Abstract
Background
Sensorineural hearing loss is caused by defects in the inner ear. In the present study, associations between chronic rhinosinusitis, outer hair cell injury, and sensorineural hearing loss were investigated.
Material/Methods
A total of 103 patients who met the inclusion criteria were recruited and allocated into a chronic rhinosinusitis group (n=82) and a simple deviated nasal septum group (n=21). Degree and type of hearing loss, including distortion product otoacoustic emissions, were used to assess the status of cochlear outer hair cells.
Results
The rate of hearing loss in the simple deviated nasal septum group was significantly lower than in the chronic rhinosinusitis group (4.76%, 1/21 vs. 24.39%, 20/82, P<0.05), among which 15 chronic rhinosinusitis patients (75%, 15/20) had hearing loss in the high frequency range. Acoustic stapedial reflexes were elicited in all patients of the 2 groups, while positive Metz was found in 3 chronic rhinosinusitis patients (15%, 3/20). The pass rate of distortion product otoacoustic emissions (DPOAEs) for chronic rhinosinusitis patients was significantly lower than in simple deviated nasal septum patients (88.10% vs. 70.73%, P<0.05). Moreover, the signal-to-noise ratio of DPOAE test results at 704 Hz, 3991 Hz, and 5649 Hz in the chronic rhinosinusitis group were significantly lower than in the simple deviated nasal septum group (P<0.05). Logistic regression analysis revealed a correlation between severity of chronic rhinosinusitis and sensorineural hearing loss (OR=1.39, P<0.05).
Conclusions
Outer hair cell injury and sensorineural hearing loss may have a common cause in chronic rhinosinusitis patients.
MeSH Keywords: Hair Cells, Auditory, Outer; Hearing Loss, Sensorineural; Otoacoustic Emissions, Spontaneous
Background
Chronic rhinosinusitis (CRS) refers to persistent inflammation of the nasal cavity and sinus mucous membranes for more than 12 weeks and is a common and frequently occurring disease found in patients attending the Department of Otolaryngology [1]. The main clinical manifestations of CRS include nasal congestion, runny nose, headache, hyposmia, anosmia, and other chronic symptoms [2]. In addition, inflammation of CRS may also cause ventilation and drainage holdback and the impairment of mucociliary clearance functions [3], which promotes invasion of pathogenic organisms and results in further aggravation of the above-mentioned symptoms [4]. Such a vicious cycle is likely to cause obstruction of the ostiomeatal complex (OMC) and subsequent microbial infection, which in turn leads to decreased oxygen tension in the sinus, retention of secretions, changes in the secretion characteristics and pH values, and increased protein and glucose levels and viscosity [5]. Furthermore, this series of reactions may cause inflammation and dysfunction of the middle ear through the eustachian tube [6]. The invaded pathogenic microbes in the paranasal sinuses and lymphocytes activated by inflammatory reactions may migrate to the inner ear, where the endolymphatic sac induces immune response antigens generated by nasal mucosa-associated lymphoid tissue [7]. The induced immune response can cause immune-mediated inner ear injury and result in sensorineural deafness. In addition, inner ear injury caused by cytokines (such as tumor necrosis factor, TNF) released during inflammation can also produce sudden deafness [8].
Studies have shown that food allergies can elevate total IgE and special IgE (sIgE) levels and cause sudden sensorineural hearing loss in patients with immune-mediated deficiency [9]. The probable mechanism is that externally-activated anticardiolipin (aCL) antibody or its activity of interacting with anti-beta2 GP1 antibody, which are insufficient in the patients with immune-mediated deficiency, can cause outer ear cell (OHC) injury [10]. In addition, diseases such as hyperlipidemia, hypertension, and diabetes are also risk factors for hearing loss [11]. These patients have high viscous blood, abnormal aggregated platelets, and small fat emboli, which can cause the blood vessels connecting to the inner ear to become very thin because of hypoxia. As a result, OHCs may be damaged by insufficient nutrition from blood and lead to hearing impairment [12,13].
The location of hearing impairment can be determined by various hearing tests. The middle ear has a certain volume and contains an ossicular chain retracted by muscles and ligaments, regarded as a special acoustic device. Hearing loss due to middle ear inflammation can be determined by measuring acoustic admittance and impedance, which reflects the physiological and pathological condition of the middle ear [14].
In contrast, otoacoustic emissions (OAEs) are sounds of cochlea’s sensory hair cell origin. OAEs can be recorded in silence, but more commonly are measured in response to acoustic stimulation. Continuous sinusoidal stimuli evoke distortion product otoacoustic emissions (DPOAEs), which can be used to assess the status of cochlear outer hair cells [15]. DPOAEs is a type of OAE examination, which can determine slight hearing loss at high frequencies. Failure of passing the DPOAE test suggests derangement, decreased numbers or loss of OHCs [16].
Therefore, DPOAE is usually applied to judge whether the hearing loss caused by tympanitis, sinusitis or other types of inflammation results from OHCs impairment. In addition to the above-mentioned hearing tests, the auditory brainstem response (ABR) is generally used to assess functional changes in auditory pathway integrity by analyzing auditory evoked potentials in the brain induced by external click stimuli [17].
To date, there is no definitive clinical proof of the utility of performing hearing tests for sensorineural hearing loss in CRS patients without complaints of hearing loss. The aim of the present study was to investigate the location and possible mechanisms of sensorineural hearing loss by performing hearing tests and measuring various biochemical immunological indexes in CRS patients.
