Abstract
The accurate quantification of cardiac output (CO) is given vital importance in modern medical practice, especially in high-risk surgical and critically ill patients. CO monitoring together with perioperative protocols to guide intravenous fluid therapy and inotropic support with the aim of improving CO and oxygen delivery has shown to improve perioperative outcomes in high-risk surgical patients. Understanding of the underlying principles of CO measuring devices helps in knowing the limitations of their use and allows more effective and safer utilization. At present, no single CO monitoring device can meet all the clinical requirements considering the limitations of diverse CO monitoring techniques. The evidence for the minimally invasive CO monitoring is conflicting; however, different CO monitoring devices may be used during the clinical course of patients as an integrated approach based on their invasiveness and the need for additional hemodynamic data. These devices add numerical trend information for anesthesiologists and intensivists to use in determining the most appropriate management of their patients and at present, do not completely prohibit but do increasingly limit the use of the pulmonary artery catheter.
Keywords: Bioreactance, cardiac output monitoring, minimally invasive monitors, thermodilution technique
Introduction
Hemodynamic optimization in critically ill patients is a complex task. Organ perfusion is not only determined by perfusion pressure but also by cardiac output (CO).[1] This necessitates accurate quantification of CO or at least precise detection of the change in the CO perioperatively as well as in the Intensive Care Unit. Invasive monitoring with a pulmonary artery catheter (PAC) was the gold standard in the past, but many alternative less invasive devices are now available. The term Minimally Invasive CO Monitoring (MICOM) collectively describes all devices that do not require insertion of a PAC to calculate CO. The use of CO monitoring along with perioperative protocols to guide intravenous fluid therapy and inotropic support with the aim of improving CO and oxygen delivery (DO2) is essential components of goal-directed therapy (GDT).[2] Studies have demonstrated that MICOM combined with GDT protocols improve perioperative outcomes in high-risk surgical patients.[3,4,5,6,7]
An understanding of the underlying principles of how CO measuring devices work as well as the errors and limitations of their use will allow for more effective and safer utilization.
The major principles and techniques of CO measurement are as follows:
The Fick principle
-
Indicator dilution techniques that include
- Thermodilution
- Pulse dye densitometry
- Lithium dilution technique
Arterial waveform analysis techniques
Transthoracic impedance and bioreactance analysis
The Doppler principle.
Fick's cardiac output measurement
Adolf Eugen Fick, in 1870, first described a method of measuring CO in humans by postulating that the total uptake or release of oxygen by the lungs is the product of blood flow through the lungs and the arteriovenous oxygen content difference.[8] According to his hypothesis, CO can be computed using the equation:
CO = VO2/([CaO2-CvO2] × 100)
VO2 is the oxygen consumption; CaO2 and CvO2 refer to the arterial and mixed venous oxygen content, respectively. Although this technique is very accurate and it is used in cardiac catheterization laboratories, it is not practical for bedside use or continuous CO (CCO) monitoring.
The NICO™ system (Novametrix Medical Systems, Wallingford, USA) utilizes a modification of the Fick principle using carbon dioxide (CO2) to obtain CO measurements in intubated, sedated, and mechanically ventilated patients. The monitor consists of a proprietary disposable rebreathing loop that is attached to the ventilator circuit, a mainstream infrared CO2 sensor, a disposable airflow sensor, and a pulse oximeter [Figure 1]. CO2 production (VCO2) is calculated as a product of CO2 concentration and airflow during a breathing cycle, and arterial CO2 content is derived from the end-tidal CO2 with adjustments for the slope of CO2 dissociation curve and the degree of dead space ventilation. The attached rebreathing loop generates a partial rebreathing state every 3 min that results in an increased end-tidal CO2 and reduced CO2 elimination. The difference between normal and rebreathing ratios is used to calculate CO with an assumption that CO does not change significantly between normal and rebreathing states. This allows the omission of the venous CO2 content measurement which is required in the Fick's equation. There are several limitations in the use of this device due to various requirements such as (a) intubation, (b) mechanical ventilation with fixed ventilator settings, and (c) minimal gas exchange abnormalities.[9] Hence, this technique may only be applied in mechanically ventilated patients with relatively stable hemodynamics.
Figure 1.
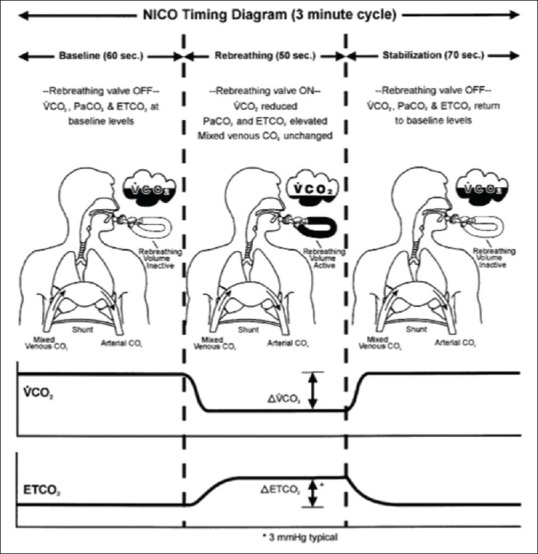
Rebreathing circuit, sequence of rebreathing, and stabilization while using NICO™ system
Indicator dilution techniques
Thermodilution cardiac output
Introduced in 1970, PAC measurement is considered the gold standard. After a predetermined amount of cold saline is injected into the proximal port of a PAC, thermal variations of the blood are measured. CO and other indices are then derived using the thermodilution curve generated using calculations based on the Stewart–Hamilton equation [Figure 2].[10,11]
Figure 2.
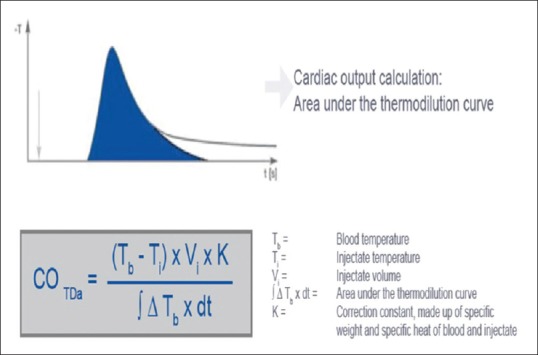
Calculation of cardiac output by measuring area under thermodilution curve using Stewart–Hamilton equation
There are many common sources of error using this technique including the temperature and volume of the injectate, timing during respiration, rate of injection, presence of shunts, and cardiac valvular abnormalities.[12] Moreover, misinterpretation of the data obtained is the most common cause leading to complications.[13] As well, many complications have been attributed to the invasiveness of the PAC leading to significant mortality and morbidity.[14,15,16,17,18,19] Therefore, the use of PACs should be restricted to use in patients with cardiac failure and patients with pulmonary hypertension requiring titration of pulmonary vasodilator therapy.[20]
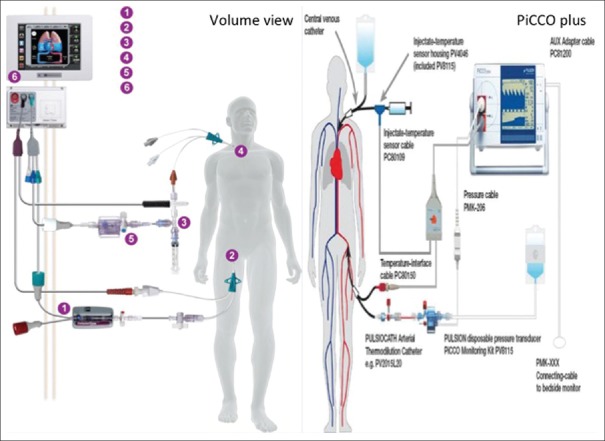
More recently, transpulmonary thermodilution has been used in an attempt to attain the accuracy of PAC thermodilution while avoiding its complications. PiCCOplus (Pulsion Medical Systems, Germany) and EV1000/VolumeView (Edwards Lifesciences, Irvine, CA, USA) use this technique for intermittent calibration of their continuous pulse pressure analysis-based CO monitors. Cold saline is injected into the superior vena cava through a central venous catheter. An arterial cannula is placed in a major artery (femoral, axillary, or brachial), which has an integrated thermistor. It measures the change in blood temperature, and computer software is used to plot a thermodilution curve of temperature change over time [Figure 3]. Cannulation through the femoral vein should be avoided as it may result in an overestimation of intrathoracic blood volume (ITBV).[21] If the femoral vein is the site planned for central venous access, the catheter should be placed on the contralateral side from the femoral arterial catheter to avoid crosstalk phenomena.[22] These monitors also calculate additional values such as the global end-diastolic volume (GEDV), ITBV, extravascular lung water (EVLW), and a pulmonary vascular permeability index from transpulmonary thermodilution.
