Association between parental history and genetic risk scores for coronary heart disease prediction: The population-based CoLaus study. Atherosclerosis. 2016;244:59–65. doi: 10.1016/j.atherosclerosis.2015.10.104. [DOI] [PubMed] [Google Scholar]
- 13.Matthews KA, Kuller LH, Sutton-Tyrrell K, Chang YF. Changes in cardiovascular risk factors during the perimenopause and postmenopause and carotid artery atherosclerosis in healthy women. Stroke. 2001;32:1104–11. doi: 10.1161/01.str.32.5.1104. [DOI] [PubMed] [Google Scholar]
- 14.Willett W, Stampfer MJ, Bain C, Lipnick R, Speizer FE, Rosner B, et al. Cigarette smoking, relative weight, and menopause. Am J Epidemiol. 1983;117:651–8. doi: 10.1093/oxfordjournals.aje.a113598. [DOI] [PubMed] [Google Scholar]
- 15.McCusker ME, Yoon PW, Gwinn M, Malarcher AM, Neff L, Khoury MJ, et al. Family history of heart disease and cardiovascular disease risk-reducing behaviors. Genet Med. 2004;6:153–8. doi: 10.1097/01.gim.0000127271.60548.89. [DOI] [PubMed] [Google Scholar]
- 16.Austin MA, Hutter CM, Zimmern RL, Humphries SE. Genetic causes of monogenic heterozygous familial hypercholesterolemia: A huge prevalence review. Am J Epidemiol. 2004;160:407–20. doi: 10.1093/aje/kwh236. [DOI] [PubMed] [Google Scholar]
- 17.Jurca R, LaMonte MJ, Durstine LJ. Physical activity and nontraditional CHD risk factors: New pathways for primordial prevention of coronary heart disease. Pres Counc Physical Fitness Sport Res. 2005;6:1–8. [Google Scholar]
- 18.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: An update. J Am Coll Cardiol. 2004;43:1731–7. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 19.Ainsworth BE, Wilcox S, Thompson WW, Richter DL, Henderson KA. Personal, social, and physical environmental correlates of physical activity in African-American women in South Carolina. Am J Prev Med. 2003;25:23–9. doi: 10.1016/s0749-3797(03)00161-2. [DOI] [PubMed] [Google Scholar]
- 20.Norman PE, Powell JT. Vitamin D and cardiovascular disease. Circ Res. 2014;114:379–93. doi: 10.1161/CIRCRESAHA.113.301241. [DOI] [PubMed] [Google Scholar]
- 21.Muscogiuri G, Annweiler C, Duval G, Karras S, Tirabassi G, Salvio G, et al. Vitamin D and cardiovascular disease: From atherosclerosis to myocardial infarction and stroke. Int J Cardiol. 2017;230:577–84. doi: 10.1016/j.ijcard.2016.12.053. [DOI] [PubMed] [Google Scholar]
- 22.Anderson JL, Vanwoerkom RC, Horne BD, Bair TL, May HT, Lappé DL, et al. Parathyroid hormone, Vitamin D, renal dysfunction, and cardiovascular disease: Dependent or independent risk factors? Am Heart J. 2011;162:331–900. doi: 10.1016/j.ahj.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–11. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168:1629–37. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dobnig H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, et al. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168:1340–9. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 26.Anderson JL, May HT, Horne BD, Bair TL, Hall NL, Carlquist JF, et al. Relation of Vitamin D deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare population. Am J Cardiol. 2010;106:963–8. doi: 10.1016/j.amjcard.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 27.Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: A prospective study. Arch Intern Med. 2008;168:1174–80. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JH, Gadi R, Spertus JA, Tang F, O’Keefe JH. Prevalence of Vitamin D deficiency in patients with acute myocardial infarction. Am J Cardiol. 2011;107:1636–8. doi: 10.1016/j.amjcard.2011.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun X, Cao ZB, Tanisawa K, Ito T, Oshima S, Ishimi Y, et al. Associations between the serum 25(OH)D concentration and lipid profiles in Japanese men. J Atheroscler Thromb. 2015;22:355–62. doi: 10.5551/jat.26070. [DOI] [PubMed] [Google Scholar]
- 30.Pfeifer M, Begerow B, Minne HW, Nachtigall D, Hansen C. Effects of a short-term Vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab. 2001;86:1633–7. doi: 10.1210/jcem.86.4.7393. [DOI] [PubMed] [Google Scholar]