Material and Methods
Participants
This study was conducted at the Otolaryngological Department of the First Affiliated Hospital of Fujian Medical University from March 2013 to February 2014. According to Guidelines for Diagnosis and Treatment of Chronic Rhinosinusitis (Kunming, 2012) and European Position Paper on Rhinosinusitis and Nasal Polyps 2012 (EPOS2012), CRS patients with clinical and post-operational pathological diagnosed CRS with a Lund-Mackey score >0 were recruited as the study group. The patients diagnosed as having simple deviated nasal septum by nasal sinus coronal computed tomography and surgery, in addition to a Lund-Mackey score=0 were recruited as the control group. All patients met the following inclusion criteria: 1) without complaint of hearing loss; 2) with sufficient clinical data; 3) with sufficient diagnostic data (including nasal coronal CT, middle ear CT, medical examination from the department of otolaryngological and hearing tests); and 4) without abnormalities in auricles, dry and unobstructed ear canal and intact tympanic membrane. Patients were excluded if they met any of the following conditions: 1) older than 60 years; 2) with a history of ear disease (such as tympanitis, trauma, perforation of tympanic membrane, ear tumors); 3) with a clear history of exposure to ototoxicity drugs; 4) a history of exposure to prolonged noises; 5) a history of systemic diseases which could affect hearing (such as hypertension, diabetes, nephropathy, thyroid disease, bronchial asthma, autoimmune diseases, anemia, sleep apnea syndrome, cervical spondylosis); 6) a history of infectious diseases which could affect hearing (such as mumps, measles, epidemic cerebrospinal meningitis, scarlet fever, diphtheria, typhoid, rubella, herpes zoster, syphilis); and 7) individuals with special occupations (such as divers, pilots and tunnel operators). As a result, a total of 103 patients who met the inclusion criteria were recruited and divided into the CRS group (n=82) or the simple deviated nasal septum group (n=21). The study was approved by the ethical committee of the First Affiliated Hospital of Fujian Medical University and written informed consent was obtained from all participants and/or their guardians.
Measurement of CRS severity and course
CRS severity was assessed by the Lund-Mackay score (LMS) [18], which was based on coronal CT scanning of patients in the bilateral maxillary sinus, anterior ethmoid sinus, posterior ethmoid sinus, frontal sinus and sphenoid sinus. The complete lucency, partial opacity and complete opacity of fluid accumulated from the sinus was scored as 0, 1, and 2 respectively. Remarkably, the ostiomeatal complex was scored as either 0 or 2 for not being obstructed or obstructed. Therefore, LMS for the right or left sinus ranged from 0~12 with a total score 0~24 for both sides. The CRS course was counted during the collecting history as the number of months from the complaint of onset until visiting the hospital.
Laboratory examinations
Serum total IgE and sIgE
Analyses of serum total immunoglobulin E (IgE) and allergen-specific immunoglobulin E (sIgE) antibodies were performed using an Allergy Screen allergen diagnosis kit (Mediwiss, Moers, Germany) and a matching detection instrument. Detection of sIgE included 10 inhalation allergens (dust mites, dust, mulberry, cat dander, dog dander, cockroaches, amaranth, molds, pollens, and tree nuts) and 9 food allergens (egg white, milk, shrimp, beef, shellfish, crab, mango, cashew, and pineapple). According to operating manual, the intensity of the reagent strip color for each allergen is proportional to the serum specific antibody level, and examination result of sIgE >0.35 IU/mL and total IgE >100.00 IU/mL can be regarded as positive.
Lipid biochemical and Immunological index
Analysis of the lipid biochemical index and immunological index from fasting venous blood was performed with an automatic biochemical analyzer AU5800 (Beckman Coulter, USA) using original reagents. Lipid biochemical indexes included serum total cholesterol (TC, normal range: 3.60–5.69 mmol/L), triglycerides, (TG 0.34–1.7 mmol/L), low-density lipoprotein cholesterol (LDL, <3.64 mmol/L), very low-density lipoprotein cholesterol (VLDLC, <0.78 mol/L), free fatty acids (FFA, 129–769 uq/L), apolipoprotein B (apoB, 0.6–1.1 g/L) and lipoprotein (a) (<300 mg/L). Dyslipidemia was considered if one or more of the above-mentioned lipid biochemical indicators were higher than the normal range. Immunological indexes include immunoglobulin A (IgA, 0.50–4.00 g/L), immunoglobulin G (IgG, 7.0–16.0 g/L), immunoglobulin M (IgM, 0.40–3.00 g/L), antinuclear antibody (ANA, 0–1.0 s/co), anti-Streptolysin O (ASO, 0–408 IU/mL), rheumatoid factor (RF, 0–15 IU/mL) and complement 3 (C3,0.90–1.80 g/L).
Audiology assessments
Audiological assessments
These were performed for each ear of a participant in a sound-treated room, including pure tone audiometry, an acoustic impedance test, tympanometry, acoustic stapedial reflex, distortion product otoacoustic emissions (DPOAE), and the auditory brainstem response (ABR).
Pure tone audiometry
Orbiter 922 Audiometer, TDH250P air-conduction earphone, and B-71 bone-conduction earphones (Madsen, Denmark) were used to test the air- and bone-conduction hearing thresholds at 250 Hz, 500 Hz, 1 kHz, 2 kHz, 4 kHz, and 8 kHz, respectively, according to the criteria of GB/16 403–1996 [19]. If the air- or bone-conduction threshold was ≥25 dB at one of the test frequencies, the patient was considered as suffering from hearing loss; if the curve of air- and bone-conduction threshold declined continually without air-bone gap, the patient was considered to have sensorineural hearing loss.
Tympanometry
A TympStar middle ear analyzer (GSI, US) was used to measure the middle ear functional condition at 226 Hz. The tympanogram was plotted with external auditory canal pressure (in daPa) as the X-axis and acoustic impedance (in mL or cc) as the Y-axis, respectively. As shown in Figure 1, the A-type of tympanogram with ± 100 mmH2O of tympanic pressure, 0.3 mL–1.6 mL of acoustic amplitude and 0.5–1.0 mL of tympanum volume was regarded as normal [9].
Figure 1.