Figure 3.
Transpulmonary thermodilution technique (volume view and PiCCO plus)
A number of potential sources of error have been identified. As compared to the PAC thermodilution technique, transpulmonary thermodilution is more vulnerable to errors due to drift and indicator recirculation yet less vulnerable to errors due to respiratory variation. As PAC thermodilution measures right heart CO whereas transpulmonary thermodilution measures left heart CO, the presence of an intracardiac or intrapulmonary shunt will lead to differing CO measurements. The magnitude of error produced due to valvular regurgitation cannot be predicted and depends on the site and severity of the regurgitation. However, a high degree of correlation between PAC and transpulmonary thermodilution has been established in various experimental and clinical settings including during cardiac surgery and in intensive care with septic and burns patients.[23,24,25,26]
Underestimation of CO has been reported due to indicator recirculation despite the fact that approximately 96%–97% of the indicator present in the pulmonary artery is recovered in the aorta.[27] The indicator recirculation means the amount of the cold injectate that leaves the blood and enters the tissues and later reenters the bloodstream. Consequently, indicator loss into the lungs, especially in patients with pulmonary edema, has been suggested as a reason for the poor correlation in some studies.[27,28] The effects of indicator loss and recirculation tend to cancel each other out; however, which one of the two parameters is more significant remains to be understood.[28,29]
Pulse dye densitometry
The pulse dye densitometry (DDG-2001 analyzer, Nihon Kohden, Tokyo, Japan) estimates the arterial concentration of indocyanine green (ICG) after an intravenous bolus injection.[30] A fingertip sensor, which emits light at wavelengths of 805 and 890 nm, is used to detect the ICG concentration noninvasively after its passage through the pulmonary circulation. The relative ratio of ICG concentration is used to calculate CO. ICG dye is nontoxic and rarely causes allergy. It is cleared from the blood exclusively through the liver without undergoing either intrahepatic conjugation or enterohepatic metabolism. PDD technique usually allows for a new measurement after 20 min once the ICG concentration decreases to 1% of its initial concentration. Studies concerning the accuracy of this technique have reported conflicting results.[30,31]
Lithium dilution technique
The LidCOplus (LidCO Ltd., Cambridge, UK) determines continuous real-time CO changes based on the pulse power analysis through the PulseCO algorithm and uses lithium dilution for intermittent calibration. This method uses 0.5–2 ml boluses (0.15 mmol/ml) of lithium chloride (to a maximum cumulative dose of 20 ml) injected through a central or peripheral venous catheter, and lithium concentration is measured by a sensor attached to the indwelling arterial cannula. The resulting lithium concentration versus time curve is used to calculate plasma flow using the Stewart–Hamilton equation [Figure 4]. A correction for packed cell volume is then applied to convert plasma flow into blood flow by dividing it by one packed cell volume.[32] Lithium can generate a high signal-to-noise ratio since it does not naturally occur in plasma. It also has a rapid redistribution time and an insignificant first-pass loss from the circulation.[33] On contrary in patients with long-term lithium treatment reduced accuracy may be seen. Furthermore, the presence of nondepolarizing neuromuscular blocking agents, especially atracurium and rocuronium, leads to an overestimation of CO due to cross-reaction of these muscle relaxants with the lithium sensor at high-peak doses.[32] In addition, this technique is contraindicated in patients weighing <40 kg and during the first trimester of pregnancy.
Figure 4.
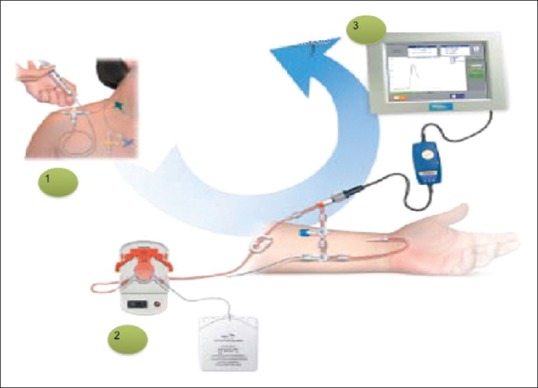
LidCOplus system
Lithium dilution CO measurement has shown good correlation with the PAC thermodilution technique in normal and in hyperdynamic conditions,[9,34] if there are constant blood flow and no indicator loss.[9,23,27,34,35,36] The mean bias between LiDCO device and thermodilution using a PAC has been found to be 0.11 L/min (2 SD 1.94 L/min).[27] Mora et al. showed a good correlation and marginal bias (0.28 L/min) between the lithium dilution and thermodilution technique in patients with impaired left ventricular function after cardiac surgery.[35] On the other hand, LiDCO device performed less well in patients undergoing coronary artery bypass graft and when used during clamping or unclamping of the aorta in comparison to PAC-based thermodilution.[28,34] Available evidence till date supports the LidCOplus technology as a reliable substitute to the more invasive thermodilution method using PAC.
Arterial waveform analysis
Otto Frank first suggested the concept of using the blood pressure waveform to measure blood flow changes in 1899.[37] He described the circulation in terms of a “Windkessel” model. although blood is incompressible, the artery itself is distensible and so the volumes of blood entering and leaving an arterial segment at any given moment may be different from those entering and leaving another segment at the same time. The volumes are only the same when they are averaged over the cardiac cycle. The total stroke volume (SV) must be equal to the forward flow in systole (Qs) plus the forward flow in diastole (Qd), assuming the aortic valve is competent (SV = Qs + Qd). At the beginning of diastole, there is no further inflow into the aorta, and so Qd is proportional to the difference between the pressure in the aorta and the pressure in the arterial beds. This is described as the end-systolic mean distending pressure (Pmd). Therefore, Qd = k × Pmd, where k is a constant of proportionality dependent on the properties of resistance and compliance as described above [Figure 5]. As the peripheral vascular resistance should not change over a single cardiac cycle, the values of Qs and Qd should be proportional to As and Ad, the area under the pressure curve during systole and diastole, respectively. Therefore,
Figure 5.
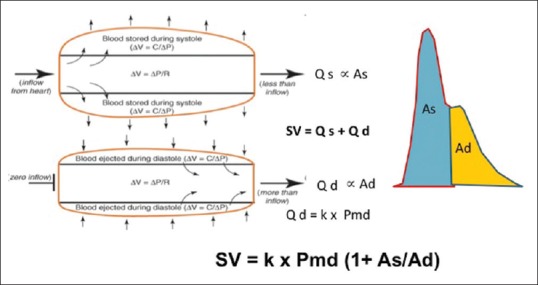
Pulse pressure analysis model to calculate the stroke volume using the arterial waveform
Qs/As = Qd/Ad or Qs = (Qd/Ad) × As = Qd (As/Ad)
Rearranging these equations:
SV = Qd (As/Ad) + Qd = Qd (1+ As/Ad)
SV = k × Pmd (1+ As/Ad)
This model depends on an additional value k, which has to be determined either by calibrating the prediction of this model to another measurement of SV (such as transesophageal echocardiography or indicator dilution) or in an uncalibrated manner by estimating its value from nomograms based on variables such as the patient's age, sex, height, and weight.
Following Otto Frank, the principle of pulse pressure analysis was described by Erlanger and Hooker in 1904, which turned attention to using aortic-arterial pulse pressure to estimate the SV.[38] The concept centered around the theory that fluctuations in blood pressure (pulse height) around a mean value are proportional to the volume of blood forced into the arterial conduit by each systole [Figure 6]. In this technique of pulse pressure analysis, the arterial waveform obtained from an arterial catheter or a finger probe is used to calculate the SV and the systemic vascular resistance (SVR). A major drawback of this technology is the fact that the compliance of the aortic wall is nonlinear and also age related. These prevent any straightforward correlations of pressure to volume.[39] In 1983, Wesseling et al. developed an algorithm to compensate for the aortic wall compliance nonlinearity. This made the calculation of the SV possible by integrating the area under the systolic phase of the arterial waveform curve.[40] Subsequently, several methods based on models representing the systemic circulation were developed for SV estimation. These approaches are generically referred to as pulse contour analysis methods.
Figure 6.
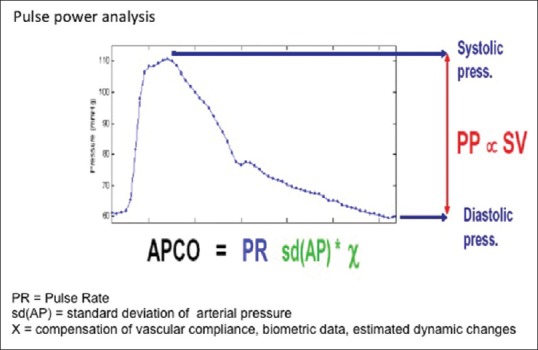
Derivation of cardiac output from the pulse pressure analysis of the arterial waveform
The other approach, nonmorphology based (does not utilize pulse contour analysis), is pulse power analysis. This approach assumes that the net power change in the heartbeat is the balance between the input of a mass of blood (SV) minus the blood mass that is lost to the periphery during the beat. It is based on the law of conservation of mass/power and in taking the whole as opposed to a portion of the beat is independent of the position of the reflected wave. A time-based autocorrelation is used; thereby, a frequency approach to measuring power (such as Fourier transformation) is avoided. Therefore, the effects of arterial damping that change frequency response and discrepancies in measurement due to site-specific waveform distortions are limited. This method does not allow measurement of absolute values of SV and only calculation of changes in it is possible. Hence, for accurate values calibration against a standard method (Lithium dilution technique) is carried out.
In all of these technologies, SV is estimated from the systolic, the diastolic, or the systolic and diastolic components of the waveform. The parameters considered are the systolic and diastolic portions of the pressure waveform, the aortic impedance and compliance and peripheral vascular resistance. The CO is calculated then by multiplying the SV with the heart rate (HR). It is important to note that all arterial waveform analysis methods rely greatly on an ideal arterial pressure tracing. This creates a situation for a potential source of error from an under-or over-damped arterial waveform. Moreover, these systems essentially require an arterial wave that is purely reflective of the forward SV. Thus, conditions that distort the arterial waveform either by artifact, physiologic, or pathophysiologic phenomenon (cardiac arrhythmias, intraaortic balloon counter-pulsation, or aortic regurgitation) will result in inaccuracies.[41]
The accuracy of CO calculation by the pulse pressure analysis technique has been extensively investigated against the gold standard PAC thermodilution method. The new generation software of Flotrac/Vigileo for CO calculation and PAC thermodilution technique has been shown correlating well in patients with a regular rhythm and stable respiratory patterns.[42] In addition, the SV variation (SVV) calculated with this method predicted fluid responsiveness in septic shock patients with reasonable accuracy.[43] In contrast, this technique appeared less accurate when hemodynamic changes were induced by norepinephrine infusion, in off-pump coronary artery bypass surgery, and in open aortic abdominal aneurysm repair as compared to the CO derived by PAC or echocardiography.[44,45,46] This indicates that a cautious approach is recommended when using this method in patients that require considerable inotropic or vasopressor support or in environments and settings where rapid hemodynamic changes occur such as in the operating room.
The commercial systems based on the arterial waveform analysis method are divided into two groups: (a) auto/noncalibrated and (b) externally calibrated.
Auto/noncalibrated devices
FloTrac sensor (Edwards Lifesciences, USA) uses an improved algorithm to derive the SV from the pulse pressure analysis of the arterial waveform derived from a standard indwelling arterial catheter. A similar approach is used by the ProAQT sensor in the Pulsioflex monitor (Pulsion Medical Systems, Germany). The system can be externally calibrated by entering a CO value measured by another method such as echocardiography to increase its accuracy
LidCO rapid system (LidCO Ltd., UK) monitor uses pulse power analysis to determine SV changes. It uses nomograms for the calculation of CO
Nexfin monitor (BMEYE, Netherlands) is a completely noninvasive monitor, which uses an inflatable cuff around the middle phalanx of the finger to derive the finger arterial pressure waveform. This is then reconstructed into a brachial arterial pressure waveform. Subsequently, a novel algorithm for pulse contour analysis (Nexfin CO-Trek) based on the systolic pressure area of this waveform and a physiological three-element Windkessel model that is individualized for each patient is used for determining a CCO. The parameters that are measured by the Nexfin HD include continuous BP (systolic, diastolic, and mean), HR, CCO, SV, SVR, and an index of the left ventricular contractility (dp/dt). This method has been validated with positive results[47]
The esCCO (Nihon Kohden, Japan): This noninvasive monitor uses Pulse Wave Transit Time (PWTT) analysis technology. The CCO derivation in esCCO is based on the principle of an inverse correlation between SV and PWTT. PWTT is defined as the time from the electrocardiogram (ECG) R-wave peak to the pulse wave rise point, which is defined as the point where the differentiated pulse wave reaches 30% of its peak amplitude. PWTT consists of three intervals [Figure 7]: (1). Preejection period (PEP) which is time from the ECG R-wave to the rise point of the aortic root pressure wave; (2). PWTT through elastic artery (T1) which is time from the rise point of the aortic root pressure wave to the rise point of the radial artery pressure wave; and (3). PWTT through peripheral arteries (T2) which is time from the rise point of the radial artery pressure wave to the rise point of the pulse oximetry wave measured by an SpO2 probe on the fingertip. PWTT is acquired from the delay between pulse oximetry waveform and the ECG-R wave signals of each cardiac cycle. Variations in these are used to calculate SV and CCO using formula:
Figure 7.
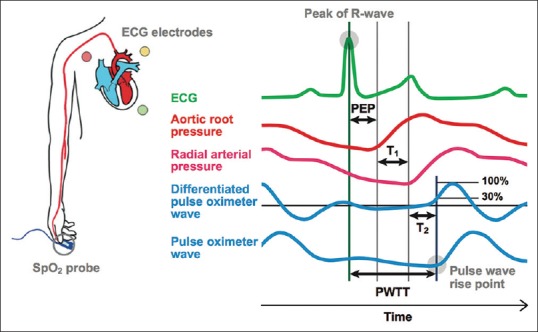
Components of pulse wave transit time. PEP: preejection period, T1: PWTT through elastic artery, and T2: PWTT through peripheral arteries, PWTT = PEP + T1 + T2
CO = K × (α × PWTT + β) × HR; where α and β are experimental constants.