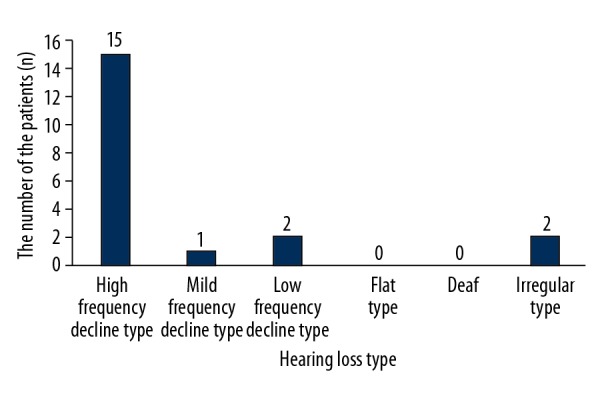
Distribution of CRS patients with sensorineural hearing loss in different hearing loss types.
Acoustic stapedial reflex
A TympStar middle ear analyzer (GSI, US) was used to measure the stapedius muscle reflex ability at 226 Hz. The acoustic reflex threshold of ear(s) on 1 side and 2 sides was tested at 0.5, 1, 2, and 4 kHz. The Metz test is considered to be positive if the difference of threshold between pure tone audiometry and the acoustic stapedial reflex threshold is <60 dB. There was at least 1 positive result of the Metz test at 0.5, 1, 2, or 4 kHz [20].
Distortion Product Optoacoustic (otoacoustic) Emissions (DPOAE)
An otoacoustic emissions instrument (SmartOAE, Intelligent Hearing, USA) with stimulus of 65/50 dB sound pressure level (SPL) (L1/L2), frequency ratio of 1.22 (f2/f1) and acoustic stimulus output of 2f1–f2 was used to conduct DPOAE. The DPOAE audiogram was constructed to record response amplitude, background noise and the signal-noise ratio (SNR) at 9 frequencies: 357, 499, 704, 1003, 1409, 2000, 2822, 3991, and 5649 Hz. Detection standard for each frequency was response amplitude ≥–10 dB SPL, and a signal-noise ratio ≥6 dB was regarded as the threshold of detection at each frequency. A pass in DPOAE required the detection at least 6 of the 9 frequencies [21].
Auditory Brainstem Response (ABR)
A spirit-evoked potential apparatus (Nicolet, USA) was used to record ABR response threshold amplitude in an audiometric test room. The intensity of acoustic stimuli started at 95 dB nHL, and was decreased or increased 10 dB nHL each time. The weakest intensity reproducibly recorded as wave v was set as the threshold of ABR wave v, which was regarded as the ABR response threshold. The test was repeated twice. The latent periods of wave I, III, and V, as well as the interval periods of I–III, III–V, and I–V, were also recorded and analyzed. An ABR response threshold of ≤30 dB nHL was considered to be normal [12].
Statistical analysis
The measurement data, such as DPOAE signal-noise ratio at various frequencies, are reported as the mean ± standard deviation and normally distributed data compared using a t test. The categorical data, such as the rate of hearing loss, DPOAE pass rate, gender, serum total IgE and sIgE (positive vs. negative), blood lipid level (normal vs. abnormal), are shown as percentages and were compared using the χ2 test.
Binary logistic regression analysis was carried out to investigate the correlation between sensorineural hearing loss and the following risk factors: serum total IgE, serum sIgE, blood lipid, immunological factors, age, gender, and CRS severity.
SPSS software (version 20.0, SPSS, IL) was used for all data analysis and a P-value <0.05 was considered to be statistically significant.
Results
Comparison of demographic data and biochemical and immune indexes between the study and control groups
The demographic information and biochemical immunological index of 103 participants are shown in Table 1, of which 82 (57 male and 25 female) and 21 (18 male and 3 female) patients were designated in the CRS group (study group) and the simple deviated nasal septum group (control group), respectively. The median age of the study and control groups was 28 years (13–40) and 26 years (17–40), respectively. In addition, there were 76 patients (92.68%, 76/82) with concurrency of deviated nasal septum in the CRS group. No significant difference was found in demographic data or each biochemical immunological indicator between the study and control groups.
Table 1.
Demographic information and biochemical immunological index of the study and control groups.
| CRS group (study group, n=82) | Simple deviated nasal septum group (control group, n=21) | P-value | |
|---|---|---|---|
| Median age (years, min–max) | 28, 13–40 | 26, 17–40 | 0.139 |
| Male (n,%) | 57, 69.5 | 18, 85.7 | 0.137 |
| Female (n,%) | 25, 30.5 | 3, 14.3 | 0.137 |
| Abnormal blood lipid (n,%) | 21, 25.6 | 3, 14.3 | 0.389 |
| Deviated nasal septum (n,%) | 76, 92.7 | 21, 100.0 | 0.342 |
| TC (mmol/L) (mean, median, min–max) |
4.95±0.67 4.71, 3.92–6.27 |
4.61±1.01 4.61, 3.01–6.51 |
0.406 |
| TG (mmol/L) (mean, median, min–max) |
1.41±0.69 1.20, 0.71–2.87 |
1.06±0.51 0.95, 0.56–2.38 |
0.18 |
| LDL-C (mmol/L) (mean, median, min–max) |
3.23±0.83 3.01, 1.94–4.41 |
2.65±0.80 2.49, 1.78–4.29 |
0.12 |
| VLDLC (mmol/L) (mean, median, min–max) |
0.73±0.36, 0.71, 0.32–1.43 | 0.6±0.27 0.45, 0.35–1.08 |
0.38 |
| FFA (uq/L) (mean, median, min–max) |
348.8±91.96, 350.5, 498–207 | 367.8±152.95 328.5, 582–153 |
0.74 |
| apoB (g/L) (mean, median, min–max) |
0.99±0.26 0.92, 1.38–0.64 |
0.87±0.28 0.80, 1.39–0.56 |
0.30 |
| Lipoprotein(a) (g/L) (mean, median, min–max) |
148.69±108.95 92.85, 338.6–57.9 |
91.41±71.22 71.8, 279–21.4 |
0.18 |
| IgA (g/L) | 2.21±1.06 | 2.10±1.27 | 0.685 |
| IgG (g/L) | 12.39±4.06 | 11.96±5.33 | 0.686 |
| IgM (g/L) | 1.33±0.61 | 1.12±0.41 | 0.139 |
| Number of positive IgE (n,%) | 29, (35.5) | 6, (28.6) | 0.558 |
| Number of positive sIgE (n,%) | 33, (40.2) | 11, (52.4) | 0.316 |
| ANA | 0.42±0.38 | 0.30±0.14 | 0.20 |
| ASO | 109.32±33.06 | 119.45±40.25 | 0.234 |
| RF | 10.28±4.06 | 10.27±5.11 | 0.992 |
| C3 | 1.02±0.56 | 1.01±0.42 | 0.939 |
Tympanometry
The tympanograms of all participants in both the study and control groups were all A-type and the stapes muscle reflex could be elicited in all patients. There was no significant difference of tympanic pressure and the peak values of acoustic compliance between the study and control groups (Table 2).
Table 2.
Comparison of tympanic pressure and peak value of acoustic compliance between the study and control groups.
| CRS group (study group, n=82) | Simple deviated nasal septum group (control group, n=21) | P-P-value | |
|---|---|---|---|
| Tympanic pressure (daPa) | 8.75±9.22 | 9.06±10.52 | 0.894 |
| Acoustic compliance (mL) | 0.52±0.15 | 0.47±0.09 | 0.148 |
Comparison of hearing tests between the study and control groups
As shown in Table 3, sensorineural hearing loss was found in 20 (30 ears) out of 82 patients (164 ears) in the study group (18.3%, 30/164), which was significantly higher than in the control group (2.4%, 1/42, P=0.007). In addition, the DPOAE pass rate of the GRS group was significantly lower than in the control group (70.73%, 48/164 vs. 88.01%, 37/42, P=0.028), suggesting that the cochlear sound-magnifying function was damaged due to cochlear OHCs impairment in some CRS patients. Notably, the SNRs of the CRS group at high frequencies of 3991 Hz and 5649 Hz were significantly lower than in the control group (18.72±8.99 vs. 24.00±7.54, P=0.001 and 15.63±9.64 vs. 20.73±9.63, P=0.003, respectively), suggesting a high-frequency decline type of hearing loss in these CRS patients (Table 4).
Table 3.
Comparison of pure tone audiometry and DPOAE between the study and control groups.
| CRS group (study group, n=164 ears, %) | Simple deviated nasal septum group (control group, n=42 ears, %) | P-P-value | |
|---|---|---|---|
| Rate of hearing loss (n, %) | 30 (18.3) | 1 (2.4) | 0.007 |
| DPOAE pass rate (n, %) | 116 (70.7) | 37 (88.1) | 0.028 |
Table 4.
Comparison of SNRs at specified frequencies between the study and control groups.
| Frequency | Amplitude | CRS group (study group, n=164 ears) | Simple deviated nasal septum group (control group, n=42 ears) | P-value |
|---|---|---|---|---|
| 357 | DP | −1.39±8.46 | 1.56±6.97 | 0.100 |
| NS | −4.68±6.44 | −3.71±5.79 | 0.510 | |
| SNR | 4.34±6.88 | 5.12±7.62 | 0.522 | |
| 499 | DP | 1.94±8.41 | 3.23±5.17 | 0.360 |
| NS | −7.72±8.43 | −8.5±7.83 | 0.690 | |
| SNR | 10.1±7.81 | 12.2±9.04 | 0.171 | |
| 704 | DP | 7.58±10.11 | 12.01±7.89 | 0.030 |
| NS | −6.24±8.91 | −9.33±8.06 | 0.130 | |
| SNR | 15.23±8.92 | 19.23±6.89 | 0.007 | |
| 1003 | DP | 6.99±9.82 | 7.79±8.01 | 0.730 |
| NS | −12.86±8.58 | −12.01±7.77 | 0.710 | |
| SNR | 19.19±7.81 | 21.36±7.63 | 0.108 | |
| 1409 | DP | 4.87±6.91 | 6.23±8.24 | 0.490 |
| NS | −11.75±9.13 | −12.13±7.25 | 0.840 | |
| SNR | 16.96±7.32 | 18.71±8.62 | 0.185 | |
| 2000 | DP | 2.21±7.01 | 3.03±9.02 | 0.710 |
| NS | −14.71±5.96 | −13.44±8.35 | 0.510 | |
| SNR | 15.83±6.1 | 17.67±8.16 | 0.107 | |
| 2822 | DP | 3.94±15.78 | 5.6±5.78 | 0.440 |
| NS | −12.89±13.69 | −13.44±6.21 | 0.780 | |
| SNR | 17.43±17.73 | 17.59±6.41 | 0.954 | |
| 3991 | DP | 4.01±8.03 | 8.27±7.9 | 0.030 |
| NS | −12.23±7.06 | −13.71±6.92 | 0.390 | |
| SNR | 18.72±8.99 | 24±7.54 | 0.001 | |
| 5649 | DP | 2.09±8.23 | 6.48±10.01 | 0.070 |
| NS | −12.36±8.76 | −11.92±9.17 | 0.840 | |
| SNR | 15.63±9.64 | 20.73±9.63 | 0.003 |
NS – noise; DP – distortion product otoacoustic emissions; SNR – signal-to-noise ratio.