Calibrated devices
The LidCOplus (LidCO Ltd., UK) device incorporates lithium dilution CO to intermittently calibrate its pulse power analysis based CCO measurement
The PiCCOplus monitor (Pulsion Medical Systems, Germany) uses transpulmonary thermodilution for intermittent calibration and continuous real-time CO is calculated by pulse pressure analysis. Calibration is required at least every 8 h in stable patients; however, during resuscitation or in patients who are hemodynamically unstable this monitor may require calibration as often as every 15 min. It has been shown to be reliable in pediatric patients as well as during one-lung ventilation and during renal replacement therapy[48,49,50]
The EV1000/VolumeView monitor (Edwards Lifesciences, Irvine, CA, USA) also uses pulse contour analysis to derive continuous real-time CO and transpulmonary thermodilution for intermittent calibrations. This monitor also displays a new variable named “the global ejection fraction” in addition to other volumetric parameters such as the EVLW and GEDV. A validation study has shown this new method better than PiCCOplus monitor in the calculation of GEDV.[51]
Transthoracic impedance and bioreactance analysis
Nyboer, in 1950, described for the time the extrapolation of SV using variations in the transthoracic electrical impedance to an alternating current that occurs synchronously with the cardiac cycle.[52] Subsequently, Kubicek et al. introduced this technique in clinical practice for CO calculation in 1966.[53] The CO is continuously derived from electrical signals received by using skin electrodes (BIOZ, Cardiodynamics, San Diego, USA) or electrodes mounted on the tracheal tube (ECOM TM, Conmed Corp, Utica, USA). These are used to determine the intra-beat-to-beat variations in transthoracic voltage in response to the applied high-frequency current across the thorax [Figure 8]. The SV is calculated using the formula:
Figure 8.
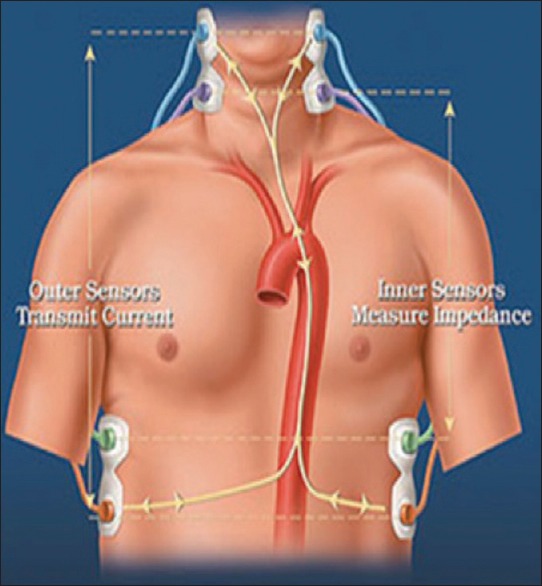
Application of electrodes in impedance cardiography
SV = ρ × L/Z0 2 × (dZ/dt) maxVET
Where ρ-resistivity of blood, L-mean distance between the inner electrodes (the thoracic length), ventricular ejection time (VET), (dZ/dt) max- the absolute of the maximum value of the first derivative during systole, and Z0-basal thoracic impedance. VET is obtained from the dZ/dt versus time curve [Figure 9].
Figure 9.
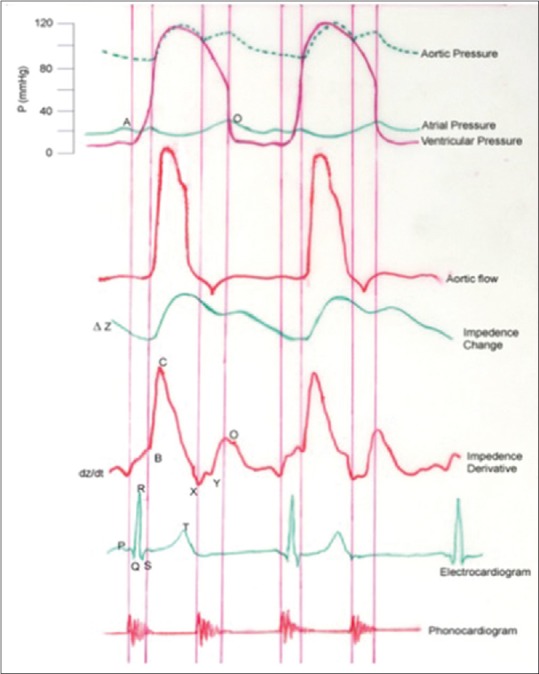
Variation of ventricular, aortic and atrial pressure, aortic flow, thoracic impedance change, and first derivative of impedance (dZ/dt) as a function of time (t). Electrocardiogram and phonocardiogram taken simultaneously are also shown. The curve depicts the cardiac events/performance. B: Opening of the Aortic Valve, X: Closure of the Aortic Valve, Y: Closure of pulmonary valve, O: Mitral valve opening/rapid ventricular filling, B-X: Ventricular Ejection Time, C: Maximal deflection of dz/dt (Peak Flow), B-C: Slope-Acceleration Contractility Index, A: Atrial Systole, and Q: Start of ventricular depolarization
Various sources of error include motion artifacts, electrical interferences, presence of arrhythmias, anatomic shunts, pleural and pericardial effusions, and foreign bodies in the chest and pulmonary edema. Despite many modifications to the mathematical algorithms, clinical validation is not robust.[54,55] A meta-analysis calculated the mean percentage of error to be 43% between this monitoring device and PAC thermodilution technique.[56] The utility of this monitor still needs to be validated.
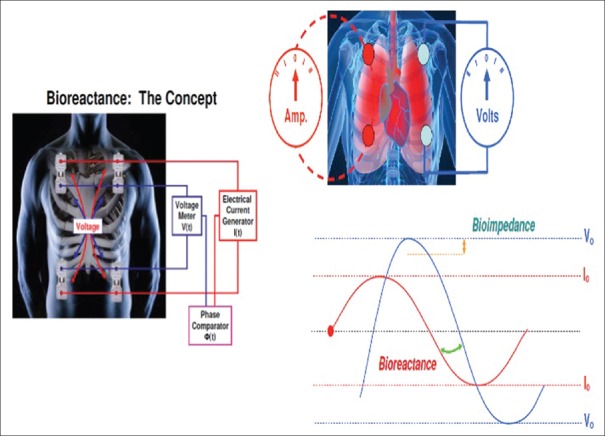
A modification of thoracic bioimpedance known as bioreactance, which refers to the overall sum of electrical resistance, capacitive and inductive properties of blood, and biological tissues has been introduced (NICOM Reliant system, Cheetah Medical Ltd., Maidenhead, Berkshire, UK). Phase shifts are induced between an applied electrical current and the resulting voltage signal due to bioreactance. Thus, in the bioreactance technique, oscillating current is delivered across thorax and frequency spectra variations in response to the cyclic blood flow out of the heart are analyzed to calculate SV in contrast to bioimpedance where transthoracic voltage amplitude changes are extrapolated for the same. Almost linear relationship has been found between the phase shifts measured continuously and the blood flow in the aorta [Figure 10]. This approach results in less interference from the patient movement, electrical noise, lead placement, respiratory effort, and body mass index due to a higher signal-to-noise ratio. In addition to CO, it also measures HR, systolic blood pressure, diastolic blood pressure, mean arterial pressure (MAP), SV, SVV, VET, arterial hemoglobin oxygen saturation (SpO2), and thoracic fluid content (TFC). In addition, the system calculates clinical parameters such as cardiac index, SV index, total peripheral resistance (TPR), TPR index (TPRI), cardiac power (CP), CP index, DO2 index, TFC delta (TFCd), and TFCd from baseline based on the above measured parameters or based on a manually entered parameters such as manually entered hemoglobin, manually entered blood oxygenation (SpO2), or manually entered MAP. However, despite impressive battery of data, this technology still has limitations concerning the measured CO accuracy during dynamic conditions which lead to all other derived parameters as information noise in practical clinical settings. In patients undergoing cardiac surgery, the correlation of this technology was not strong during the immediate postoperative period when compared with PAC.[57]
Figure 10.
Bioimpedance, the analysis of transthoracic voltage amplitude changes in response to high-frequency current
Doppler cardiac output monitoring devices
Transthoracic Doppler or esophageal probes can be used noninvasively to estimate CO; the latter was first introduced in the 1970s for this purpose. The ultrasound emitted by the probe is reflected and has frequency shift depending on the velocity of red blood cells in the descending aorta. The velocity of blood is calculated using the equation v = speed of sound × cosθ × transmitted frequency × frequency shift/2 (θ = angle of incidence between the beam and reflecting blood). The stroke distance is calculated by multiplying the red cell velocity by the measured ejection time [Figure 11]. This multiplied by the cross-sectional area (CSA) quantifies the SV that passes through at the level of Doppler interrogation. CSA is derived either by nomogram based on age, weight, and height (Deltex monitor) or directly measured with the transducer (Hemosonic) using M-mode. The actual SV at the level of LV outflow is then estimated by assuming that the descending aorta receives 70% of the total CO. Another noninvasive ultrasound probe USCOM™ (USCOM, Sydney, Australia) measures CO using a suprasternal probe. This has been used in stable ICU patients and has shown good correlation with the PAC.[58,59]
Figure 11.