Hearing loss has been divided into 6 types, (high-, mid-, and low-frequency decline types, as well as flat-type, deaf-type, and irregular-type). Among the CRS patients with sensorineural hearing loss, 15 (75%) exhibited the high-frequency decline type (Figure 1).
The tympanograms of all participants in both the study and control groups did not reveal significant differences. However, 3 out 20 patients (15%, 3/20) suffering from hearing loss in the CRS group were positive in the Metz test, suggesting impairment of cochlear OHCs in these 3 patients, in contrast to normal middle ear structure in the other CRS patients with hearing loss. Finally, all the participants showed normal results in the ABR test and no difference was found between the study and control groups (Table 5), suggesting intact functions from the retrocochlear auditory nerve to the brainstem.
Table 5.
Comparison of ABR analysis between the study and control groups.
| CRS group (study group, n=164 ears) |
Simple deviated nasal septum group (control group, n=42 ears) |
P-value | |
|---|---|---|---|
| Wave I latent periods (mean, max, min, median, ms) |
1.41±0.21 1.72–1.11, 1.50 |
1.33±0.24 1.85–1.04, 1.23 |
0.21 |
| Wave III latent periods (mean, max, min, median, ms) |
3.47±0.25 4.00–3.09, 3.46 |
3.37±0.20 3.84–3.08, 3.35 |
0.16 |
| Wave V latent periods (mean, max, min, median, ms) |
5.40±0.14 5.70–5.12, 5.41 |
5.32±0.29 5.90–4.83, 5.32 |
0.22 |
| I–III interval (mean, max, min, median, ms) |
2.07±0.21 2.50–1.60, 2.11 |
2.06±0.19 2.36–1.60, 2.10 |
0.78 |
| III–V interval (mean, max, Min, median, ms) |
1.93±0.15 2.19–1.67, 1.88 |
1.94±0.16 2.26–1.67, 1.95 |
0.79 |
| I–V interval (mean, max, min, median, ms) |
3.96±0.28 4.36–3.03, 3.99 |
3.99±0.19 4.30–3.05, 4.04 |
0.74 |
| Response threshold of wave V (mean, max, min, median, dBnHL) |
18.84±2.93 25–15, 20 |
17.50±4.13 30–15, 15 |
0.20 |
To further investigate the relationship between total IgE and sIgE with sensorineural hearing loss in CRS patients, the number of increased (positive detection) and normal levels of total IgE and sIgE in CRS patients with hearing loss was compared with CRS patients without hearing loss. As shown in Table 6, the rate of positive total IgE and sIgE detection in CRS patients with sensorineural hearing loss was not significantly different from those of CRS patients with normal hearing.
Table 6.
Comparison of total IgE and sIgE between patients with and without sensorineural hearing loss in the experimental group.
| Increased level | Normal level | Total patient number | P-value | ||
|---|---|---|---|---|---|
| Total IgE | Hearing loss | 7 | 13 | 20 | 0.596 |
| Normal hearing | 22 | 40 | 62 | ||
| sIgE | Hearing loss | 9 | 11 | 20 | 0.794 |
| Normal hearing | 24 | 38 | 62 |
Logistic regression analysis of correlated factors of sensorineural hearing loss
Logistic regression analysis was carried out to identify factors correlated with sensorineural hearing loss. As shown in Table 7, among the 7 possible factors based on analysis of clinical data, the results indicated a significant correlation between sensorineural hearing loss and the severity of CRS (OR=1.39, P=0.014), but not with the other factors (age, gender, total IgE, sIgE, blood lipid, and course of CRS).
Table 7.
Logistic regression analysis of 7 possible correlated factors of sensorineural hearing loss.
| Factors | OR | P-value |
|---|---|---|
| Age | 0.050 | 0.095 |
| Gender | 0.507 | 0.285 |
| Serum total IgE | 0.736 | 0.679 |
| sIgE | 0.650 | 0.540 |
| Blood lipid | 2.081 | 0.242 |
| CRS severity | 1.39 | 0.014 |
| CRS course (Month) | 1.033 | 0.078 |
Discussion
This study was designed to investigate the characteristics and mechanisms of hearing loss in CRS patients by using a combination of various hearing tests and correlation analysis of possible factors affecting audiology. As far as we are aware, this is the first study to analyze the correlation between sensorineural hearing loss and CRS patients who have not complained of hearing loss.
CRS is clinically defined as chronic inflammation of mucosa of the nasal cavity and paranasal sinuses lasting for ≥12 weeks. Chronic deferment and deterioration of CRS can be caused by many mechanisms, including microbial infection, super-antigen origin, bacterial biofilm, anatomic abnormalities of the nasal cavity and paranasal sinus, allergic reactions, neurogenic origin, dysfunction of the nasal mucociliary system, and other internal and external factors resulting in decreased immunity [22]. In addition, the morphological and/or functional changes of the eustachian tubes of CRS patient caused by inflammation spread, stimulation of inflammatory secretions, and accumulation of paranasal sinus secretions can cause conduction deafness through retrograde infection of the middle ear [23]. A recent population-based study revealed an association between sudden sensorineural hearing loss and CRS, especially in CRS patients <44 years of age [8]. Furthermore, nasosinusitis can be induced by impaired ventilation and drainage in patients with a deviated nasal septum (especially those with high deviation) due to a moved middle turbinate under consistent pressure [24]; therefore, chronic rhinosinusitis is often associated with nasal septum deviation. In the present study, patients with a simple deviated nasal septum and a Lund-Mackey score of 0 were designated as the control group and were compared to the CRS study group to exclude the effects of anatomic abnormality of the nasal structure on audiological alterations [24]. The results of pure tone audiometry combined with acoustic stapedial reflex, DPOAE and ABR tests indicated that the hearing loss in both the study and control groups was sensorineural and excluded the possibilities of conduction deafness and brainstem auditory route abnormalities. The occurrence and rate of otoacoustic emission was dependent on the intact structure of the cochlea and middle ear, which is related to active movement of cochlear OHCs and cochlear active energy release. Combined with results of the DPOAE and Metz tests, the sensorineural hearing loss that occurred in CRS patients was likely caused by impaired functions of cochlear OHCs [25]. In our study, CRS patients had a significantly lower DPOAE pass rate compared with the control group (70.73% vs. 88.10%, P=0.028). Moreover, CRS patients had significant lower SNRs at high frequencies (3991 Hz and 5649 Hz), which was consistent with the high-decline type of hearing loss detected in CRS patients by pure tone audiometry. Unexpectedly, the CRS group also showed significantly lower SNRs at 704 Hz compared with the control group (15.23±8.92 vs. 19.23±6.89, P=0.007). Further studies involving a larger cohort of patients will be required to explore the reason(s) for this phenomenon.