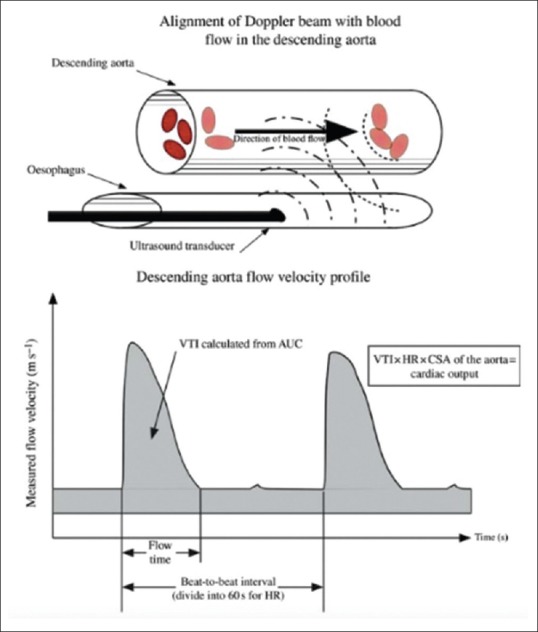
The esophageal aortic Doppler probe into the esophagus manipulated to achieve the optimal velocity-time curve. The velocity time integral is calculated from the area under the curve. The cardiac output is calculated from the product of the velocity time integral, heart rate, and cross-sectional area of the aorta
Although Doppler ultrasound is a noninvasive and easy to set up monitor, it has several limitations which preclude its use in many settings. First, the devices measure blood flow in the descending aorta and then extrapolate that into LV outflow on the assumption that a fixed proportion of flow between the cephalic vessels and the descending aorta exists; however, this may be altered in hemodynamically unstable patients. Moreover, the CSA of the aorta is not fixed, and the use of a nomogram may produce erroneous results. Second, these devices are operator dependent and may require 10–12 insertions for an accurate reading with an inter- and intra-observer variability of 10%–12%.[60,61] Third, the probe position needs to be very accurate and a misalignment of more than 20° can lead to misinterpretations.[60,62]
The overwhelming data in support of esophageal Doppler that showed decrease in mortality and morbidity in various studies led the National Institute for Health and Clinical Excellence to release guidelines in 2011 advocating its use.[63]
Minimally invasive cardiac output monitoring and goal-directed therapy: Clinical relevance
In general, all the MICOM devices target to evaluate SV, either as an absolute value or in terms of relative change. In addition, some of the derived indices depicted on these devices aim to predict fluid responsiveness in terms of SV increase in response to a fluid challenge. In clinical practice; however, the approach should be goal-directed for a particular patient rather than SV per se. There is no single best method for validating MICOM devices. Various studies have used approaches such as graded lower body negative pressure to simulate hemorrhagic hypovolemia in healthy volunteers or comparisons in relatively stable clinical conditions with the gold standard using Bland–Altman analysis.[64] The clinical setting is different from experimental conditions as acute and chronic comorbidities all contribute to significant variation. Clinical situations often demand more information in the dynamic responses to an intervention in place of any absolute values. Till date, there is a paucity of literature to address whether MICOM will provide appropriate information to guide clinical management in the real clinical situations.[64]
Optimal cardiac volume loading is usually considered using the Frank-Starling physiological model of the heart. As per this model, subjects nearer the top of the Frank-Starling curve will not be able to increase their SV any further in response to fluid challenge. However, “a sustained response” and “an appropriate fluid challenge” are difficult to define in the real clinical settings. Various studies have used variety of different bolus volumes with little evidence to justify their choices. The increment in SV to a fluid bolus challenge needs to be sufficient to be distinct from the underlying variation in the patient's CO and from measurement variation. The consequence of these factors would suggest that fluid optimization still remains something of an art and will depend on the clinician who interprets the data.
In an attempt to make fluid responsiveness evaluation more reliable, MICOM devices derived indices such as pulse pressure variation (PPV), SVV, and systolic pressure variation make an estimate of likely fluid responsiveness on the basis of heart-lung interactions. However, all the above indices are inaccurate if the tidal volume and intrathoracic pressures are not constant.[65] Tidal volumes of 8 ml/kg have been suggested as the minimum ventilating volume for improved accuracy, and this may not be possible in all clinical situations.[66] The patient position is another factor that changes the optimal threshold value for the PPV and SVV.[67] The obvious conclusion of these studies is that the clinician should not rely on an isolated value from a CO monitor and the information must be interpreted in the light of proper clinical perspective.
Despite these considerations, there is reasonably strong supportive evidence for MICOM improving patient outcome as a part of optimization protocols, especially in colorectal surgery.[5,6] Goepfert et al. reported reduced complications and length of stay in ICU in cardiac patients with individually optimized hemodynamic therapy using GEDVI derived from transcardiopulmonary thermodilution.[68] Evidence-based handling of the cardiovascular system may be subjective and precise fluid therapy according to a protocol may be difficult to master and will be seen as prejudiced. Hence, the positive outcomes associated with the goal-directed protocols may arise from the individualistic approach to fluid administration. Table 1 summaries some of the GDT protocols and the indices used.[69] Whether the intended endpoints or means to achieve them are based on any particular science remains to be proven. One study found that just less than one-quarter of all surgical procedures meet criteria for minimally invasive monitoring.[66] At present, there are limited data to demonstrate that the benefits seen in the clinical trials actually translate into patients’ benefit in routine clinical practice.[70] Moreover, recent meta-analysis by Joosten et al. challenged the validity of noninvasive methods over PAC. They reported a huge percentage error with noninvasive technique (of 47%) which is much higher than the acceptable limit of 30%.[71] Using techniques that are not even within the acceptable limit of accuracy (when the utility of standard method is itself questionable), puts these noninvasive techniques under a lot of scrutiny. This indicates that when CO monitoring is obligatory, it is better to use PAC rather than relying on these noninvasive techniques.
Table 1.
Recommended indices to direct goal-directed therapy with the use of various minimally invasive cardiac output devices
| MICO device | Indices used for GDT | Variation recommended | Intervention recommended |
|---|---|---|---|
| Esophageal Doppler | FTc | FTc <0.35 s | 200 ml fluid challenge over 10 min |
| SV | SV increase >10% | ||
| Vigileo-FloTrac system (In OR, PPV tidal volume >8 ml/kg) | PPV | PPV/SVV >13% | 200 ml fluid challenge over 15 min |
| SVV | SV increase >10% | ||
| Pulse oximeter pleth variability | PVI | PVI >15% for >5 min | 200 ml fluid challenge over 15 min |
| Vigileo-FloTrac system (GDT group) | SVV | SVV >12% | 250 ml of 5% albumin bolus, may repeat up to 20 ml/kg |
| CO | Monitor CO change | ||
| NICE protocol by the National Health Service in the UK | SV |
SV >10% by 200-250 fluid challenge over 5-10 min | Give volume |
| BP | SV<10% by 200-250 fluid challenge over 5–10 min | Give inotropes/no fluids | |
| Central venous saturation (ScvO2) monitoring protocol | ScvO2 | ScvO2 <70% | If SVV >12% give fluids |
| SaO2 | SaO2 >95% | If SVV <10% give inotrope | |
| Hb | Hb >10 g % | ||
| P (v-a) CO2 | P (v-a) CO2 >6 mmHg | ||
| SVV |
CO: Cardiac output, MICO: Minimally invasive cardiac output, GDT: Goal-directed therapy, FTc: Corrected flow time, SV: Stroke volume, PPV: Pulse pressure variation, SVV: SV variation, PVI: Pleth variability index, BP: Blood pressure, OR: Odds ratio, NICE: National Institute for Health and Clinical Excellence, Hb: Hemoglobin
Although the available evidence for the MICOM appears to be conflicting, at least the monitoring appears to be safe with a catheter-related infection rate of only 0.78% with femoral arterial cannulation for transpulmonary thermodilution measurement that is comparable with routine intensive care monitoring.[72] In comparison, the complication rate following PAC insertion is between 4% and 10%, PAC-related bacteremia occurring in 0.7%–1.8%, and arrhythmias requiring treatment in 3%.[73,74,75]
Conclusion
It is obvious from the existing literature that different CO monitoring techniques have their own limitations. Hence, no single device can meet all clinical requirements. Different devices may be used as an integrative model along a typical clinical patient trail based on their invasiveness and the need for additional hemodynamic data. We need further evidence of complications and accuracy of MICOM devices when used in routine practice. As a supplement to the medical history, clinical examination, and other monitoring modalities, MICOM devices add numerical trend information for anesthesiologists and intensivists to use in determining the most appropriate management of their patients. They can facilitate the delivery of personalized fluid regimes with the goal of improving patient outcomes. These devices, at present, do not completely prevent but do progressively confine the use of the PAC. As with any device or piece of equipment, there is variability in the quality of the measured and derived data. What really matters for all these devices and for devices, in general, is how the generated data are interpreted by the people that use them.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Reuter DA, Goetz AE. Arterial pulse contour analysis: Applicability to clinical routine. In: Pinsky MR, Payen D, editors. Functional Hemodynamic Monitoring. Update in Intensive Care and Emergency Medicine. Vol. 42. New York: Springer-Verlag; 2005. pp. 175–81. [Google Scholar]
- 2.Headley JM. Pulses, pressure, and flow: Emerging trends in less invasive cardiovascular monitoring. AACN News. 2005;22:14–7. [Google Scholar]
- 3.Cecconi M, Rhodes A. Within five years cardiac output monitoring will be included in the minimum monitoring standards for major surgery. Bull R Coll Anaesth. 2012;76:31–3. [Google Scholar]
- 4.Price JD, Sear JW, Venn RM. Perioperative fluid volume optimization following proximal femoral fracture. Cochrane Database Syst Rev. 2004;(1):CD003004. doi: 10.1002/14651858.CD003004.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Giglio MT, Marucci M, Testini M, Brienza N. Goal-directed haemodynamic therapy and gastrointestinal complications in major surgery: A meta-analysis of randomized controlled trials. Br J Anaesth. 2009;103:637–46. doi: 10.1093/bja/aep279. [DOI] [PubMed] [Google Scholar]
- 6.Abbas SM, Hill AG. Systematic review of the literature for the use of oesophageal Doppler monitor for fluid replacement in major abdominal surgery. Anaesthesia. 2008;63:44–51. doi: 10.1111/j.1365-2044.2007.05233.x. [DOI] [PubMed] [Google Scholar]
- 7.Pearse R, Dawson D, Fawcett J, Rhodes A, Grounds RM, Bennett ED, et al. Early goal-directed therapy after major surgery reduces complications and duration of hospital stay. A randomised, controlled trial [ISRCTN38797445] Crit Care. 2005;9:R687–93. doi: 10.1186/cc3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fick A. On the measurement of blood mass in the heart ventricules. Sitzber Physik Med Ges Wurzburg. 1870;36:16–28. [Google Scholar]
- 9.Gueret G, Kiss G, Rossignol B, Bezon E, Wargnier JP, Miossec A, et al. Cardiac output measurements in off-pump coronary surgery: Comparison between NICO and the Swan-Ganz catheter. Eur J Anaesthesiol. 2006;23:848–54. doi: 10.1017/S0265021506000573. [DOI] [PubMed] [Google Scholar]
- 10.Pinsky MR. Hemodynamic evaluation and monitoring in the ICU. Chest. 2007;132:2020–9. doi: 10.1378/chest.07-0073. [DOI] [PubMed] [Google Scholar]
- 11.Button D, Weibel L, Reuthebuch O, Genoni M, Zollinger A, Hofer CK, et al. Clinical evaluation of the FloTrac/Vigileo system and two established continuous cardiac output monitoring devices in patients undergoing cardiac surgery. Br J Anaesth. 2007;99:329–36. doi: 10.1093/bja/aem188. [DOI] [PubMed] [Google Scholar]
- 12.Nishikawa T, Dohi S. Errors in the measurement of cardiac output by thermodilution. Can J Anaesth. 1993;40:142–53. doi: 10.1007/BF03011312. [DOI] [PubMed] [Google Scholar]
- 13.Jain M, Canham M, Upadhyay D, Corbridge T. Variability in interventions with pulmonary artery catheter data. Intensive Care Med. 2003;29:2059–62. doi: 10.1007/s00134-003-1924-7. [DOI] [PubMed] [Google Scholar]
- 14.Buhre W, Rossaint R. Perioperative management and monitoring in anaesthesia. Lancet. 2003;362:1839–46. doi: 10.1016/S0140-6736(03)14905-7. [DOI] [PubMed] [Google Scholar]
- 15.Ahmed H, Kaufman D, Zenilman ME. A knot in the heart. Am Surg. 2008;74:235–6. [PubMed] [Google Scholar]
- 16.Chen LC, Huang PH. Entrapment of a Swan-Ganz catheter. J Chin Med Assoc. 2007;70:213–4. doi: 10.1016/S1726-4901(09)70360-0. [DOI] [PubMed] [Google Scholar]
- 17.George RB, Olufolabi AJ, Muir HA. Critical arrhythmia associated with pulmonary artery catheterization in a parturient with severe pulmonary hypertension. Can J Anaesth. 2007;54:486–7. doi: 10.1007/BF03022037. [DOI] [PubMed] [Google Scholar]
- 18.Bremer HC, Kreisel W, Roecker K, Dreher M, Koenig D, Kurz-Schmieg AK, et al. Phosphodiesterase 5 inhibitors lower both portal and pulmonary pressure in portopulmonary hypertension: A case report. J Med Case Rep. 2007;1:46. doi: 10.1186/1752-1947-1-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rex S, Busch T, Vettelschoss M, de Rossi L, Rossaint R, Buhre W, et al. Intraoperative management of severe pulmonary hypertension during cardiac surgery with inhaled iloprost. Anesthesiology. 2003;99:745–7. doi: 10.1097/00000542-200309000-00033. [DOI] [PubMed] [Google Scholar]