The well-known factors associated with sensorineural hearing loss include congenital deafness, senile deafness, deafness due to infectious diseases or systemic diseases, ototoxic deafness, traumatic deafness, autoimmune deafness, noise-induced deafness and stress-induced deafness. Patients with the above-mentioned diseases were excluded from our study, and logistic regression analysis was performed to investigate the influence of other possible risk factors on sensorineural hearing loss. The results unequivocally demonstrated that the severity of CRS was associated with sensorineural hearing loss (OR=1.39, P<0.05).
To date, the molecular mechanisms of sensorineural hearing loss caused by impaired inner ear structure and functions in CRS patients have not been completely elucidated. Allergic reaction is one important mechanism involved in the development of CRS [9,10] and 40–80% of CRS patients suffer from allergies [26]. Some scholars believe that allergic reactions can increase the release of various inflammatory mediators, resulting in increased vascular permeability and nasal mucosa edema, which leads to obstruction of the paranasal sinus, blockage of sinus drainage, retention of mucoid discharges, propagation of microbial pathogens, and, finally, CRS [27]. Corbo et al. proposed that allergic reactions could retard the nasal mucociliary function of cleaning and cause CRS, based on their study of nasal ciliary beat frequency and mucociliary clearance in 200 patients with allergic rhinitis [22]. There are numerous immunity-related plasma cells, mast cells, and other tissue components capable of producing lysosomes, leukocytes with phagocytosis, and fibrinolysis functions, and myoblasts with repairing functions located in the nasal mucosa lamina propria and submucosal layer [28]. The nasal-associated lymphoid tissue (NALT) is composed of special lymphoid tissue from the nasal cavity to the pharynx, which is called Waldeyer’s ring. It contains affluent antigen presenting cells and CD4+ T cells and is the main site of antigen-specific immune responses after intranasal immunization [29,30]. Therefore, allergic rhinitis can lead to sensorineural hearing loss by affecting the connection between the nasal mucosa and inner ears [31–33]. In our study, the CRS group did not display any significant difference in immunologic indexes compared with the control group, and CRS patients with sensorineural hearing loss had no significant difference in total IgE or sIgE levels compared with CRS patients without sensorineural hearing loss. Therefore, a further study with a larger sample size is warranted to investigate the correlation between sensorineural hearing loss with IgE-mediated type I hypersensitivity and type III hypersensitivity reactions.
In addition to allergic reaction, CRS is also likely to cause sensorineural hearing loss by inner ear function impairment through the release of various regional cytokines. The endolymphatic sac contains abundant complement factors, lymphocytes, and macrophages, and the cytokines and adhesion molecules produced play important roles in the immune responses of the inner ear. Immune responses can be enhanced by TNF-α produced by stimulated macrophages and lymphocytes in response to captured cross-antigens between pathogenic microorganism and body tissues in the endolymphatic sac during nasal cavity paranasal sinus infections [13,18,19,31]. As a consequence, TNF-α can activate the downstream NF-κB, MAPKs, and START pathways and cause apoptosis and degeneration of cells, leading to sensorineural hearing loss. Additionally, TNF-α may also cause an abnormal microcirculation in the inner ear through stimulation of the sphingosine 1-phosphate signaling pathway [34]. Analysis of blood inflammatory factors in patients with idiopathic sudden sensorineural hearing loss (ISSNHL) revealed a significant relationship between TNF-α levels and the outcome of sensorineural hearing loss [35]. Numerous clinical research studies have reported that treatment with TNF-α inhibitors can improve the curative effect of ISSNHL [3,35]. Moreover, IL-6 expression was detected in spiral ligament, stria vascularis, and spiral ganglion neurons of the inner ear [36]. As an important cytokine involved in acute inflammation, IL-6 has dual functions in the proliferation of plasma cells capable of producing antibodies and antioxidants by inducing the anti-apoptosis gene (such as bcl gene family). Previous studies have shown that the expression of IL-6 was decreased during the early stages of the cochlear inflammatory response and can cause ISSNHL through START3 and NF-κB signal pathway activation [37,38]. Tsinaslanidou et al. found that increased IL-6 expression was positively correlated with repair of hair cell functions [39]. During the late stage of the cochlear inflammatory response, the occurrence of ISSNHL caused by impaired inner ear structure and functions can also be related to elevated expression of IL-2 and IL-8. Studies have revealed that IL-2, secreted and released from the endolymphatic sac, can regulate sICAM-1 and cause immunological cells to enter the inner ear through altered venae spiralis modioli characteristics. The resulting fibrotic lesions of inner ear sclerotin may lead to inner ear degenerative diseases and the occurrence of ISSNHL [40,41]. Iguchi and Anniko demonstrated that IL-8 induced by interactions between leucocytes and vascular endothelial cells affected inner ear functions as a result of the accumulation of inflammatory cells [42].
Conclusions
A logistic regression analysis in the present study revealed that the severity of CRS was correlated with sensorineural hearing loss (OR: 1.39, P=0.014). In addition, the DPOAE pass rates were significantly lower in some CRS patients (P=0.028), suggesting that their cochlear sound magnifying function was damaged due to cochlear OHCs impairment. We propose that CRS and impairment of cochlear functions by damaged outer hair cells in sensorineural hearing loss patients might have a common causative factor. The molecular mechanisms remain to be investigated with a larger cohort of patients and analysis of related cytokines in future studies.
Footnotes
Source of support: Departmental sources
Conflict of interests
None.
References
- 1.Benninger MS, Ferguson BJ, Hadley JA, et al. Adult chronic rhinosinusitis: Definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol Head Neck Surg. 2003;129(3 Suppl):S1–S32. doi: 10.1016/s0194-5998(03)01397-4. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharyya N. Clinical and symptom criteria for the accurate diagnosis of chronic rhinosinusitis. Laryngoscope. 2006;116(7 Pt 2 Suppl 110):1–22. doi: 10.1097/01.mlg.0000224508.59725.19. [DOI] [PubMed] [Google Scholar]
- 3.Van Zele T, Claeys S, Gevaert P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006;61(11):1280–89. doi: 10.1111/j.1398-9995.2006.01225.x. [DOI] [PubMed] [Google Scholar]
- 4.Sun Y, Dong Z, Yang Z. [Study on the clearence function of mucociliary system in nasal middle meatus]. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 2002;16(10):530–32. [in Chinese] [PubMed] [Google Scholar]
- 5.Huang M, Yang P, Wang Z. [Clinical, pathological and CT imaging studies of fungal sinusitis]. Chinese Journal of Medical Imaging Technology. 2000;16(8):637–38. [in Chinese] [Google Scholar]
- 6.MacArthur CJ, Hausman F, Kempton JB, et al. Inner ear tissue remodeling and ion homeostasis gene alteration in murine chronic otitis media. Otol Neurotol. 2013;34(2):338–46. doi: 10.1097/MAO.0b013e31827b4d0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gloddek B, Arnold W. The endolymphatic sac receives antigenetic information from the organs of the mucosa-associated lymphatic system. Acta Otolaryngol. 1998;118(3):333–36. doi: 10.1080/00016489850183403. [DOI] [PubMed] [Google Scholar]
- 8.Hung SH, Lin HC, Kao LT, et al. Sudden sensorineural hearing loss is associated with chronic rhinosinusitis: Population-based study. J Laryngol Otol. 2016;130(6):521–25. doi: 10.1017/S0022215116000906. [DOI] [PubMed] [Google Scholar]
- 9.Keles E, Sapmaz E, Godekmerdan A. The role of allergy in the etiopathogenesis of idiopathic sudden sensorineural hearing loss. Eur Arch Otorhinolaryngol. 2013;270(6):1795–801. doi: 10.1007/s00405-012-2189-y. [DOI] [PubMed] [Google Scholar]
- 10.Toubi E, Ben-David J, Kessel A, et al. Immune-mediated disorders associated with idiopathic sudden sensorineural hearing loss. Ann Otol Rhinol Laryngol. 2004;113(6):445–49. doi: 10.1177/000348940411300605. [DOI] [PubMed] [Google Scholar]
- 11.Lee JS, Choi HG, Jang JH, et al. Analysis of predisposing factors for hearing loss in adults. J Korean Med Sci. 2015;30(8):1175–82. doi: 10.3346/jkms.2015.30.8.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirano K, Ikeda K, Kawase T, et al. Prognosis of sudden deafness with special reference to risk factors of microvascular pathology. Auris Nasus Larynx. 1999;26(2):111–15. doi: 10.1016/s0385-8146(98)00072-8. [DOI] [PubMed] [Google Scholar]
- 13.Kojima Y, Ito S, Furuya N. Hearing improvement after therapy for hyperlipidemia in patients with chronic-phase sudden deafness. Ann Otol Rhinol Laryngol. 2001;110(2):105–8. doi: 10.1177/000348940111000202. [DOI] [PubMed] [Google Scholar]
- 14.Hunter LL, Margolis RH. Multifrequency tympanometry: Current clinical application. Am J Audiol. 1992;1(3):33–43. doi: 10.1044/1059-0889.0103.33. [DOI] [PubMed] [Google Scholar]
- 15.Kemp DT. Stimulated acoustic emissions from within the human auditory system. J Acoust Soc Am. 1978;64(5):1386–91. doi: 10.1121/1.382104. [DOI] [PubMed] [Google Scholar]
- 16.Kemp DT. Otoacoustic emissions, their origin in cochlear function, and use. Br Med Bull. 2002;63:223–41. doi: 10.1093/bmb/63.1.223. [DOI] [PubMed] [Google Scholar]
- 17.Zhou X, Cao M. [Bone-conduction auditory brainstem response]. Chinese Scientific Journal of Hearing and Speech Rehabilitation. 2012;(1):31–34. [in Chinese] [Google Scholar]
- 18.Lund VJ, Mackay IS. Staging in rhinosinusitus. Rhinology. 1993;31(4):183–84. [PubMed] [Google Scholar]
- 19.Standardization CTCoa. China National Standard. 1996. Acoustics – Audiometric test methods – Basic pure tone air and bone conduction threshold audiometry GB/T 16403–1996. [Google Scholar]
- 20.Bergenius J, Borg E, Hirsch A. Stapedius reflex test, brainstem audiometry and opto-vestibular tests in diagnosis of acoustic neurinomas. A comparison of test sensitivity in patients with moderate hearing loss. Scand Audiol. 1983;12(1):3–9. doi: 10.3109/01050398309076218. [DOI] [PubMed] [Google Scholar]