- 20.Rex S, Schaelte G, Metzelder S, Flier S, de Waal EE, Autschbach R, et al. Inhaled iloprost to control pulmonary artery hypertension in patients undergoing mitral valve surgery: A prospective, randomized-controlled trial. Acta Anaesthesiol Scand. 2008;52:65–72. doi: 10.1111/j.1399-6576.2007.01476.x. [DOI] [PubMed] [Google Scholar]
- 21.He Q, Feng Z, Wang JH, Tang PX, Chang ZG, Liu YL, et al. Influence of the venous catheter site on data of pulse indicator continuous cardiac output monitoring. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2009;21:601–3. [PubMed] [Google Scholar]
- 22.Michard F. Looking at transpulmonary thermodilution curves: The cross-talk phenomenon. Chest. 2004;126:656–7. doi: 10.1378/chest.126.2.656-a. [DOI] [PubMed] [Google Scholar]
- 23.Goedje O, Hoeke K, Lichtwarck-Aschoff M, Faltchauser A, Lamm P, Reichart B, et al. Continuous cardiac output by femoral arterial thermodilution calibrated pulse contour analysis: Comparison with pulmonary arterial thermodilution. Crit Care Med. 1999;27:2407–12. doi: 10.1097/00003246-199911000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Buhre W, Weyland A, Kazmaier S, Hanekop GG, Baryalei MM, Sydow M, et al. Comparison of cardiac output assessed by pulse-contour analysis and thermodilution in patients undergoing minimally invasive direct coronary artery bypass grafting. J Cardiothorac Vasc Anesth. 1999;13:437–40. doi: 10.1016/s1053-0770(99)90216-1. [DOI] [PubMed] [Google Scholar]
- 25.Marx G, Schuerholz T, Sümpelmann R, Simon T, Leuwer M. Comparison of cardiac output measurements by arterial trans-cardiopulmonary and pulmonary arterial thermodilution with direct Fick in septic shock. Eur J Anaesthesiol. 2005;22:129–34. doi: 10.1017/s0265021505000244. [DOI] [PubMed] [Google Scholar]
- 26.Della Rocca G, Costa MG, Coccia C, Pompei L, Di Marco P, Vilardi V, et al. Cardiac output monitoring: Aortic transpulmonary thermodilution and pulse contour analysis agree with standard thermodilution methods in patients undergoing lung transplantation. Can J Anaesth. 2003;50:707–11. doi: 10.1007/BF03018714. [DOI] [PubMed] [Google Scholar]
- 27.Böck J, Deuflhard P, Hoeft A, Korb H, Wolpers HG, Steinmann J, et al. Thermal recovery after passage of the pulmonary circulation assessed by deconvolution. J Appl Physiol (1985) 1988;64:1210–6. doi: 10.1152/jappl.1988.64.3.1210. [DOI] [PubMed] [Google Scholar]
- 28.Beattie C, Moores C, Thomson AJ, Nimmo AF. The effect of anaesthesia and aortic clamping on cardiac output measurement using arterial pulse power analysis during aortic aneurysm repair. Anaesthesia. 2010;65:1194–9. doi: 10.1111/j.1365-2044.2010.06558.x. [DOI] [PubMed] [Google Scholar]
- 29.von Spiegel T, Hoeft A. Transpulmonary indicator methods in intensive medicine. Anaesthesist. 1998;47:220–8. doi: 10.1007/s001010050550. [DOI] [PubMed] [Google Scholar]
- 30.Baulig W, Bernhard EO, Bettex D, Schmidlin D, Schmid ER. Cardiac output measurement by pulse dye densitometry in cardiac surgery. Anaesthesia. 2005;60:968–73. doi: 10.1111/j.1365-2044.2005.04296.x. [DOI] [PubMed] [Google Scholar]
- 31.Hori T, Yamamoto C, Yagi S, Iida T, Taniguchi K, Hasegawa T, et al. Assessment of cardiac output in liver transplantation recipients. Hepatobiliary Pancreat Dis Int. 2008;7:362–6. [PubMed] [Google Scholar]
- 32.Reuter DA, Huang C, Edrich T, Shernan SK, Eltzschig HK. Cardiac output monitoring using indicator-dilution techniques: Basics, limits, and perspectives. Anesth Analg. 2010;110:799–811. doi: 10.1213/ANE.0b013e3181cc885a. [DOI] [PubMed] [Google Scholar]
- 33.Rhodes A, Sunderland R. Arterial pulse pressure analysis: The LiDCOplus system. In: Pinsky MR, Payen D, editors. Functional Hemodynamic Monitoring Update in Intensive Care and Emergency Medicine. Berlin: Springer; 2005. pp. 183–92. [Google Scholar]
- 34.Costa MG, Della Rocca G, Chiarandini P, Mattelig S, Pompei L, Barriga MS, et al. Continuous and intermittent cardiac output measurement in hyperdynamic conditions: Pulmonary artery catheter vs. lithium dilution technique. Intensive Care Med. 2008;34:257–63. doi: 10.1007/s00134-007-0878-6. [DOI] [PubMed] [Google Scholar]
- 35.Mora B, Ince I, Birkenberg B, Skhirtladze K, Pernicka E, Ankersmit HJ, et al. Validation of cardiac output measurement with the liDCO™ pulse contour system in patients with impaired left ventricular function after cardiac surgery. Anaesthesia. 2011;66:675–81. doi: 10.1111/j.1365-2044.2011.06754.x. [DOI] [PubMed] [Google Scholar]
- 36.Broch O, Renner J, Höcker J, Gruenewald M, Meybohm P, Schöttler J, et al. Uncalibrated pulse power analysis fails to reliably measure cardiac output in patients undergoing coronary artery bypass surgery. Crit Care. 2011;15:R76. doi: 10.1186/cc10065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frank O. Die Grundform des arteriellen pulses. Erste Abh Mathematische Analyse Z Biol. 1899;37:485–526. [Google Scholar]
- 38.Erlanger J, Hooker DR. An experimental study of blood pressure and of pulse – Pressure in man. Johns Hopkins Hosp Rep. 1904;12:145. [Google Scholar]
- 39.Van Lieshout JJ, Wesseling KH. Editorial II: Continuous cardiac output by pulse contour analysis? Br J Anaesth. 2001;86:467–8. doi: 10.1093/bja/86.4.467. [DOI] [PubMed] [Google Scholar]
- 40.Wesseling KH, de Wit B, Weber JAP, Smith NT. A simple device for the continuous measurement of cardiac output. Adv Cardiovasc Phys. 1983;5:16–52. [Google Scholar]
- 41.Breukers RM, Sepehrkhouy S, Spiegelenberg SR, Groeneveld AB. Cardiac output measured by a new arterial pressure waveform analysis method without calibration compared with thermodilution after cardiac surgery. J Cardiothorac Vasc Anesth. 2007;21:632–5. doi: 10.1053/j.jvca.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 42.Mayer J, Boldt J, Poland R, Peterson A, Manecke GR., Jr Continuous arterial pressure waveform-based cardiac output using the floTrac/Vigileo: A review and meta-analysis. J Cardiothorac Vasc Anesth. 2009;23:401–6. doi: 10.1053/j.jvca.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 43.Khwannimit B, Bhurayanontachai R. Prediction of fluid responsiveness in septic shock patients: Comparing stroke volume variation by FloTrac/Vigileo and automated pulse pressure variation. Eur J Anaesthesiol. 2012;29:64–9. doi: 10.1097/EJA.0b013e32834b7d82. [DOI] [PubMed] [Google Scholar]
- 44.Monnet X, Anguel N, Jozwiak M, Richard C, Teboul JL. Third-generation floTrac/Vigileo does not reliably track changes in cardiac output induced by norepinephrine in critically ill patients. Br J Anaesth. 2012;108:615–22. doi: 10.1093/bja/aer491. [DOI] [PubMed] [Google Scholar]
- 45.Jeong YB, Kim TH, Roh YJ, Choi IC, Suh JH. Comparison of uncalibrated arterial pressure waveform analysis with continuous thermodilution cardiac output measurements in patients undergoing elective off-pump coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 2010;24:767–71. doi: 10.1053/j.jvca.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 46.Kusaka Y, Yoshitani K, Irie T, Inatomi Y, Shinzawa M, Ohnishi Y, et al. Clinical comparison of an echocardiograph-derived versus pulse counter-derived cardiac output measurement in abdominal aortic aneurysm surgery. J Cardiothorac Vasc Anesth. 2012;26:223–6. doi: 10.1053/j.jvca.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 47.Martina JR, Westerhof BE, van Goudoever J, de Beaumont EM, Truijen J, Kim YS, et al. Noninvasive continuous arterial blood pressure monitoring with nexfin®. Anesthesiology. 2012;116:1092–103. doi: 10.1097/ALN.0b013e31824f94ed. [DOI] [PubMed] [Google Scholar]