- 21.Burke SR, Rogers AR, Neely ST, et al. Influence of calibration method on distortion-product otoacoustic emission measurements: I. test performance. Ear Hear. 2010;31(4):533–45. doi: 10.1097/AUD.0b013e3181d86b3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu HY, Nikolova EB, Beagley KW, Russell MW. Induction of antibody-secreting cells and T-helper and memory cells in murine nasal lymphoid tissue. Immunology. 1996;88(4):493–500. doi: 10.1046/j.1365-2567.1996.d01-690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomiyama S, Harris JP. The role of the endolymphatic sac in inner ear immunity. Acta Otolaryngol. 1987;103(3–4):182–88. [PubMed] [Google Scholar]
- 24.Flint PW, Haughey BH, Lund V, et al. Cummings otolaryngology: Head and neck surgery. Elsevier/Saunders; 2015. [Google Scholar]
- 25.Hopkins K. Deafness in cochlear and auditory nerve disorders. Handb Clin Neurol. 2015;129:479–94. doi: 10.1016/B978-0-444-62630-1.00027-5. [DOI] [PubMed] [Google Scholar]
- 26.Ma C, Billings P, Harris JP, Keithley EM. Characterization of an experimentally induced inner ear immune response. Laryngoscope. 2000;110(3 Pt 1):451–56. doi: 10.1097/00005537-200003000-00024. [DOI] [PubMed] [Google Scholar]
- 27.Harris JP, Woolf NK, Ryan AF. Elaboration of systemic immunity following inner ear immunization. Am J Otolaryngol. 1985;6(3):148–52. doi: 10.1016/s0196-0709(85)80077-6. [DOI] [PubMed] [Google Scholar]
- 28.Yan Z, Wang JB, Gong SS, Huang X. Cell proliferation in the endolymphatic sac in situ after the rat Waldeyer ring equivalent immunostimulation. Laryngoscope. 2003;113(9):1609–14. doi: 10.1097/00005537-200309000-00038. [DOI] [PubMed] [Google Scholar]
- 29.Kuper CF, Koornstra PJ, Hameleers DM, et al. The role of nasopharyngeal lymphoid tissue. Immunol Today. 1992;13(6):219–24. doi: 10.1016/0167-5699(92)90158-4. [DOI] [PubMed] [Google Scholar]
- 30.Tomassen P, Van Zele T, Zhang N, et al. Pathophysiology of chronic rhinosinusitis. Proc Am Thorac Soc. 2011;8(1):115–20. doi: 10.1513/pats.201005-036RN. [DOI] [PubMed] [Google Scholar]
- 31.Lasisi AO, Abdullahi M. The inner ear in patients with nasal allergy. J Natl Med Assoc. 2008;100(8):903–5. doi: 10.1016/s0027-9684(15)31403-6. [DOI] [PubMed] [Google Scholar]
- 32.Lombardi C, Tansini A, Passalacqua G. Seasonal sensorineural hearing loss associated with allergic rhinitis: A case report. J Allergy Clin Immunol. 2006;117(2):468–69. doi: 10.1016/j.jaci.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 33.Singh S, Nagarkar AN, Bansal S, et al. Audiological manifestations of allergic rhinitis. J Laryngol Otol. 2011;125(9):906–10. doi: 10.1017/S0022215111001137. [DOI] [PubMed] [Google Scholar]
- 34.Scherer EQ, Yang J, Canis M, et al. TNFα enhances microvascular tone and reduces blood flow in the cochlea via enhanced S1P signaling. Stroke. 2010;41(11):2618–24. doi: 10.1161/STROKEAHA.110.593327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demirhan E, Eskut NP, Zorlu Y, et al. Blood levels of TNF-alpha, IL-10, and IL-12 in idiopathic sudden sensorineural hearing loss. Laryngoscope. 2013;123(7):1778–81. doi: 10.1002/lary.23907. [DOI] [PubMed] [Google Scholar]
- 36.Fujioka M, Kanzaki S, Okano HJ, et al. Proinflammatory cytokines expression in noise-induced damaged cochlea. J Neurosci Res. 2006;83(4):575–83. doi: 10.1002/jnr.20764. [DOI] [PubMed] [Google Scholar]
- 37.Masuda M, Kanzaki S, Minami S, et al. Correlations of inflammatory biomarkers with the onset and prognosis of idiopathic sudden sensorineural hearing loss. Otol Neurotol. 2012;33(7):1142–50. doi: 10.1097/MAO.0b013e3182635417. [DOI] [PubMed] [Google Scholar]
- 38.Kim MG, Jung YG, Eun YG. Effect of steroid, carbogen inhalation, and lipoprostaglandin E1 combination therapy for sudden sensorineural hearing loss. Am J Otolaryngol. 2011;32(2):91–95. doi: 10.1016/j.amjoto.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 39.Tsinaslanidou Z, Tsaligopoulos M, Angouridakis N, et al. The expression of TNFα, IL-6, IL-2 and IL-8 in the serum of patients with idiopathic sudden sensorineural hearing loss: Possible prognostic factors of response to corticosteroid treatment. Audiology & Neurotology Extra. 2016;6:9–19. [Google Scholar]
- 40.Garcia Berrocal JR, Ramirez-Camacho R. Immune response and immunopathology of the inner ear: An update. J Laryngol Otol. 2000;114(2):101–7. doi: 10.1258/0022215001905021. [DOI] [PubMed] [Google Scholar]
- 41.Garcia Berrocal JR, Ramirez-Camacho R, Vargas JA, Millan I. Does the serological testing really play a role in the diagnosis immune-mediated inner ear disease? Acta Otolaryngol. 2002;122(3):243–48. doi: 10.1080/000164802753648105. [DOI] [PubMed] [Google Scholar]
- 42.Iguchi H, Anniko M. Interleukin 8 can affect inner ear function. J Otorhinolaryngol Relat Spec. 1998;60(4):181–89. doi: 10.1159/000027591. [DOI] [PubMed] [Google Scholar]