- 48.Tibby SM, Hatherill M, Marsh MJ, Morrison G, Anderson D, Murdoch IA, et al. Clinical validation of cardiac output measurements using femoral artery thermodilution with direct fick in ventilated children and infants. Intensive Care Med. 1997;23:987–91. doi: 10.1007/s001340050443. [DOI] [PubMed] [Google Scholar]
- 49.Trepte C, Haas S, Meyer N, Gebhardt M, Goepfert MS, Goetz AE, et al. Effects of one-lung ventilation on thermodilution-derived assessment of cardiac output. Br J Anaesth. 2012;108:922–8. doi: 10.1093/bja/aes032. [DOI] [PubMed] [Google Scholar]
- 50.Sakka SG, Hanusch T, Thuemer O, Wegscheider K. The influence of venovenous renal replacement therapy on measurements by the transpulmonary thermodilution technique. Anesth Analg. 2007;105:1079–82. doi: 10.1213/01.ane.0000280440.08530.fb. [DOI] [PubMed] [Google Scholar]
- 51.Kiefer N, Hofer CK, Marx G, Geisen M, Giraud R, Siegenthaler N, et al. Clinical validation of a new thermodilution system for the assessment of cardiac output and volumetric parameters. Crit Care. 2012;16:R98. doi: 10.1186/cc11366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nyboer J. Plethysmography. Impedance. In: Glasser O, editor. Medical Physics. Vol. 2. Chicago, IL: Year Book Publication; 1950. pp. 736–43. [Google Scholar]
- 53.Kubicek WG, Karnegis JN, Patterson RP, Witsoe DA, Mattson RH. Development and evaluation of an impedance cardiac output system. Aerosp Med. 1966;37:1208–12. [PubMed] [Google Scholar]
- 54.de Waal EE, Konings MK, Kalkman CJ, Buhre WF. Assessment of stroke volume index with three different bioimpedance algorithms: Lack of agreement compared to thermodilution. Intensive Care Med. 2008;34:735–9. doi: 10.1007/s00134-007-0938-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gujjar AR, Muralidhar K, Banakal S, Gupta R, Sathyaprabha TN, Jairaj PS, et al. Non-invasive cardiac output by transthoracic electrical bioimpedence in post-cardiac surgery patients: Comparison with thermodilution method. J Clin Monit Comput. 2008;22:175–80. doi: 10.1007/s10877-008-9119-y. [DOI] [PubMed] [Google Scholar]
- 56.Peyton PJ, Chong SW. Minimally invasive measurement of cardiac output during surgery and critical care: A meta-analysis of accuracy and precision. Anesthesiology. 2010;113:1220–35. doi: 10.1097/ALN.0b013e3181ee3130. [DOI] [PubMed] [Google Scholar]
- 57.Guzzi L, Jaffe MB, Orr JA. Clinical evaluation of a new non-invasive method of cardiac output measurement – Preliminary results in CABG patients. Anesthesiology. 1998;89:A543. [Google Scholar]
- 58.Tan HL, Pinder M, Parsons R, Roberts B, van Heerden PV. Clinical evaluation of USCOM ultrasonic cardiac output monitor in cardiac surgical patients in Intensive Care Unit. Br J Anaesth. 2005;94:287–91. doi: 10.1093/bja/aei054. [DOI] [PubMed] [Google Scholar]
- 59.Chand R, Mehta Y, Trehan N. Cardiac output estimation with a new Doppler device after off-pump coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 2006;20:315–9. doi: 10.1053/j.jvca.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 60.Lefrant JY, Bruelle P, Aya AG, Saïssi G, Dauzat M, de La Coussaye JE, et al. Training is required to improve the reliability of esophageal Doppler to measure cardiac output in critically ill patients. Intensive Care Med. 1998;24:347–52. doi: 10.1007/s001340050578. [DOI] [PubMed] [Google Scholar]
- 61.Valtier B, Cholley BP, Belot JP, de la Coussaye JE, Mateo J, Payen DM, et al. Noninvasive monitoring of cardiac output in critically ill patients using transesophageal Doppler. Am J Respir Crit Care Med. 1998;158:77–83. doi: 10.1164/ajrccm.158.1.9707031. [DOI] [PubMed] [Google Scholar]
- 62.Freund PR. Transesophageal Doppler scanning versus thermodilution during general anesthesia. An initial comparison of cardiac output techniques. Am J Surg. 1987;153:490–4. doi: 10.1016/0002-9610(87)90800-2. [DOI] [PubMed] [Google Scholar]
- 63.Ghosh S, Arthur B, Klein AA. NICE guidance on cardioQ(TM) oesophageal Doppler monitoring. Anaesthesia. 2011;66:1081–3. doi: 10.1111/j.1365-2044.2011.06967.x. [DOI] [PubMed] [Google Scholar]
- 64.Chikhani M, Moppett IK. Minimally invasive cardiac output monitoring: What evidence do we need? Br J Anaesth. 2011;106:451–3. doi: 10.1093/bja/aer056. [DOI] [PubMed] [Google Scholar]
- 65.Pinsky MR. Probing the limits of arterial pulse contour analysis to predict preload responsiveness. Anesth Analg. 2003;96:1245–7. doi: 10.1213/01.ANE.0000055821.40075.38. [DOI] [PubMed] [Google Scholar]
- 66.Maguire S, Rinehart J, Vakharia S, Cannesson M. Technical communication: Respiratory variation in pulse pressure and plethysmographic waveforms: Intraoperative applicability in a North American academic center. Anesth Analg. 2011;112:94–6. doi: 10.1213/ANE.0b013e318200366b. [DOI] [PubMed] [Google Scholar]
- 67.Biais M, Bernard O, Ha JC, Degryse C, Sztark F. Abilities of pulse pressure variations and stroke volume variations to predict fluid responsiveness in prone position during scoliosis surgery. Br J Anaesth. 2010;104:407–13. doi: 10.1093/bja/aeq031. [DOI] [PubMed] [Google Scholar]
- 68.Goepfert MS, Richter HP, Zu Eulenburg C, Gruetzmacher J, Rafflenbeul E, Roeher K, et al. Individually optimized hemodynamic therapy reduces complications and length of stay in the Intensive Care Unit: A prospective, randomized controlled trial. Anesthesiology. 2013;119:824–36. doi: 10.1097/ALN.0b013e31829bd770. [DOI] [PubMed] [Google Scholar]
- 69.Suehiro K, Joosten A, Alexender B, Cannesson M. Fluid resuscitation and management in anesthesia: Guiding goal directed therapy. Curr Anesthesiol Rep. 2014;4:360–75. [Google Scholar]
- 70.Saugel B, Cecconi M, Wagner JY, Reuter DA. Noninvasive continuous cardiac output monitoring in perioperative and intensive care medicine. Br J Anaesth. 2015;114:562–75. doi: 10.1093/bja/aeu447. [DOI] [PubMed] [Google Scholar]
- 71.Joosten A, Desebbe O, Suehiro K, Murphy LS, Essiet M, Alexander B, et al. Accuracy and precision of non-invasive cardiac output monitoring devices in perioperative medicine: A systematic review and meta-analysis. Br J Anaesth. 2017;118:298–310. doi: 10.1093/bja/aew461. [DOI] [PubMed] [Google Scholar]
- 72.Belda FJ, Aguilar G, Teboul JL, Pestaña D, Redondo FJ, Malbrain M, et al. Complications related to less-invasive haemodynamic monitoring. Br J Anaesth. 2011;106:482–6. doi: 10.1093/bja/aeq377. [DOI] [PubMed] [Google Scholar]
- 73.Binanay C, Califf RM, Hasselblad V, O’Connor CM, Shah MR, Sopko G, et al. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: The ESCAPE trial. JAMA. 2005;294:1625–33. doi: 10.1001/jama.294.13.1625. [DOI] [PubMed] [Google Scholar]
- 74.Harvey S, Harrison DA, Singer M, Ashcroft J, Jones CM, Elbourne D, et al. Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC-man): A randomised controlled trial. Lancet. 2005;366:472–7. doi: 10.1016/S0140-6736(05)67061-4. [DOI] [PubMed] [Google Scholar]
- 75.Hadian M, Pinsky MR. Evidence-based review of the use of the pulmonary artery catheter: Impact data and complications. Crit Care. 2006;10(Suppl 3):S8. doi: 10.1186/cc4834. [DOI] [PMC free article] [PubMed] [Google Scholar]